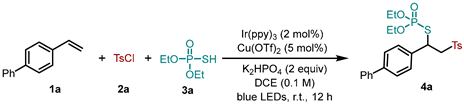
Abstract
Both sulfonyl and phosphorothioate are important privileged structural motifs which are widely presented in pharmaceuticals and agrochemicals. Herein, we describe an efficient approach to synthesizing sulfonyl-containing phosphorothioates by merging photoredox and copper catalysis at room temperature. This protocol is compatible with a wide range of substrates and can be applied to the late-stage modification of complex molecules. Control experiments are conducted to demonstrate the generation of the sulfonyl radical in the transformation.
1. Introduction
In recent years, phosphorothioates have received continued attention owing to their remarkable biological activities and broad applications in the pharmaceutical and agrochemical industries [1,2,3,4]. These compounds not only can be used as potential therapeutic molecules, anticancer agents, antivirals and pesticides but also serve as valuable building blocks in organic synthesis [5,6,7,8,9]. In this context, numerous methodologies have been reported for their synthesis [10]. On the other hand, as a prominent class of organosulfur compounds, sulfones play a pivotal role in various drugs, pesticides and natural products [11,12,13,14,15,16,17]. To date, methods for the construction of sulfonyl compounds from sulfonyl chlorides, sodium sulfinates or sulfur dioxide surrogates have attracted considerable attention, and significant progress has been made in this area [18,19,20,21,22,23,24]. However, despite these achievements, the preparation of sulfonyl-containing phosphorothioates has been rarely explored.
In the last few decades, the direct vicinal difunctionalization of alkenes has emerged as a powerful strategy to access complex molecules, which could introduce two functional groups into the double bond simultaneously [25,26,27,28,29,30,31,32]. Accordingly, substantial efforts have been devoted to the construction of sulfonyl-containing compounds via the difunctionalization of alkenes, such as hydrosulfonation, oxysulfonation, aminosulfonylation and selenosulfonation [33,34,35]. However, only limited examples have been developed for the thiosulfonylation of alkenes involving the simultaneous installation of two C–S bonds (Scheme 1a). For example, Xu et al. reported a thiosulfonylation reaction of alkenes in the presence of a dual gold/photoredox catalysis system [36,37]. Lian and co-workers established a thiocyanatosulfonation of α,β-unsaturated amides/esters using TMSNCS, aryldiazonium tetrafluoroborates and sulfur dioxide to access β-thiocyanated sulfone compounds [38]. Our group also disclosed an intramolecular thiosulfonylation of alkenes to deliver sulfonated [3,1]-benzothiazepines under metal-free conditions [39]. Given the importance of phosphorothioates and sulfones in organic and medicinal chemistry, we hypothesized that the introduction of these two functional groups into alkenes using the vicinal difunctionalization strategy would contribute to synthetic community and drug discovery. Herein, we describe a mild and efficient method to synthesize sulfonyl-containing phosphorothioates via the thiosulfonylation of alkenes using sulfonyl chlorides and phosphorothioic acids (Scheme 1b). In the presence of photoredox and copper catalysis, this practical reaction features a broad substrate scope and can be readily applied to the late-stage functionalization of bioactive molecules.
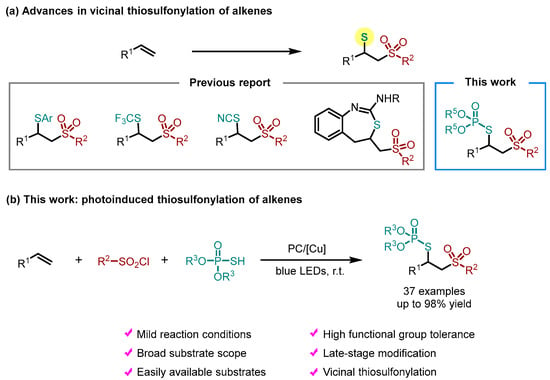
Scheme 1.
The development of alkene thiosulfonylation.
