Mesoporous-Layered Double Oxide/MCM-41 Composite with Enhanced Catalytic Performance for Cyclopentanone Aldol Condensation
Abstract
:1. Introduction
2. Results and Discussions
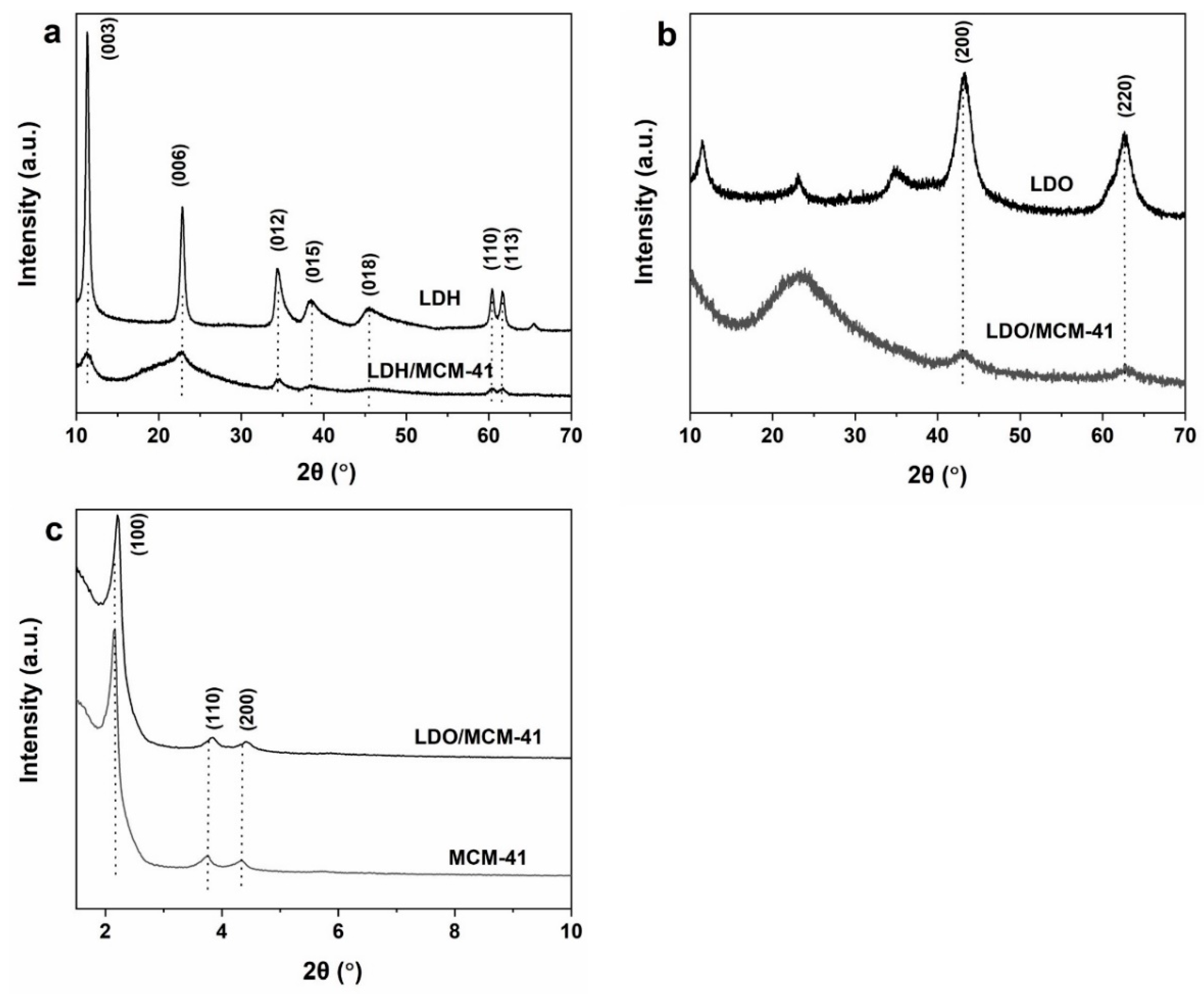
2.1. XRD
2.2. N2 Sorption
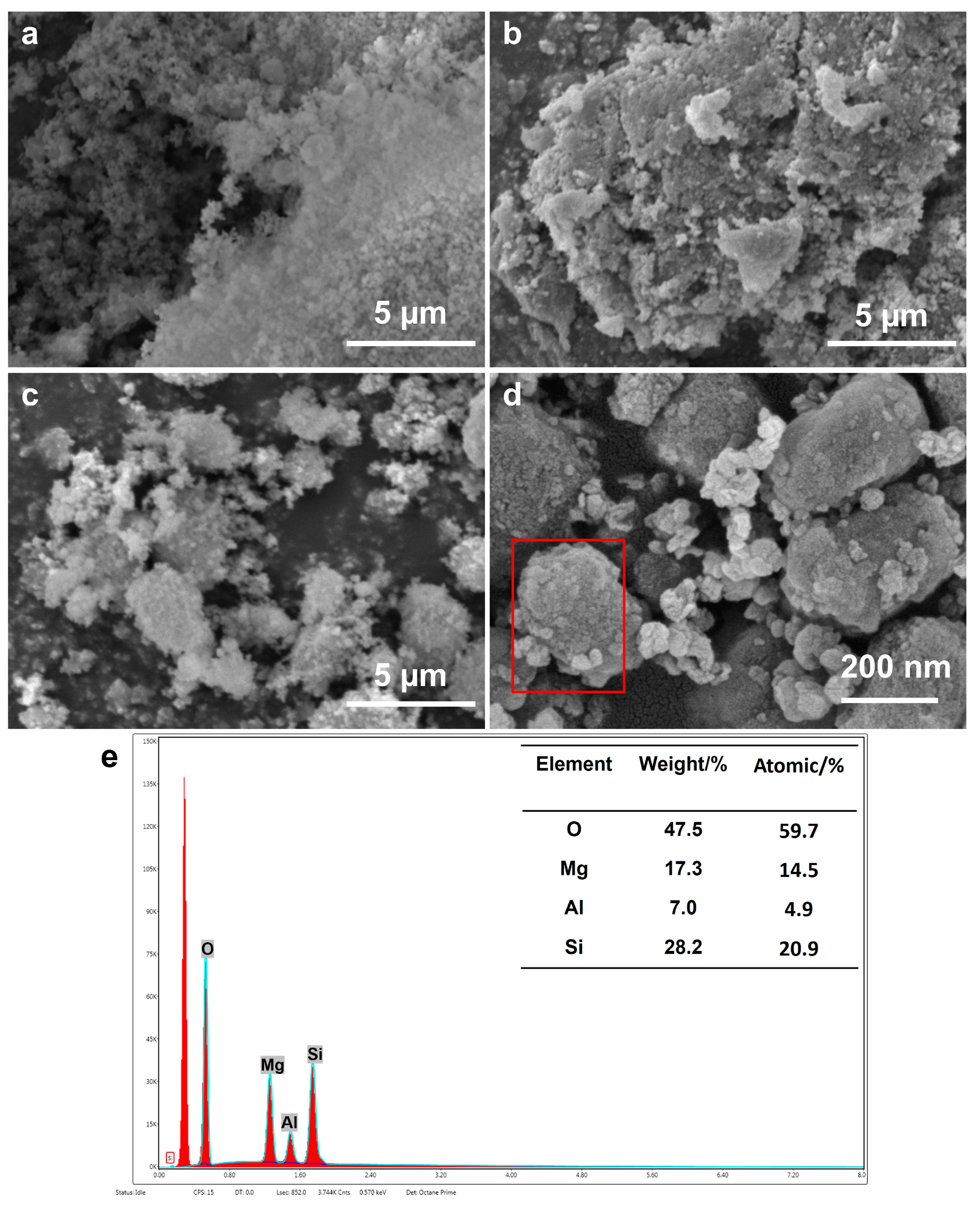
2.3. SEM
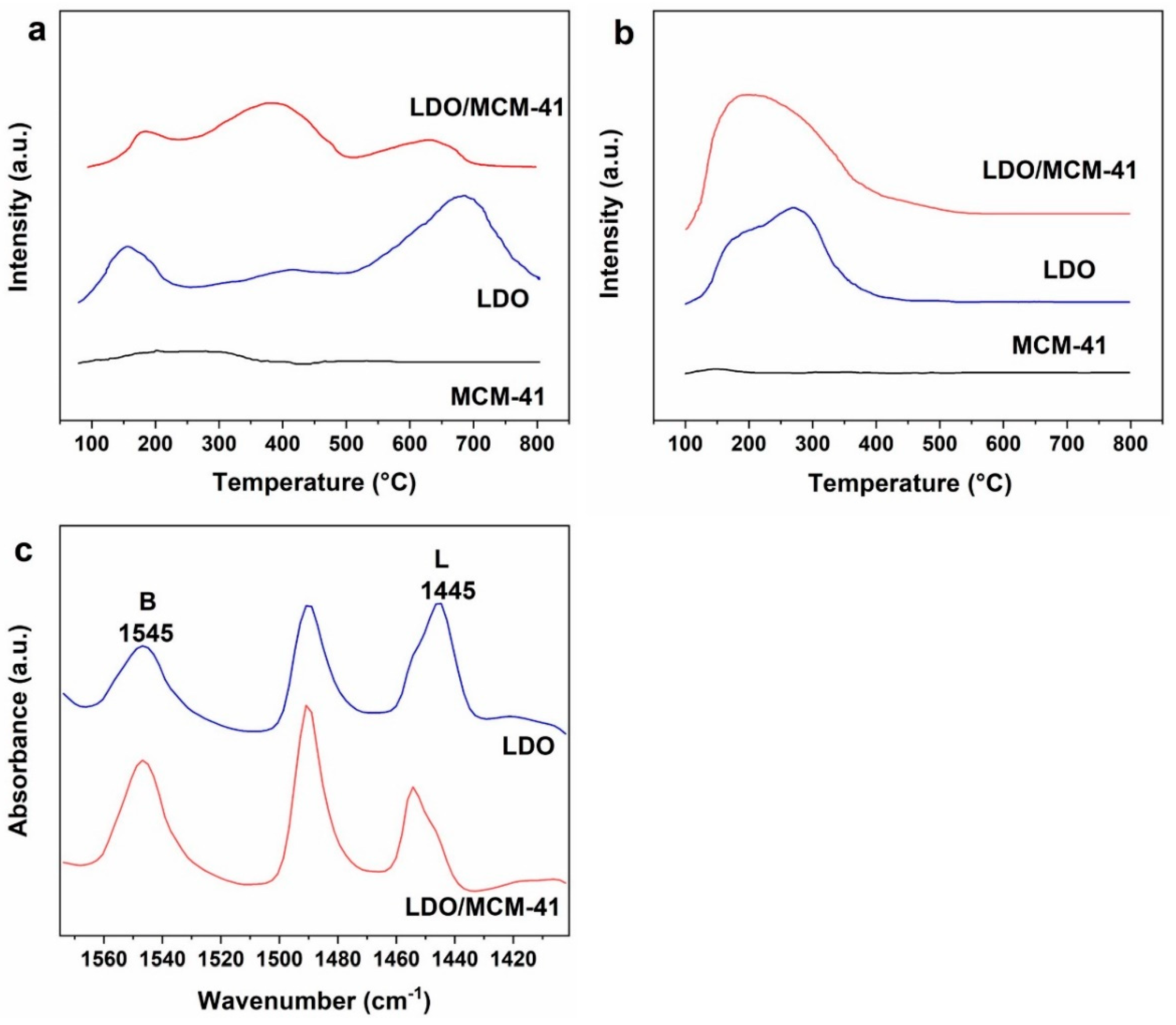
2.4. TPD and Pyridine-FTIR
2.5. Activity Evaluation
2.6. Stability
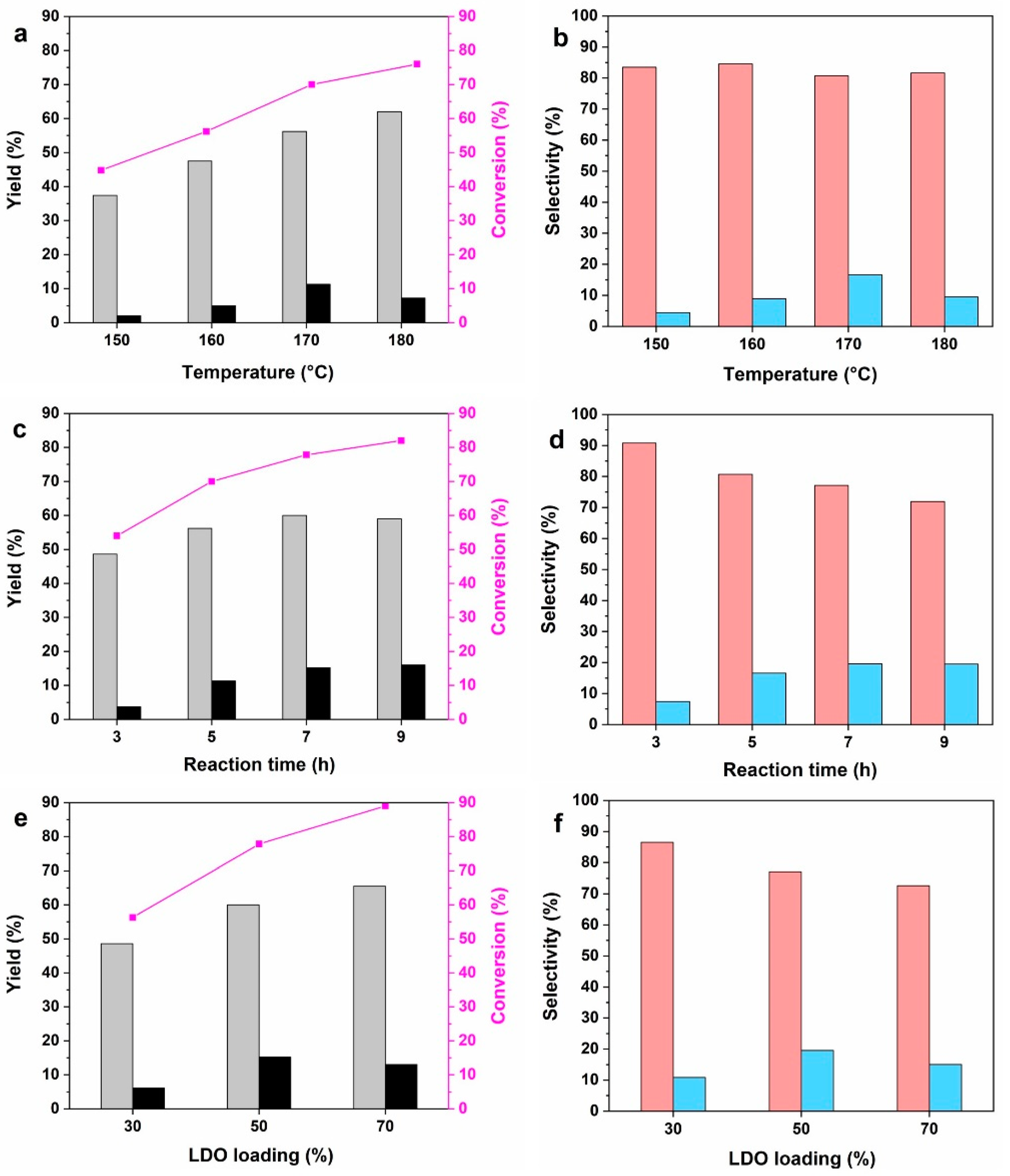
2.7. Effect of Reaction Parameters
3. Experiments
3.1. Catalyst Preparation
3.2. Characterization
3.3. Aldol Condensation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Q.; Zhang, Q.; Chen, L.; Ma, L. A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies. Fuel Process. Technol. 2020, 208, 106485. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-phase furfural-acetone aldol condensation over basic mixed oxides. Appl. Catal. B Environ. 2012, 113–114, 201–211. [Google Scholar] [CrossRef]
- Meemanah, T.; Chotirattanachote, A.; Ahmad, J.; Rashid, U.; Ngamcharussrivichai, C. Bifunctional acid–base strontium–titanium mixed oxides supported on SBA-15 for selective synthesis of renewable branched bio-jet fuel precursor. Fuel 2023, 351, 128895. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Zhang, Q.; Liu, Q.; Wang, C.; Ma, L. Synthesis of jet fuel range cycloalkanes with cyclopentanone and furfural. Energy Fuels 2020, 34, 7149. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 2013, 50, 2572–2574. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59. [Google Scholar] [CrossRef]
- Meng, X.; Su, H.; Song, R.; Su, J.; Bian, J. Solvent-Free Aldol Condensation of Cyclopentanone with Natural Clay-Based Catalysts: Origin of Activity & Selectivity. Catalysts 2023, 13, 530. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Díaz, E.; Ordóñez, S. Cyclopentanone as an Alternative Linking Reactant for Heterogeneously Catalyzed Furfural Aldol Condensation. ChemCatChem 2017, 9, 1765–1770. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárova, K.; Liptaj, T.; Štolcová, M.; Prónayová, N.; Soták, T. Cyclopentanone: A raw material for production of C15 and C17 fuel precursors. Biomass Bioenergy 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Wan, J.; Fu, L.; Yang, H.; Wang, K.; Xi, F.; Pan, L.; Li, Y.; Liu, Y. TiO2–ZrO2 Composite oxide as an acid–base bifunctional catalyst for self-condensation of cyclopentanone. Ind. Eng. Chem. Res. 2020, 59, 19918. [Google Scholar] [CrossRef]
- Ngo, D.T.; Sooknoi, T.; Resasco, D.E. Improving stability of cyclopentanone aldol condensation MgO-based catalysts by surface hydrophobization with organosilanes. Appl. Catal. B Environ. 2018, 237, 835. [Google Scholar] [CrossRef]
