Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of Vesicles
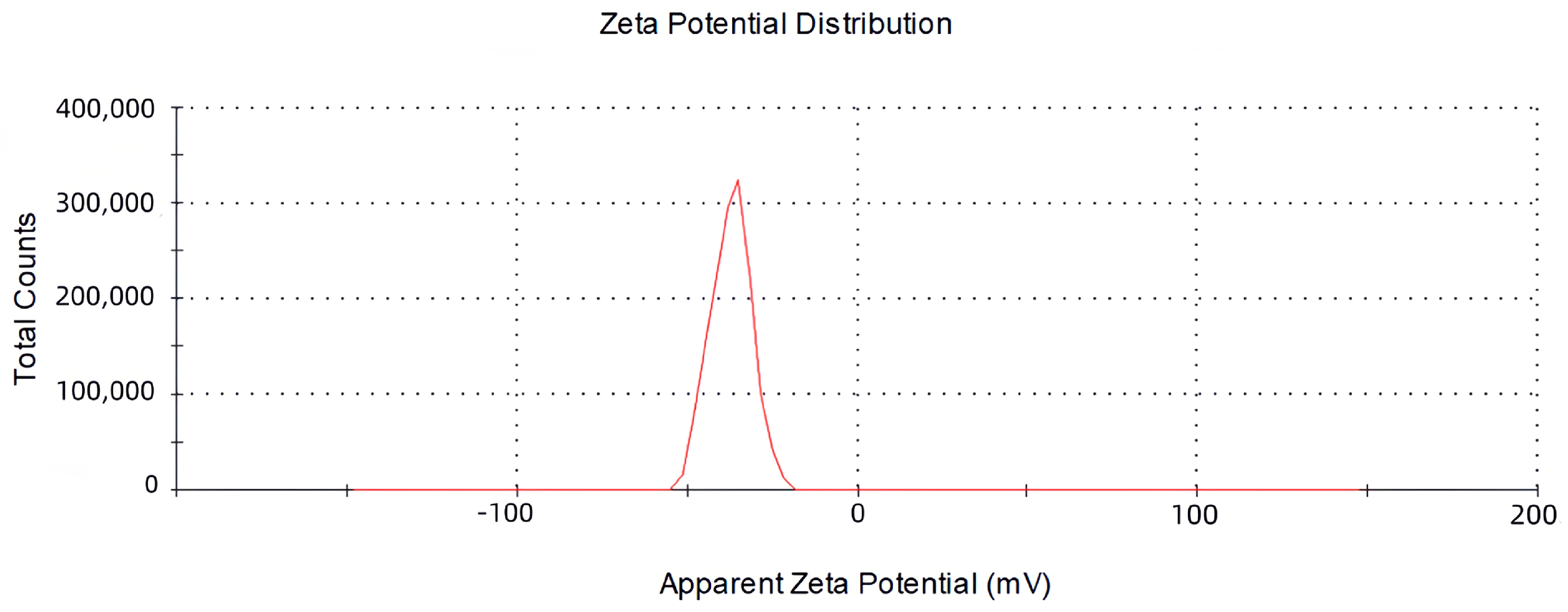
2.2. The Size, Zeta Potential and Polydispersity Index (PDI) of Vesicles
2.3. Differential Scanning Calorimetry (DSC)
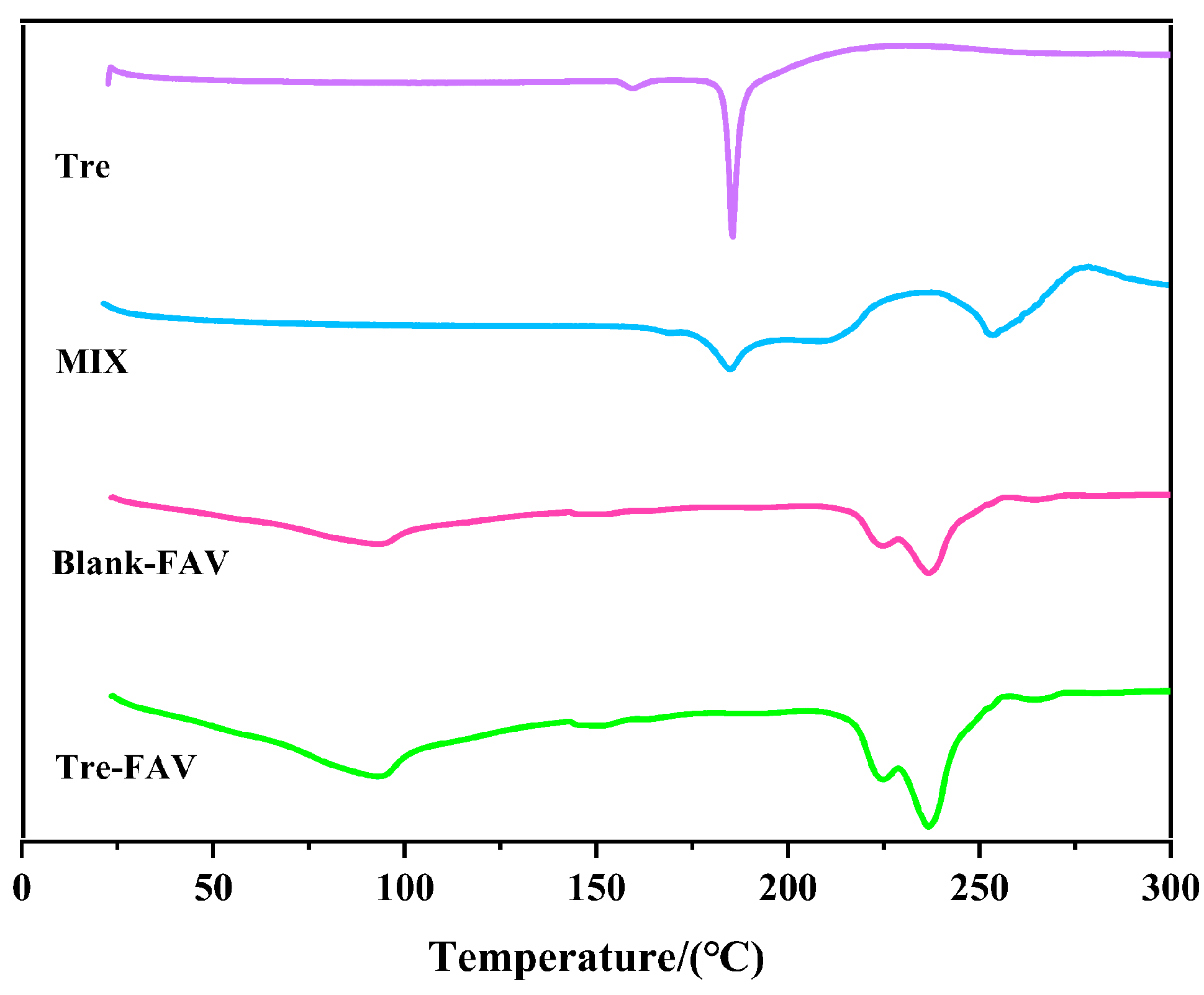
2.4. Fourier Transform Infrared Spectrometer (FTIR)
2.5. Stability Studies of Vesicles
2.6. In Vitro Transdermal Studies
2.6.1. In Vitro Transdermal Permeation Studies of Vesicular Dispersion
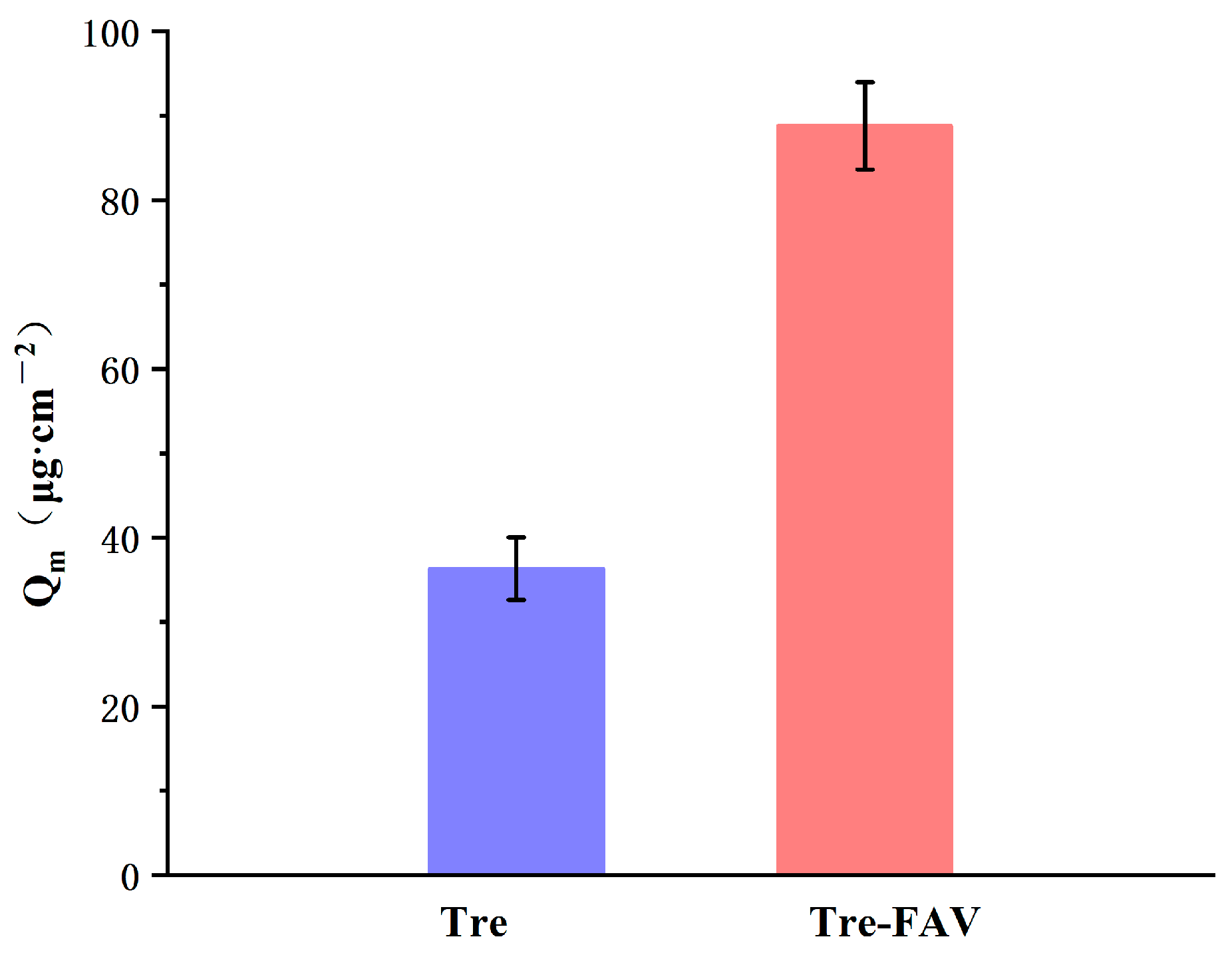
2.6.2. Skin Retention of Vesicular Dispersion
2.7. In-Vivo Pharmacodynamic Study
2.7.1. Establishment of Psoriatic Animal Models
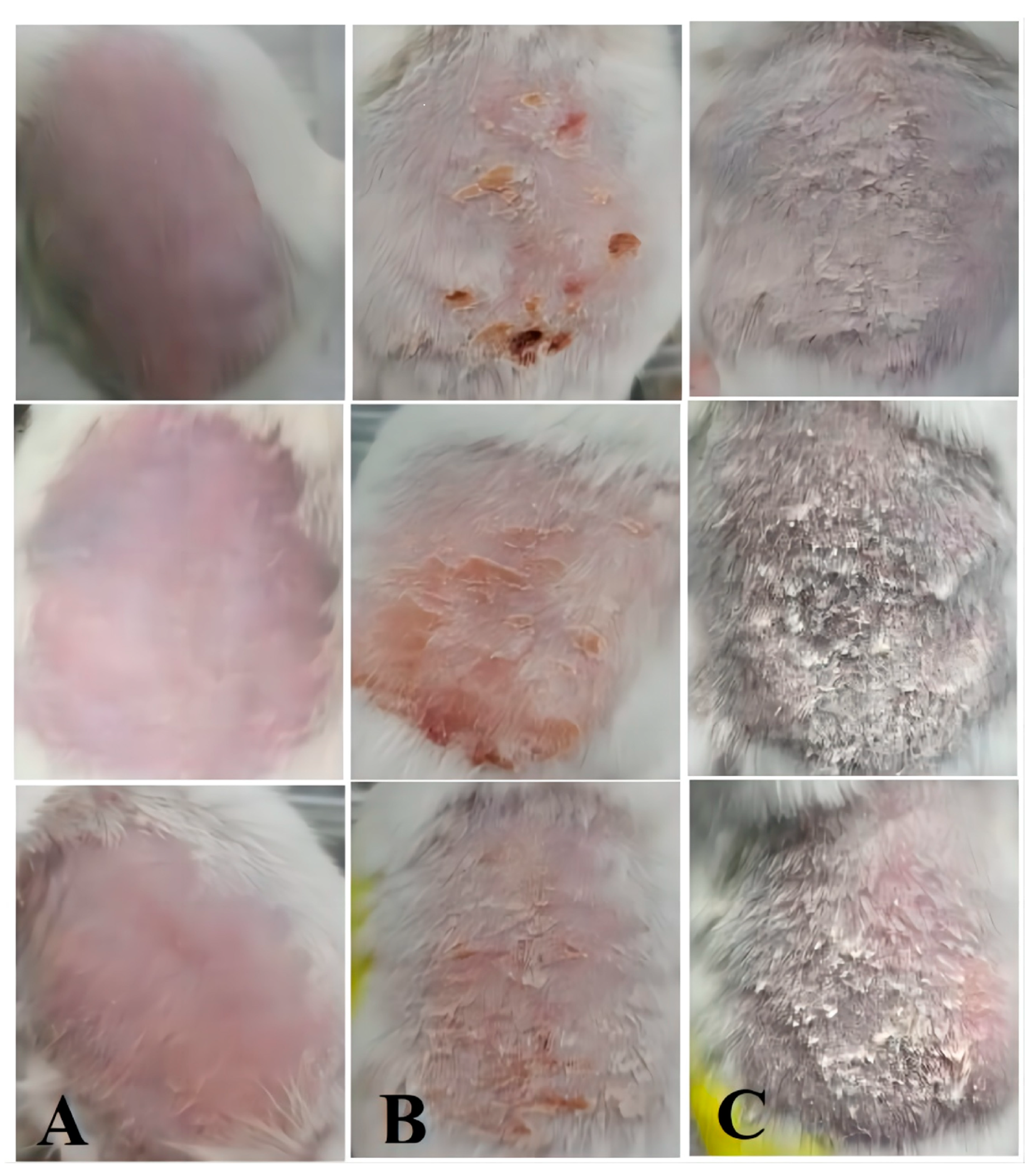
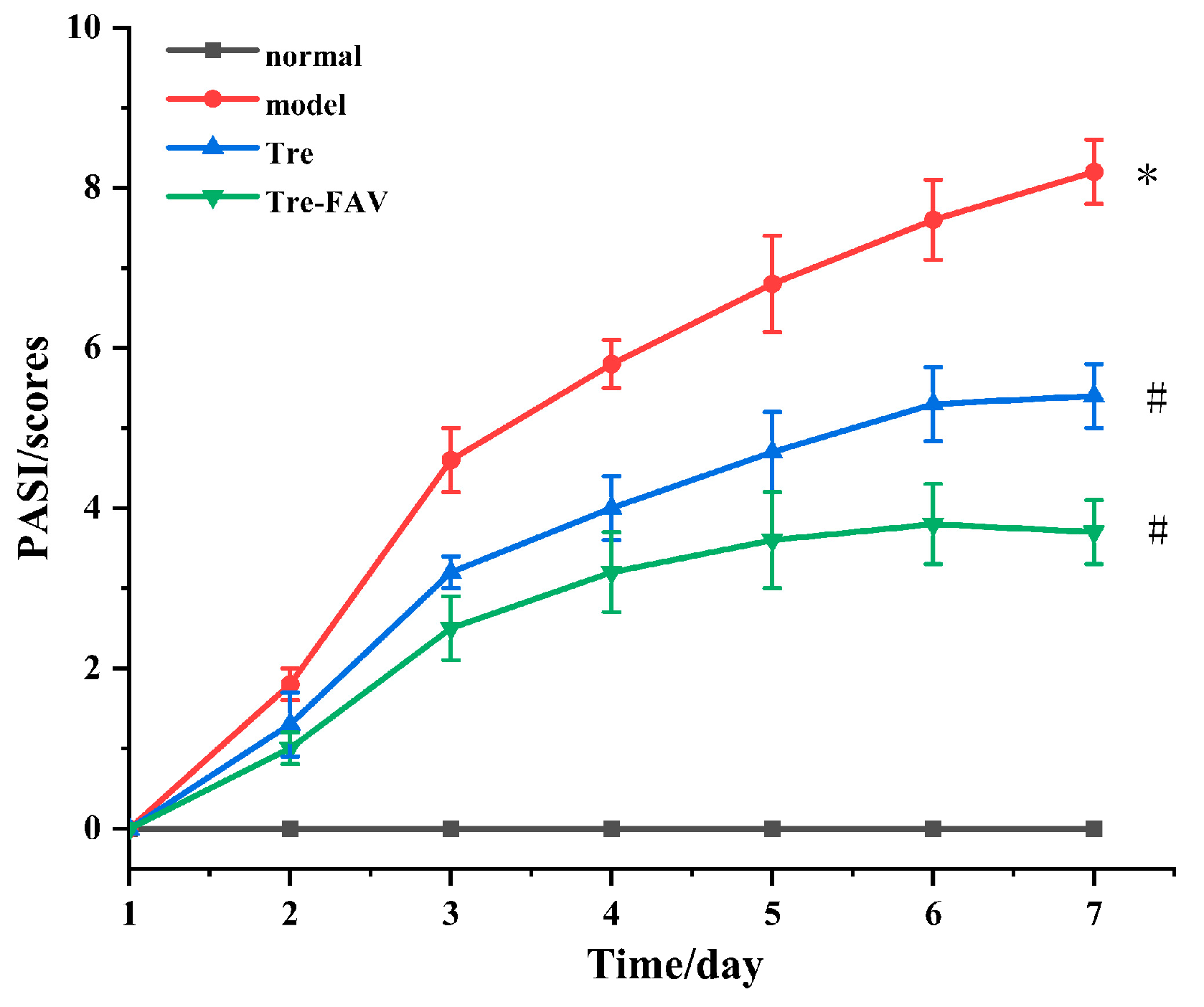
2.7.2. Psoriasis Area Severity Index (PASI) Evaluation
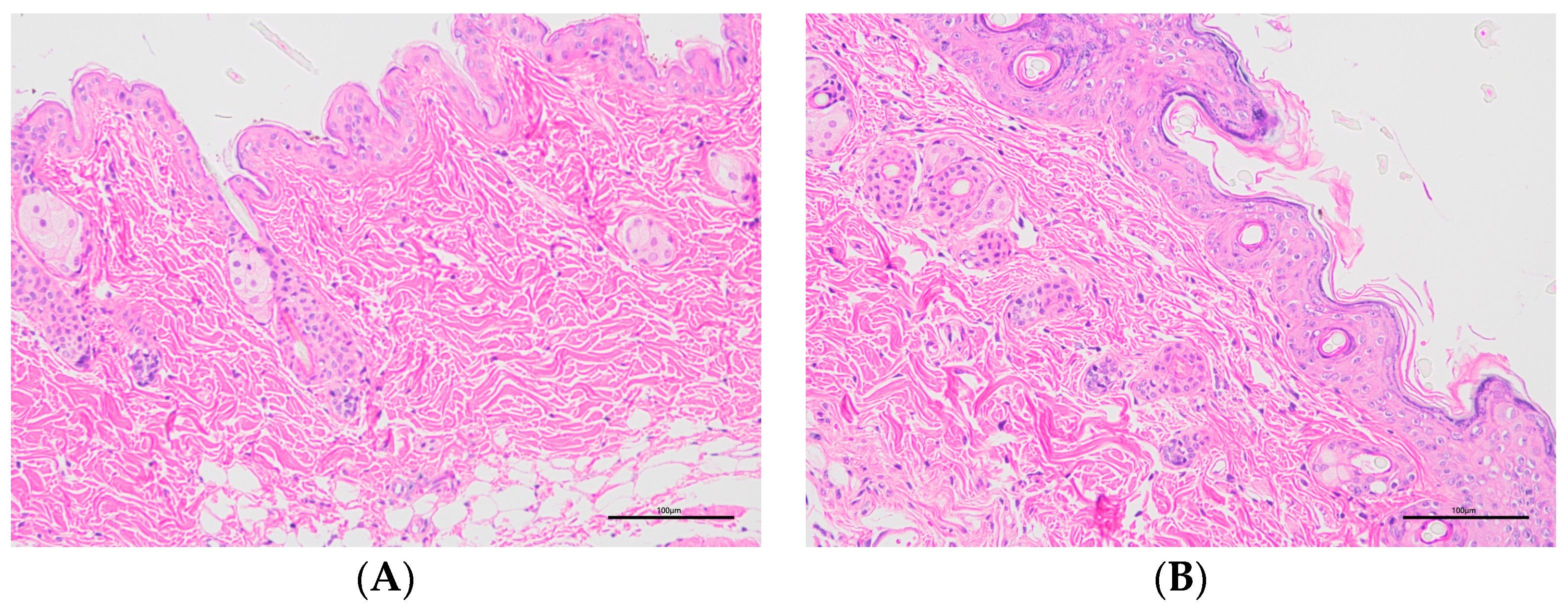
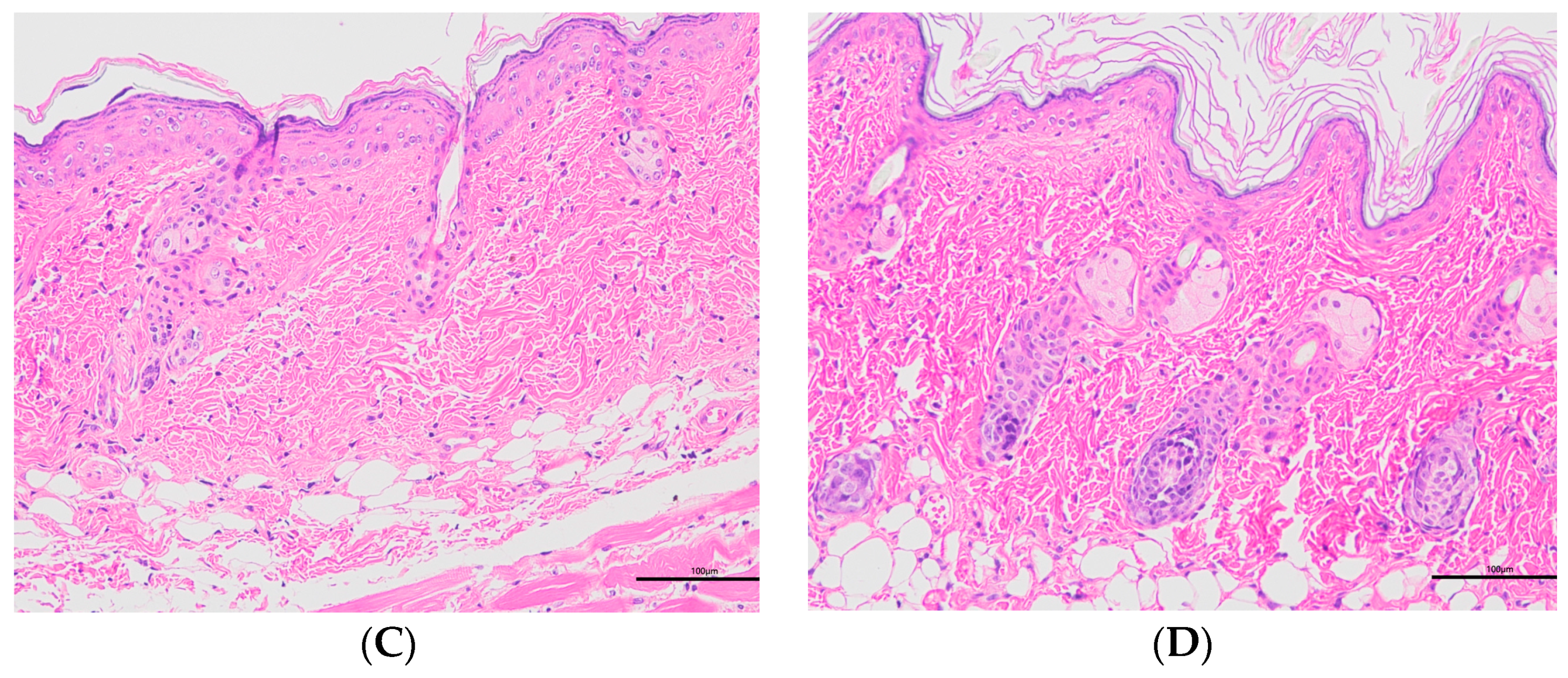
2.7.3. Histopathology
2.7.4. Weight Ratio of Spleen to Body (Spleen/Body wt%)
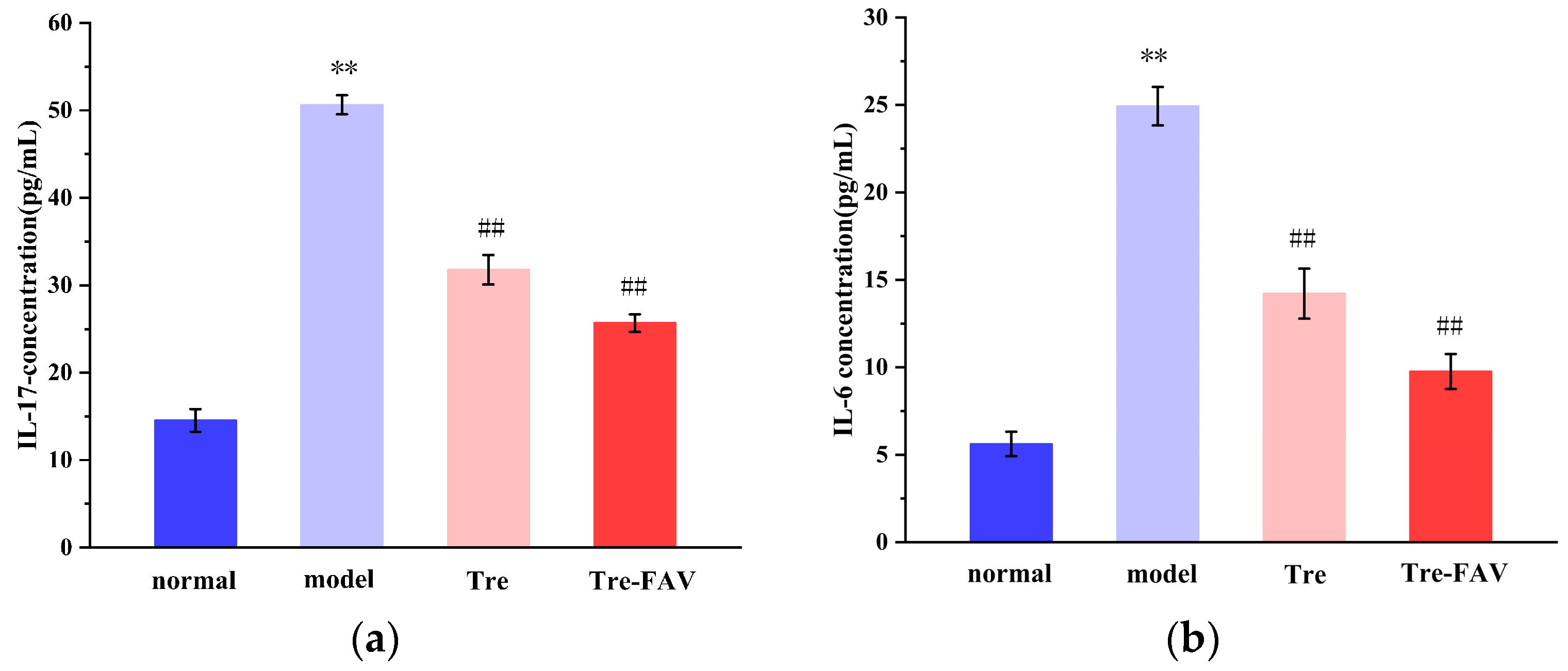
2.7.5. The Levels of IL-17 and IL-6 Detection
2.8. Discussion
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Preparation of Vesicular Formulations
3.4. Characterisation of Oleic Acid Vesicular Formulations
3.4.1. Morphology of Vesicles
3.4.2. The Size, Zeta Potential and Polydispersity Index (PDI) of Vesicles
3.4.3. Encapsulation Efficiency
3.4.4. Differential Scanning Calorimetry (DSC)
3.4.5. Fourier Transform Infrared Spectrometer (FTIR)
3.4.6. Stability Studies of Vesicles
3.5. In Vitro Transdermal Study
3.5.1. Processing of the Isolated Mouse Skin
3.5.2. Ex Vivo Skin Permeation Study
3.5.3. In Vitro Skin Retention Experiments
3.6. In Vivo Pharmacodynamic Studies
3.6.1. Establishment of Psoriatic Animal Models