2. Results and Discussion
We began our study with the use of 4-vinylbiphenyl 1a, tosyl chloride 2a and O,O-diethyl S-hydrogen phosphorothioate 3a in the presence of Ir(ppy)3 and Cu(OTf)2 along with K2HPO4 as the base in DCE under 30 W blue LED irradiation at room temperature. Gratifyingly, we found that the desired product 4a could be obtained with an excellent yield of 99% (93% isolated yield, Table 1, entry 1). The control experiments showed that no reaction occurred in the absence of Ir(ppy)3 or light irradiation, confirming that both the photocatalyst and visible light were essential for this transformation (Table 1, entries 2–3). Without Cu(OTf)2 in the reaction system, the yield of 4a was diminished significantly (Table 1, entry 4). When changing the photocatatlyst to Ir(ppy)2(dtbbpy)PF6, the corresponding product was achieved with a comparable yield (Table 1, entry 5). Similarly, the use of other copper catalysts such as CuCl2 and Cu(CH3CN)4PF6 also underwent the reaction efficiently (Table 1, entries 6–7). Replacing K2HPO4 with other bases (KHCO3, K2CO3 and Na2CO3) produced inferior results (Table 1, entries 8–10). Finally, the effect of solvent was examined, and the reaction in THF and CH3CN resulted in moderate yields (Table 1, entries 11–12).

Table 1.
Optimization of the reaction conditions a.
With the optimized reaction conditions established, we turned our attention to investigating the scope and generality of the three-component vicinal difunctionalization of alkenes (Figure 1 and Supplementary Materials). To verify the scalability of this protocol, the model reaction was carried out on a 6 mmol scale to obtain the product 4a with a 78% yield. In general, a range of alkenes bearing both electron-donating and electron-withdrawing substituents on the aromatic ring reacted smoothly to provide the products 4b–4l with moderate to good yields. Functional groups such as methyl, methoxy, fluoro, chloro, trifluoromethyl and ester were all well tolerated in this transformation. Notably, a heteroaromatic alkene containing a thiazole group could also be used to provide the corresponding product 4m at a 63% yield. It is worth mentioning that different types of alkenes including 1,2-dihydronaphthalene, α-methyl styrene, 1,3-enyne and 1,3-diene could furnish the desired compounds 4n–4q in 48–86% yields.

Figure 1.
Substrate scope for the photoinduced synthesis of sulfonyl-containing phosphorothioates a,b. a Reactions conditions: alkene 1 (0.2 mmol), sulfonyl chloride 2 (0.4 mmol), phosphorothioate 3 (0.4 mmol), Ir(ppy)3 (2 mol%), Cu(OTf)2 (5 mol%), K2HPO4 (0.4 mmol), DCE (2 mL), irradiation with 30 W blue LEDs at room temperature for 12 h. b Isolated yield based on alkene 1.
Then, we explored the substrate scope of commercially available aromatic and aliphatic sulfonyl chlorides. As expected, various sulfonyl chlorides with substituents on the aromatic ring underwent the reaction efficiently, giving the corresponding sulfonyl-containing phosphorothioates 5a–5h with moderate to good yields. Remarkably, the aliphatic sulfonyl chlorides were also suitable substrates to deliver the desired products 5i–5m in reasonable yields. In addition, the scope of other alkoxy- or aryloxy-substituted phosphorothioic acids was evaluated and it was found that O,O-dimethoxy, diisopropoxy and diphenoxy phosphorothioic acid reacted well to afford products 5n–5p in 57–97% yields.
Furthermore, the robustness of this three-component protocol was evaluated via the late-stage functionalization of bioactive molecules. As shown in Scheme 2, the alkenes derived from indomethacin, estrone, ciprofibrate and febuxostat were found to be compatible under the standard conditions to deliver the desired products 6a–6d in 55–94% yields.

Scheme 2.
Late-stage functionalization of bioactive molecules.
