Designing Inorganic–Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of DESs
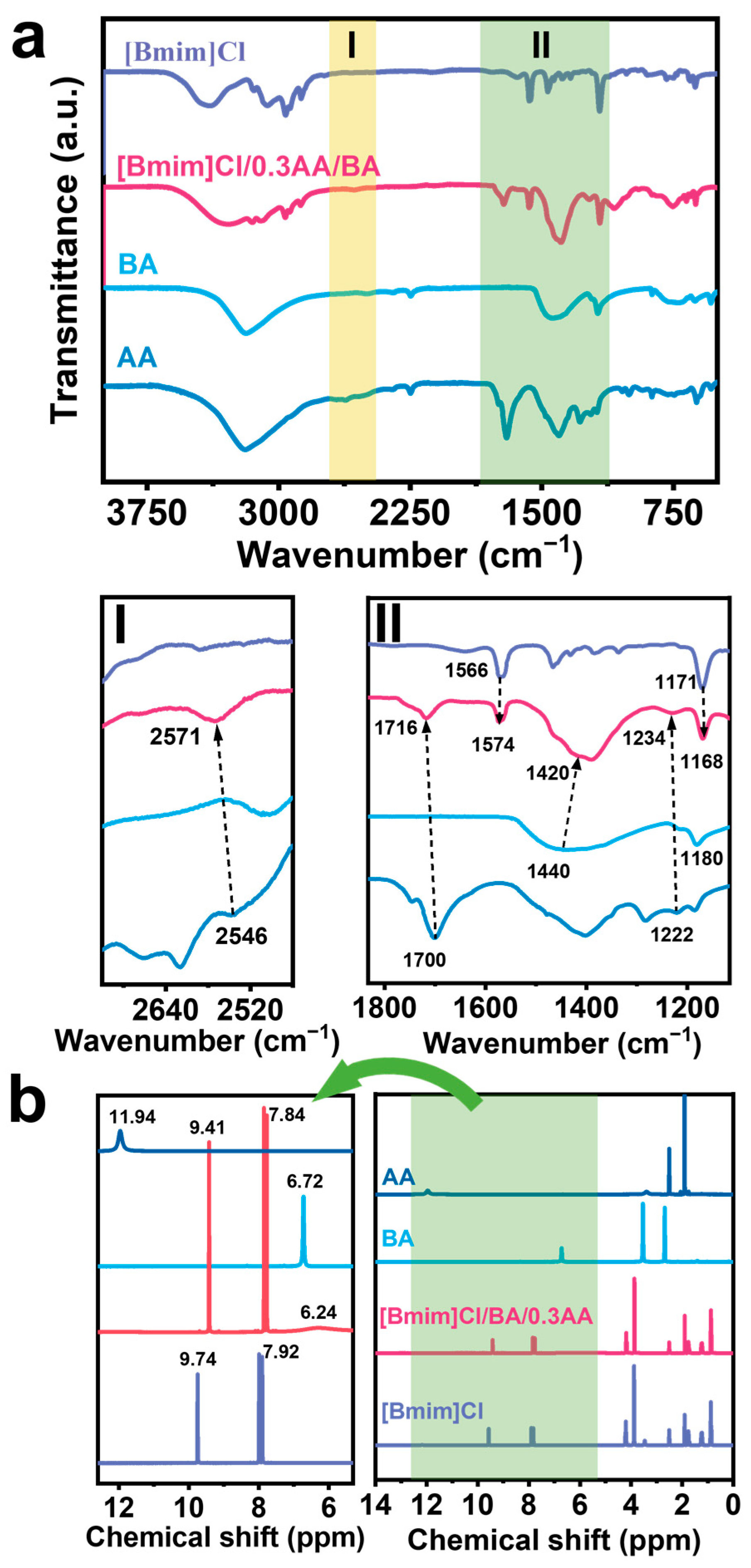
2.2. Characterization of the DADES [Bmim]Cl/BA/0.3AA
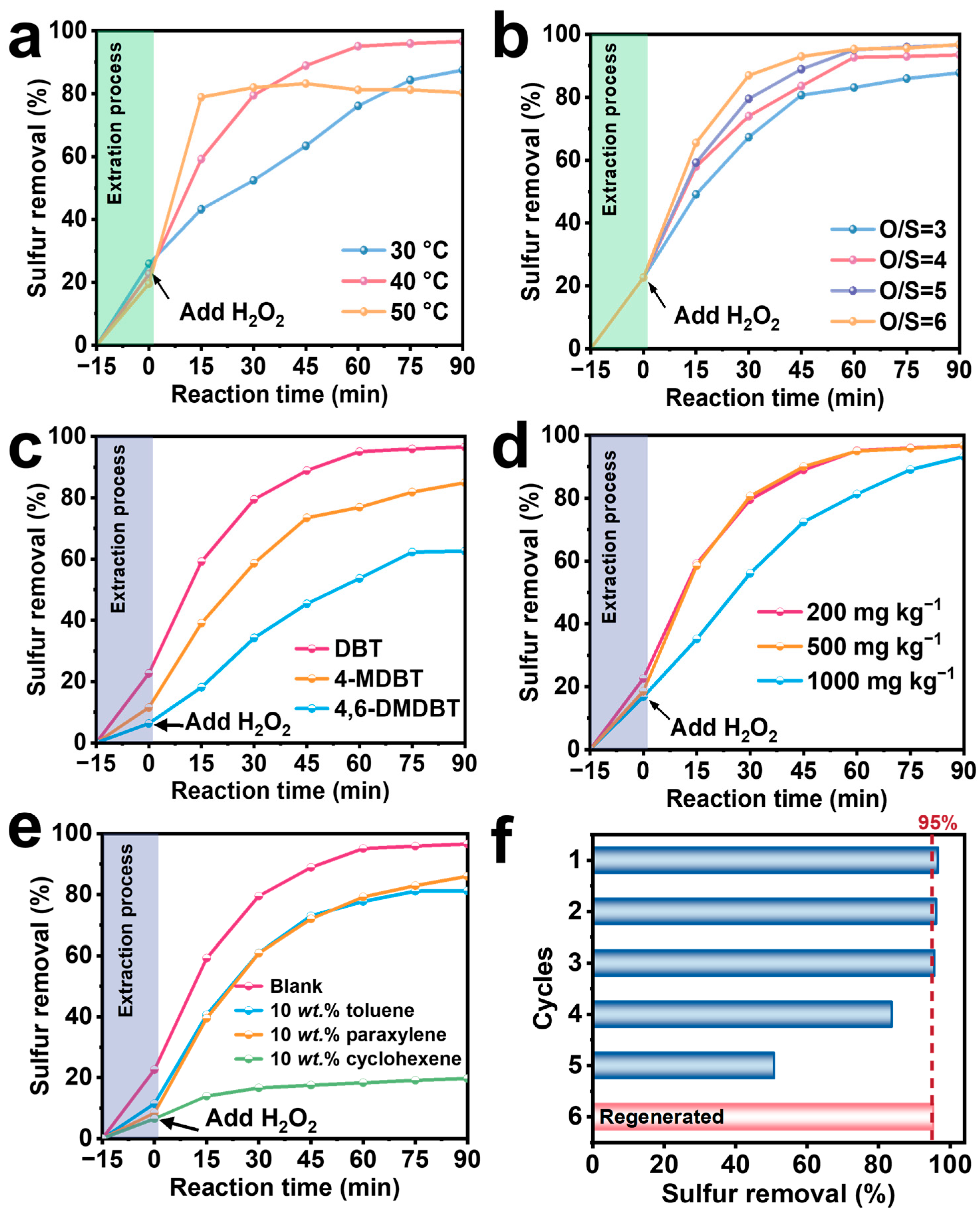
2.3. Evaluation of Desulfurization Performance
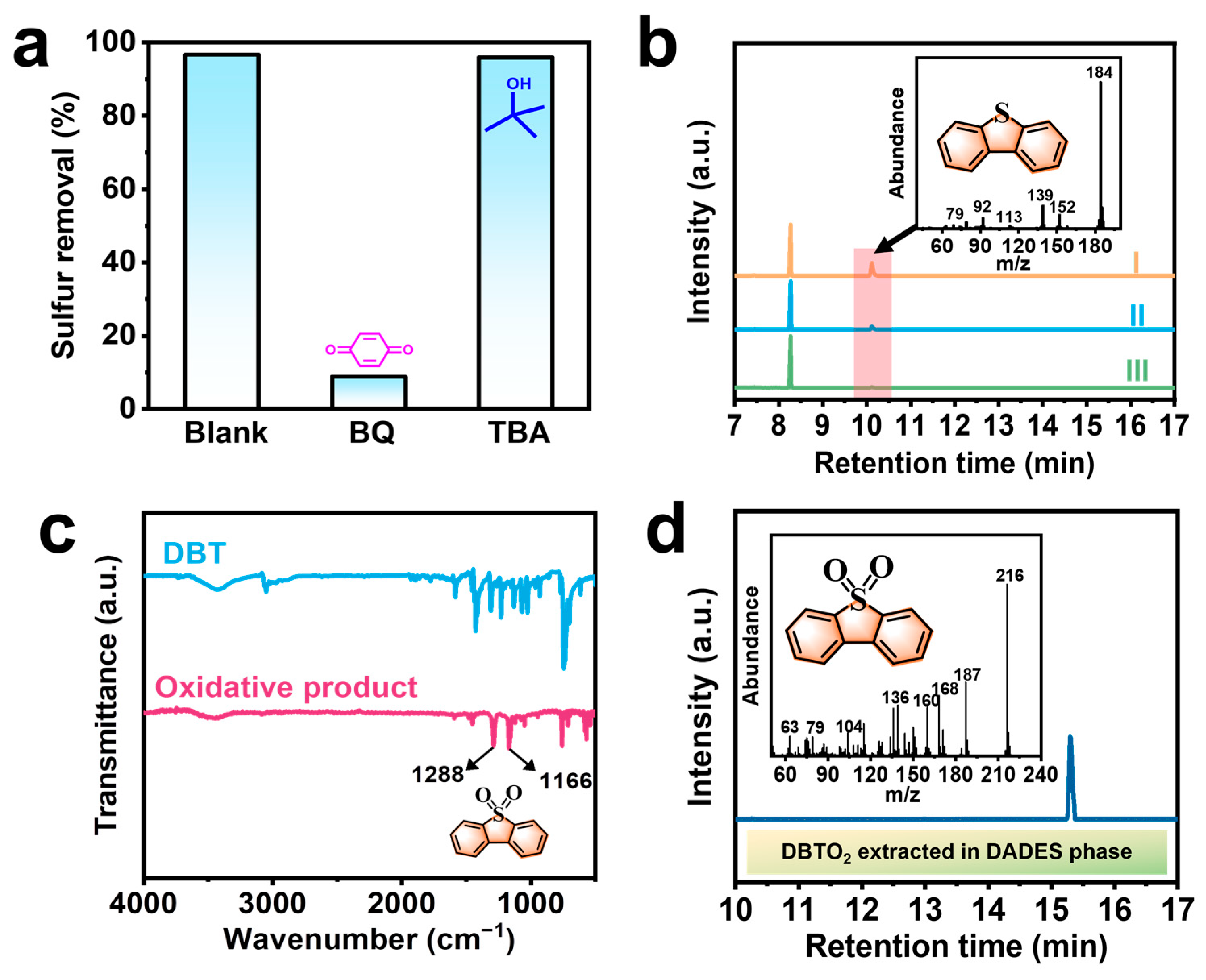
2.4. Possible EODS Reaction Mechanism over [Bmim]Cl/BA/0.3AA
3. Experimental Section
3.1. Materials
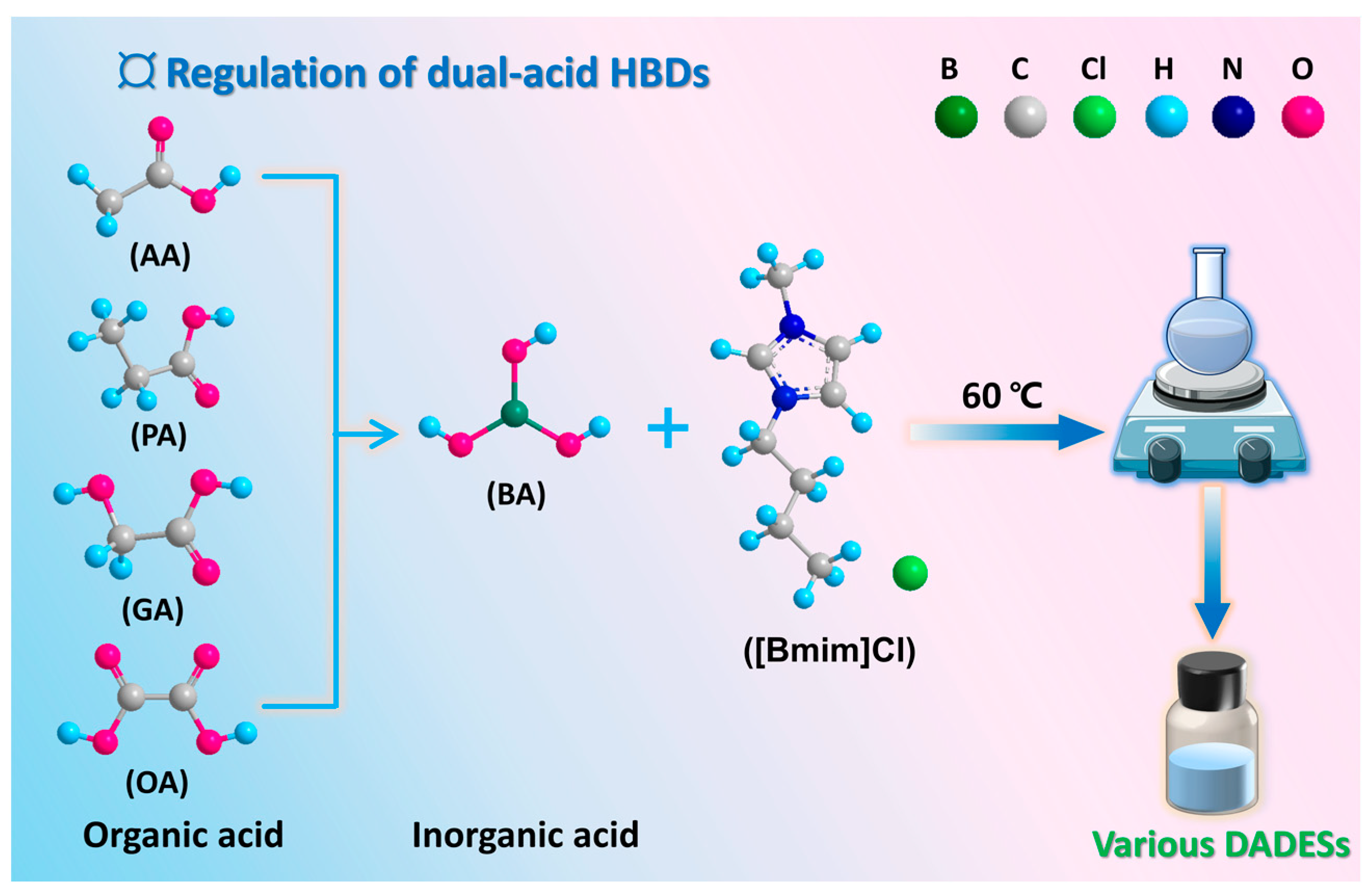
3.2. Preparation of DESs
3.3. Miscibility of DES and Model Oil
3.4. Desulfurization Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argam, V.A.; Ekaterina, A.E.; Raman, A.M.; David, A.D.; Maxim, O.L.; Ivan, S.L.; Alexey, A.S.; Alexander, V.A.; Anna, M.T.; Artur, M.A.; et al. Catalytic aerobic desulfurization of fuels in the presence of nanosized mixed carbide FeWC. Chem. Eng. J. 2023, 464, 142641. [Google Scholar]
- Ghosh, P.; Mukherji, S. Environmental Contamination by Heterocyclic Polynuclear Aromatic Hydrocarbons and their Microbial Degradation. Bioresour. Technol. 2021, 341, 125860. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, S.; Gao, Y.; Zhao, Y.; Goto, M.; Han, L.-B. Selective PP and POP Bond Formations through Copper-Catalyzed Aerobic Oxidative Dehydrogenative Couplings of H-Phosphonates. Angew. Chem. Int. Ed. 2010, 122, 7004–7007. [Google Scholar] [CrossRef]
- Ali, R.; Fallah, S.; Mohamadi, Z. Anatase titania-vanadium polyphosphomolybdate as an efficient and reusable nano catalyst for desulphurization of gas oil. J. Serb. Chem. Soc. 2014, 79, 1099–1110. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hayyan, M.; Hashim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Toutov, A.A.; Salata, M.; Fedorov, A.; Yang, Y.F.; Liang, Y.; Cariou, R.; Betz, K.N.; Couzijn, E.P.A.; Shabaker, J.W.; Houk, K.N.; et al. A potassium tert-butoxide and hydrosilane system for ultra-deep desulfurization of fuels. Nat. Energy 2017, 2, 17008. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, W.; Chao, Y.; Zhang, J.; Zhang, P.; Zhu, H.; Li, C.; Chen, Z.; Li, H.; Dai, S. A template-free solvent-mediated synthesis of high surface area boron nitride nanosheets for aerobic oxidative desulfurization. Chem. Commun. 2016, 52, 144–147. [Google Scholar] [CrossRef]