3.6.2. Psoriasis Area Severity Index (PASI) Evaluation
3.6.3. Histopathologic Examination
3.6.4. Weight Ratio of Spleen to Body (Spleen/Body wt%)
3.6.5. The Levels of IL-17 and IL-6 Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, X.; Chen, X.X.; Kang, X.D.; Ran, M.; Wang, Y.L.; Zhao, Z.K.; Li, C.X. Identification of potential key molecules and signaling pathways for psoriasis based on weighted gene co-expression network analysis. World J. Clin. Cases 2022, 10, 5965–5983. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Elaissari, A.; Fessi, H. Advances in psoriasis physiopathology and treatments: Up to date of mechanistic insights and perspectives of novel therapies based on innovative skin drug delivery systems (ISDDS). J. Control. Release 2016, 239, 182–202. [Google Scholar] [CrossRef]
- Chen, H.; Su, Z.; Pan, X.; Zheng, X.; Li, H.; Ye, Z.; Tang, B.; Lu, Y.; Zheng, G.; Lu, C. Phytochemicals: Targeting autophagy to treat psoriasis. Phytomedicine 2023, 120, 155041. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.; Lu, Q. The Inflammatory Response in Psoriasis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Dan, D.; Srivastava, N. Psoriasis: Striving for Potential Biomarkers. ASSAY Drug Dev. Technol. 2023, 21, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Chan, A.R.; Hessami-Booshehri, M. Psoriasis in patients of color: Differences in morphology, clinical presentation, and treatment. Cutis 2020, 106, 7–10, E10. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Aberrations in Lipid Expression and Metabolism in Psoriasis. Int. J. Mol. Sci. 2021, 22, 6561. [Google Scholar] [CrossRef]