To illustrate the mechanism for this visible-light-mediated reaction, some additional control experiments were performed (Scheme 3). First, when three equivalents of radical scavenger (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) were added to the model reaction under the standard conditions, the formation of product 4a was completely suppressed, suggesting that a free radical pathway might be involved in this reaction. Equally, a radical clock experiment was performed using vinyl cyclopropane 7, and the ring-opening product 8 was obtained with a 24% yield. Taken together, these findings support the generation of sulfonyl radicals under light irradiation. We also conducted a reaction between tosyl chloride 2a and O,O-diethyl S-hydrogen phosphorothioate 3a in the presence of K2HPO4, but the formation of benzenethiosulfonate 9 was not detected. This result indicated that both sulfonyl radicals and sulfenyl radicals were not likely generated by the homolytic cleavage of benzenethiosulfonates.
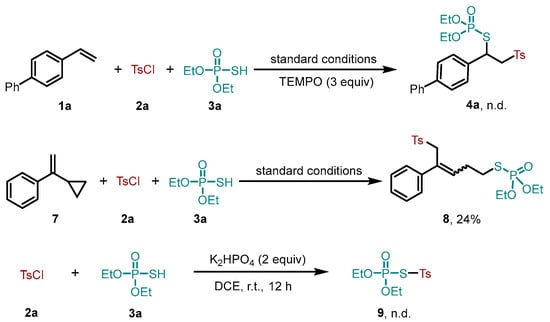
Scheme 3.
Control experiments.
On the basis of the above results and the literature reports, a plausible mechanism for this three-component protocol is depicted in Scheme 4. Initially, the visible-light-excited Ir(III)* undergoes single-electron transfer with sulfonyl chloride 2 to generate the sulfonyl radical A, which then adds to the double bond of alkene 1 to form the alkyl radical intermediate B. Meanwhile, the reaction of the copper catalyst with phosphorothioic acid 3 affords the CuI complex C, followed by single-electron oxidation with Ir (IV) to give the CuII intermediate D and regenerate the photocatalyst. Subsequently, the alkyl radical intermediate B is captured by the CuII intermediate D to form the CuIII species E. Finally, the desired product is delivered via the reductive elimination of E, along with releasing the CuI catalyst.

Scheme 4.
Proposed reaction mechanism.
3. Materials and Methods
3.1. General Information
Unless otherwise stated, all commercial reagents were used as received. All solvents were dried and distilled according to the standard procedures. Flash column chromatography was performed using silica gel (60 Å pore size, 32–63 μm, standard-grade). Analytical thin-layer chromatography was performed using glass plates pre-coated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Thin-layer chromatography plates were visualized using exposure to ultraviolet light. The nuclear magnetic resonance (NMR) spectra were recorded in parts per million from internal tetramethylsilane on the δ scale. The 1H, 13C and 19F NMR spectra were recorded in CDCl3 using a Bruker DRX 400 spectrometer (Bruker, Fallanden, Switzerland) operating at 400 MHz, 100 MHz and 376 MHz, respectively. All chemical shift values are quoted in ppm and coupling constants quoted in Hz. High-resolution mass spectrometry (HRMS) spectra were obtained using a micrOTOF II instrument.
3.2. General Procedure for the Synthesis of Sulfonyl-Containing Phosphorothioates
An oven-dried flask was charged with alkene 1 (0.2 mmol, 1.0 equiv), sulfonyl chloride 2 (0.4 mmol, 2 equiv), K2HPO4 (0.4 mmol, 2 equiv), Cu(OTf)2 (5 mol%) and Ir(ppy)3 (2 mol%) under a nitrogen atmosphere. Then, anhydrous DCE (2 mL) and O,O-diethyl S-hydrogen phosphorothioate 3 (0.4 mmol, 2 equiv) were added to the flask. The mixture was placed around 30 W blue LEDs at a distance of ~5 cm and stirred under blue light irradiation for 12 h at room temperature. After completion of the reaction as monitored using TLC analysis, the mixture was filtered through a celite pad, and washed using ethyl acetate; then, the filtrate was evaporated and the residue was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 2:1) to give the corresponding product.