- Li, G.; Dissanayake, S.; Suib, S.L.; Resasco, D.E. Activity and stability of mesoporous CeO2 and ZrO2 catalysts for the self-condensation of cyclopentanone. Appl. Catal. B Environ. 2019, 267, 118373. [Google Scholar] [CrossRef]
- Pramod, C.; Upendar, K.; Mohan, V.; Sarma, D.S.; Dhar, G.M.; Prasad, P.S.; Raju, B.D.; Rao, K. Hydrotalcite-SBA-15 composite material for efficient carbondioxide capture. J. CO2 Util. 2015, 12, 109–115. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Zadsirjan, V.; Farzaneh, V. SBA-15/hydrotalcite nanocomposite as an efficient support for the immobilization of heteropolyacid: A triply-hybrid catalyst for the synthesis of 2-amino-4H-pyrans in water. Appl. Surf. Sci. 2017, 426, 8819. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Improvement on the Catalytic Performance of Mg-Zr Mixed Oxides for Furfural-Acetone Aldol Condensation by Supporting on Mesoporous Carbons. ChemSusChem 2013, 6, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Balaga, R.; Yan, P.; Ramineni, K.; Du, H.; Xia, Z.; Marri, M.R.; Zhang, Z.C. The role and performance of isolated zirconia sites on mesoporous silica for aldol condensation of furfural with acetone. Appl. Catal. A Gen. 2022, 648, 118901. [Google Scholar] [CrossRef]