- Xia, L.; Li, R.; Wang, Y.; Lin, Z.; Zheng, J.; Li, X.; Lu, Q.; Zhang, J.; Jin, H.; Fu, L.; et al. Efficacy, safety, and cost-effectiveness of all-trans retinoic acid/Clobetasol Propionate Compound Ointment in the treatment of mild to moderate psoriasis vulgaris: A randomized, single-blind, multicenter clinical trial. Dermatol. Ther. 2018, 31, e12632. [Google Scholar] [CrossRef]
- Pinter, A.; van de Kerkhof, P. The role of topical therapies along the psoriasis patient journey: An overview from the Symposium ‘Tailoring topical psoriasis treatments to patients’ needs and expectations’ of the 30th EADV Congress 2021. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef]
- Nițescu, D.A.; Mușetescu, A.; Nițescu, M.; Costescu, M.; Coman, O.A. Experimental research in topical psoriasis therapy (Review). Exp. Ther. Med. 2021, 22, 971. [Google Scholar] [CrossRef] [PubMed]
- Shinde, G.; Desai, P.; Shelke, S.; Patel, R.; Bangale, G.; Kulkarni, D. Mometasone furoate-loaded aspasomal gel for topical treatment of psoriasis: Formulation, optimization, in vitro and in vivo performance. J. Dermatol. Treat. 2022, 33, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Rapalli, V.K.; Gorantla, S.; Saha, R.N.; Dubey, S.K.; Puri, A.; Singhvi, G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020, 26, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef]
- Lu, K.J.; Wang, W.; Xu, X.L.; Jin, F.Y.; Qi, J.; Wang, X.J.; Kang, X.Q.; Zhu, M.L.; Huang, Q.L.; Yu, C.H.; et al. A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomater. Sci. 2019, 7, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.; Daulatabad, D. Topical Tretinoin in the Treatment of Nail Psoriasis. Indian Dermatol. Online J. 2022, 13, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Wysowski, D.K.; Pitts, M.; Beitz, J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J. Am. Acad. Dermatol. 