- Ahmed, I.; Kim, C.-U.; Jhung, S.H. Metal-free oxidative desulfurization with molecular oxygen by using N-enriched porous carbons derived from ionic liquid-loaded covalent-organic polymer. Chem. Eng. J. 2022, 450, 138416. [Google Scholar] [CrossRef]
- Saleh, T.A. Carbon nanotube-incorporated alumina as a support for MoNi catalysts for the efficient hydrodesulfurization of thiophenes. Chem. Eng. J. 2021, 404, 126987. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Tao, Z.; Li, B.; Zhao, D.; Gao, H.; Zhu, Z.; Wang, G.; Shu, X. Defect engineering and post-synthetic reduction of Cu based metal–organic frameworks towards efficient adsorption desulfurization. Chem. Eng. J. 2023, 455, 140487. [Google Scholar] [CrossRef]
- Li, C.P.; Li, D.; Zou, S.S.; Li, Z.; Yin, J.M.; Wang, A.L.; Cui, Y.N.; Yao, Z.L.; Zhao, Q. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem. 2013, 15, 2793–2799. [Google Scholar] [CrossRef]
- Xun, S.; Wu, C.; Tang, L.; Yuan, M.; Chen, H.; He, M.; Zhu, W.; Li, H. One-pot in-situ synthesis of coralloid supported VO2 catalyst for intensified aerobic oxidative desulfurization. Chin. J. Chem. Eng. 2022, 56, 136–140. [Google Scholar] [CrossRef]
- An, X.; Jiang, W.; He, J.; Zhu, L.; Xu, L.; Li, H.; Zhu, W.; Li, H. Modulating Electronic Characteristics of Nickel Molybdate via an Effective Manganese-Doping Strategy to Enhance Oxidative Desulfurization Performance. Inorg. Chem. 2022, 61, 21067–21075. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Wendi, F.; Jinrui, Z.; Xinyu, L.; Xinmiao, Z.; Chao, W.; Jing, H.; Wei, J.; Hongping, L.; Huaming, L. UiO-66(Zr)-based porous ionic liquids for highly efficient extraction coupled catalytic oxidative desulfurization. Chem. Eng. J. 2023, 470, 144290. [Google Scholar]
