Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds
Abstract
:1. Introduction
2. Results and Discussion
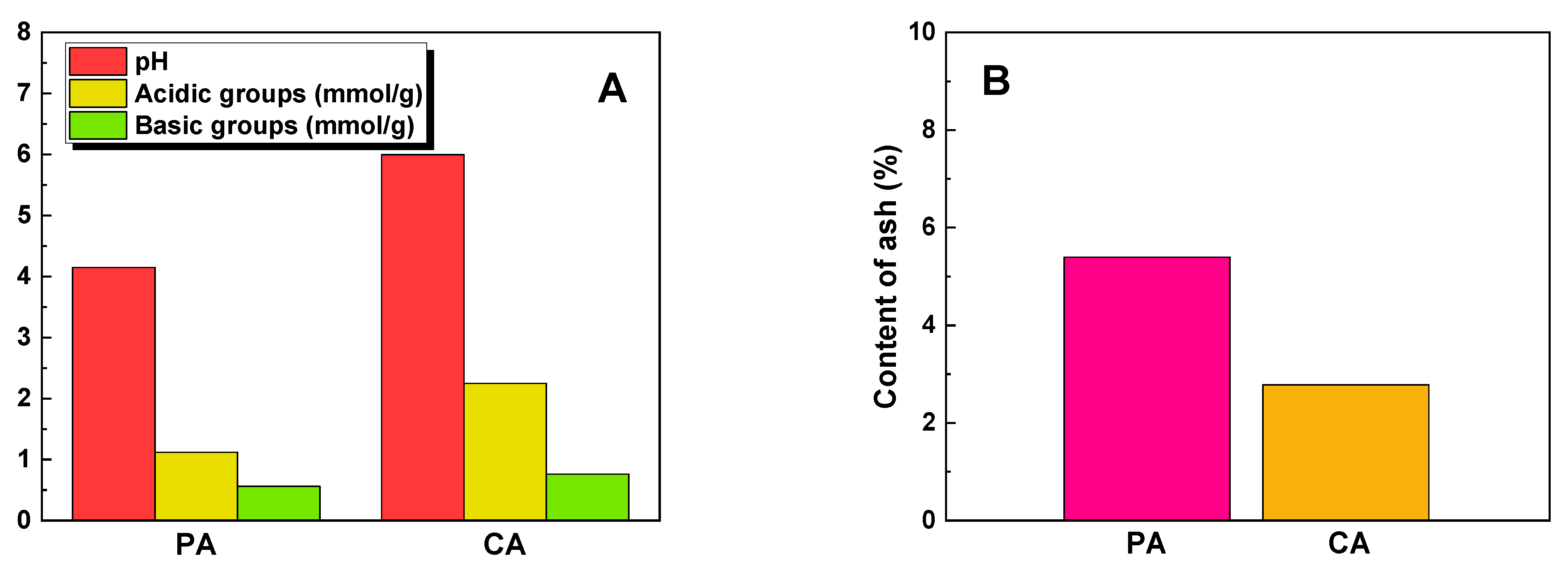
2.1. Characterization of the Biochar Samples
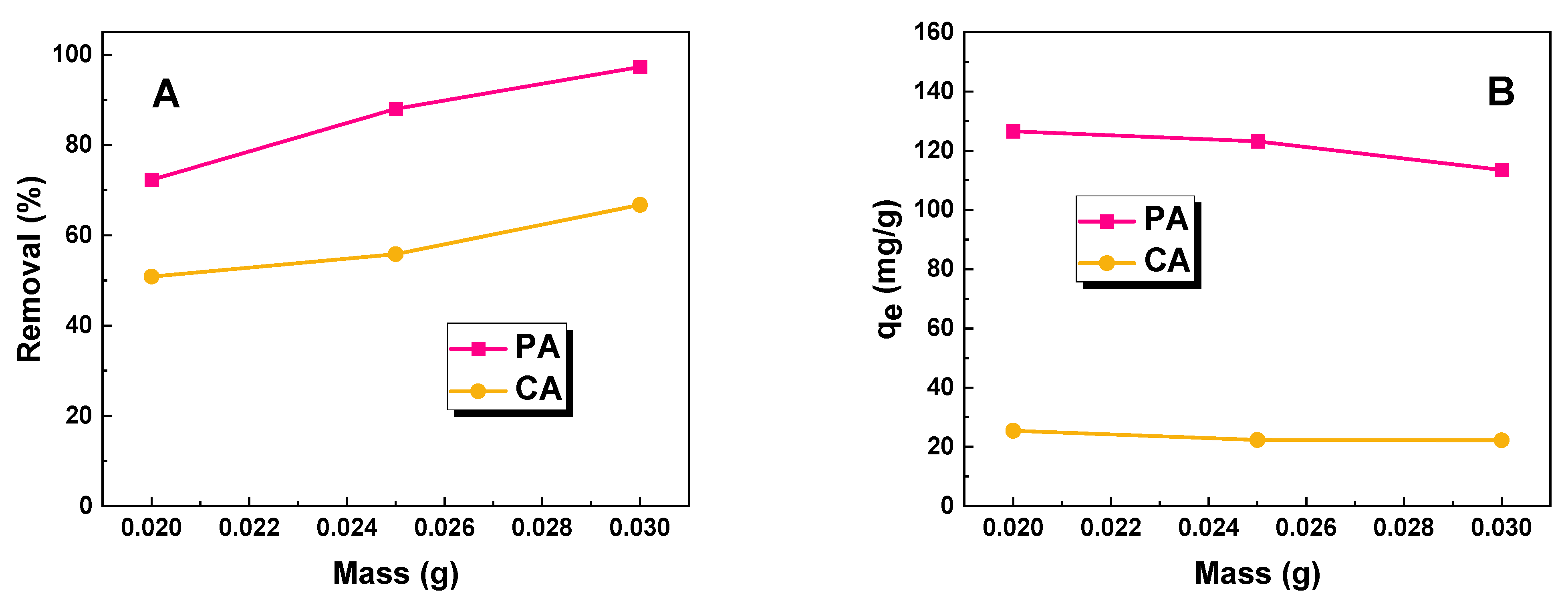
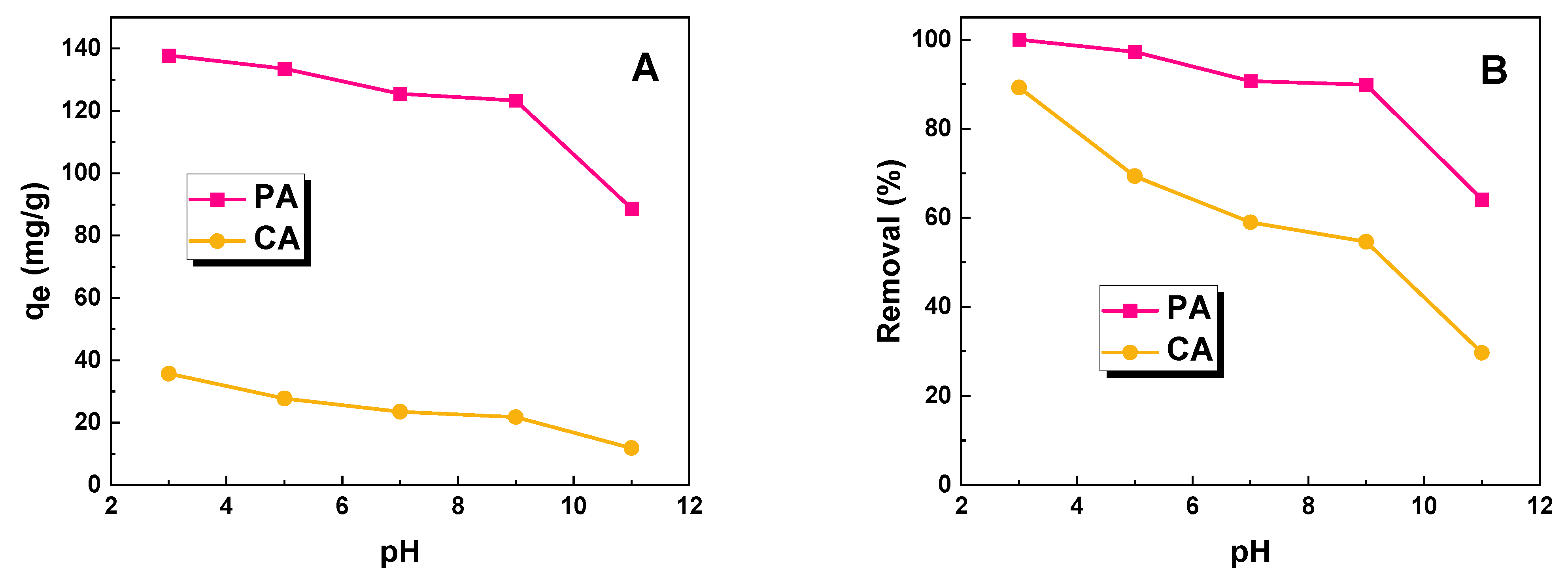
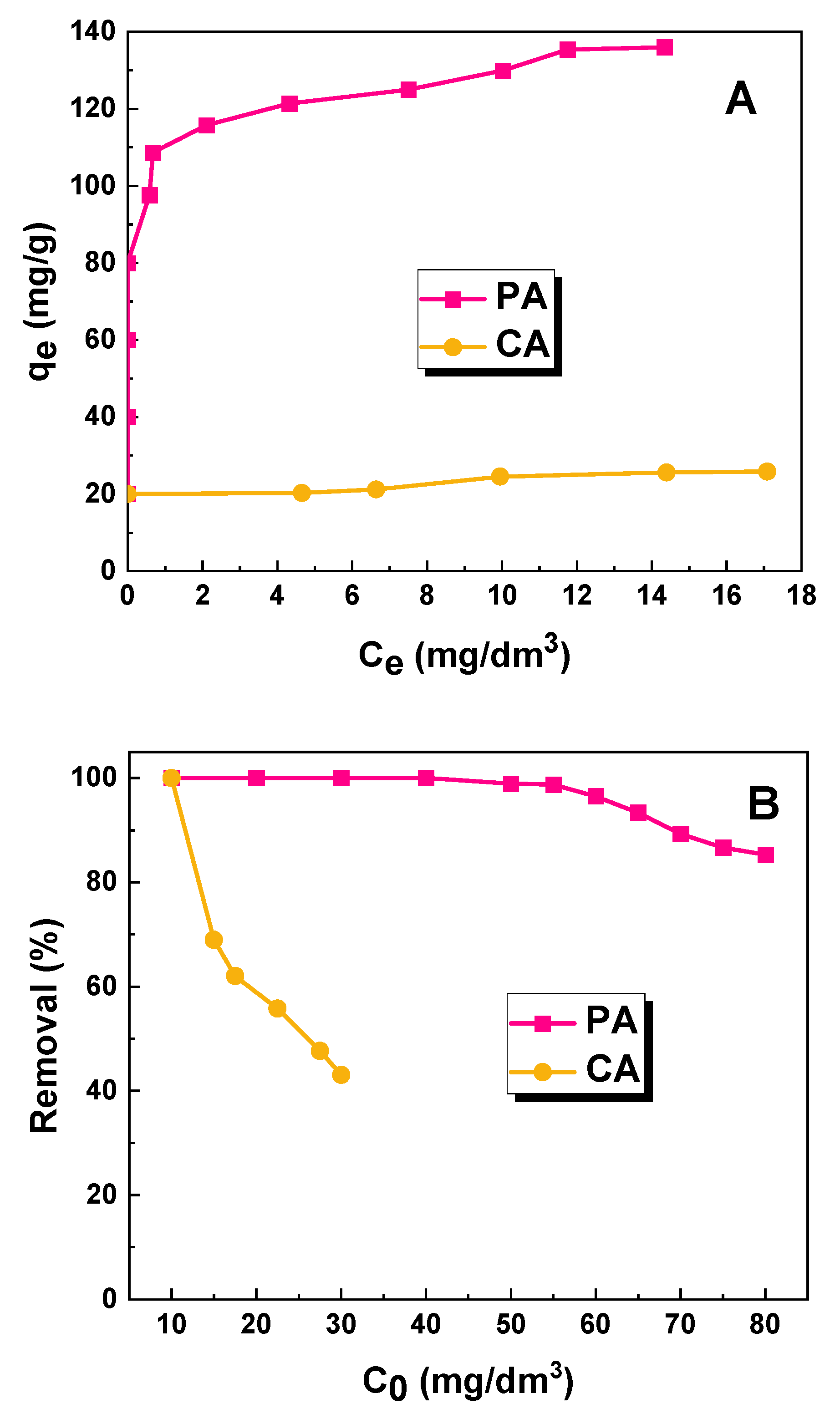
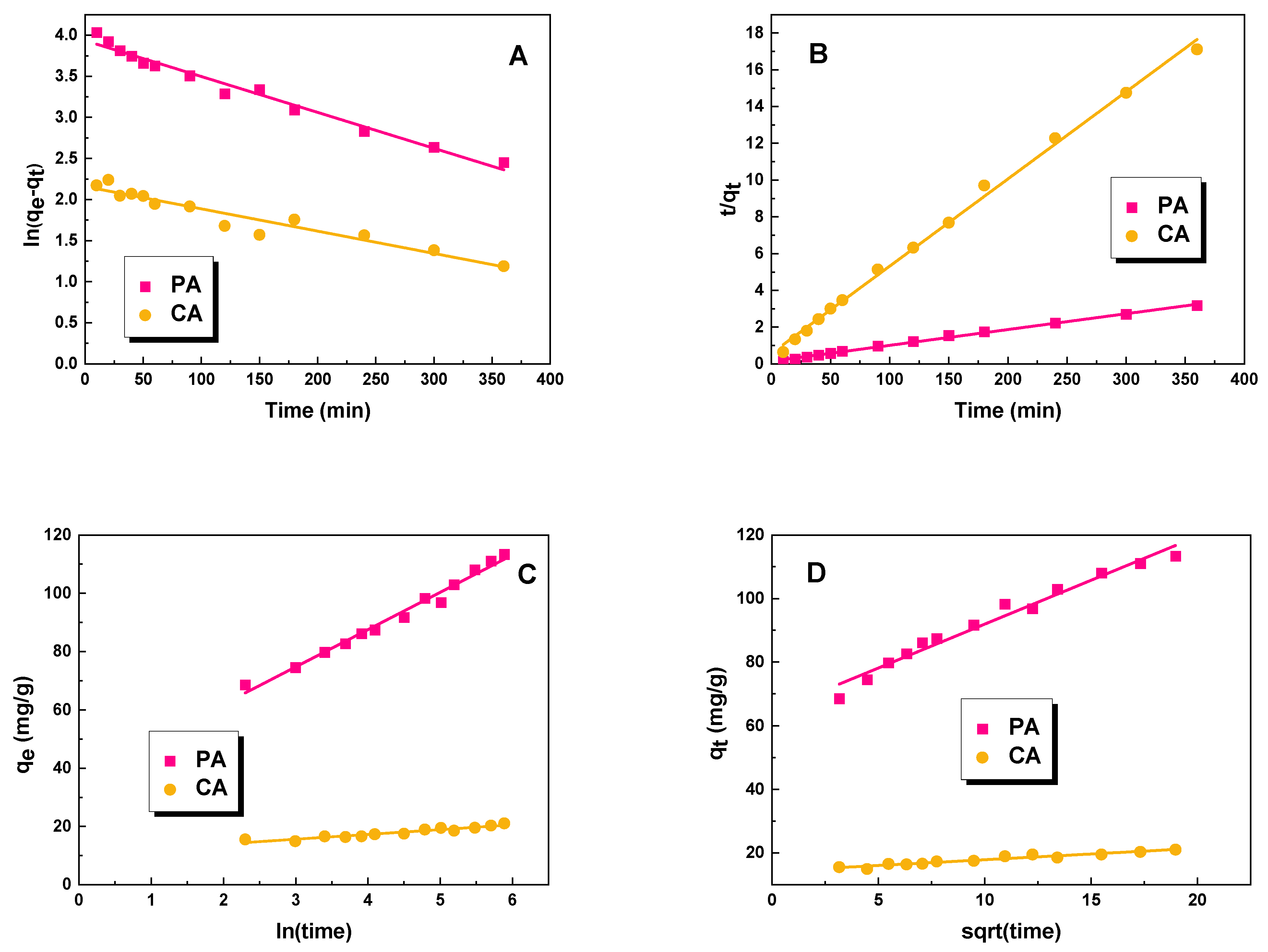
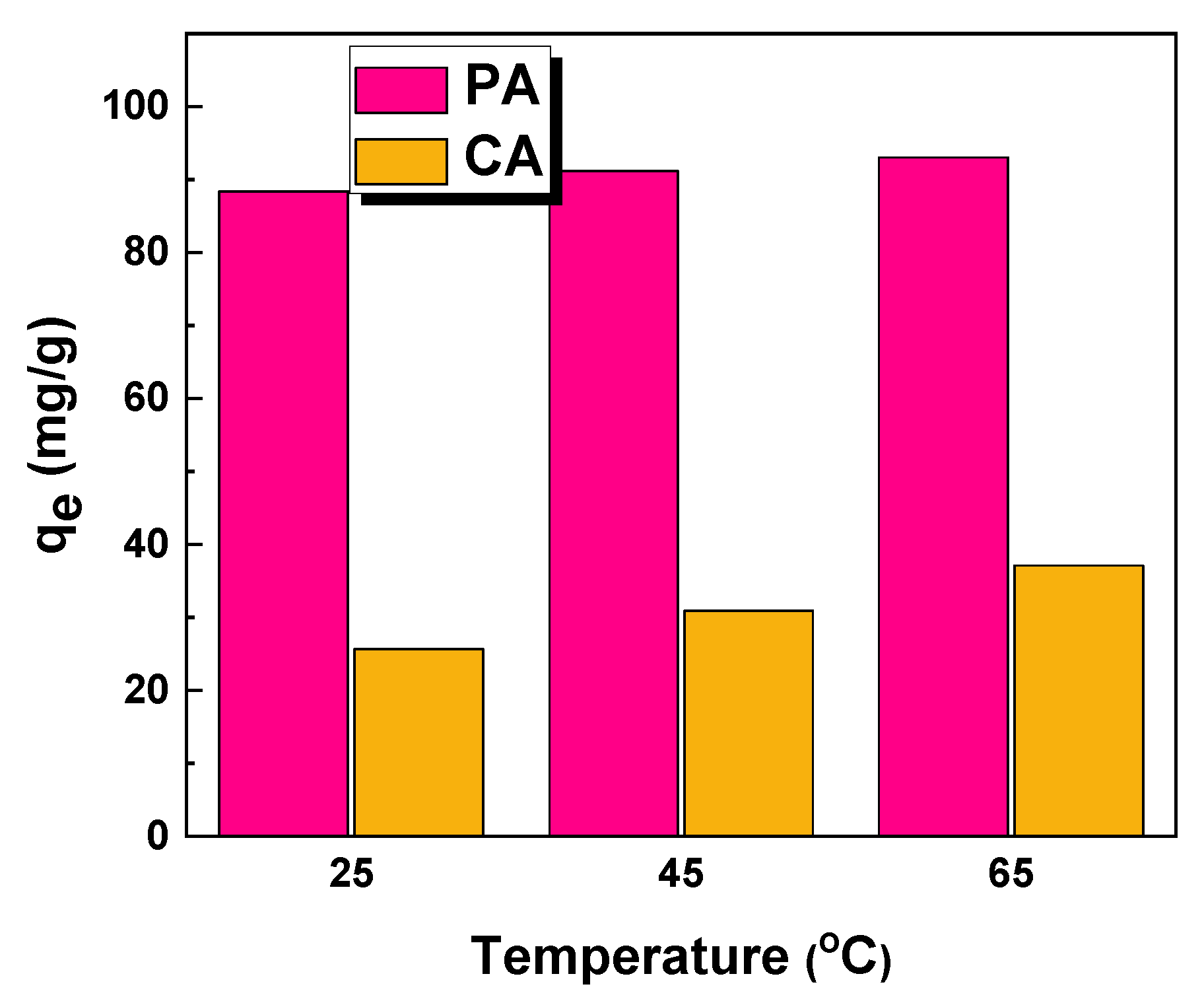
2.2. Adsorption Studies
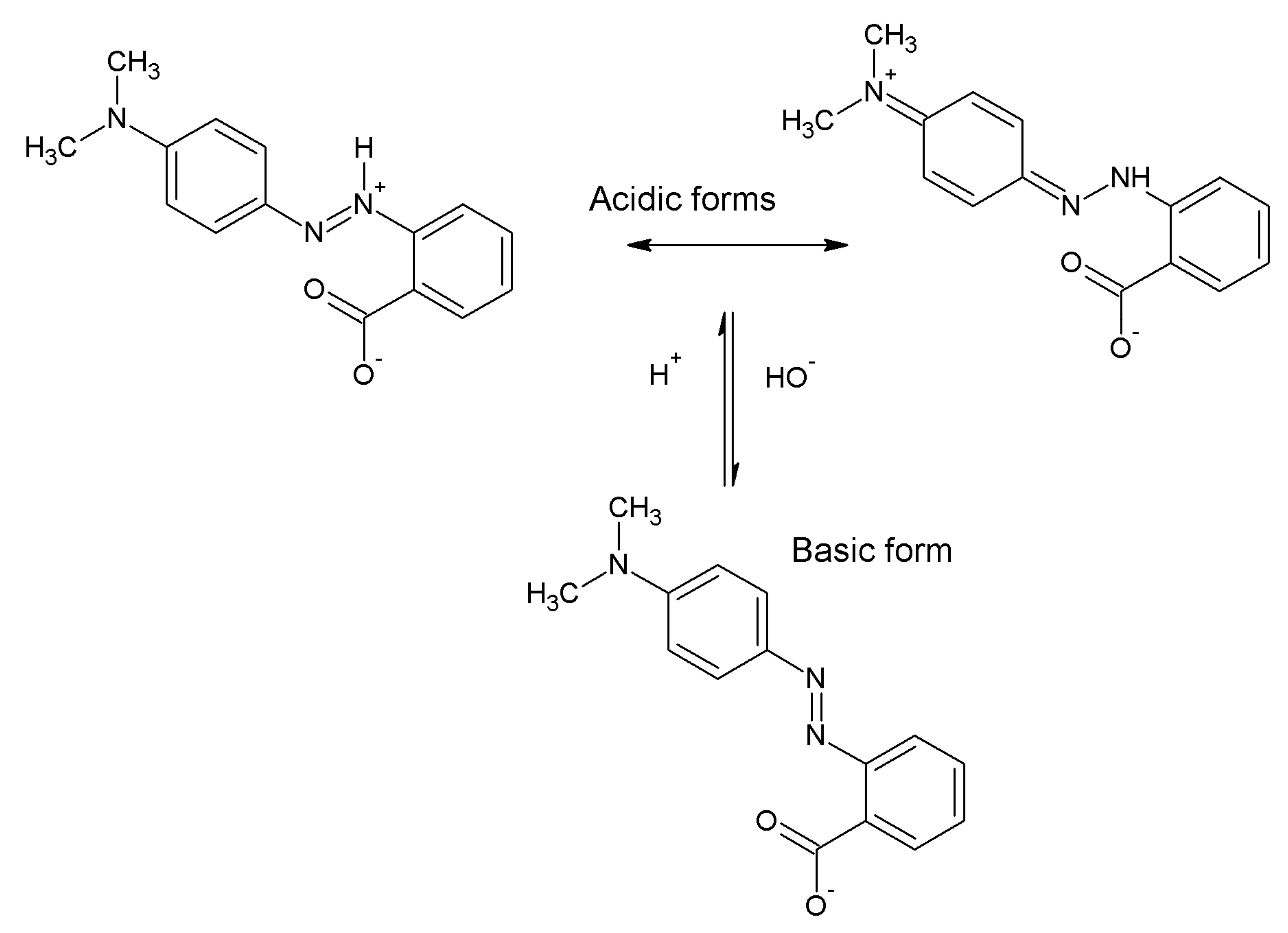
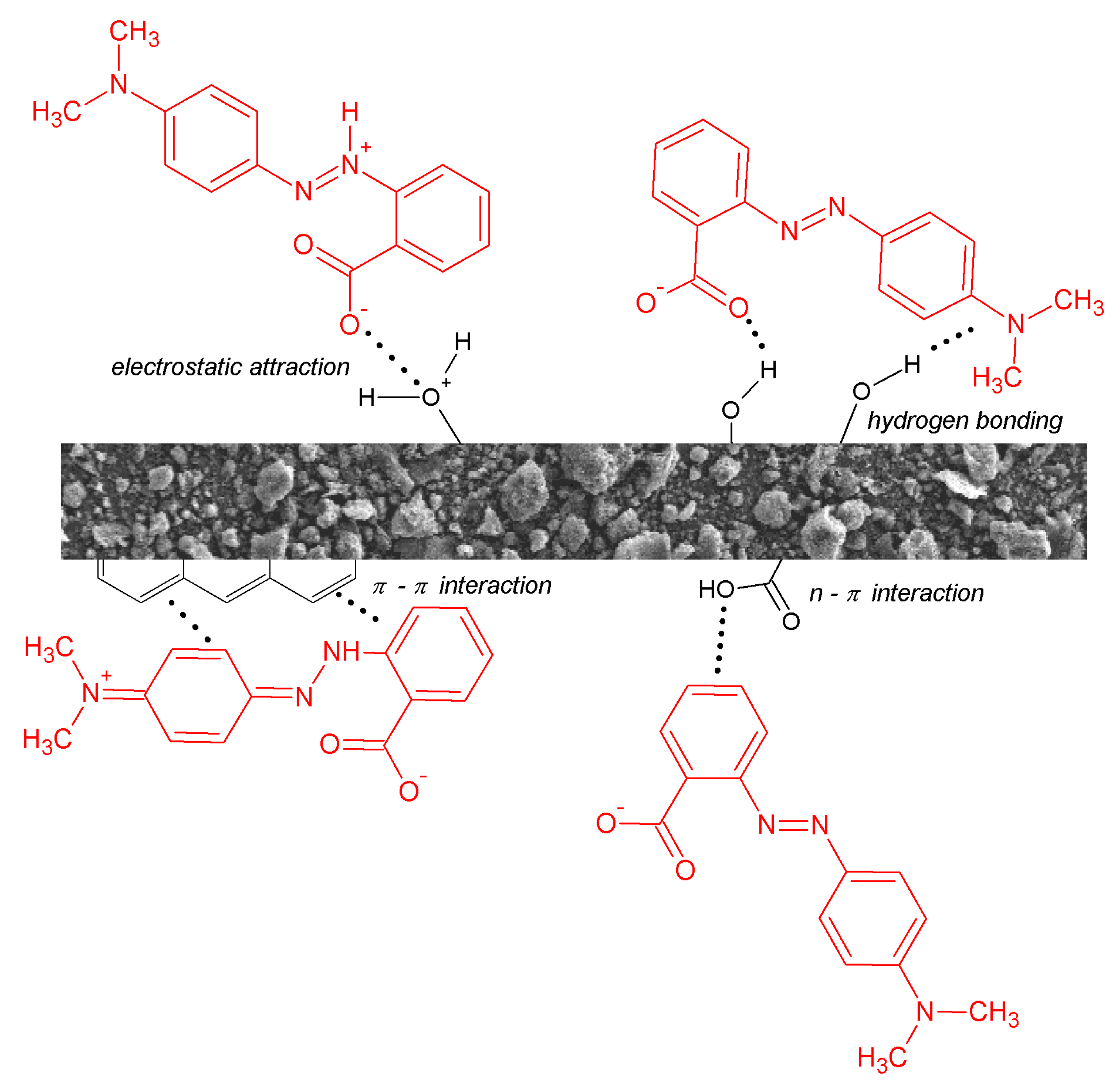
2.3. Mechanism of Adsorption
3. Materials and Methods
3.1. Precursor and Biochar Samples Preparation
3.2. Characterization of Resulted Biochar Samples
3.3. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Albuquerque, A.F.; Vendemiatti, J.A.; Morales, D.A.; Ormond, A.B.; Freeman, H.S.; Umbuzeiro, G. Chlorine disinfection of dye wastewater: Implications for a commercial azo dye mixture. Sci. Total Environ. 2013, 442, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, S.; Castro-Muñoz, R. Advanced biomaterials and alternatives tailored as membranes for water treatment and the latest innovative European water remediation projects: A review. Case Stud. Chem. Environ. Eng. 2022, 5, 100205. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Jiang, W.; Xiang, X.; Li, S.; Zhang, X. Synthesis, characterization and machine learning based performance prediction of straw activated carbon. J. Clean. Prod. 2019, 212, 1210–1223. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater: The perspective role of nanomaterials and nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar]
- Serafin, J.; Dziejarski, B. Activated carbons—Preparation, characterization and their application in CO2 capture: A review. Environ. Sci. Pollut. Res. 2023, 1–55, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Guerreiro, M.C. Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers. Química Nova 2011, 34, 472–476. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Demiral, H.; Uzun, I. Preparation and characterization of activated carbons from poplar wood (Populus L.). Surf. Interface Anal. 2010, 42, 1338–1341. [Google Scholar] [CrossRef]
- Hussaro, K. Preparation of activated carbon from palm oil shell by chemical activation with Na2CO3 and ZnCl2 as impregnated agents for H2S adsorption. Am. J. Environ. Sci. 2014, 10, 336–346. [Google Scholar] [CrossRef]
- Wolski, R.; Bazan-Wozniak, A.; Pietrzak, R. Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials. Molecules 2023, 28, 6712. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mahardiani, L.; Saputro, S.; Baskoro, F.; Zinki, N.M.; Taufiq, M. Facile synthesis of carboxylated activated carbon using green approach for water treatment. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 012003. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nowicki, P.; Pietrzak, R. Removal of NO2 from gas stream by activated bio-carbons from physical activation of residue of supercritical extraction of hops. Chem. Eng. Res. Des. 2021, 166, 67–73. [Google Scholar] [CrossRef]
- Alzahrani, H.R.; Kumakli, H.; Ampiah, E.; Mehari, T.; Thornton, A.J.; Babyak, C.M.; Fakayode, S.O. Determination of macro, essential trace elements, toxic heavy metal concentrations, crude oil extracts and ash composition from Saudi Arabian fruits and vegetables having medicinal values. Arab. J. Chem. 2017, 10, 906–913. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methylene Blue and Methyl Red from Aqueous Solutions Using Activated Carbons Obtained by Chemical Activation of Caraway Seed. Molecules 2023, 28, 6306. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. J. Chem. Eng. 2020, 393, 124785. [Google Scholar] [CrossRef]
- Rajoriya, S.; Saharan, V.K.; Pundir, A.S.; Nigam, M.; Roy, K. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. Curr. Opin. Green Sustain. Chem. 2021, 4, 100180. [Google Scholar] [CrossRef]