2001, 45, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; McCaffery, P.J.; Drager, U.C.; De Luca, L.M. Retinoids in embryonal development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef]
- Cuomo, F.; Ceglie, S.; Miguel, M.; Lindman, B.; Lopez, F. Oral delivery of all-trans retinoic acid mediated by liposome carriers. Colloids Surf. B Biointerfaces 2021, 201, 111655. [Google Scholar] [CrossRef]
- Kumar, L.; Verma, S.; Kumar, S.; Prasad, D.N.; Jain, A.K. Fatty acid vesicles acting as expanding horizon for transdermal delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 251–260. [Google Scholar] [CrossRef]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. 10-Hydroxy Decanoic Acid-Based Vesicles as a Novel Topical Delivery System: Would It Be a Better Platform Than Conventional Oleic Acid Ufasomes for Skin Cancer Treatment? Pharmaceutics 2023, 15, 1461. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Utreja, P. Corrigendum to: Oleic Acid Vesicles as a New Approach for Transdermal Delivery of Econazole Nitrate: Development, Characterization, and In-vivo Evaluation in Wistar Rats. Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 30–49, Corrigendum in Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Mondal, D.; Sarkar, N. Dynamics of the vesicles composed of fatty acids and other amphiphile mixtures: Unveiling the role of fatty acids as a model protocell membrane. Biophys. Rev. 2020, 12, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bhardwaj, A.; Vij, M.; Bajpai, P.; Goutam, N.; Kumar, L. Oleic acid vesicles: A new approach for topical delivery of antifungal agent. Artif. Cells Nanomed. Biotechnol. 2014, 42, 95–101. [Google Scholar] [CrossRef]