- An, X.; Xu, L.; Xu, L.; Zhu, L.; She, J.; He, J.; Jiang, W.; Zhu, W.; Li, H. Boosting Catalytic Oxidative Desulfurization Performance over Yolk-Shell Nickel Molybdate Fabricated by Defect Engineering. Inorg. Chem. 2023, 62, 9199–9208. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jin, X.; Xiang, G.; Xin, A.; Yupeng, L.; Linhua, Z.; Wenshuai, Z.; Huaming, L. In situ fabrication of hollow silica confined defective molybdenum oxide for enhanced catalytic oxidative desulfurization of diesel fuels. Fuel 2021, 305, 121470. [Google Scholar]
- Zhu, D.; Zhu, K.; Xu, L.; Huang, H.; He, J.; Zhu, W.; Li, H.; Jiang, W. Co-based metal-organic frameworks confined N-hydroxyphthalimide for enhancing aerobic desulfurization of diesel fuels. Fuel 2024, 356, 129599. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Wang, X.; Liu, P.; Fan, M.; Yan, X.; Ma, Z.; Zhang, Y.; Dai, C. Synergistic effect of dual hydrogen-donor deep eutectic solvent for performance improvement of fracturing-oil expulsion fluids. Chem. Eng. J. 2023, 468, 143728. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Fu, W.; Ran, H.; Jiang, W.; Li, H.; Zhu, W.; Li, H.; Zhang, M. Highly efficient extractive desulfurization by specific functionalized porous ionic liquids via C–H···π interactions. Fuel Process. Technol. 2023, 243, 107687. [Google Scholar] [CrossRef]
- Goeltz, J.C.; Matsushima, L.N. Metal-free redox active deep eutectic solvents. Chem. Commun. 2017, 53, 9983–9985. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Landy, D.; Greige-Gerges, H. Microextraction of bioactive compounds using deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Chi, M.; Su, T.; Sun, L.; Zhu, Z.; Liao, W.; Ren, W.; Zhao, Y.; Lü, H. Biomimetic oxygen activation and electron transfer mechanism for oxidative desulfurization. Appl. Catal. B 2020, 275, 119134. [Google Scholar] [CrossRef]
- Xu, L.; Yin, J.; Luo, Y.; Liu, H.; Li, H.; Zhu, L.; He, J.; Jiang, W.; Zhu, W.; Li, H. Rational Design of Caprolactam-Based Deep Eutectic Solvents for Extractive Desulfurization of Diesel Fuel and Mechanism Study. ACS Sustain. Chem. Eng. 2022, 10, 4551–4560. [Google Scholar]
