Functionalized Biochar from the Amazonian Residual Biomass Murici Seed: An Effective and Low-Cost Basic Heterogeneous Catalyst for Biodiesel Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Sodium Percentage
2.2. Characterization of Materials
2.2.1. Elemental Analysis (CHNS)
2.2.2. TG/DTG Thermogravimetric Analysis
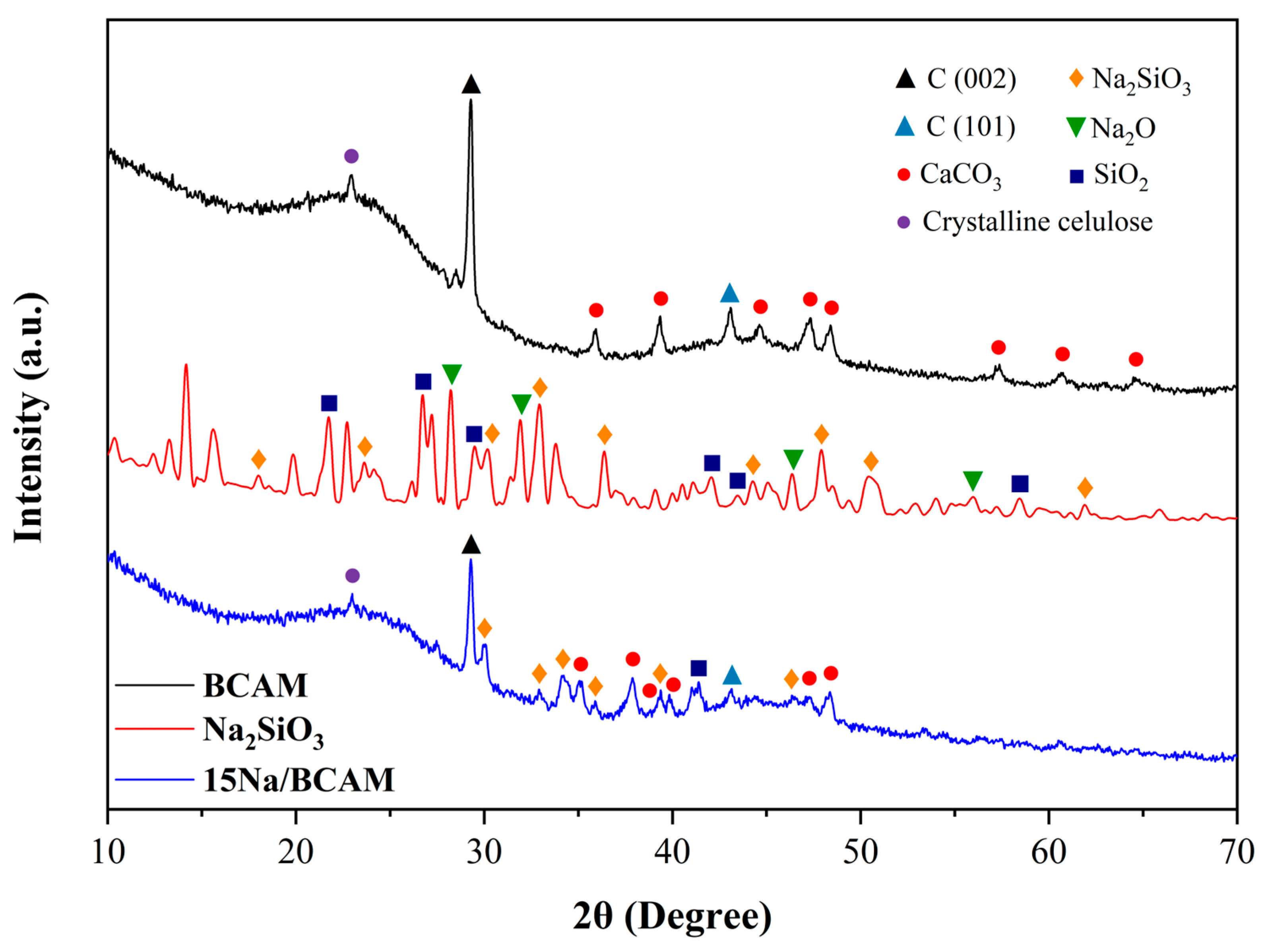
2.2.3. X-ray Diffraction (XRD)
2.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
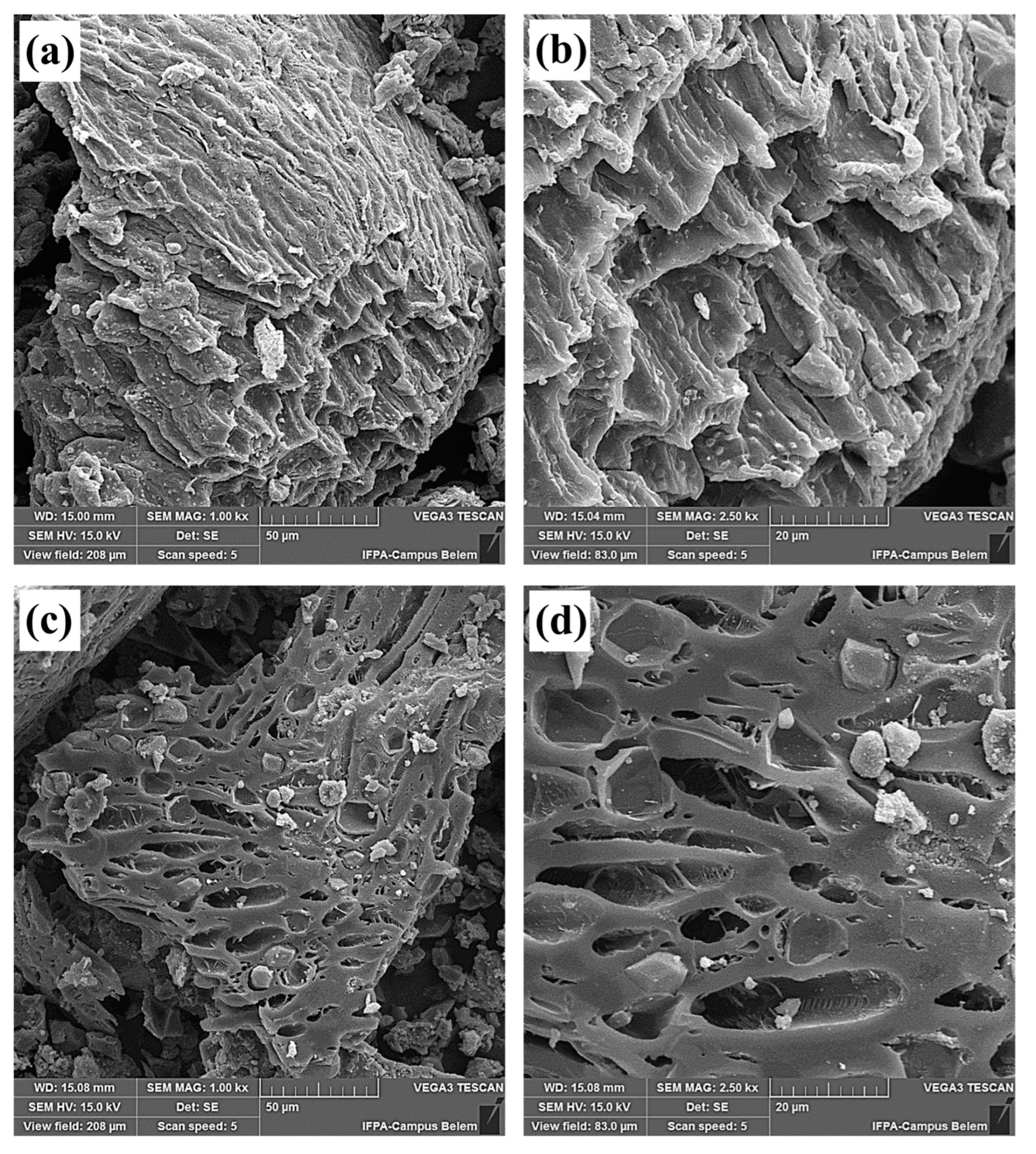
2.2.5. Scanning Electron Microscopy (SEM)
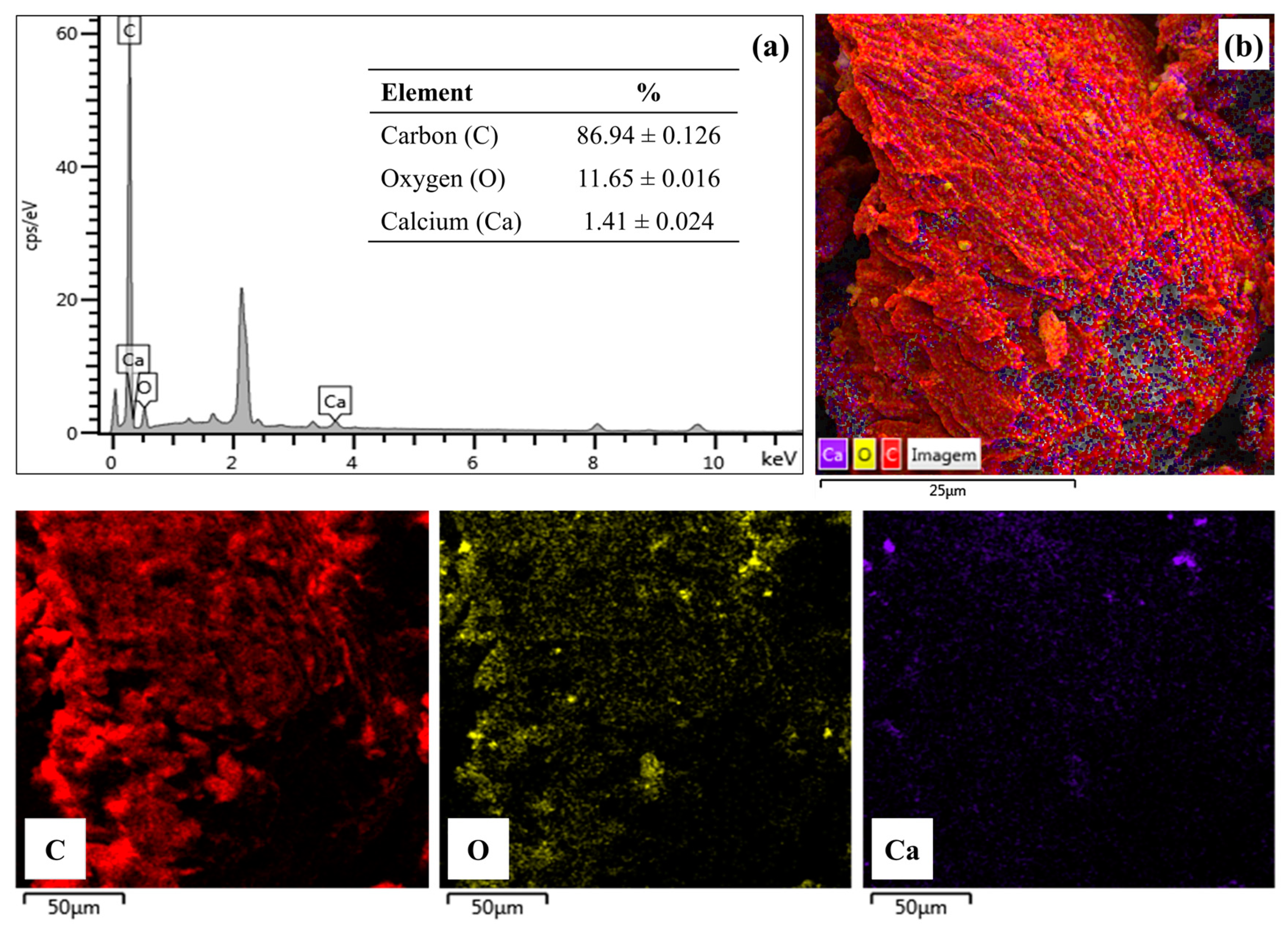
2.2.6. Energy Dispersion X-ray Spectroscopy (EDS)
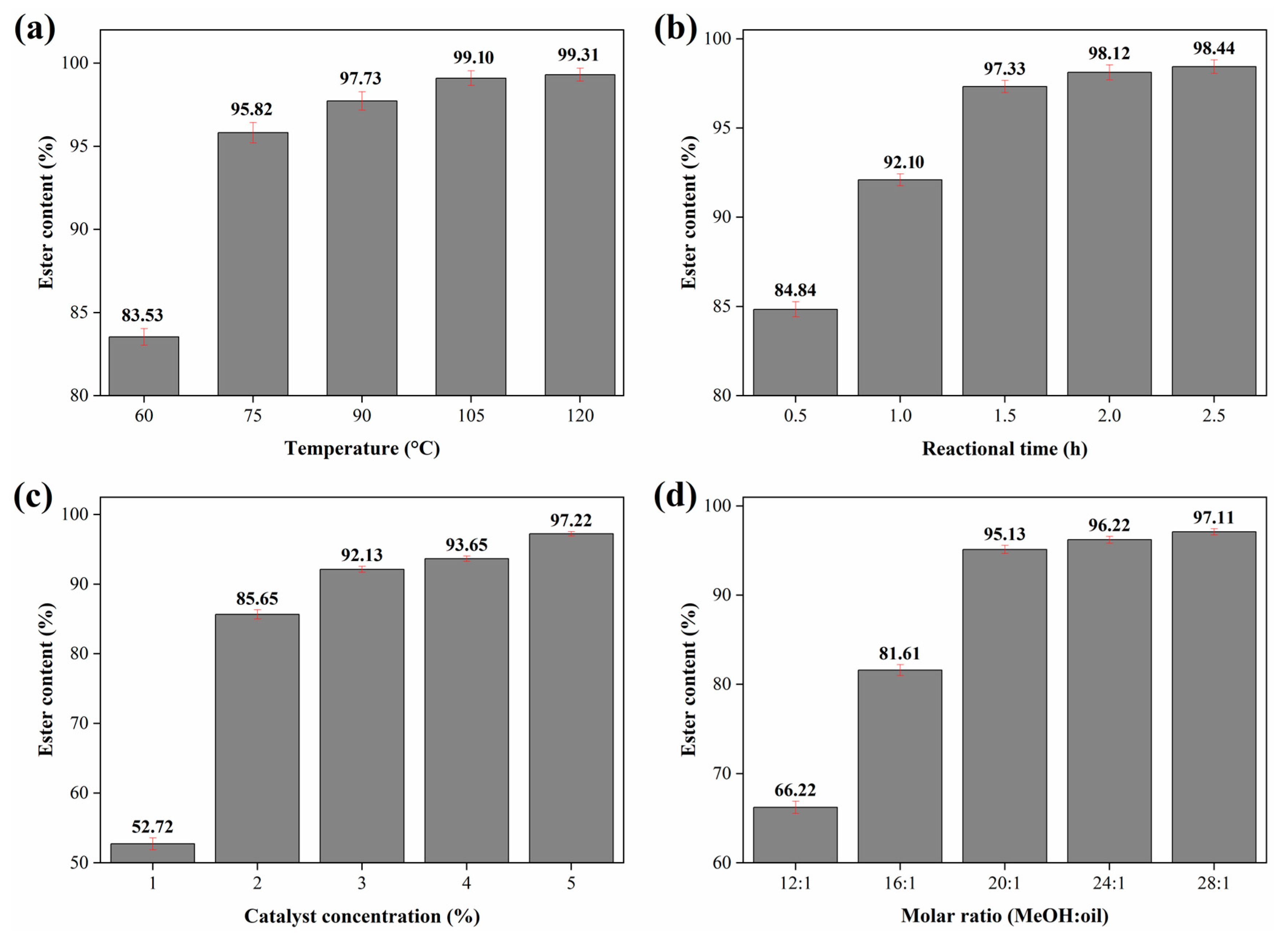
2.3. Study of the Influence of Reaction Parameters for Biodiesel Synthesis
2.4. Physical and Chemical Properties of Biodiesel
2.5. Catalyst Reuse Study
2.6. Catalyst Regeneration Study
2.7. Comparison of Catalyst 15Na/BCAM with Different Biochar-Based Catalysts
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Transesterification Reaction
3.5. Characterization of Biodiesel
3.5.1. Ester Content
3.5.2. Physicochemical Properties of Biodiesel
3.6. Catalyst Recovery
3.7. Catalyst Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, K.S.; Mdlovu, N.V.; Chan, H.Y.; Wu, K.C.W.; Wu, J.C.S.; Huang, Y.T. Preparation and characterization of mesoporous polymer-based solid acid catalysts for biodiesel production via transesterification of palmitic oils. Catal. Today 2022, 397–399, 145–154. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Esterification and transesterification over SrO–ZnO/Al2O3 as a novel bifunctional catalyst for biodiesel production. Renew. Energy 2020, 158, 388–399. [Google Scholar] [CrossRef]
- Soares, S.; Fernandes, G.M.; Moraes, L.M.B.; Batista, A.D.; Rocha, F.R.P. Single-phase determination of calcium and magnesium in biodiesel using smartphone-based digital images. Fuel 2022, 307, 121837. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.H.; Yao, M.; Fang, Z.; Wang, Y.T. Production of biodiesel and hydrogen from plant oil catalyzed by magnetic carbon-supported nickel and sodium silicate. Green Chem. 2016, 18, 3302–3314. [Google Scholar] [CrossRef]
- Bambase, M.E.; Almazan, R.A.R.; Demafelis, R.B.; Sobremisana, M.J.; Dizon, L.S.H. Biodiesel production from refined coconut oil using hydroxide-impregnated calcium oxide by cosolvent method. Renew. Energy 2021, 163, 571–578. [Google Scholar] [CrossRef]
