The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Charactrization of AYP
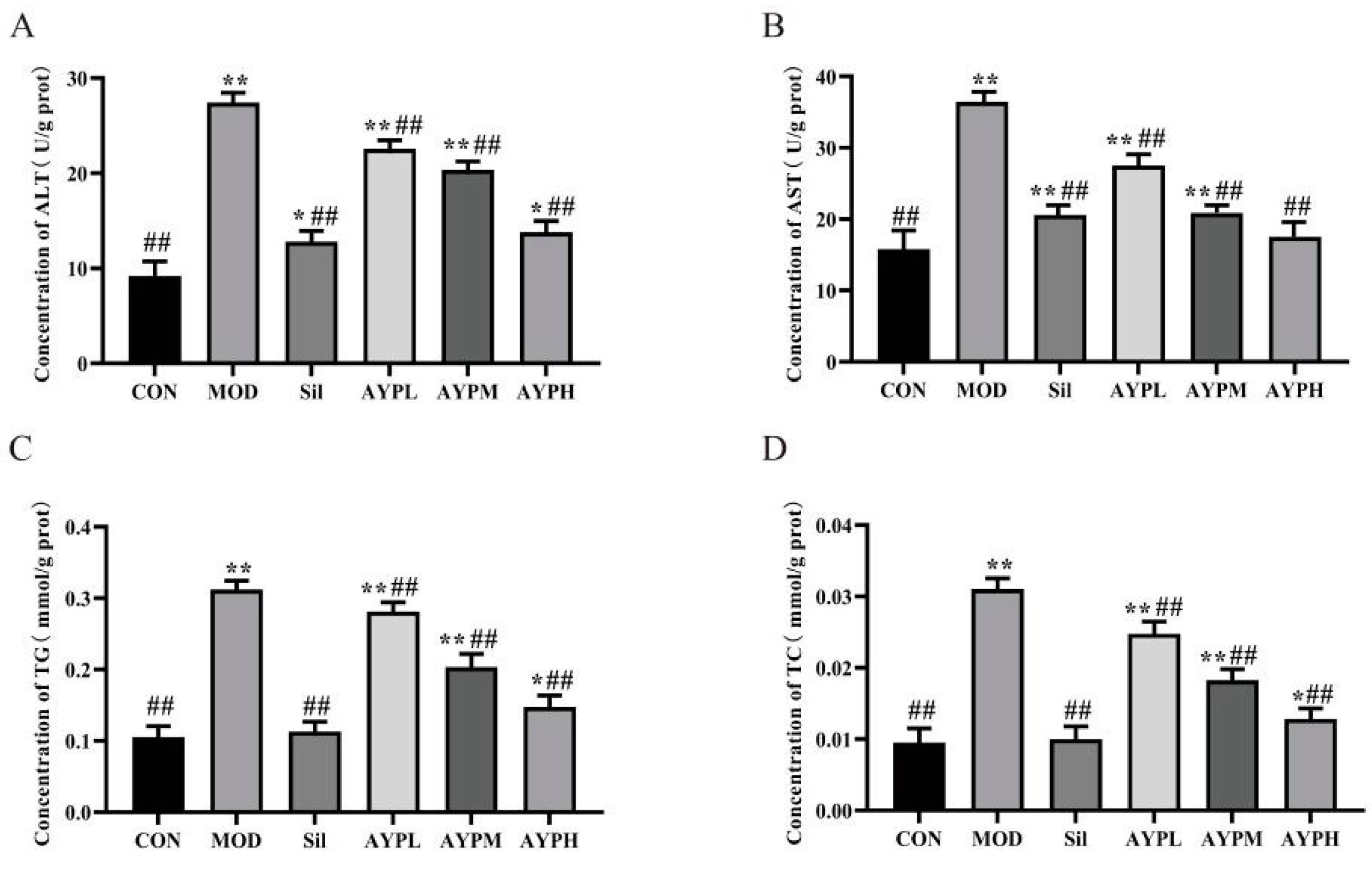
2.2. The Effect of AYP on the Liver Damage of Mice
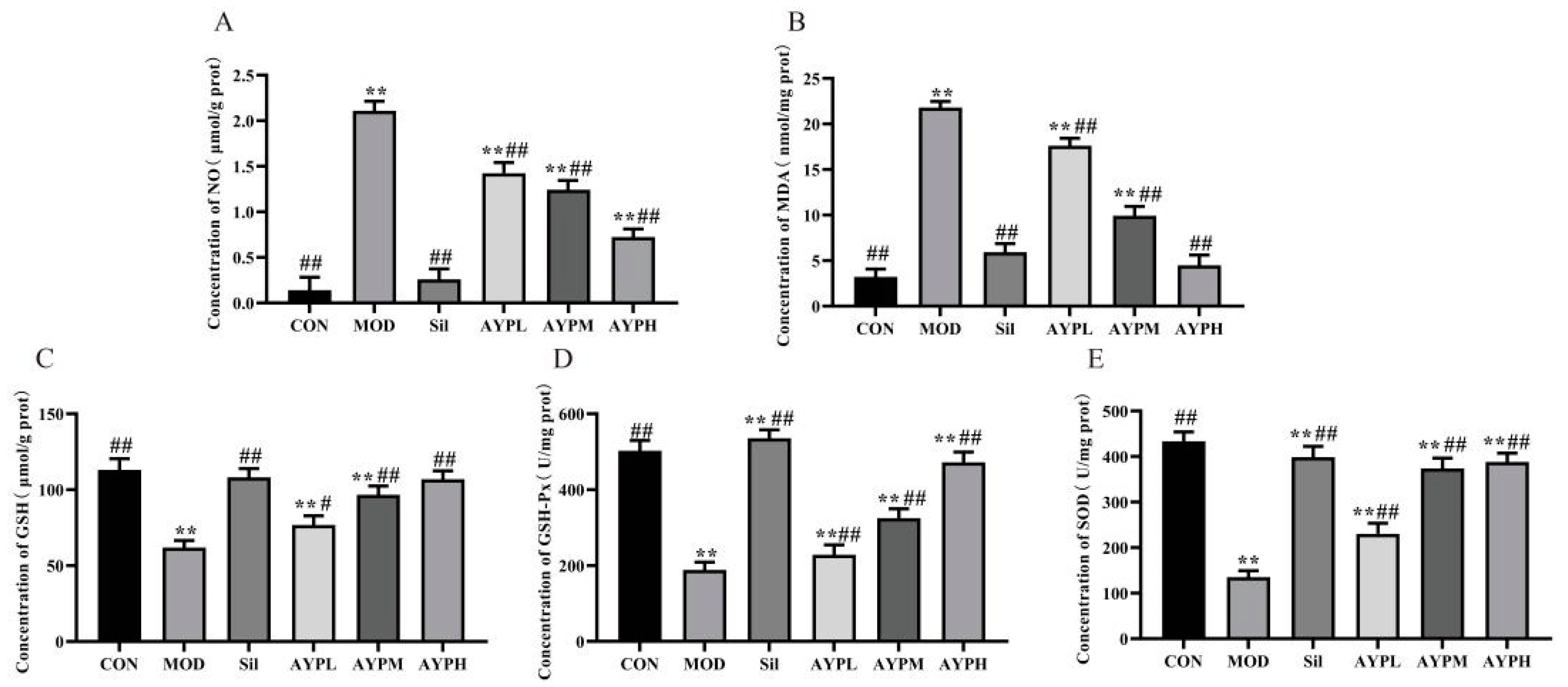
2.3. The Effect of AYP on the Oxidation Indicators in Mice Liver
2.4. The Effect of AYP on the Secretion of Serum Cytokines in Mice
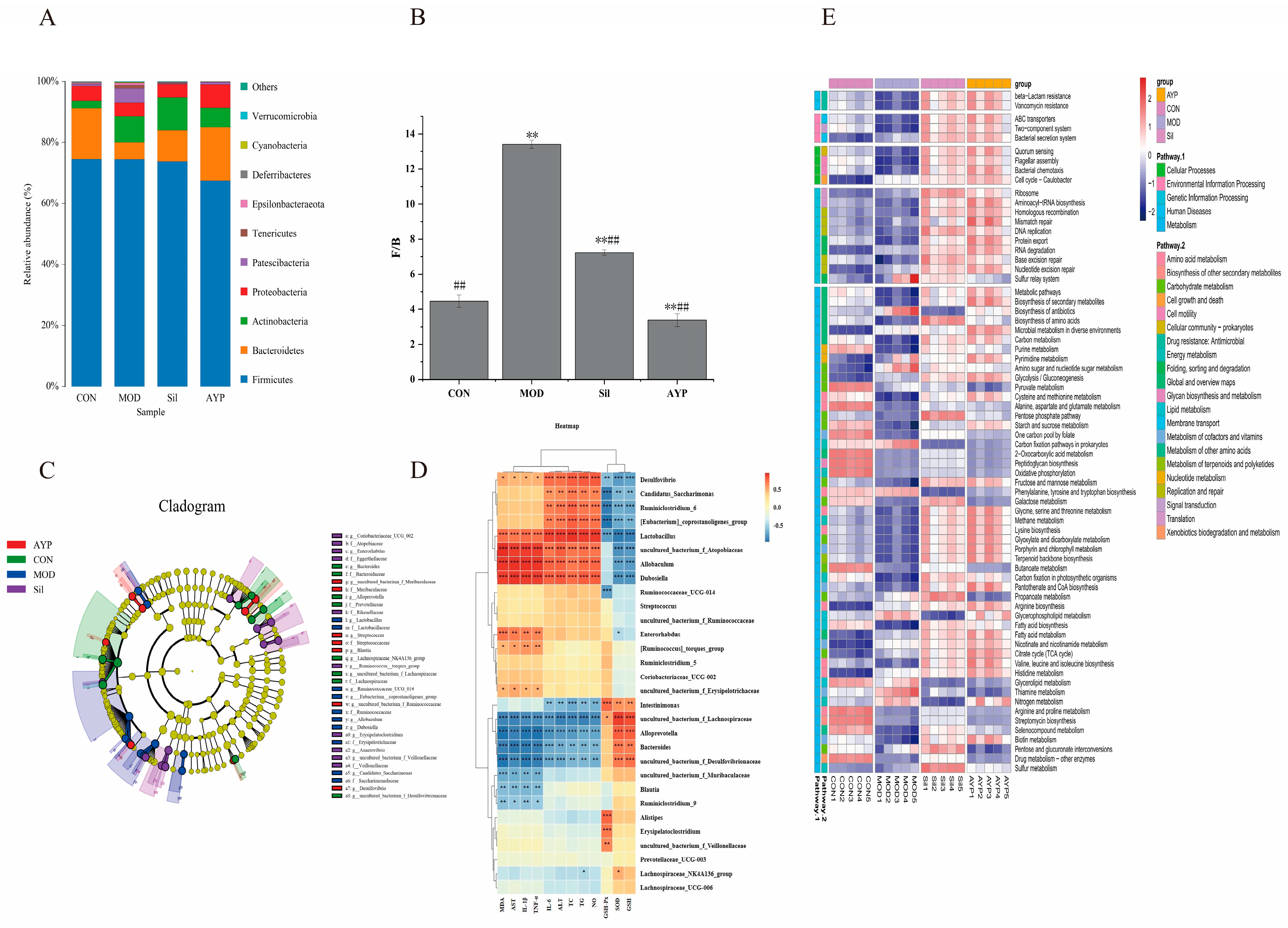
2.5. The Effect of AYP on the Intestinal Microbiota of Mice
2.5.1. The Effect of AYP on the Intestinal Bacteria of Mice
2.5.2. The Effect of AYP on the Intestinal Fungi of Mice
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction and Purification
3.3. Structural and Polysicochemical Analysis of AYP
3.4. Animal Experiment
3.4.1. Experimental Animals and Procedures
3.4.2. Measurements of Liver Damage
3.4.3. Measurements of Liver Oxidative Stress, and Serum Cytokines
3.4.4. Analysis of the Intestinal Bacteria and Fungi
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Xu, Y.; Ye, M.; Ye, W.; Xiao, J.; Zhou, H.; Zhang, W.; Shu, Y.; Huang, Y.; Chen, Y. Resveratrol in liquor exacerbates alcoholic liver injury with a reduced therapeutic effect in mice: An unsupervised herbal wine habit is risky. Nutrients 2022, 14, 4752. [Google Scholar] [CrossRef]
- Evangelou, E.; Suzuki, H.; Bai, W.; Pazoki, R.; Gao, H.; Matthews, P.M.; Elliott, P. Alcohol consumption in the general population is associated with structural changes in multiple organ systems. Elife 2021, 10, e65325. [Google Scholar] [CrossRef]
- Deutsch-Link, S.; Jiang, Y.; Peery, A.F.; Barritt, A.S.; Bataller, R.; Moon, A.M. Alcohol-associated liver disease mortality increased from 2017–2020 and accelerated during the COVID-19 pandemic. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 20, 2142–2144. [Google Scholar] [CrossRef]
- Wang, X.; Lan, Y.; Zhu, Y.; Li, S.; Liu, M.; Song, X.; Zhao, H.; Liu, W.; Zhang, J.; Wang, S.; et al. Hepatoprotective effects of Auricularia cornea var. Li. Polysaccharides against the alcoholic liver diseases through different metabolic pathways. Sci. Rep. 2018, 8, 7574. [Google Scholar] [CrossRef]
- Li, H.; Xie, Z.; Zhang, Y.; Liu, Y.; Niu, A.; Liu, Y.; Zhang, L.; Guan, L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Sun, S.; Wang, K.; Sun, L.; Cheng, B.; Qiao, S.; Dai, H.; Shi, W.; Ma, J.; Liu, H. Therapeutic manipulation of gut microbiota by polysaccharides of Wolfiporia cocos reveals the contribution of the gut fungi-induced PGE2 to alcoholic hepatic steatosis. Gut Microbes 2020, 12, 1830693. [Google Scholar] [CrossRef]
- Bjørkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019, 10, 663–675. [Google Scholar] [CrossRef]
- Yang, A.M.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Investig. 2017, 127, 2829–2841. [Google Scholar] [CrossRef]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef]