- Khadka, B.; Bhattarai, A. UV-VIS studies on interaction between sodium dioctylsulfosuccinate (aot) and methyl red. Rev. Roum. Chim. 2020, 65, 989–996. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Wolski, R.; Paluch, D.; Nowicki, P.; Pietrzak, R. Removal of organic dyes from aqueous solutions by activated carbons prepared from residue of supercritical extraction of marigold. Materials 2022, 15, 3655. [Google Scholar] [CrossRef] [PubMed]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Ortíz, Á.; Figueroa-Lopez, K.J.; Ortega-Toro, R. Kinetics and adsorption equilibrium in the removal of azo-anionic dyes by modified cellulose. Sustainability 2022, 14, 3640. [Google Scholar] [CrossRef]
- Khan, T.A.; Nouman, M.; Dua, D.; Khan, S.A.; Alharthi, S.S. Adsorptive scavenging of cationic dyes from aquatic phase by H3PO4 activated Indian jujube (Ziziphus mauritiana) seeds based activated carbon: Isotherm, kinetics, and thermodynamic study. J. Saudi Chem. Soc. 2022, 26, 101417. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Malash, G.F.; El-Khaiary, M.I. Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem. Eng. J. 2010, 163, 256–263. [Google Scholar] [CrossRef]
- Chen, T.; Da, T.; Ma, Y. Reasonable calculation of the thermodynamic parameters from adsorption equilibrium constant. J. Mol. Liq. 2021, 322, 114980. [Google Scholar] [CrossRef]