- Shu, C.; Liu, X.; Xie, W.; Cai, S.; Li, W.; Wang, M. Reductive desulfurization of aromatic sulfides with nickel boride in deep eutectic solvents. New J. Chem. 2021, 45, 10502–10509. [Google Scholar] [CrossRef]
- Guan, S.Q.; Li, Z.L.; Xu, B.E.; Wu, J.C.; Han, J.; Guan, T.T.; Wang, J.L.; Li, K.X. Deep eutectic solvents with multiple catalytic sites for highly efficient extractive and oxidative desulfurization. Fuel 2023, 333, 126329. [Google Scholar] [CrossRef]
- Sun, L.L.; Zhu, Z.G.; Su, T.; Liao, W.P.; Hao, D.M.; Chen, Y.Y.; Zhao, Y.C.; Ren, W.Z.; Ge, H.; Lu, H.Y. Novel acidic eutectic mixture as peroxidase mimetics for oxidative desulfurization of model diesel. Appl. Catal. B 2019, 255, 117747. [Google Scholar] [CrossRef]
- Yang, B.; Fan, K.; Yu, S.; Peng, X.; Zhang, T.; Zhang, L.; Zhang, F.; Wu, H.; Guo, J.; Liu, B. Oxidative desulfurization of thiophene derivatives with L-proline/benzene sulfonic acid deep eutectic solvent and their interaction: An experimental and computational study. J. Clean. Prod. 2023, 406, 136878. [Google Scholar] [CrossRef]
- Guan, S.; Li, Z.; Xu, B.; Wu, J.; Han, J.; Guan, T.; Wang, J.; Li, K. Deep Eutectic Solvents with Excellent Catalytic Ability for Extractive and Oxidative Desulfurization under Room Temperature. ACS Sustain. Chem. Eng. 2023, 11, 6292–6301. [Google Scholar]
- Jiang, W.; Zhu, K.; Li, H.; Zhu, L.; Hua, M.; Xiao, J.; Wang, C.; Yang, Z.; Chen, G.; Zhu, W.; et al. Synergistic effect of dual Brønsted acidic deep eutectic solvents for oxidative desulfurization of diesel fuel. Chem. Eng. J. 2020, 394, 124831. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, H.; Li, H.; Zhu, L.; Tao, R.; Zhu, W.; Li, H.; Dai, S. Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel. Green Chem. 2019, 21, 3074–3080. [Google Scholar] [CrossRef]
- Zhang, W.D.; Li, Y.; Wang, K.; Yue, Y.; Tie, M.; Gu, X.J.; Xue, Y.L. Effect of different ionic liquids and organic solvents on the structural and physicochemical properties of cellulose-protein complexes extracted from Se-enriched peanut leaves. Int. J. Biol. Macromol. 2022, 217, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, Ö.; Yıldız, Ö.; Çakır, M.