- Allampati, S.; Mullen, K.D. Long-term management of alcoholic liver disease. Clin. Liver Dis. 2016, 20, 551–562. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, J.; Di, L.; Li, J.; Hu, L.; Qiao, H.; Wang, L.; Feng, Y. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohydr. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Zhang, K.; Lu, Y.; Wu, Q.; Chen, J.; Li, Y.; Wu, Q.; Chen, Y. Extraction, purification, structural characterization, and gut microbiota relationship of polysaccharides: A review. Int. J. Biol. Macromol. 2022, 213, 967–986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Li, Y.; Liu, R.; Zeng, M. Mediation of the microbiome-gut axis by oyster (Crassostrea gigas) polysaccharides: A possible protective role in alcoholic liver injury. Int. J. Biol. Macromol. 2021, 182, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hua, H.; Guo, Y.; Qian, H.; Liu, J.; Cheng, Y. Study on the hepatoprotective effect of sporidiobolus pararoseus polysaccharides under the “gut microbiome-amino acids metabolism” network. Food Biosci. 2022, 49, 101928. [Google Scholar] [CrossRef]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/Nf-κb/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Guo, M. Effects of ultrasound treatment on extraction and rheological properties of polysaccharides from Auricularia cornea var. Li. Molecules 2019, 24, 939. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Qi, Y.; Wu, D.; Liu, X.; Liu, C.; Gao, Y.; Shi, J.; Fang, L.; Min, W. Impact of Auricularia cornea var. Li polysaccharides on the physicochemical, textual, flavor, and antioxidant properties of set yogurt. Int. J. Biol. Macromol. 2022, 206, 148–158. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, L.; Jiang, G.; Ren, L.; Wang, L.; Liu, X. Anti-diabetic activity of polysaccharides from Auricularia cornea var. Li. Foods 2022, 11, 1464. [Google Scholar] [CrossRef]
- MZhao, M.; Shi, W.; Chen, X.; Liu, Y.; Yang, Y.; Kong, X. Regulatory effects of auricularia cornea var. Li. Polysaccharides on immune system and gut microbiota in cyclophosphamide-induced mice. Front. Microbiol. 2022, 13, 1056410. [Google Scholar]
- Wang, Y.; Guo, M. Purification and structural characterization of polysaccharides isolated from Auricularia cornea var. Li. Carbohydr. Polym. 2020, 230, 115680. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Cheng, L.; Shi, R.; Qayum, A.; Bilawal, A.; Gantumur, M.A.; Hussain, M.A.; Jiang, Z.; Tian, B. Comparison in bioactivity and characteristics of Ginkgo biloba seed polysaccharides from four extract pathways. Int. J. Biol. Macromol. 2020, 159, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae pi80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Fariña, J.I.; Viñarta, S.C.; Cattaneo, M.; Figueroa, L.I.C. Structural stability of Sclerotium rolfsii atcc 201126 β-glucan with fermentation time: A chemical, infrared spectroscopic and enzymatic approach. J. Appl. Microbiol. 