- Chaudhary, G.; Gupta, N.; Singh, A.P. Synthesis and application of Cu(II) immobilized MCM-41 based solid Lewis acid catalyst for aminolysis reaction under solvent-free condition. Polyhedron 2021, 207, 115348. [Google Scholar] [CrossRef]
- Prabu, M.; Manikandan, M.; Kandasamy, P.; Kalaivani, P.R.; Rajendiran, N.; Raja, T. Synthesis of Biodiesel using the Mg/Al/Zn Hydrotalcite/SBA-15 Nanocomposite Catalyst. ACS Omega 2019, 4, 3500–3507. [Google Scholar] [CrossRef]
- Creasey, J.J.; Parlet, C.M.A.; Manayil, J.C.; Isaacs, M.A.; Wilson, J.; Lee, A.F. Facile route to conformal hydrotalcite coatings over complex architectures: A hierarchically ordered na-noporous base catalyst for FAME production. Green Chem. 2015, 17, 2398. [Google Scholar] [CrossRef]
- Velazquez-Herrera, F.D.; Gonzalez-Rodal, D.; Fetter, G.; Perez-Mayoral, E. Enhanced catalytic performance of highly mesoporous hydrotalcite/SBA-15 composites in-volved in chromene multicomponent synthesis. Microporous Mesoporous Mater. 2020, 309, 110569. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y.; Zhao, N. Synthesis of CuAl hydrotalcite-SBA-15 composites and CO2 capture using the sorbent. Appl. Surf. Sci. 2019, 481, 337. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Xiao, G.; Liu, F. Blooming-forming cyanobacteria pyrolysis over Ni-Al layered double oxides/MCM-41 for nitriles under nitrogen and methanol atmosphere. Biomass Convers. Biorefinery 2019, 10, 1063–1070. [Google Scholar] [CrossRef]
- Mi, J.; Lan, Z.; Chen, J.; Cao, Y.; Jiang, L. MgAl-LDO mixed oxide derived from layered double hydroxide: A potential support for CoMo sulfur-resistant water-gas shift catalyst. Catal. Commun. 2016, 78, 44. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Promoted nickel-based catalysts on modified mesoporous silica support: The role of yttria and magnesia on CO2 methanation. Microporous Mesoporous Mater. 2020, 306, 110455. [Google Scholar] [CrossRef]
- Matthias, T.; Katsumi, K.; Alexander, V.N.; James, P.O.; Francisco, R.; Jean, R.; Kenneth, S.W.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Abdellattif, M.H.; Mokhtar, M. MgAl-Layered double hydroxide solid base catalysts for henry reaction: A green protocol. Catalysts 2018, 8, 133. [Google Scholar] [CrossRef]
- Shao, S.; Dong, W.; Li, X.; Zhang, H.; Xiao, R.; Cai, Y. Solvent-free synthesis of jet fuel by aldol condensation and hydroprocessing of cyclopentanone as bio-mass-derivates. J. Clean. Prod. 2020, 250, 119459. [Google Scholar] [CrossRef]