- Sabzevar, A.M.; Ghahramaninezhad, M.; Shahrak, M.N. Enhanced biodiesel production from oleic acid using TiO2-decorated magnetic ZIF-8 nanocomposite catalyst and its utilization for used frying oil conversion to valuable product. Fuel 2021, 288, 119586. [Google Scholar] [CrossRef]
- Rezania, S.; Mahdinia, S.; Oryani, B.; Cho, J.; Kwon, E.E.; Bozorgian, A.; Nodeh, H.R.; Darajeh, N.; Mehranzamir, K. Biodiesel production from wild mustard (Sinapis arvensis) seed oil using a novel heterogeneous catalyst of LaTiO3 nanoparticles. Fuel 2022, 307, 121759. [Google Scholar] [CrossRef]
- Mares, E.K.L.; Gonçalves, M.A.; da Luz, P.T.S.; Rocha Filho, G.N.; Zamian, J.R.; Conceição, L.R.V. Acai seed ash as a novel basic heterogeneous catalyst for biodiesel synthesis: Optimization of the biodiesel production process. Fuel 2021, 299, 120887. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wang, J.; Yang, J.; Fu, L.; Cai, Z.; Fu, Y. A novel in situ transesterification of yellow horn seeds to biodiesel using a dual function switchable solvent. Fuel 2022, 312, 122974. [Google Scholar] [CrossRef]
- Conceição, L.R.V.; Carneiro, L.M.; Giordani, D.S.; Castro, H.F. Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew. Energy 2017, 113, 119–128. [Google Scholar] [CrossRef]
- Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crop. Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Quevedo-Amador, R.A.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Badawi, M.; Bonilla-Petriciolet, A. Functionalized hydrochar-based catalysts for biodiesel production via oil transesterification: Optimum preparation conditions and performance assessment. Fuel 2022, 312, 122731. [Google Scholar] [CrossRef]
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renew. Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Ning, Y.; Niu, S.; Wang, Y.; Zhao, J.; Lu, C. Sono-modified halloysite nanotube with NaAlO2 as novel heterogeneous catalyst for biodiesel production: Optimization via GA_BP neural network. Renew. Energy 2021, 175, 391–404. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M.; et al. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Gonçalves, M.A.; Mares, E.K.L.; da Luz, P.T.S.; Zamian, J.R.; Rocha Filho, G.N.; Conceição, L.R.V. Statistical optimization of biodiesel production from waste cooking oil using magnetic acid heterogeneous catalyst MoO3/SrFe2O4. Fuel 2021, 304, 121463. [Google Scholar] [CrossRef]