3.3. General Procedure for the Scale-Up Experiment
An oven-dried flask was charged with 4-vinyl-1,1′-biphenyl 1a (6 mmol, 1.0 equiv), 4-methylbenzenesulfonyl chloride 2a (12 mmol, 2 equiv), K2HPO4 (12 mmol, 2 equiv), Cu(OTf)2 (5 mol%) and Ir(ppy)3 (2 mol%) under a nitrogen atmosphere. Then, anhydrous DCE (60 mL) and O,O-diethyl S-hydrogen phosphorothioate 3a (6 mmol, 2 equiv) were added to the flask. The mixture was placed around 30 W blue LEDs at a distance of ~5 cm and stirred under blue light irradiation for 12 h at room temperature. After completion of the reaction as monitored using TLC analysis, the mixture was filtered through a celite pad, and washed with ethyl acetate; then, the filtrate was evaporated and the residue was purified using flash column chromatography on silica gel (petroleum ether/ethyl acetate = 2:1) to give the corresponding product 4a with a 78% yield (2.342 g).
4. Conclusions
In conclusion, we have developed a visible-light-mediated three-component reaction of alkenes, sulfonyl chlorides and phosphorothioates under blue LED irradiation at room temperature. This efficient protocol shows a broad substrate scope, high functional group tolerance and excellent chemoselectivity. Under the mild reaction conditions, a variety of sulfonyl-containing phosphorothioates were obtained with moderate to good yields. Moreover, the gram-scale synthetic capability and late-stage functionalization of the bioactive molecules demonstrated the applicability of this methodology.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28237869/s1, including the characterization data and 1H, 13C and 19F NMR spectra of products 4, 5, 6.
Author Contributions
Conceptualization, J.W., G.L. and F.-S.H.; methodology, X.W.; software, F.-S.H.; validation, M.C. and S.Z.; formal analysis, F.-S.H.; investigation, X.W., M.C. and S.Z.; resources, J.W. and F.-S.H.; data curation, X.W. and F.-S.H.; writing—original draft preparation, F.-S.H.; writing—review and editing, J.W., G.L. and F.-S.H.; visualization, X.W. and M.C.; supervision, J.W., G.L. and F.-S.H.; project administration, X.W., M.C. and S.Z.; funding acquisition, J.W. and F.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Zhejiang Province (LQ21B020002 and LZ23B020001), the National Natural Science Foundation of China (22101199), the Open Fund of Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers (E22307) and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained in the article tables and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, N.S.; Frederiksen, J.K.; Piccirilli, J.A. Synthesis, Properties, and Applications of Oligonucleotides Containing an RNA Dinucleotide Phosphorothiolate Linkage. Acc. Chem. Res. 2011, 44, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Yang, T.; Mishra, S.; Cronin, C.; Chakraborty, S.; Shen, J.-B.; Liang, B.T.; Jacobson, K.A. 5′-Phosphate and 5′-Phosphonate Ester Derivatives of (N)-Methanocarba Adenosine with in Vivo Cardioprotective Activity. J. Med. Chem. 2013, 56, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, X.; KuoLee, R.; Chen, W. Synthesis and immunostimulatory properties of the phosphorothioate analogues of cdiGMP. Bioorg. Med. Chem. Lett. 2008, 18, 5631–5634. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, S.; Rapozzi, V.; Quadrifoglio, F.; Xodo, L. Anti-gene Effect in Live Cells of AG Motif Triplex-Forming Oligonucleotides Containing an Increasing Number of Phosphorothioate Linkages. Biochemistry 2001, 40, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.F.; Uhlenbeck, O.C. Determination of RNA-protein contacts using thiophosphate substitutions. Biochemistry 1989, 28, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Murdock, L.L.; Hopkins, T.L. Insecticidal, anticholinesterase, and hydrolytic properties of O,O-dialkyl S-aryl phosphorothiolates in relation to structure. J. Agric. Food Chem. 1968, 16, 954–958. [Google Scholar] [CrossRef]