- Tran, H.V.; Hoang, L.T.; Huynh, C.D. An investigation on kinetic and thermodynamic parameters of methylene blue adsorption onto graphene-based nanocomposite. Chem. Phys. 2020, 535, 110793. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Paluch, D.; Wolski, R.; Cielecka-Piontek, J.; Nosal-Wiercińska, A.; Pietrzak, R. Biocarbons obtained from fennel and caraway fruits as adsorbents of methyl red sodium salt from water system. Materials 2022, 15, 8177. [Google Scholar] [CrossRef]
- Canales-Flores, R.A.; Prieto-García, F. Taguchi optimization for production of activated carbon from phosphoric acid impregnated agricultural waste by microwave heating for the removal of methylene blue. Diam. Relat. Mater. 2020, 109, 108027. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of pseudo-first-order, pseudo-second-order and modified pseudo-first-order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Cielecka-Piontek, J.; Nosal-Wiercińska, A.; Pietrzak, R. Adsorption of organic compounds on adsorbents obtained with the use of microwave heating. Materials 2022, 15, 5664. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Dziejarski, B. Application of isotherms models and error functions in activated carbon CO2 sorption processes. Microporous Mesoporous Mater. 2023, 354, 112513. [Google Scholar] [CrossRef]
- Tien, C.; Ramarao, B.V. On the significance and utility of the Lagergren model and the pseudo second-order model of batch adsorption. Sep. Sci. Technol. 2017, 52, 975–986. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]



| Sample | Surface Area 1 (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Iodine Number (mg/g) | ||
|---|---|---|---|---|---|---|
| Total | Micropore | Total | Micropore | |||
| PA | 89 | 29 | 0.139 | 0.081 | 6.27 | 465 |
| CA | 345 | 302 | 0.349 | 0.165 | 4.04 | 371 |
| Element | Sample | ||
|---|---|---|---|
| Precursor [%] | PA [%] | CA [%] | |
| Na | 0.231 | 0.012 | 0.005 |
| K | 3.154 | 0.041 | 0.026 |
| Ca | 2.430 | 0.279 | 0.063 |
| Mg | 0.971 | 0.289 | 0.022 |
| Fe | 0.192 | 0.064 | 0.047 |
| Mn | 0.027 | 0.006 | 0.002 |
| Al | 0.184 | 0.066 | 0.030 |
| Cu | 0.002 | 0.003 | 0.003 |
| Cd | 0.001 | 0.001 | 0.001 |
| Cr | 0.002 | 0.001 | 0.001 |
| Pb | 0.001 | 0.002 | 0.002 |
| Ni | 0.001 | 0.002 | 0.001 |
| P | 2.199 | 0.234 | 0.033 |
| S | 0.472 | 0.060 | 0.022 |
| Si | 0.221 | 0.135 | 0.041 |
| Zn | 0.005 | 0.012 | 0.011 |
| SiO2 | 71 | 69 | 70 |
| Precursor | Activator | Precursor: Activator Ratio | Activation Time (min) | Activation Temperature (°C) | Sorption Capacity (mg/g) | Source |
|---|---|---|---|---|---|---|
| Fennel seeds | CO2 | - | 30 | 800 | 135 | This study |
| Na2CO3 | 1:2 | 45 | 700 | 26 | ||
| Caraway seeds | Na2CO3 | 1:2 | 45 | 700 | 28 | [18] |