- Santos, H.C.L.; Gonçalves, M.A.; Viegas, A.C.; Figueira, B.A.M.; da Luz, P.T.S.; Rocha Filho, G.N.; Conceição, L.R.V. Tungsten oxide supported on copper ferrite: A novel magnetic acid heterogeneous catalyst for biodiesel production from low quality feedstock. RSC Adv. 2022, 12, 34614–34626. [Google Scholar] [CrossRef]
- De, A.; Boxi, S.S. Application of Cu impregnated TiO2 as a heterogeneous nanocatalyst for the production of biodiesel from palm oil. Fuel 2020, 265, 117019. [Google Scholar] [CrossRef]
- Martínez-Klimov, M.E.; Ramírez-Vidal, P.; Tejeda, P.R.; Klimova, T.E. Synergy between sodium carbonate and sodium titanate nanotubes in the transesterification of soybean oil with methanol. Catal. Today 2020, 353, 119–125. [Google Scholar] [CrossRef]
- Boonphayak, P.; Khansumled, S.; Yatongchai, C. Synthesis of CaO-SiO2 catalyst from lime mud and kaolin residue for biodiesel production. Mater. Lett. 2021, 283, 128759. [Google Scholar] [CrossRef]
- Jamil, F.; Kumar, P.S.M.; Al-Haj, L.; Myint, M.T.Z.; Al-Muhtaseb, A.H. Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Convers. Manag. X 2021, 10, 100047. [Google Scholar] [CrossRef]
- Cao, X.; Sunab, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Low, Y.W.; Yee, K.F. A review on lignocellulosic biomass waste into biochar-derived catalyst: Current conversion techniques, sustainable applications and challenges. Biomass Bioenerg. 2021, 154, 106245. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Bert, F.S. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Sudhakar, M.P.; Ahamed, T.S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Corrêa, A.P.L.; Bastos, R.R.C.; Rocha Filho, G.N.; Zamian, J.R.; Conceição, L.R.V. Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction. RSC Adv. 2020, 10, 20245–20256. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.R.C.; Corrêa, A.P.L.; da Luz, P.T.S.; Rocha Filho, G.N.; Zamian, J.R.; Conceição, L.R.V. Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Convers. Manag. 2020, 205, 112457. [Google Scholar] [CrossRef]
- Lima, R.P.; da Luz da Luz, P.T.S.; Braga, M.; dos Santos Batista, P.R.; da Costa, C.E.F.; Zamian, J.R.; do Nascimento, L.A.S.; Rocha Filho, G.N. Murumuru (Astrocaryum murumuru Mart.) butter and oils of buriti (Mauritia flexuosa Mart.) and pracaxi (Pentaclethra macroloba (Willd.) Kuntze) can be used for biodiesel production: Physico-chemical properties and thermal and kinetic studies. Ind. Crop. Prod. 2017, 97, 536–544. [Google Scholar] [CrossRef]
- Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, L.; Xing, S.; Luo, W.; Wang, Z.; Pengmei, L.V. Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J. Clean. Prod. 2018, 199, 772–780. [Google Scholar] [CrossRef]
- Zhao CPengmei Lv Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Jitjamnong, J.; Thunyaratchatanon, C.; Luengnaruemitchai, A.; Kongrit, N.; Kasetsomboon, N.; Sopajarn, A.; Chuaykarn, N.; Khantikulanon, N. Response surface optimization of biodiesel synthesis over a novel biochar-based heterogeneous catalyst from cultivated (Musa sapientum) banana peels. Biomass Conv. Bioref. 2021, 11, 2795–2811. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Pittman, J.C.U.; Mohan, D. Ciprofloxacin and acetaminophen sorption onto banana peel biochars: Environmental and process parameter influences. Environ. Res. 2021, 201, 111218. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Oh, J.I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Rajendran, N.; Kang, D.; Han, J.; Gurunathan, B. Process optimization, economic and environmental analysis of biodiesel production from food waste using a citrus fruit peel biochar catalyst. J. Clean. Prod. 2022, 365, 132712. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- Fernández, J.V.; Faria, D.N.; Santoro, M.C.; Mantovaneli, R.; Cipriano, D.F.; Brito, G.M.; Carneiro, M.T.W.D.; Schettino, M.A.; Gonzalez, J.L.; Freitas, J.C.C. Use of Unmodifed Cofee Husk Biochar and Ashes as Heterogeneous Catalysts in Biodiesel Synthesis. Bioenerg. Res. 2022, 16, 1746–1757. [Google Scholar] [CrossRef]
- Pires, F.C.S.; Silva, A.P.S.; Salazar, M.A.R.; Costa, W.A.; Costa, H.S.C.; Lopes, A.S.; Rogez, H.; Junior, R.N.C. Determination of process parameters and bioactive properties of the murici pulp (Byrsonima crassifolia) extracts obtained by supercritical extraction. J. Supercrit. Fluids 2019, 146, 128–135. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Paracampo, N.E.N.P.; Mattietto, R.A.; Nascimento, W.M.O.; Junior, R.A.G.; Resende, M.D.V. Avaliação Nutricional da Polpa de Frutos Provenientes de Clones de Muricizeiro; Boletim de Pesquisa e Desenvolvimento; Embrapa Amazônia Oriental: Belém, Brazil, 2020. [Google Scholar]
- Ferreira, M.G.R. Murici (Byrsonima crassifolia (L.) Rich.), Embrapa. 2005. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/859538/murici-byrsonima-crassifolia-l-rich (accessed on 15 May 2023).