- Sun, Y.; Chi, J.; Ye, X.; Wang, S.; Liang, J.; Yue, P.; Xiao, H.; Gao, X. Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. LWT 2021, 139, 110554. [Google Scholar] [CrossRef]
- More, N.B.; Sharma, N.; Pulivendala, G.; Bale, S.; Godugu, C. Natural product topical therapy in mitigating imiquimod-induced psoriasis-like skin inflammation-underscoring the anti-psoriatic potential of Nimbolide. Indian J. Pharmacol. 2021, 53, 278–285. [Google Scholar] [PubMed]
- Wang, S.; Zhu, L.; Xu, Y.; Qin, Z.; Xu, A. Salvianolic acid B ameliorates psoriatic changes in imiquimod-induced psoriasis on BALB/c mice by inhibiting inflammatory and keratin markers via altering phosphatidylinositol-3-kinase/protein kinase B signaling pathway. Korean J. Physiol. Pharmacol. 2020, 24, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ji, F.; Zhao, Y.; Hai, X. Arsenic trioxide liposome gels for the treatment of psoriasis in mice. J. Liposome Res. 2023. [Google Scholar] [CrossRef]
- Xi, L.; Lin, Z.; Qiu, F.; Chen, S.; Li, P.; Chen, X.; Wang, Z.; Zheng, Y. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Acta Pharm. Sin. B 2022, 12, 339–352. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, Y.; Tan, G.; Han, Y.; Tang, Z.; Xu, W.; Zeng, F.; Guo, Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-α-induced ICAM-1 expression via NF-κB/MAPK in HaCaT cells. Cell. Physiol. Biochem. 2015, 35, 1335–1346. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Y.; Wang, M.; Wang, Y.; Wang, W.; Pang, M.; Xu, Y. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int. J. Pharm. 