| Green tea leaves | Na2CO3 | 1:2 | 60 | 700 | 70 | [19] |
| Biogas Plant Waste | CO2 | - | 45 | 800 | 31 | [12] |
| Marigold | CO2 | - | 60 | 800 | 102 | [22] |
| Isotherms | Parameters | Sample | |
|---|---|---|---|
| PA | CA | ||
| qexp (mg/g) | 135 | 26 | |
| Langmuir | KL (dm3/mg) | 1.261 | 0.473 |
| R2 | 0.887 | 0.940 | |
| qm (mg/g) | 130 | 29 | |
| Freundlich | KF (mg/g(dm3/mg)1/n) | 45.04 | 14.97 |
| R2 | 0.998 | 0.997 | |
| 1/n | 0.047 | 0.191 | |
| Dubinin–Radushkevich | E (kJ/mol) | 1119.04 | 649.46 |
| R2 | 0.733 | 0.882 | |
| qm (mg/g) | 133 | 26 | |
| Temkin | BT (J/mol) | 3.43 | 9.70 |
| R2 | 0.887 | 0.971 | |
| AT (L/mg) | 1.00 | 1.05 | |
| Halsey | KH | 104.47 | 15.36 |
| R2 | 0.926 | 0.943 | |
| nH | 10.03 | 5.76 | |
| Sips | KS (L/g) | 998.49 | 13.03 |
| R2 | 0.787 | 0.695 | |
| aS (L/g) | 0.61 | 4.48 | |
| βS | 0.77 | 0.29 | |
| Kinetics Model | Parameters | Sample | |
|---|---|---|---|
| PA | CA | ||
| qe (mg/g) | 113 | 21 | |
| Pseudo-first-order | k1 (1/min) | 1.22 × 10−5 | 7.53 × 10−6 |
| R2 | 0.979 | 0.925 | |
| qe/cal (mg/g) | 51 | 8 | |
| Pseudo-second-order | k2 (g/mg × min) | 24.56 × 10−3 | 3.52 × 10−3 |
| R2 | 0.997 | 0.997 | |
| qe/cal (mg/g) | 116 | 21 | |
| Elovich | α (mg/g × min) | 0.078 | 0.607 |
| R2 | 0.985 | 0.909 | |
| β (g/mg) | 2.17 × 10−2 | 1.09 × 10−3 | |