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- Lim, X.Y.; Yek, P.N.Y.; Liew, R.K.; Chiong, M.C.; Mahari, W.A.W.; Peng, W.; Chong, C.T.; Lin, C.Y.; Aghbashlo, M.; Tabatabaei, M.; et al. Engineered biochar produced through microwave pyrolysis as a fuel additive in biodiesel combustion. Fuel 2022, 312, 122839. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 13, 325–333. [Google Scholar] [CrossRef]
- Liew, R.K.; Nam, W.L.; Chong, M.Y.; Phang, X.Y.; Su, M.H.; Yek, P.N.Y.; Ma, N.L.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Oil palm waste: An abundant and promising feedstock for microwave pyrolysis conversion into good quality biochar with potential multi-applications. Process Saf. Environ. Protect. 2018, 115, 57–69. [Google Scholar] [CrossRef]
- Guo, F.; Peng, Z.G.; Dai, J.Y.; Xiu, Z.L. Calcined sodium silicate as solid base catalyst for biodiesel production. Fuel Process. Technol. 2010, 91, 322–328. [Google Scholar] [CrossRef]
- Oliveira, K.G.; Lima, R.R.S.; Longe, C.; Bicudo, T.C.; Sales, R.V.; Carvalho, L.S. Sodium and potassium silicate-based catalysts prepared using sand silica concerning biodiesel production from waste oil. Arab. J. Chem. 2022, 15, 103603. [Google Scholar] [CrossRef]
- Ogbu, I.M.; Ajiwe, V.I.E.; Okoli, C.P. Performance evaluation of carbon-based heterogeneous acid catalyst derived from Hura crepitans seed pod for esterification of high FFA vegetable oil. Bioenerg. Res. 2018, 11, 772–783. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Biodiesel production from edible oils using algal biochar/CaO/K2CO3 as a heterogeneous and recyclable catalyst. Renew. Energy 2021, 168, 1207–1216. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Wang, C.H. Biomass gasification bottom ash as a source of CaO catalyst for biodiesel production via transesterification of palm oil. Energy Convers. Manag. 2015, 92, 234–243. [Google Scholar] [CrossRef]
- Syazwani, O.N.; Teo, S.H.; Islam, A.; Taufiq-Yapa, Y.H. Transesterification activity and characterization of natural CaO derived from waste venus clam (Tapes belcheri S.) material for enhancement of biodiesel production. Process Saf. Environ. Protect. 2017, 105, 303–315. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Ikram, M.; Jadhav, A.H.; Ali, M.W.; Ul-Hamid, A.; Nam, H.; Lakshminarayana, P.; Kumar, A.; Bahari, M.B.; et al. Synthesis and catalytic properties of calcium oxide obtained from organic ash over a titanium nanocatalyst for biodiesel production from dairy scum. Chemosphere 2021, 290, 133296. [Google Scholar] [CrossRef]