2009, 106, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jie, Z.; Ying, X.; Yue, Q.; Zhou, Y.; Sun, L. Structural characterization of alkali-soluble polysaccharides from Panax ginseng c. A. Meyer. R. Soc. Open Sci. 2018, 5, 171644. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Cheng, R.F.; Sun, M.K.; Hu, Q.R.; Deng, Z.; Zhang, B.; Li, H. Hovenia acerba lindl. Peduncles and seeds extracts ameliorate alcoholic liver injury by activating the Nrf2/HO-1 signalling pathway in LO2 cells and mice. Food Biosci. 2023, 51, 102224. [Google Scholar] [CrossRef]
- Che, Z.; Song, Y.; Xu, C.; Li, W.; Dong, Z.; Wang, C.; Ren, Y.; So, K.F.; Tipoe, G.L.; Wang, F.; et al. Melatonin alleviates alcoholic liver disease via egfr-brg1-tert axis regulation. Acta Pharm. Sin. B 2023, 13, 100–112. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, L.; Zhao, Q.; Zhang, L.; Chen, J.; Liu, B.; Zhao, B. Preliminary characterizations, antioxidant and hepatoprotective activity of polysaccharide from cistanche deserticola. Int. J. Biol. Macromol. 2016, 93 Pt A, 678–685. [Google Scholar] [CrossRef]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L. Corrigendum to “ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake” [Curr. Res. Food Sci. 5(2022) 515–530]. Curr. Res. Food Sci. 2022, 5, 1108. [Google Scholar] [CrossRef] [PubMed]
- Ekakitie, L.I.; Okpoghono, J.; Orororo, O.C.; Ekakitie, O.A. Ameliorative prowess of bee honey in the tissues of rats administered aluminium nitrate. Sci. Afr. 2021, 12, e00782. [Google Scholar] [CrossRef]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Parvez, F.; Rahman, M.M.; Khan, F.; Subhan, N.; Alam, M.A. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating ampk-akt-enos pathway in the isoprenaline-induced oxidative stress model. Sci. Rep. 2020, 10, 14659. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Q.; Wang, L.; Zhao, M.; Zhao, B. Protective effect of polysaccharide from maca (Lepidium meyenii) on Hep-G2 cells and alcoholic liver oxidative injury in mice. Int. J. Biol. Macromol. 2017, 99, 63–70. [Google Scholar] [CrossRef]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]
- Cai, L.; Zou, S.; Liang, D.; Luan, L. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis radix. Carbohydr. Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef]
- Yang, K.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κb signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, H.H.L.; Kim, Y.; Lee, H.B.; Lee, E.; Park, J.H.; Park, H.Y. Corchorus olitorius L. Ameliorates alcoholic liver disease by regulating gut-liver axis. J. Funct. Foods 2021, 85, 104648. [Google Scholar] [CrossRef]
- Namachivayam, A.; Gopalakrishnan, A.V. A review on molecular mechanism of alcoholic liver disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef]
