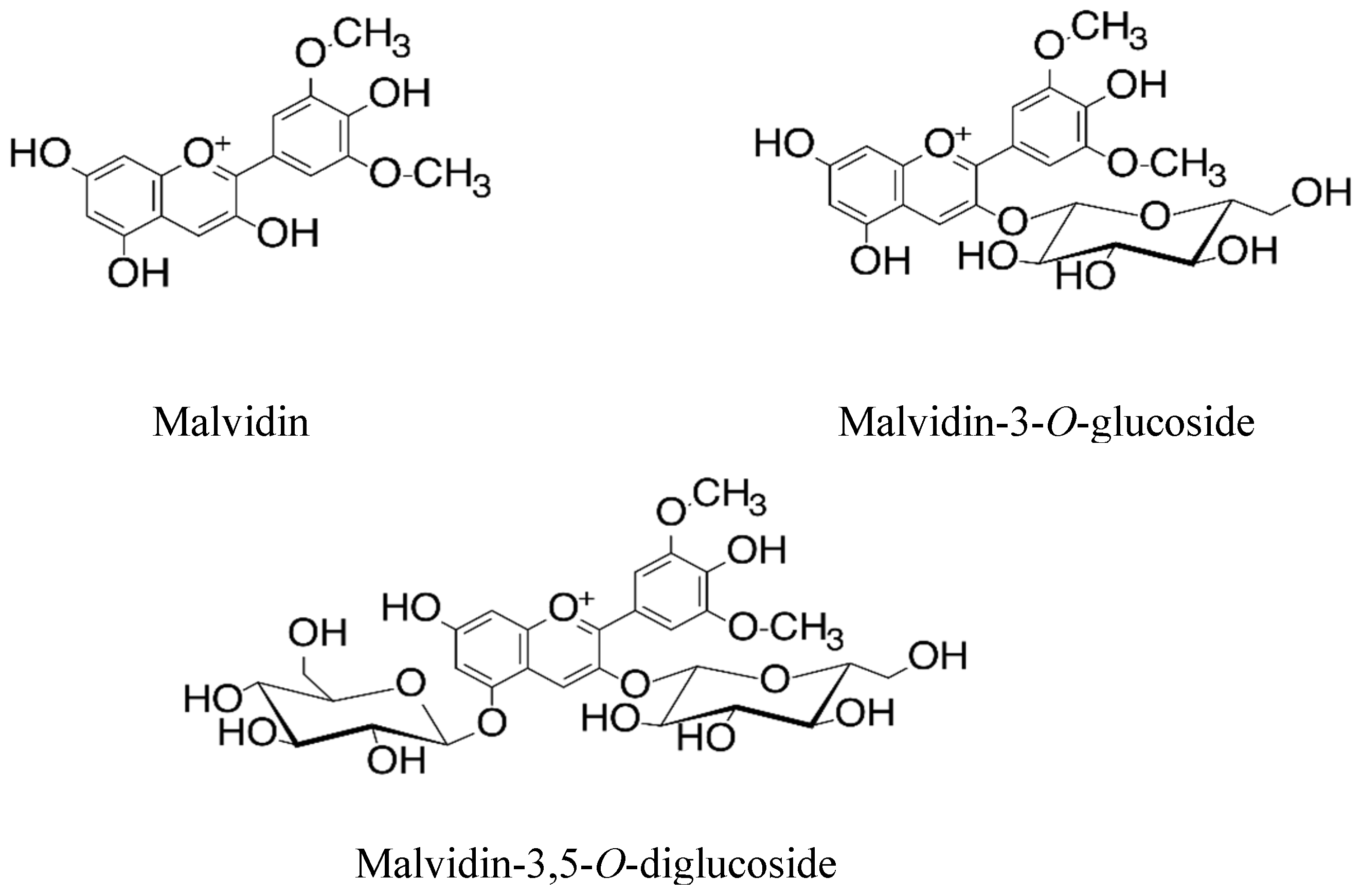
Malvidin and Its Mono- and Di-Glucosides Forms: A Study of Combining Both In Vitro and Molecular Docking Studies Focused on Cholinesterase, Butyrylcholinesterase, COX-1 and COX-2 Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acetylcholinesterase and Buthyrylcholinesterase Inhibition
2.2. Anti-Inflammatory Activity—COX Inhibition
| Compound | IC50 (µM) | ||
|---|---|---|---|
| COX-1 | COX-2 | Selectivity Index COX-2/COX-1 | |
| Mv | 12.45 ± 0.70 d | 2.76 ± 0.16 d | 0.22 |
| Mv 3-glc | 74.78 ± 0.06 b | 39.92 ± 3.02 b | 0.53 |
| Mv 3,5-diglc | 90.36 ± 1.92 a | 66.45 ± 1.93 a | 0.83 |
| Indomethacin * | 18.32 ± 0.40 c | 15.22 ± 1.36 c | 0.83 |
2.3. Computational Outcomes
2.3.1. Inhibitory Activity towards AChE and BChE
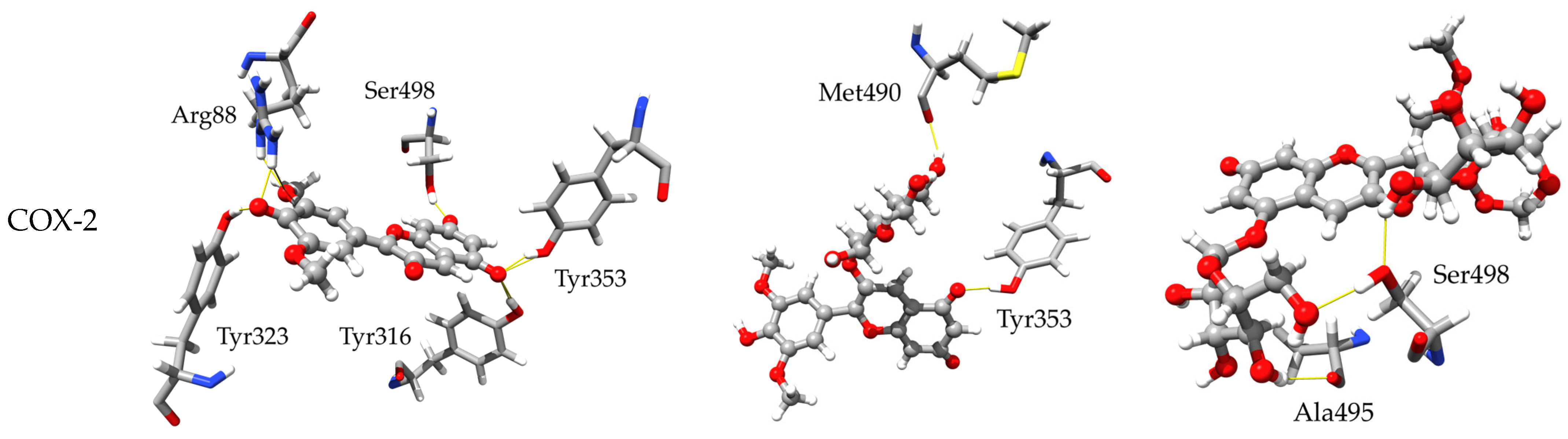
2.3.2. Inhibitory Activity towards COX-1 and COX-2
3. Materials and Methods
3.1. Materials
3.2. Acetylcholinesterase and Butyrylcholinesterase Inhibition Assay
3.3. Cyclooxygenase (COX) Inhibition Assay
3.4. Computational Studies
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase Inhibitors from Plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Marino, T.; Prejanò, M.; Russo, N. Antioxidant and Copper-Chelating Power of New Molecules Suggested as Multiple Target Agents against Alzheimer’s Disease. A Theoretical Comparative Study. Phys. Chem. Chem. Phys. 2022, 24, 16353–16359. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a Potential Pharmacological Agent to Manage Memory Deficit, Oxidative Stress and Alterations in Ion Pump Activity Induced by Experimental Sporadic Dementia of Alzheimer’s Type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.; Liu, W.; Zhang, L.; Tian, Q.; Li, H.; Li, M. MiR-485-3p Serves as a Biomarker and Therapeutic Target of Alzheimer’s Disease via Regulating Neuronal Cell Viability and Neuroinflammation by Targeting AKT3. Mol. Genet. Genom. Med. 2021, 9, e1548. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Lee, S.; Lee, H.J.; Min, J.W.; Iwatsubo, T.; Teunissen, C.E.; Cho, H.J.; Ryu, J.H. Targeting MicroRNA-485-3p Blocks Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2021, 22, 13136. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential Applications of Curcumin and Curcumin Nanoparticles: From Traditional Therapeutics to Modern Nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Arshad, L.; Areeful Haque, M.; Bukhari, S.N.A.; Jantan, I. An Overview of Structure-Activity Relationship Studies of Curcumin Analogs as Antioxidant and Anti-Inflammatory Agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic Mono-Carbonyl Curcumin Analogues Attenuate Oxidative Stress in Mouse Models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, H.U.; Khan, F.A.; Shah, A.; Wadood, A.; Ahmad, S.; Almehmadi, M.; Alsaiari, A.A.; Shah, F.U.; Kamran, N. Anti-Alzheimer and Antioxidant Effects of Nelumbo Nucifera L. Alkaloids, Nuciferine and Norcoclaurine in Alloxan-Induced Diabetic Albino Rats. Pharmaceuticals 2022, 15, 1205. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase Activities of Selected Polyphenols—A Short Report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Jom, K.N.; Jongruaysup, B.; Tabtimsri, S.; Pruesapan, K.; Thangsiri, S.; Inthachat, W.; Siriwan, D.; Charoenkiatkul, S.; et al. Comparison of Phytochemicals, Antioxidant, and In Vitro Anti-Alzheimer Properties of Twenty-Seven Morus Spp. Cultivated in Thailand. Molecules 2020, 25, 2600. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Büttner, R.; Schulz, A.; Reuter, M.; Akula, A.K.; Mindos, T.; Carlstedt, A.; Riecken, L.B.; Baader, S.L.; Bauer, R.; Morrison, H. Inflammaging Impairs Peripheral Nerve Maintenance and Regeneration. Aging Cell 2018, 17, e12833. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF Cytokines in Aging, Multiple Sclerosis, and Dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; HadjiakhoonDi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic Potential of Flavonoids in Inflammatory Bowel Disease: A Comprehensive Review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Kim, H.P. The Long Search for Pharmacologically Useful Anti-Inflammatory Flavonoids and Their Action Mechanisms: Past, Present, and Future. Biomol. Ther. 2022, 30, 117. [Google Scholar] [CrossRef]
- Fagundes, F.L.; Pereira, Q.C.; Zarricueta, M.L.; Dos Santos, R.d.C. Malvidin Protects against and Repairs Peptic Ulcers in Mice by Alleviating Oxidative Stress and Inflammation. Nutrients 2021, 13, 3312. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase Inhibitory and Antioxidant Cyanidin Glycosides in Cherries and Berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Strugała-Danak, P.; Spiegel, M.; Hurynowicz, K.; Gabrielska, J. Interference of Malvidin and Its Mono- and Di-Glucosides on the Membrane—Combined in Vitro and Computational Chemistry Study. J. Funct. Foods 2022, 99, 105340. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Xie, J.; Cui, H.; Mozafari, M.R.; Chen, W. Potential Micro-/Nano-Encapsulation Systems for Improving Stability and Bioavailability of Anthocyanins: An Updated Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 3362–3385. [Google Scholar] [CrossRef]
- Strugała, P.; Loi, S.; Bazanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef]
- Roncato, J.F.F.; Camara, D.; Brussulo Pereira, T.C.; Quines, C.B.; Colomé, L.M.; Denardin, C.; Haas, S.; Ávila, D.S. Lipid Reducing Potential of Liposomes Loaded with Ethanolic Extract of Purple Pitanga (Eugenia uniflora) Administered to Caenorhabditis elegans. J. Liposome Res. 2019, 29, 274–282. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Yusuf, E.; Wojdyło, A.; Oszmiański, J.; Nowicka, P. Nutritional, Phytochemical Characteristics and In Vitro Effect on α-Amylase, α-Glucosidase, Lipase, and Cholinesterase Activities of 12 Coloured Carrot Varieties. Foods 2021, 10, 808. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tsai, P.F.; Wu, J.S.B. Protective Effect of Anthocyanidins against Sodium Dithionite-Induced Hypoxia Injury in C6 Glial Cells. J. Agric. Food Chem. 2014, 62, 5603–5608. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Yang, Q.; Lu, Y.; Wang, G.; Yang, H.; Dong, J.; Zhang, H. Malvidin Alleviates Mitochondrial Dysfunction and ROS Accumulation through Activating AMPK-α/UCP2 Axis, Thereby Resisting Inflammation and Apoptosis in SAE Mice. Front. Pharmacol. 2023, 13, 1038802. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Shahid Nadeem, M.; Afzal, M.; Alzarea, S.I.; Sayyed, N.; et al. Antiamnesic Potential of Malvidin on Aluminum Chloride Activated by the Free Radical Scavenging Property. ACS Omega 2022, 7, 24231–24240. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins Inhibit Cyclooxygenase-2 Expression in LPS-Evoked Macrophages: Structure-Activity Relationship and Molecular Mechanisms Involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-KappaB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-KappaB Activation along with Their Inhibitory Effect on INOS Expression and NO Production in Activated Macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar] [CrossRef]
- Baumann, J.; Bruchhausen, F.V.; Wurm, G. Flavonoids and Related Compounds as Inhibition of Arachidonic Acid Peroxidation. Prostaglandins 1980, 20, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Dutta, K.; Sen, S.; Dey, B.K.; Gogoi, P. Perceived Benefits and Risk of NSAIDs in Relation to Its Association with Cancer: A Comprehensive Review. J. Pharm. Res. Int. 2021, 33, 236–244. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Wang, X.-N.; Wang, J.; Sui, Z.-Q. Malvidin and Its Glycosides from Vaccinium Ashei Improve Endothelial Function by Anti-Inflammatory and Angiotensin I-Converting Enzyme Inhibitory Effects. Nat. Prod. Commun. 2018, 13, 49–52. [Google Scholar] [CrossRef]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and Anti-Inflammatory Activities of Target Anthocyanins Di-Glucosides Isolated from Syzygium Cumini Pulp by High Speed Counter-Current Chromatography. J. Food Biochem. 2020, 44, 1050–1062. [Google Scholar] [CrossRef]
- Bastin, A.R.; Sadeghi, A.; Abolhassani, M.; Doustimotlagh, A.H.; Mohammadi, A. Malvidin Prevents Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Peripheral Blood Mononuclear Cells. IUBMB Life 2020, 72, 1504–1514. [Google Scholar] [CrossRef]