- Wang, S.; Hao, P.; Li, S.; Zhang, A.; Guan, Y.; Zhang, L. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by calcined silicates. Appl. Catal. A Gen. 2017, 542, 174–181. [Google Scholar] [CrossRef]
- Gui, X.; Chen, S.; Yun, Z. Continuous production of biodiesel from cottonseed oil and methanol using a column reactor packed with calcined sodium silicate base catalyst. Chin. J. Chem. Eng. 2016, 24, 499–505. [Google Scholar] [CrossRef]
- Das, A.; Shi, D.; Halder, G.; Rokhum, S.L. Microwave-assisted synthesis of glycerol carbonate by transesterification of glycerol using Mangifera indica peel calcined ash as catalyst. Fuel 2022, 330, 125511. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Li, S.; Shi, J. A biomimetic silicification approach to synthesize CaO–SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 2015, 153, 48–55. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Khalil, N.N.A.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Mashuri, S.I.S.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Daramola, M.O.; Mtshali, K.; Senokoane, L.; Fayemiwo, O.M. Influence of operating variables on the transesterification of waste cooking oil to biodiesel over sodium silicate catalyst: A statistical approach. J. Taibah Univ. Sci. 2016, 10, 675–684. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Rice husk-derived sodium silicate as a highly efficient and low-cost basic heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2016, 119, 453–462. [Google Scholar] [CrossRef]
- ASTM International D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM: West Conshohocken, PA, USA, 2006.
- ASTM D1298; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM: West Conshohocken, PA, USA, 2005.
- ASTM D664; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM: West Conshohocken, PA, USA, 2018.
- ASTM D6371; Standard Test Method for Cold Filter Plugging Point of Diesel and Heating Fuels. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM D93; Standard Test Methods for Flash Point by Pensky-Martens Closed CupTester. ASTM: West Conshohocken, PA, USA, 2013.
- ASTM D130; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM: West Conshohocken, PA, USA, 2018.