- Kasagami, T.; Miyamoto, T.; Yamamoto, I. Activated transformations of organophosphorus insecticides in the case of non-AChE inhibitory oxons. Pest Manag. Sci. 2002, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Duschmale, J.; Hansen, H.F.; Duschmale, M.; Koller, E.; Albaek, N.; Moller, M.R.; Jensen, K.; Koch, T.; Wengel, J.; Bleicher, K. In Vitro and in Vivo Properties of Therapeutic Oligonucleotides Containing Non-Chiral 3′ and 5′ Thiophosphate Linkages. Nucleic Acids Res. 2020, 48, 63–74. [Google Scholar] [CrossRef]
- Jones, D.J.; O’Leary, E.M.; O’Sullivan, T.P. Synthesis and application of phosphonothioates, phosphonodithioates, phosphorothioates, phosphinothioates and related compounds. Tetrahedron Lett. 2018, 59, 4279–4292. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Yang, R.; Song, X.; Xiao, Q. Recent Advances in the Direct Synthesis of Sulfur-Containing Organophosphorus Compounds via Radical Processes. Adv. Syn. Catal. 2023, 365, 2280–2298. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Man, H.-W.; Schafer, P.; Wong, L.M.; Patterson, R.T.; Corral, L.G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M.A.; Tang, Y.; et al. Discovery of (S)-N-[2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl] acetamide (apremilast), A Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-alpha Inhibitor. J. Med. Chem. 2009, 52, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Wu, H.; Kobayashi, J.; Ohizumi, Y.; Hirata, Y.; Higashijima, T.; Miyazawa, T. Agelasidine-A, A Novel Sesquiterpene Possessing Antispasmodic Activity from the Okinawa Sea Sponge Agelas sp. Tetrahedron Lett. 1983, 24, 4105–4108. [Google Scholar] [CrossRef]
- Woo, S.Y.; Kim, J.H.; Moon, M.K.; Han, S.H.; Yeon, S.K.; Choi, J.W.; Jang, B.K.; Song, H.J.; Kang, Y.G.; Kim, J.W.; et al. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson’s disease therapy. J. Med. Chem. 2014, 57, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, J.; Li, X.; Zhu, J.; Volodine, A.; Wang, X.; Yang, J.; Van Puyvelde, P.; Van der Bruggen, B. New Promising Polymer for Organic Solvent Nanofiltration: Oxidized Poly (Arylene Sulfide Sulfone). J. Membr. Sci. 2018, 549, 438–445. [Google Scholar] [CrossRef]
- Kausar, A.; Zulfiqar, S.; Sarwar, M.I. Recent Developments in Sulfur-Containing Polymers. Polym. Rev. 2014, 54, 185–267. [Google Scholar] [CrossRef]
- Julia, M.; Paris, J.-M. Syntheses a l’aide de Sulfones v(+)-Methode de Synthese Generale de Doubles Liaisons. Tetrahedron Lett. 1973, 14, 4833–4836. [Google Scholar] [CrossRef]
- Joseph, D.; Idris, M.A.; Chen, J.; Lee, S. Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds. ACS Catal. 2021, 11, 4169–4204. [Google Scholar] [CrossRef]
- Qiu, G.; Zhou, K.; Gao, L.; Wu, J. Insertion of sulfur dioxide via a radical process: An efficient route to sulfonyl compounds. Org. Chem. Front. 2018, 5, 691–705. [Google Scholar] [CrossRef]
- Ge, D.; Chen, J.-W.; Xu, P.; Pan, J.; Chu, X.-Q. 1,n-Thiosulfonylation using thiosulfonates as dual functional reagents. Chin. Chem. Lett. 2022, 33, 4732–4739. [Google Scholar] [CrossRef]
- Zhen, J.; Du, X.; Xu, X.; Li, Y.; Yuan, H.; Xu, D.; Xue, C.; Luo, Y. Visible-Light-Mediated Late-Stage Sulfonylation of Boronic Acids via N–S Bond Activation of Sulfonamides. ACS Catal. 2022, 12, 1986–1991. [Google Scholar] [CrossRef]