- Scarpellini, E.; Mariana, F.; Marinella, L.; Carlo, R.; Giammarco, F.; Ludovico, A.; Adriano, D.S. Gut microbiota and alcoholic liver disease. Rev. Recent Clin. Trials 2016, 11, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumpetis, T.; Sood, G. Pathogenesis of alcoholic liver disease: An update. Clin. Liver Dis. 2019, 23, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.G.; Schnabl, B. From intestinal dysbiosis to alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Casafont Morencos, F.; De las Heras Castano, G.; Martin Ramos, L.; López Arias, M.J.; Ledesma, F.; Pons Romero, F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 1996, 41, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef] [PubMed]
- Seidling, W.; Hamberg, L.; Máliš, F.; Salemaa, M.; Kutnar, L.; Czerepko, J.; Kompa, T.; Buriánek, V.; Dupouey, J.L.; Vodálová, A.; et al. Comparing observer performance in vegetation records by efficiency graphs derived from rarefaction curves. Ecol. Indic. 2020, 109, 105790. [Google Scholar] [CrossRef]
- Albillos, A.; De Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Cheng, L.; Zhang, X.; Liu, Y.; Zhang, R.; Weng, P.; Wu, Z. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Res. Int. 2021, 149, 110675. [Google Scholar] [CrossRef]
- Eom, T.; Ko, G.; Kim, K.C.; Kim, J.S.; Unno, T. Dendropanax morbifera leaf extracts improved alcohol liver injury in association with changes in the gut microbiota of rats. Antioxidants 2020, 9, 911. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.; Guo, X.; Huang, L.; Yang, J.; Yu, Q.; Chen, Y.; Xie, J. Cyclocarya paliurus polysaccharide alleviates liver inflammation in mice via beneficial regulation of gut microbiota and TLR4/MAPK signaling pathways. Int. J. Biol. Macromol. 2020, 160, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Petrosino, J.; Ajami, N.; Feng, W.; Wang, Y.; Liu, Y.; Beier, J.I.; Barve, S.S.; Yin, X.; Wei, X.; et al. Mcclain, Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am. J. Pathol. 2016, 186, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Liu, Y.; Li, W.; Niu, A.; Ren, P.; Liu, Y.; Jiang, C.; Inam, M.; Guan, L. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. Polysaccharides on properties and gut microbiota. Carbohydr. Polym. 2022, 281, 119055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, W.; Liu, C.; Wang, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur. J. Pharmacol. 2022, 928, 175096. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Li, J.V.; Zhou, N.Y.; Tang, H.; Wang, Y. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Montandon, S.A.; Loizides-Mangold, U.; Gaïa, N.; Lazarevic, V.; De Vito, C.; Perroud, E.; Bochaton-Piallat, M.L.; Dibner, C.; Schrenzel, J.; et al. The GLP-1r agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. 