- Gonçalves, M.A.; Mares, E.K.L.; da Luz, P.T.S.; Zamian, J.R.; Rocha Filho, G.N.; Castro, H.F.; Conceição, L.R.V. Biodiesel synthesis from waste cooking oil using heterogeneous acid catalyst: Statistical optimization using linear regression model. J. Renew. Sustain. Energy 2021, 13, 04310. [Google Scholar] [CrossRef]
- Borah, M.J.; Sarmah, H.J.; Bhuyan, N.; Mohanta, D.; Deka, D. Application of Box-Behnken design in optimization of biodiesel yield using WO3/graphene quantum dot (GQD) system and its kinetics analysis. Biomass Conv. Bioref. 2022, 12, 221–232. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, L.; Wei, G.; Yuan, Y.; Huang, Z. Synthesis, characterization and application of a novel carbon-doped mix metal oxide catalyst for production of castor oil biodiesel. J. Clean. Prod. 2022, 373, 133768. [Google Scholar] [CrossRef]
- Wan, Z.; Hameed, B.H. Transesterification of palm oil to methyl ester on activated carbon supported calcium oxide catalyst. Biores. Technol. 2011, 102, 2659–2664. [Google Scholar] [CrossRef]
- Alsharifi, M.; Znad, H.; Hena, S.; Ang, M. Biodiesel production from canola oil using novel Li/TiO2 as a heterogeneous catalyst prepared via impregnation method. Renew. Energy 2017, 114, 1077–1089. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Silva, C.; Weschenfelder, T.A.; Rovani, S.; Corazza, F.C.; Corazza, M.L.; Dariva, C.; Oliveira, J.V. Continuous production of fatty acid ethyl esters from soybean oil in compressed ethanol. Ind. Eng. Chem. Res. 2007, 46, 5304–5309. [Google Scholar] [CrossRef]







| Sample | C | H | N | O a | Na b | Si b |
|---|---|---|---|---|---|---|
| Murici seed | 45.17 ± 0.04 | 2.88 ± 0.02 | 2.82 ± 0.01 | 49.07 | - | - |
| BCAM | 71.72 ± 0.03 | 1.49 ± 0.01 | 1.98 ± 0.01 | 24.76 | - | - |
| Catalyst 15Na/BCAM | 53.85 ± 0.03 | 1.33 ± 0.01 | 0.87 ± 0.01 | 28.45 | 9.53 | 5.82 |
| Biodiesel Properties | Unit | Test Methods | ASTM D6751 Limits | Present Study |
|---|---|---|---|---|
| Kinematic viscosity (at 40 °C) | mm2 s−1 | ASTM D445 [61] | 1.9–6.0 | 4.47 |
| Density (at 20 °C) | g cm−3 | ASTM D1298 [62] | 0.875–0.900 | 0.880 |
| Acid value | mg KOH g−1 | ASTM D664 [63] | 0.5 max | 0.20 |
| Cold filtre plugging point | °C | ASTM D6371 [64] | NS | 0.0 |
| Flash point | °C | ASTM D93 [65] | 130 min | 150 |
| Copper strip corrosion | - | ASTM D130 [66] | 3 max | 1a |
| Sample | Basicity (mmol g−1) |
|---|---|
| Catalyst fresh | 3.911 ± 0.031 |
| Catalyst after 5° reactional cycle | 1.420 ± 0.011 |