- He, F.-S.; Bao, P.; Yu, F.; Zeng, L.-H.; Deng, W.-P.; Wu, J. Copper-Catalyzed Regioselective 1,4-Selenosulfonylation of 1,3-Enynes to Access Cyanoalkylsulfonylated Allenes. Org. Lett. 2021, 23, 7472–7476. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, W.; Xie, W.; Chen, Q.; Wu, J. Generation of (E)-β-trifluoromethyl vinylsulfonohydrazides under photocatalysis and their anti-bacteria activity. Chin. Chem. Lett. 2023, 34, 107984. [Google Scholar] [CrossRef]
- He, F.-S.; Bao, P.; Tang, Z.; Yu, F.; Deng, W.-P.; Wu, J. Photoredox-catalyzed α-sulfonylation of ketones from sulfur dioxide and thianthrenium salts. Org. Lett. 2022, 24, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov Functionalization of Alkenes and Alkynes: Recent Developments and Trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef] [PubMed]
- He, F.-S.; Ye, S.; Wu, J. Recent Advances in Pyridinium Salts as Radical Reservoirs in Organic Synthesis. ACS Catal. 2019, 9, 8943–8960. [Google Scholar] [CrossRef]
- Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Intermolecular Radical Carboamination of Alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef]
- Derosa, J.; Apolinar, O.; Kang, T.; Tran, V.T.; Engle, K.M. Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Zhu, X.; Li, Y.; Zhao, X.; Shi, S.; Zhu, F.; Lin, J.; Gao, X. Photoredox and Copper-Catalyzed Sulfonylphosphorothiolation of Alkenes toward β-Sulfonyl Phosphorothioates. Adv. Synth. Catal. 2022, 364, 3316–3320. [Google Scholar] [CrossRef]
- Liang, R.-B.; Zhu, C.-M.; Song, P.-Q.; Zhao, L.-M.; Tong, Q.-X.; Zhong, J.-J. External oxidant-free and selective thiofunctionalization of alkenes enabled by photoredox-neutral catalysis. Org. Chem. Front. 2022, 9, 4536–4541. [Google Scholar] [CrossRef]
- Lu, M.; Liang, R.-B.; Zhu, C.-M.; Tong, Q.-X.; Zhong, J.-J. Photoredox Synthesis of Thio-Functionalized Cyclic Ethers Using N-Sulfenyl Phthalimides as a Thiyl-Radical Precursor. Chin. J. Chem. 2023, 41, 1823–1828. [Google Scholar] [CrossRef]
- Du, X.; Zhen, J.-S.; Xu, X.-H.; Yuan, H.; Li, Y.-H.; Zheng, Y.; Xue, C.; Luo, Y. Hydrosulfonylation of Alkenes with Sulfonyl Imines via Ir/Cu Dual Photoredox Catalysis. Org. Lett. 2022, 24, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Aerobic Oxysulfonylation of Alkenes Leading to Secondary and Tertiary β-Hydroxysulfones. Angew. Chem. Int. Ed. 2013, 52, 7156–7159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.-L.; Deng, Y.-X.; Li, Z.-J.; Zhao, S.-Y. Copper(I)-Catalyzed Three-Component Selenosulfonation of Maleimides with Sulfonyl Hydrazides and Diselenides via Radical Relay. J. Org. Chem. 2022, 87, 15661–15669. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Dual gold and photoredox catalysis: Visible light-mediated intermolecular atom transfer thiosulfonylation of alkenes. Chem. Sci. 2017, 8, 2610–2615. [Google Scholar] [CrossRef]
- Gadde, K.; Mampuys, P.; Guidetti, A.; Ching, H.Y.V.; Herrebout, W.A.; Doorslaer, S.V.; Tehrani, K.A.; Maes, B.U.W. Thiosulfonylation of Unactivated Alkenes with Visible-Light Organic Photocatalysis. ACS Catal. 2020, 10, 8765–8779. [Google Scholar] [CrossRef]
- Jia, X.; Luo, L.; Huang, C.; Zhang, X.; Lian, Z. Iron-Catalyzed Sulfonylthiocyanation of α, β-Unsaturated Amides/Esters via the Insertion of Sulfur Dioxide. Org. Lett. 2022, 24, 7560–7565. [Google Scholar] [CrossRef]
- He, F.-S.; Wu, Y.; Zhang, J.; Xia, H.; Wu, J. Thiosulfonylation of alkenes with the insertion of sulfur dioxide under non-metallic conditions. Org. Chem. Front. 2018, 5, 2940–2944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).