Ö.; Gözmen, B.; Giray, E.S. Influence of the addition of various ionic liquids on coal extraction with NMP. Fuel 2018, 212, 12–18. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Li, H.; Xue, J.; Xiong, J.; Chang, Y.; Liu, H.; Zhao, Z. Fast Oxidative Removal of Refractory Aromatic Sulfur Compounds by a Magnetic Ionic Liquid. Chem. Eng. Technol. 2014, 37, 36–42. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef]
- Thornazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an acidic scale in room temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, N.-N.; Jiang, H.; Zheng, W.-T.; Chen, Y.; Huang, K.; Liu, F. 1-ethyl-3-methylimidazolium chloride plus imidazole deep eutectic solvents as physical solvents for remarkable separation of H2S from CO2. Sep. Purif. Technol. 2021, 276, 119313. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel bronsted acidic ionic liquids and their use as dual solvent-catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef]
- Xu, L.; Jia, H.; Zhu, D.; Huan, F.; Liu, R.; Jiang, W.; Zhu, W.; Li, H. Hydrogen bonding boosted oxidative desulfurization by ZnCl2/boric acid/polyethylene glycol-based ternary deep eutectic solvents. J. Mol. Liq. 2022, 368, 120725. [Google Scholar] [CrossRef]
- Dharaskar, S.A.; Varma, M.N.; Shende, D.Z.; Yoo, C.K.; Wasewar, K.L. Synthesis, characterization and application of 1-butyl-3 methylimidazolium chloride as green material for extractive desulfurization of liquid fuel. Sci. World J. 2013, 2013, 395274. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, J.; Qazi, A.B.; Zhang, L.; Liu, X. Metal-Based Ionic Liquids in Oxidative Desulfurization: A Critical Review. Environ. Sci. Technol. 2021, 55, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, B.; Sun, Y.; Hao, L.; Huang, Z.; Zhang, L. Synthesis and oxidative desulfurization of novel lactam-based Brønsted-Lewis acidic ionic liquids. Chem. Eng. J. 2016, 306, 131–138. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, L.; Gao, R.; Hu, G.; Zhao, J. Deep desulfurization of fuels using supported ionic liquid-polyoxometalate hybrid as catalyst: A comparison of different types of ionic liquids. J. Hazard. Mater. 2021, 401, 123267. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jhung, S.H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks. J. Hazard. Mater. 2015, 301, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, B.N.; Baek, Y.S.; Kim, S.; Choi, C.H.; Jhung, S.H. Oxidative denitrogenation of liquid fuel over W2N@carbon catalyst derived from a phosphotungstinic acid encapsulated metal–azolate framework. Appl. Catal. B 2021, 285, 119842. [Google Scholar] [CrossRef]
- Yingying, Z.; Junshuang, W.; Xiguang, C.; Ning, W.; Jing, T. Green and low-cost deep eutectic solvents for efficient extraction of basic and non-basic nitrides in simulated oils. Sep. Purif. Technol. 2023, 325, 124714. [Google Scholar]
- Bhadra, B.N.; Song, J.Y.; Uddin, N.; Khan, N.A.; Kim, S.; Choi, C.H.; Jhung, S.H. Oxidative denitrogenation with TiO2@porous carbon catalyst for purification of fuel: Chemical aspects. Appl. Catal. B 2019, 240, 215–224. [Google Scholar] [CrossRef]
- Aghbolagh, Z.S.; Khorrami, M.R.K.; Rahmatyan, M.S. Sum of ranking differences in studies on high performance of catalytic oxidative denitrogenation and desulfurization of model fuel using efficient organic–inorganic hybrid nanocatalyst. J. Indian Chem. Soc. 2021, 19, 219–230. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Carnaroglio, D.; Mantegna, S.; Tabasso, S.; Moreira, E.M.; Cravotto, G. Fuel desulfurization/denitrification via adsorption or extraction: A complementary approach to oxidative treatments. Energy Sources Part A 2016, 38, 2290–2298. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Alade, O.S. Catalysed oxidation of quinoline in model fuel and the selective extraction of quinoline-N-oxide with imidazoline-based ionic liquids. Egypt. J. Pet. 2018, 27, 159–168. [Google Scholar] [CrossRef]
- Li, J.; Zeng, K.; Xu, H.; Jiang, F.; Zhang, J. Denitrification of fuel oil by hydrogen-sulfate pyrazolium-based ionic liquids. ChemistrySelect 2017, 2, 11469–11473. [Google Scholar] [CrossRef]