- Hasni, M.; Prado, G.; Rouchaud, J.; Grange, P.; Devillers, M.; Delsarte, S. Liquid phase aldol condensation of cyclopentanone with valeraldehyde catalysed by oxynitrides possessing tuneable acid–base properties. J. Mol. Catal. A Chem. 2006, 247, 116–123. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Yu, W.; Wu, Y.; Qin, Y.; Sun, Z.; Song, L. Effects of surface acidities of MCM-41 modified with MoO3 on adsorptive desulfurization of gasoline. Appl. Surf. Sci. 2012, 263, 1–7. [Google Scholar] [CrossRef]
- Parangi, T.F.; Patel, R.M.; Chudasama, U.V. Synthesis and characterization of mesoporous Si-MCM-41 materials and their application as solid acid catalysts in some esterification reactions. Bull. Mater. Sci. 2014, 37, 609–615. [Google Scholar] [CrossRef]
- Shao, S.; Ma, L.; Li, X.; Zhang, H.; Xiao, R. Preparation of activated carbon with heavy fraction of bio-oil from rape straw pyrolysis as carbon source and its performance in the aldol condensation for aviation fuel as carrier. Ind. Crop. Prod. 2023, 192, 115912. [Google Scholar] [CrossRef]
- Wang, S.; Goulas, K.; Iglesia, E. Condensation and esterification reactions of alkanals, alkanones, and alkanols on TiO2: Elementary steps, site requirements, and synergistic effects of bifunctional strategies. J. Catal. 2016, 340, 302–320. [Google Scholar] [CrossRef]