- Chao, C.Y.; Liu, W.H.; Wu, J.J.; Yin, M.C. Phytochemical Profile, Antioxidative and Anti-Inflammatory Potentials of Gynura Bicolor DC. J. Sci. Food Agric. 2015, 95, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Marchi, P.; Paiotti, A.P.R.; Neto, R.A.; Oshima, C.T.F.; Ribeiro, D.A. Concentrated Grape Juice (G8000TM) Reduces Immunoexpression of INOS, TNF-Alpha, COX-2 and DNA Damage on 2,4,6-Trinitrobenzene Sulfonic Acid-Induced-Colitis. Environ. Toxicol. Pharmacol. 2014, 37, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F. Antioxidant and Anti-Inflammatory Effects in RAW264.7 Macrophages of Malvidin, a Major Red Wine Polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological Activity of Japanese Quince Extract and Its Interactions with Lipids, Erythrocyte Membrane, and Human Albumin. J. Membr. Biol. 2016, 249, 393. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jin, H. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Properties of Functionalized Tetrahydroacridines and Related Analogs. Med. Chem. 2014, 4, 688–696. [Google Scholar] [CrossRef]
- Jang, M.S.; Pezzuto, J.M. Assessment of Cyclooxygenase Inhibitors Using in Vitro Assay Systems. Methods Cell Sci. 1997, 19, 25–31. [Google Scholar] [CrossRef]
- Strugała, P.; Urbaniak, A.; Kuryś, P.; Włoch, A.; Kral, T.; Ugorski, M.; Hof, M.; Gabrielska, J. Antitumor and Antioxidant Activities of Purple Potato Ethanolic Extract and Its Interaction with Liposomes, Albumin and Plasmid DNA. Food Funct. 2021, 12, 1271–1290. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and Functional Basis of Cyclooxygenase Inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-Selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Ho, K.Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A New Crystal Form of Human Acetylcholinesterase for Exploratory Room-Temperature Crystallography Studies. Chem. Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.P.; Bondi, R.W.; Michalski, P.J. Model-Based Target Pharmacology Assessment (MTPA): An Approach Using PBPK/PD Modeling and Machine Learning to Design Medicinal Chemistry and DMPK Strategies in Early Drug Discovery. J. Med. Chem. 2021, 64, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Krzyżek, P.; Dworniczek, E.; Adamski, R.; Sroka, Z. In Silico Screening and In Vitro Assessment of Natural Products with Anti-Virulence Activity against Helicobacter Pylori. Molecules 2021, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges: The RESP Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- He, X.; Man, V.H.; Yang, W.; Lee, T.S.; Wang, J. A Fast and High-Quality Charge Model for the next Generation General AMBER Force Field. J. Chem. Phys. 2020, 153, 114502. [Google Scholar] [CrossRef]
- Case Ross, C.; Walker, D.A.; Darden Junmei Wang, T. Amber 2016 Reference Manual Principal Contributors to the Current Codes; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A Global View on Proteins and Their Functional Interactions in 630 Organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An Automated Pipeline for the Setup of Poisson–Boltzmann Electrostatics Calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Søndergaard, C.R.; Olsson, M.H.M.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Downloads—GROMACS 2023.3 Documentation. Available online: https://manual.gromacs.org/current/download.html (accessed on 21 November 2023).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]