- Huber, M.; Poller, M.J.; Tochtermann, J.; Korth, W.; Jess, A.; Albert, J. Revealing the nitrogen reaction pathway for the catalytic oxidative denitrification of fuels. Chem. Commun. 2023, 59, 4079–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, Z.; Li, J.; Zhang, Y.; Lin, F.; Liu, Y.; Li, C. Catalytic oxidation of thiophene and its derivatives via dual activation for ultra-deep desulfurization of fuels. J. Catal. 2012, 287, 5–12. [Google Scholar] [CrossRef]
- Lu, H.; Li, P.; Deng, C.; Ren, W.; Wang, S.; Liu, P.; Zhang, H. Deep catalytic oxidative desulfurization (ODS) of dibenzothiophene (DBT) with oxalate-based deep eutectic solvents (DESs). Chem. Commun. 2015, 51, 10703–10706. [Google Scholar] [CrossRef]
- Rajendran, A.; Fan, H.-X.; Cui, T.-Y.; Feng, J.; Li, W.-Y. Octamolybdates containing MoV and MoVI sites supported on mesoporous tin oxide for oxidative desulfurization of liquid fuels. J. Clean. Prod. 2021, 334, 130199. [Google Scholar] [CrossRef]
- Junzhen, G.; Baozhen, L.; Danfeng, Z.; Liang, C.; Haibin, Y.; Zhi, H.; Zhaohui, L.; Mu, Y.; Ge, W. Preparation of porous hollow spherical MoOX/C catalyst for efficient extraction and oxidative desulfurization. Chem. Eng. J. 2023, 474, 145853. [Google Scholar]
- Olajire, A.A.; Kareem, A.; Olaleke, A. Green synthesis of bimetallic Pt@Cu nanostructures for catalytic oxidative desulfurization of model oil. J. Nanostruct. Chem. 2017, 7, 159–170. [Google Scholar] [CrossRef]
- Jiang, W.; An, X.; Xiao, J.; Yang, Z.; Liu, J.; Chen, H.; Li, H.; Zhu, W.; Li, H.; Dai, S. Enhanced Oxygen Activation Achieved by Robust Single Chromium Atom-Derived Catalysts in Aerobic Oxidative Desulfurization. ACS Catal. 2022, 12, 8623–8631. [Google Scholar] [CrossRef]
- Mao, C.; Zhao, R.; Li, X. Phenylpropanoic acid-based DESs as efficient extractants and catalysts for the removal of sulfur compounds from oil. Fuel 2017, 189, 400–407. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, W.; Xun, S.; Li, H.; Gu, Q.; Zhao, Z.; Wang, Q. Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids. Chem. Eng. J. 2013, 220, 328–336. [Google Scholar] [CrossRef]