- Liang, D.; Li, G.; Liu, Y.; Wu, J.; Zhang, X. Controllable self-aldol condensation of cyclopentanone over MgO-ZrO2 mixed oxides: Origin of activity & selectivity. Catal. Commun. 2016, 81, 33. [Google Scholar]
- Wan, J.; Yang, H.; Fu, L.; Lin, W.; Hu, Q.; Xi, F.; Pan, L.; Li, Y.; Liu, Y. The Cyclopentanone Self-condensation Over Calcined and Uncalcined TiO2–ZrO2 with Different Acidic Properties. Catal. Lett. 2021, 152, 806–820. [Google Scholar] [CrossRef]
- Ngo, D.T.; Tan, Q.; Wang, B.; Resasco, D.E. Aldol Condensation of Cyclopentanone on Hydrophobized MgO. Promotional Role of Water and Changes in the Rate-Limiting Step upon Organosilane Functionalization. ACS Catal. 2019, 9, 2831–2841. [Google Scholar] [CrossRef]
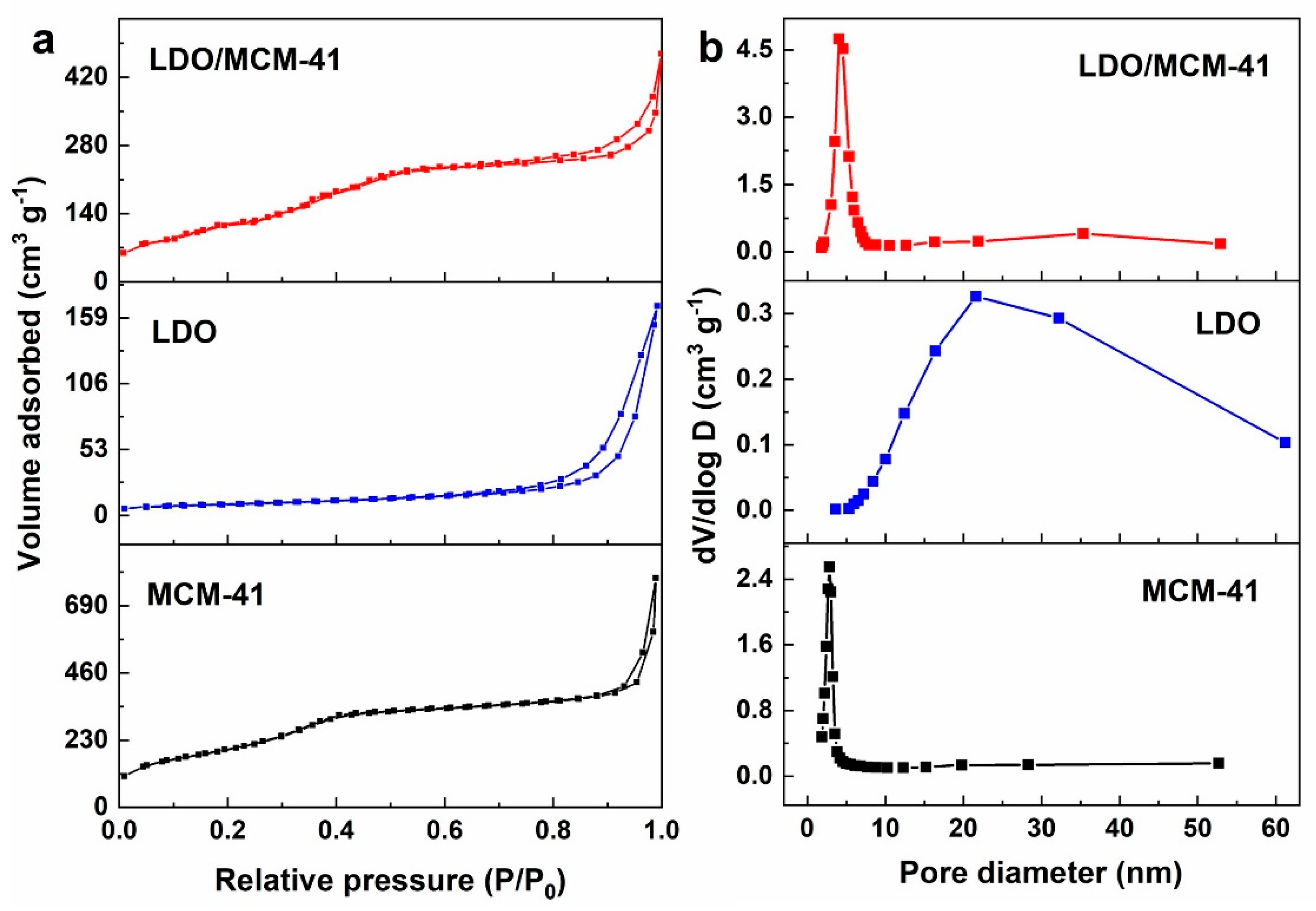

| Catalyst | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| MCM-41 | 738 | 0.65 | 3.5 |
| LDO | 150 | 0.18 | 15.2 |
| LDO/MCM-41 | 541 | 0.50 | 6.1 |
| Catalyst | Basic Sites (mmol g−1) | Total Acid Sites (mmol g−1) | B/L Ratio | |||
|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | |||
| MCM-41 | 0.01 | 0 | 0 | 0.01 | 0.01 | - a |
| LDO | 0.16 | 0.10 | 0.36 | 0.62 | 0.60 | 1.40 |
| LDO/MCM-41 | 0.06 | 0.25 | 0.12 | 0.43 | 0.77 | 2.37 |
| Catalyst | Conversion (%) | C10 Yield (%) | C15 Yield (%) | Carbon Balance (%) | TOF (h−1) c |