2021, 605, 120826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Sun, S.; Jiang, L. The therapeutic effect of glycyrrhizic acid compound ointment on imiquimod-induced psoriasis-like disease in mice. PLoS ONE 2023, 18, e0290637. [Google Scholar] [CrossRef]
- Lauffer, F.; Eyerich, K.; Boehncke, W.H.; Asadullah, K.; Beissert, S.; Ghoreschi, K.; Schön, M.P. Cytokines of the IL-17 family in psoriasis. JDDG J. Der Dtsch. Dermatol. Ges. 2020, 18, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zeng, Y.; Liu, Z.; Zhu, R.; Wang, W. Curcumin Alleviates Epidermal Psoriasis-Like Dermatitis and IL-6/STAT3 Pathway of Mice. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, M.F.; Khafagy, E.S.; Moglad, E.H.; Selim Abu Lila, A. Formulation optimization, in vitro and in vivo evaluation of niosomal nanocarriers for enhanced topical delivery of cetirizine. Saudi Pharm. J. 2023, 31, 101734. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; El-Korany, S.M.; Sayed, S. Corneal targeted fenticonazole nitrate-loaded novasomes for the management of ocular candidiasis: Preparation, in vitro characterization, ex vivo and in vivo assessments. Drug Deliv. 2022, 29, 2428–2441. [Google Scholar] [CrossRef]
- Ghodke, J.; Ekonomou, S.I.; Weaver, E.; Lamprou, D.; Doran, O.; Stratakos, A.C. The Manufacturing and Characterisation of Eugenol-Enclosed Liposomes Produced by Microfluidic Method. Foods 2023, 12, 2940. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.K.; Handa, V.; Kathuria, H. Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery. Medicines 2018, 5, 103. [Google Scholar] [CrossRef]