2021, 227, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Kang, S.; Zhang, J.; Zhao, H.; Peng, Y.; Yang, M.; Zheng, Y.; Shao, J.; Yue, X. Whey protein and xylitol complex alleviate type 2 diabetes in c57bl/6 mice by regulating the intestinal microbiota. Food Res. Int. 2022, 157, 111454. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, K.; Yang, Z.; Li, J.; Chen, G.; Wu, Q.; Lv, X.; Hu, W.; Rao, P.; Ai, L.; et al. Monascuspiloin from Monascus-fermented red mold rice alleviates alcoholic liver injury and modulates intestinal microbiota. Foods 2022, 11, 3048. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Ren, P.; Che, Y.; Zhou, J.; Wang, W.; Yang, Y.; Guan, L. Polysaccharide from flammulina velutipes residues protects mice from pb poisoning by activating AKT/GSK3Β/Nrf-2/HO-1 signaling pathway and modulating gut microbiota. Int. J. Biol. Macromol. 2023, 230, 123154. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core altered microorganisms in colitis mouse model: A comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Role of gut dysbiosis in liver diseases: What have we learned so far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fang, B.; Kang, C.; Zhao, Y.; Qiang, X.; Zhang, X.; Wang, Y.; Zhong, T.; Xiao, J.; Wang, M. Hepatoprotective mechanism of ginsenoside rg1 against alcoholic liver damage based on gut microbiota and network pharmacology. Oxidative Med. Cell. Longev. 2022, 2022, 5025237. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Puri, P.; Muthiah, M.D.; Daitya, K.; Brown, R.; Chalasani, N.; Liangpunsakul, S.; Shah, V.H.; Gelow, K.; Siddiqui, M.S.; et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology 2020, 72, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, W.; Jiang, X.; Xia, J.; Lv, L.; Li, S.; Zhuge, A.; Wu, Z.; Wang, Q.; Wang, S.; et al. Multi-omics analysis reveals the protection of gasdermin d in concanavalin a-induced autoimmune hepatitis. Microbiol. Spectr. 2022, 10, e171722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Li, T.; Lyu, S.; Liu, X.; Du, Z.; Shang, X.; Zhang, T. Integrated microbiome and metabolomic analysis reveal the repair mechanisms of ovalbumin on the intestine barrier of colitis mice. J. Agric. Food. Chem. 2023, 71, 8894–8905. [Google Scholar] [CrossRef] [PubMed]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus gg treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Litwinowicz, K.; Gamian, A. Microbiome alterations in alcohol use disorder and alcoholic liver disease. Int. J. Mol. Sci. 2023, 24, 2461. [Google Scholar] [CrossRef]
- Ciocan, D.; Voican, C.S.; Wrzosek, L.; Hugot, C.; Rainteau, D.; Humbert, L.; Cassard, A.M.; Perlemuter, G. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment. Pharmacol. Ther. 2018, 48, 961–974. [Google Scholar] [CrossRef]
- Kurdi, P.; Kawanishi, K.; Mizutani, K.; Yokota, A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacterial. J. Bacteriol. 2006, 188, 1979–1986. [Google Scholar] [CrossRef]