| Compound | Inhibition of AChE (%) | Inhibition of BChE (%) |
|---|---|---|
| Mv | 19.5 ± 2.3 b | 10.2 ± 2.2 b |
| Mv 3-glc | 26.3 ± 3.1 a | 22.1 ± 3.0 a |
| Mv 3,5-diglc | 11.9 ± 4.5 c | 13.4 ± 2.4 b |
| Neostigmine | 50.0 ± 1.2 | 50.0 ± 4.2 |
| Compound | fM | AChE | BChE |
|---|---|---|---|
| Mv | 39.56% | 2.58 × 10−7 | 1.52 × 10−6 |
| Mv− | 52.15% | 1.89 × 10−7 | 1.19 × 10−6 |
| Mv2− | 8.08% | 1.16 × 10−7 | 6.41 × 10−7 |
| Mv3− | 0.13% | 8.04 × 10−8 | 6.42 × 10−7 |
| Ki-overall | 2.10 × 10−7 | 1.28 × 10−6 | |
| Mv 3-glc | 3.49% | 2.77 × 10−6 | 5.39 × 10−7 |
| Mv 3-glc− | 78.18% | 2.42 × 10−6 | 5.99 × 10−7 |
| Mv 3-glc2− | 18.33% | 2.88 × 10−6 | 4.12 × 10−7 |
| Ki-overall | 2.52 × 10−6 | 5.63 × 10−7 | |
| Mv 3,5-diglc | 39.19% | 4.51 × 10−3 | 3.70 × 10−7 |
| Mv 3,5-diglc– | 60.70% | 1.63 × 10−4 | 8.99 × 10−8 |
| Ki-overall | 1.87 × 10−3 | 1.99 × 10−7 |
| Compound | fM | COX-1 | COX-2 |
|---|---|---|---|
| Mv | 39.56% | 9.08 × 10−4 | 5.42 × 10−5 |
| Mv− | 52.15% | 8.18 × 10−4 | 6.68 × 10−5 |
| Mv2− | 8.08% | 6.41 × 10−4 | 4.85 × 10−5 |
| Mv3− | 0.13% | 5.46 × 10−4 | 3.92 × 10−5 |
| Ki-overall | 8.38 × 10−4 | 6.02 × 10−5 | |
| Mv 3-glc | 3.49% | 3.43 × 102 | 2.41 × 10−3 |
| Mv 3-glc− | 78.18% | 2.89 × 102 | 1.15 × 10−1 |
| Mv 3-glc2− | 18.33% | 2.64 × 102 | 2.90 × 10−1 |
| Ki-overall | 2.87 × 102 | 1.43 × 10−1 | |
| Mv 3,5-diglc | 39.19% | 1.34 × 1011 | 1.98 × 109 |
| Mv 3,5-diglc− | 60.70% | 1.12 × 1011 | 3.20 × 1012 |
| Ki-overall | 1.21 × 1011 | 1.95 × 1012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strugała-Danak, P.; Spiegel, M.; Gabrielska, J. Malvidin and Its Mono- and Di-Glucosides Forms: A Study of Combining Both In Vitro and Molecular Docking Studies Focused on Cholinesterase, Butyrylcholinesterase, COX-1 and COX-2 Activities. Molecules 2023, 28, 7872. https://doi.org/10.3390/molecules28237872
Strugała-Danak P, Spiegel M, Gabrielska J. Malvidin and Its Mono- and Di-Glucosides Forms: A Study of Combining Both In Vitro and Molecular Docking Studies Focused on Cholinesterase, Butyrylcholinesterase, COX-1 and COX-2 Activities. Molecules. 2023; 28(23):7872. https://doi.org/10.3390/molecules28237872
Chicago/Turabian StyleStrugała-Danak, Paulina, Maciej Spiegel, and Janina Gabrielska. 2023. "Malvidin and Its Mono- and Di-Glucosides Forms: A Study of Combining Both In Vitro and Molecular Docking Studies Focused on Cholinesterase, Butyrylcholinesterase, COX-1 and COX-2 Activities" Molecules 28, no. 23: 7872. https://doi.org/10.3390/molecules28237872
APA StyleStrugała-Danak, P., Spiegel, M., & Gabrielska, J. (2023). Malvidin and Its Mono- and Di-Glucosides Forms: A Study of Combining Both In Vitro and Molecular Docking Studies Focused on Cholinesterase, Butyrylcholinesterase, COX-1 and COX-2 Activities. Molecules, 28(23), 7872. https://doi.org/10.3390/molecules28237872