|---|---|---|---|---|---|
| LDO (0.1 g, 2 h) a | 42.8 | 37.9 | - d | 98 | 41 |
| LDO (0.05 g, 4 h) b | 34.0 | 27.2 | - d | 96 | 32 |
| LDO/MCM-41(0.1 g, 2 h) a | 18.0 | 15.1 | 1.2 | 100 | 23 |
| LDO/MCM-41(0.05 g, 4 h) b | 16.5 | 13.0 | 1.0 | 100 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Shang, N.; Wang, J.; Liu, H. Mesoporous-Layered Double Oxide/MCM-41 Composite with Enhanced Catalytic Performance for Cyclopentanone Aldol Condensation. Molecules 2023, 28, 7920. https://doi.org/10.3390/molecules28237920
Yang J, Shang N, Wang J, Liu H. Mesoporous-Layered Double Oxide/MCM-41 Composite with Enhanced Catalytic Performance for Cyclopentanone Aldol Condensation. Molecules. 2023; 28(23):7920. https://doi.org/10.3390/molecules28237920
Chicago/Turabian StyleYang, Jinfan, Ning Shang, Jiachen Wang, and Huimin Liu. 2023. "Mesoporous-Layered Double Oxide/MCM-41 Composite with Enhanced Catalytic Performance for Cyclopentanone Aldol Condensation" Molecules 28, no. 23: 7920. https://doi.org/10.3390/molecules28237920
APA StyleYang, J., Shang, N., Wang, J., & Liu, H. (2023). Mesoporous-Layered Double Oxide/MCM-41 Composite with Enhanced Catalytic Performance for Cyclopentanone Aldol Condensation. Molecules, 28(23), 7920. https://doi.org/10.3390/molecules28237920