- Konkit, M.; Kim, K.; Kim, J.H.; Kim, W. Protective effects of lactococcus chungangensis cau 28 on alcohol-metabolizing enzyme activity in rats. J. Dairy. Sci. 2018, 101, 5713–5723. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhou, J.; Liu, S.; Liu, Y.; Yang, Y.; Wang, W.; Che, Y.; Inam, M.; Guan, L. The protective mechanism of a novel polysaccharide from lactobacillus-fermented Nostoc commune vauch. On attenuating cadmium-induced kidney injury in mice. Int. J. Biol. Macromol. 2023, 226, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, T.; Karkkainen, O. Changes in the human metabolome associated with alcohol use: A review. Alcohol Alcohol. 2019, 54, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Song, Z.; Han, H.; Ge, X.; Desert, R.; Athavale, D.; Komakula, S.S.B.; Magdaleno, F.; Chen, W.; Lantvit, D.; et al. Intestinal osteopontin protects from alcohol-induced liver injury by preserving the gut microbiome and the intestinal barrier function. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 813–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, P.; Hu, L.; Yang, L.; Chu, H.; Hou, X. Modulating phenylalanine metabolism by L. Acidophilus alleviates alcohol-related liver disease through enhancing intestinal barrier function. Cell Biosci. 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, Y.; Wang, X.; Luo, L.; Sun, K.; Zeng, L. Gut microbiome and metabolome response of pu-erh tea on metabolism disorder induced by chronic alcohol consumption. J. Agric. Food. Chem. 2020, 68, 6615–6627. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.A.; Ghanim, B.Y.; Al-Natour, M.; Arqoub, D.A.; Abdallah, Q.; Abdelrazig, S.; Alkrad, J.A.; Kim, D.H.; Qinna, N.A. Potential biomarkers and metabolomics of acetaminophen-induced liver injury during alcohol consumption: A preclinical investigation on c57/bl6 mice. Toxicol. Appl. Pharmacol. 2023, 465, 116451. [Google Scholar] [CrossRef] [PubMed]
- Clugston, R.D.; Gao, M.A.; Blaner, W.S. The hepatic lipidome: A gateway to understanding the pathogenesis of alcohol-induced fatty liver. Curr. Mol. Pharmacol. 2017, 10, 195–206. [Google Scholar] [CrossRef]
- Cao, P.; Wu, Y.; Li, Y.; Xiang, L.; Cheng, B.; Hu, Y.; Jiang, X.; Wang, Z.; Wu, S.; Si, L.; et al. The important role of glycerophospholipid metabolism in the protective effects of polyphenol-enriched tartary buckwheat extract against alcoholic liver disease. Food Funct. 2022, 13, 10415–10425. [Google Scholar] [CrossRef]
- Yadav, S.; Dwivedi, A.; Tripathi, A.; Tripathi, A.K. Therapeutic potential of short-chain fatty acid production by gut microbiota in neurodegenerative disorders. Nutr. Res. 2022, 106, 72–84. [Google Scholar] [CrossRef]