- Kumar, L.; Utreja, P. Oleic Acid Vesicles for Transdermal Delivery of Propranolol Hydrochloride: Development and Characterization. Curr. Drug Ther. 2020, 15, 238–248. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Murthy, S.N.; Singh, M.R. Design, characterization and skin permeating potential of Fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids 2016, 101, 56–63, Corrigendum in Steroids 2016, 106, 93. [Google Scholar] [CrossRef]
- Shehata, T.M.; Ibrahim, M.M.; Elsewedy, H.S. Curcumin Niosomes Prepared from Proniosomal Gels: In Vitro Skin Permeability, Kinetic and In Vivo Studies. Polymers 2021, 13, 791. [Google Scholar] [CrossRef]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, H.J.; Yang, C.M.; Liu, L.; Sun, X.Y.; Wang, J.; Chen, S.T.; Lu, Y.; Hu, M.Q.; Yan, G.; et al. Inflammatory and Immunomodulatory Effects of Tripterygium wilfordii Multiglycoside in Mouse Models of Psoriasis Keratinocytes. Chin. J. Integr. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Qiu, H.Y.; Zhang, M.D.; Hou, J.J.; Zhou, M.L.; Wu, Y. Lenalidomide attenuates IMQ-induced inflammation in a mouse model of psoriasis. Biomed. Pharmacother. 2022, 156, 113883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, L.; Lyu, Y.; Wu, D.; Zhu, Y.; Li, J.; Jiang, D.; Xin, X.; Yin, L. Topical Delivery of ROS-Responsive Methotrexate Prodrug Nanoassemblies by a Dissolvable Microneedle Patch for Psoriasis Therapy. Int. J. Nanomed. 2023, 18, 899–915. [Google Scholar] [CrossRef]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, J.; Wang, S.; Li, H.; Zhou, X. Tryptanthrin ameliorates imiquimod-induced psoriasis in mice by suppressing inflammation and oxidative stress via NF-κB/MAPK/Nrf2 pathways. J. Nat. Med. 2023, 77, 188–201. [Google Scholar] [CrossRef]