| Precursor | Catalyst | Synthesis of Catalyst | Reactional Conditions | Ester Content (%) | Cycles | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonization | Functionalization | T (°C) | t (h) | Catalyst (wt%) | RM (MeOH:oil) | |||||||
| T (°C) | t (h) | T (°C) | t (h) | |||||||||
| Avocado seeds | 20 wt%. Ca loaded | 900 | 2 | 900 | 2 | 99.5 | 5.0 | 7.3 | 15.6:1 | 99.50 | 3 | [29] |
| Banana peel | 30K/BP-600 | 600 | 2 | 600 | 4 | 65.0 | 2.0 | 4.0 | 15:1 | 98.91 | 5 | [32] |
| Pomelo peel | 25K/AP-600 | 600 | 2 | 600 | 3 | 65.0 | 2.5 | 5.0 | 8:1 | 98.00 | 8 | [31] |
| Date seeds | SrO-carbon | 400 | 5 | 450 | 4 | 65.0 | 1.5 | 4.0 | 15:1 | 94.27 | 9 | [21] |
| Sargassum oligocystum | Biochar/CaO/K2CO3 | 350 | 2 | 500 | 3 | 65.0 | 3.3 | 4.0 | 18:1 | 98.83 | 9 | [49] |
| Rice husk | 30Ca/RB700 | 700 | 3 | 700 | 4 | 65.0 | 3.0 | 8.0 | 9:1 | 94.40 | 10 | [30] |
| Urea | CaO-MgO-800-5 | 800 | 5 | 800 | 5 | 70.0 | 4.4 | 8.0 | 21:1 | 91.10 | 4 | [69] |
| Commercial activated carbon | CaO/AC | − | − | 450 | 1 | 190.0 | 1.35 | 5.5 | 15:1 | 80.98 | 3 | [70] |
| Murici seed | 15Na/BCAM | 600 | 1 | 400 | 1 | 75 | 1.5 | 5.0 | 20:1 | 97.20 | 10 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.S.; Gonçalves, M.A.; da Rocha Filho, G.N.; da Conceição, L.R.V. Functionalized Biochar from the Amazonian Residual Biomass Murici Seed: An Effective and Low-Cost Basic Heterogeneous Catalyst for Biodiesel Synthesis. Molecules 2023, 28, 7980. https://doi.org/10.3390/molecules28247980
Ribeiro TS, Gonçalves MA, da Rocha Filho GN, da Conceição LRV. Functionalized Biochar from the Amazonian Residual Biomass Murici Seed: An Effective and Low-Cost Basic Heterogeneous Catalyst for Biodiesel Synthesis. Molecules. 2023; 28(24):7980. https://doi.org/10.3390/molecules28247980
Chicago/Turabian StyleRibeiro, Thaissa Saraiva, Matheus Arrais Gonçalves, Geraldo Narciso da Rocha Filho, and Leyvison Rafael Vieira da Conceição. 2023. "Functionalized Biochar from the Amazonian Residual Biomass Murici Seed: An Effective and Low-Cost Basic Heterogeneous Catalyst for Biodiesel Synthesis" Molecules 28, no. 24: 7980. https://doi.org/10.3390/molecules28247980
APA StyleRibeiro, T. S., Gonçalves, M. A., da Rocha Filho, G. N., & da Conceição, L. R. V. (2023). Functionalized Biochar from the Amazonian Residual Biomass Murici Seed: An Effective and Low-Cost Basic Heterogeneous Catalyst for Biodiesel Synthesis. Molecules, 28(24), 7980. https://doi.org/10.3390/molecules28247980