- Qin, X.; Gu, Y.; Liu, T.; Wang, C.; Zhong, W.; Wang, B.; Cao, H. Gut mycobiome: A promising target for colorectal cancer. Biochim. Biophys. Acta -Rev. Cancer 2021, 1875, 188489. [Google Scholar] [CrossRef]
- Shankar, J. Food habit associated mycobiota composition and their impact on human health. Front. Nutr. 2021, 8, 773577. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; He, Y.; Kulyar, M.F.E.A.; Iqbal, M.; Li, K.; Liu, J. Longitudinal characterization of the gut bacterial and fungal communities in yaks. J. Fungi 2021, 7, 559. [Google Scholar] [CrossRef]
- Hartmann, P.; Lang, S.; Zeng, S.; Duan, Y.; Zhang, X.; Wang, Y.; Bondareva, M.; Kruglov, A.; Fouts, D.E.; Stärkel, P.; et al. Dynamic changes of the fungal microbiome in alcohol use disorder. Front. Physiol. 2021, 12, 699253. [Google Scholar] [CrossRef]
- Urubschurov, V.; Büsing, K.; Freyer, G.; Herlemann, D.P.; Souffrant, W.B.; Zeyner, A. New insights into the role of the porcine intestinal yeast, Kazachstania slooffiae, in intestinal environment of weaned piglets. FEMS Microbiol. Ecol. 2017, 93, fiw245. [Google Scholar] [CrossRef]
- Arfken, A.M.; Frey, J.F.; Summers, K.L. Temporal dynamics of the gut bacteriome and mycobiome in the weanling pig. Microorganisms 2020, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Mo, H.; Mu, Q.; Liu, P.; Liu, G.; Yu, W. The gut mycobiome characterization of gestational diabetes mellitus and its association with dietary intervention. Front. Microbiol. 2022, 13, 892859. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Urubschurov, V.; Büsing, K.; Souffrant, W.B.; Schauer, N.; Zeyner, A. Porcine intestinal yeast species, Kazachstania slooffiae, a new potential protein source with favourable amino acid composition for animals. J. Anim. Physiol. Anim. Nutr. 2018, 102, e892–e901. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Hou, Q.; Xu, X.; Ren, J.; Ma, L.; Yan, X. Core-predominant gut fungus Kazachstania slooffiae promotes intestinal epithelial glycolysis via lysine desuccinylation in pigs. Microbiome 2023, 11, 31. [Google Scholar] [CrossRef]
- Jiang, L.; Stärkel, P.; Fan, J.G.; Fouts, D.E.; Bacher, P.; Schnabl, B. The gut mycobiome: A novel player in chronic liver diseases. J. Gastroenterol. 2021, 56, 1–11. [Google Scholar] [CrossRef]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida albicans and Staphylococcus species: A threatening twosome. Front. Microbiol. 2019, 10, 2162. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Xi, L.; Song, Y.; Han, J.; Qin, X. Microbiome analysis reveals the significant changes in gut microbiota of diarrheic baer’s pochards (Aythya baeri). Microb. Pathog. 2021, 157, 105015. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Zhang, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia—A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int. J. Anal. Chem. 2011, 2011 Pt 1, 283658. [Google Scholar] [CrossRef]