- He, J.; Wu, P.; Chen, L.; Li, H.; Hua, M.; Lu, L.; Wei, Y.; Chao, Y.; Zhou, S.; Zhu, W.; et al. Dynamically-generated TiO2 active site on MXene Ti3C2: Boosting reactive desulfurization. Chem. Eng. J. 2021, 416, 129022. [Google Scholar] [CrossRef]
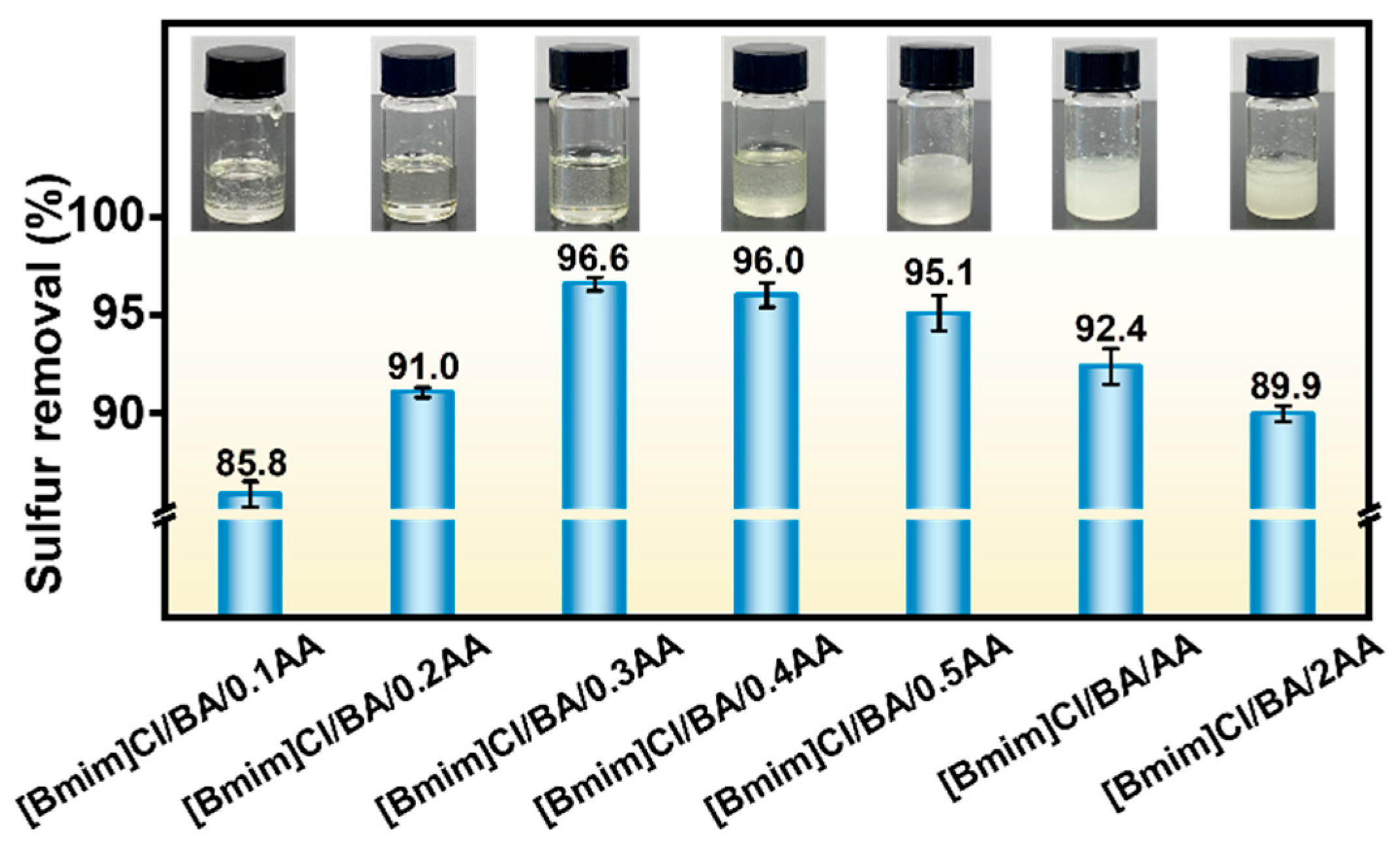




| Entry | Sample | Molar Ratio | Sulfur Removal (%) | |
|---|---|---|---|---|
| EDS a | EODS b | |||
| 1 | BA c | – | – | 3.6 |
| 2 | AA d | – | – | 2.3 |
| 3 | [Bmim]Cl e | – | 9.5 | 13.1 |
| 4 | [Bmim]Cl/BA | 1:1 | 9.8 | 75.0 |
| 5 | [Bmim]Cl/AA | 1:0.3 | 24.1 | 21.9 |
| 6 | [Bmim]Cl/BA/AA | 1:1:0.3 | 22.0 | 96.6 |
| 8 | BA/AA | 1:0.3 | White solid | |
| Entry | Simple | Amax | [In] (%) | [InH+] (%) | H0 |
|---|---|---|---|---|---|
| I | 4-nitroaniline | 1.323 | 100 | – | |
| II | [Bmim]Cl/BA | 1.242 | 93.88 | 6.12 | 2.17 |
| III | [Bmim]Cl/0.3AA | 1.230 | 92.97 | 7.03 | 2.11 |
| IV | [Bmim]Cl/BA/0.3AA | 1.198 | 90.55 | 9.45 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Xu, L.; Zhang, B.; Zhu, L.; He, J.; Li, H.; Li, H.; Jiang, W. Designing Inorganic–Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization. Molecules 2023, 28, 7743. https://doi.org/10.3390/molecules28237743
Zhu D, Xu L, Zhang B, Zhu L, He J, Li H, Li H, Jiang W. Designing Inorganic–Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization. Molecules. 2023; 28(23):7743. https://doi.org/10.3390/molecules28237743
Chicago/Turabian StyleZhu, Dongao, Lixian Xu, Beibei Zhang, Linhua Zhu, Jing He, Hongping Li, Huaming Li, and Wei Jiang. 2023. "Designing Inorganic–Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization" Molecules 28, no. 23: 7743. https://doi.org/10.3390/molecules28237743
APA StyleZhu, D., Xu, L., Zhang, B., Zhu, L., He, J., Li, H., Li, H., & Jiang, W. (2023). Designing Inorganic–Organic Dual-Acid Deep Eutectic Solvents for Synergistically Enhanced Extractive and Oxidative Desulfurization. Molecules, 28(23), 7743. https://doi.org/10.3390/molecules28237743