| Intraparticle diffusion | kid (mg/g × min1/2) | 2.76 | 0.36 |
| R2 | 0.973 | 0.933 | |
| C (mg/g) | 64.33 | 14.23 | |
| Sample | Temperature (°C) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/mol × K) |
|---|---|---|---|---|
| PA | 25 | −8.85 | 63.06 | 239.95 |
| 45 | −12.33 | |||
| 65 | −18.56 | |||
| CA | 25 | −3.20 | 42.88 | 153.48 |
| 45 | −5.13 | |||
| 65 | −9.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paluch, D.; Bazan-Wozniak, A.; Wolski, R.; Nosal-Wiercińska, A.; Pietrzak, R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules 2023, 28, 7786. https://doi.org/10.3390/molecules28237786
Paluch D, Bazan-Wozniak A, Wolski R, Nosal-Wiercińska A, Pietrzak R. Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules. 2023; 28(23):7786. https://doi.org/10.3390/molecules28237786
Chicago/Turabian StylePaluch, Dorota, Aleksandra Bazan-Wozniak, Robert Wolski, Agnieszka Nosal-Wiercińska, and Robert Pietrzak. 2023. "Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds" Molecules 28, no. 23: 7786. https://doi.org/10.3390/molecules28237786
APA StylePaluch, D., Bazan-Wozniak, A., Wolski, R., Nosal-Wiercińska, A., & Pietrzak, R. (2023). Removal of Methyl Red from Aqueous Solution Using Biochar Derived from Fennel Seeds. Molecules, 28(23), 7786. https://doi.org/10.3390/molecules28237786