- Li, W.; Yang, B.; Joe, G.H.; Shimizu, Y.; Saeki, H. Glycation with uronic acid-type reducing sugar enhances the anti-inflammatory activity of fish myofibrillar protein via the maillard reaction. Food Chem. 2023, 407, 135162. [Google Scholar] [CrossRef]
- Li, C.; Dong, Z.; Zhang, B.; Huang, Q.; Liu, G.; Fu, X. Structural characterization and immune enhancement activity of a novel polysaccharide from Moringa oleifera leaves. Carbohydr. Polym. 2020, 234, 115897. [Google Scholar] [CrossRef]
- Su, L.; Xin, C.; Yang, J.; Dong, L.; Mei, H.; Dai, X.; Wang, Q. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, F.; Wang, X.; Feng, Y.; Wang, Y. MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of Akkermansia muciniphila. Int. J. Biol. Macromol. 2021, 196, 23–34. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, Y.; Wei, C.; Tan, L.; Li, C.; Zhang, Y.; Xu, F.; Zhu, K.; Wu, G.; Cao, J. Protective effects of polysaccharide from Artocarpus heterophyllus lam. (Jackfruit) pulp on non-alcoholic fatty liver disease in high-fat diet rats via ppar and ampk signaling pathways. J. Funct. Foods 2022, 95, 105195. [Google Scholar] [CrossRef]





| ACE | Chao1 | Simpson | Shannon | |
|---|---|---|---|---|
| CON | 521.429 ± 14.231 | 533.166 ± 9.466 | 0.979 ± 0.004 ## | 6.645 ± 0.178 ## |
| MOD | 533.133 ± 13.329 | 538.946 ± 10.888 | 0.961 ± 0.003 ** | 6.326 ± 0.114 ** |
| Sil | 464.096 ± 10.631 **## | 468.206 ± 13.210 **## | 0.969 ± 0.000 **## | 6.235 ± 0.01 ** |
| AYP | 481.839 ± 7.224 **## | 484.193 ± 6.489 **## | 0.981 ± 0.0027 ## | 6.890 ± 0.056 *## |
| ACE | Chao1 | Simpson | Shannon | |
|---|---|---|---|---|
| CON | 595.656 ± 127.861 | 454.586 ± 112.503 | 0.967 ± 0.007 * | 6.680 ± 0.117 ** |
| MOD | 647.149 ± 294.838 | 506.445 ± 130.516 | 0.986 ± 0.0106 | 7.169 ± 0.228 |
| Sil | 715.839 ± 96.040 | 389.859 ± 30.940 | 0.923 ± 0.011 ** | 6.109 ± 0.133 ** |
| AYP | 604.215 ± 135.579 | 405.881 ± 54.869 | 0.967 ± 0.014 * | 6.730 ± 0.258 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Jia, Z.; An, C.; Ren, P.; Yang, Y.; Wang, W.; Su, L. The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota. Molecules 2023, 28, 8003. https://doi.org/10.3390/molecules28248003
Wang T, Jia Z, An C, Ren P, Yang Y, Wang W, Su L. The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota. Molecules. 2023; 28(24):8003. https://doi.org/10.3390/molecules28248003
Chicago/Turabian StyleWang, Tianci, Zikun Jia, Canghai An, Ping Ren, Yiting Yang, Wanting Wang, and Ling Su. 2023. "The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota" Molecules 28, no. 24: 8003. https://doi.org/10.3390/molecules28248003
APA StyleWang, T., Jia, Z., An, C., Ren, P., Yang, Y., Wang, W., & Su, L. (2023). The Protective Effect of Auricularia cornea var. Li. Polysaccharide on Alcoholic Liver Disease and Its Effect on Intestinal Microbiota. Molecules, 28(24), 8003. https://doi.org/10.3390/molecules28248003