- Sulthana, S.; Chary, P.S.; Bhavana, V.; Pardhi, E.; Singh, S.B.; Mehra, N.K. Development and evaluation emulgel for effective management of the imiquimod-induced psoriasis. Inflammopharmacology 2023, 31, 301–320. [Google Scholar] [CrossRef]





| Time/d | Size/nm | Zeta Potential/mv | EE% (Tre) |
|---|---|---|---|
| 1 | 126.37 ± 1.290 | −28.90 ± 1.920 | 84.26 ± 0.816 |
| 7 | 135.57 ± 1.361 | −27.33 ± 0.651 | 83.10 ± 1.054 |
| 15 | 143.90 ± 1.453 | −27.57 ± 0.569 | 81.83 ± 0.451 |
| 30 | 146.33 ± 0.709 | −26.60 ± 0.608 | 80.77 ± 0.681 |
| Time/d | Size/nm | Zeta Potential/mv | EE% (Tre) |
|---|---|---|---|
| 1 | 121.22 ± 1.311 | −26.71 ± 1.560 | 83.77 ± 0.656 |
| 7 | 136.87 ± 1.421 | −26.23 ± 0.551 | 81.81 ± 1.334 |
| 15 | 144.50 ± 1.213 | −26.11 ± 0.361 | 80.23 ± 0.321 |
| 30 | 157.31 ± 0.679 | −25.16 ± 0.558 | 77.17 ± 0.881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, C.; Zou, B.; Fu, L.; Ren, S.; Zhang, X. Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis. Molecules 2023, 28, 7868. https://doi.org/10.3390/molecules28237868
Zhao Y, Wang C, Zou B, Fu L, Ren S, Zhang X. Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis. Molecules. 2023; 28(23):7868. https://doi.org/10.3390/molecules28237868
Chicago/Turabian StyleZhao, Yaxin, Chao Wang, Bohang Zou, Lin Fu, Shushan Ren, and Xiangyu Zhang. 2023. "Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis" Molecules 28, no. 23: 7868. https://doi.org/10.3390/molecules28237868
APA StyleZhao, Y., Wang, C., Zou, B., Fu, L., Ren, S., & Zhang, X. (2023). Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis. Molecules, 28(23), 7868. https://doi.org/10.3390/molecules28237868





