Quality and Microbiome Analysis of Pickled Swimming Crabs (Portunus trituberculatus) during Storage at Two Alternative Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sensory Analysis
2.2. Microbiological Analysis
2.3. pH Analysis
2.4. TVB-N Evaluation
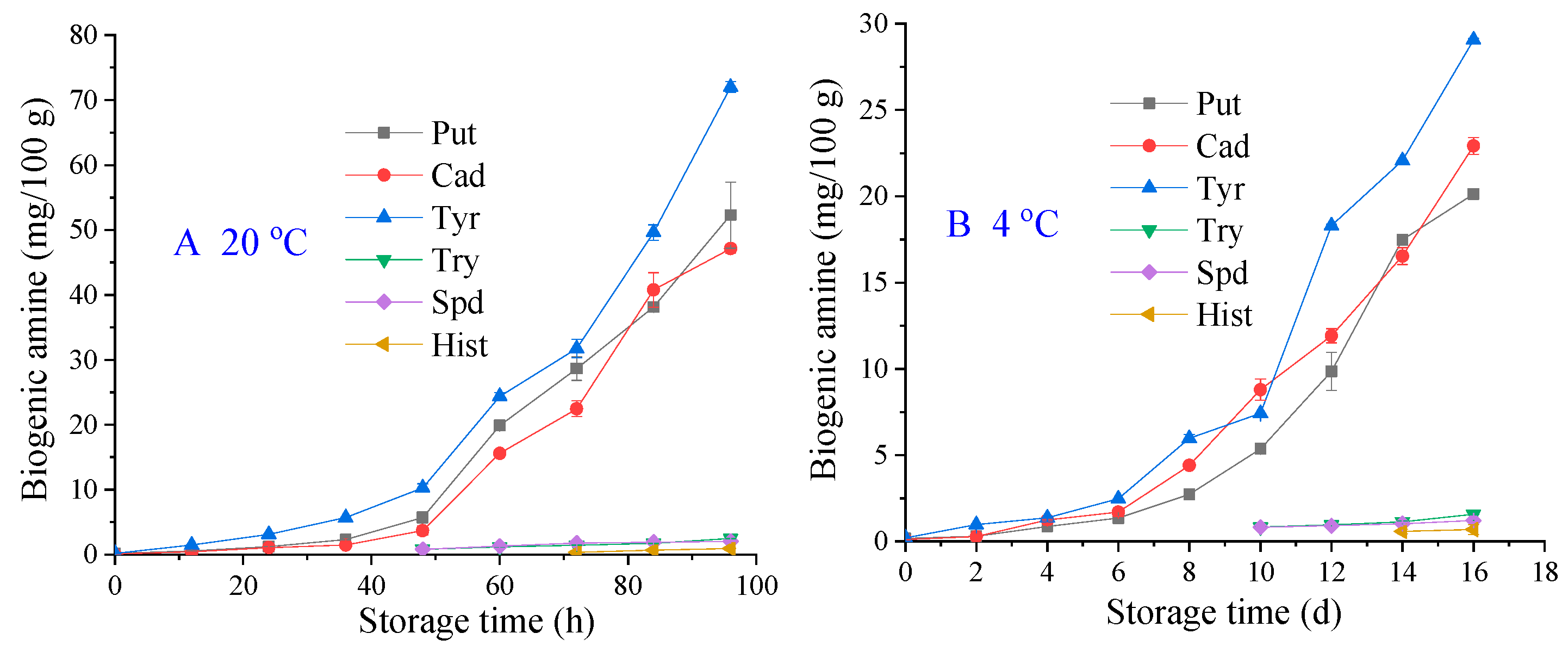
2.5. BAs Analysis
2.6. Composition of Bacterial Community
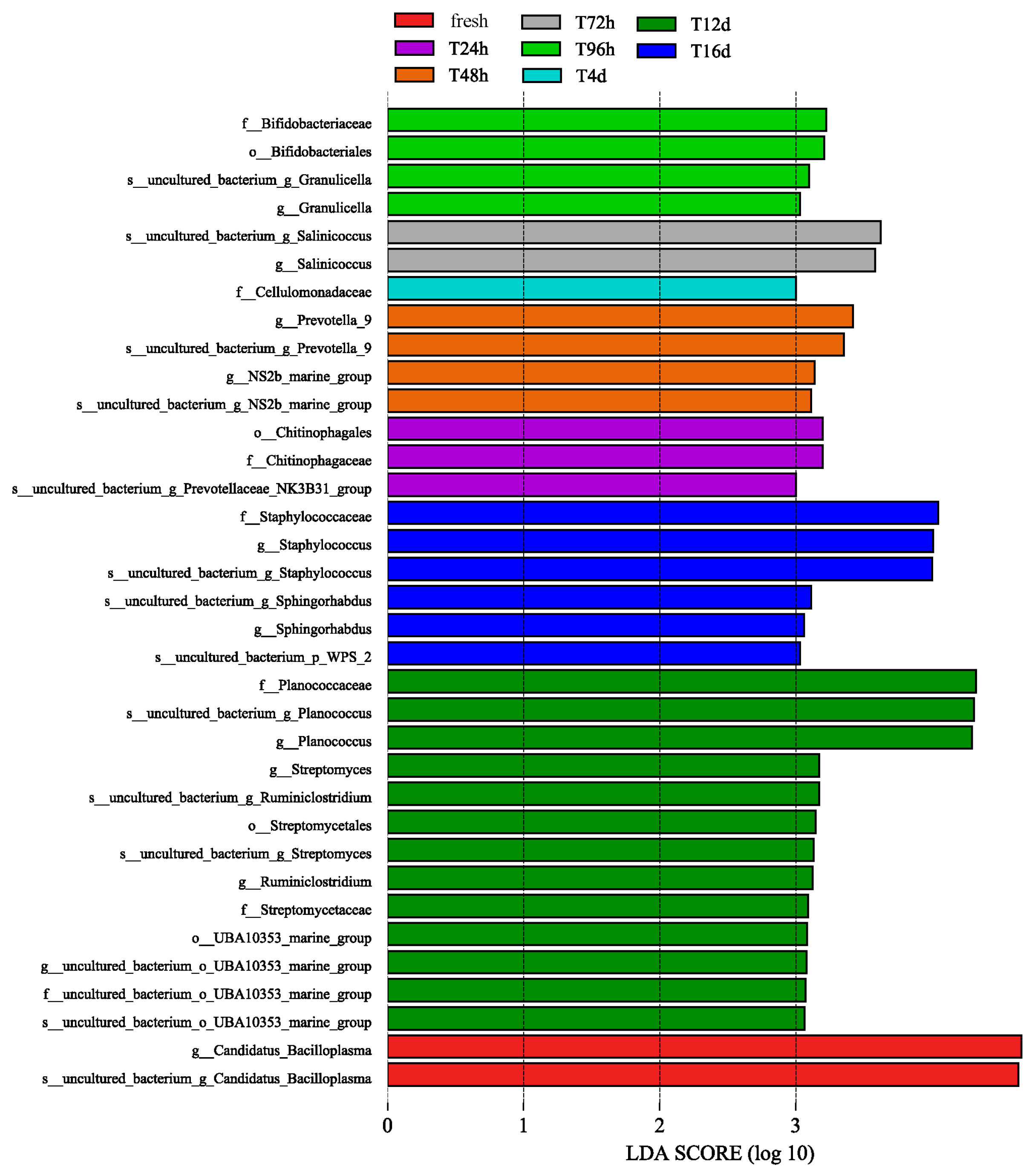
2.7. Bacterial Diversity and Difference
3. Materials and Methods
3.1. Raw Materials and Pickled Swimming Crab Processing Procedure
3.2. Sensory Analysis
3.3. Total Volatile Base Nitrogen (TVB-N) Determination
3.4. Microbiological Analysis
3.5. pH Analysis
3.6. Biogenic Amines (BAs) Analysis
3.7. DNA Extraction and High-Throughput Sequencing
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fishery Bureau of the Ministry of Agriuclture (FBMA). China Fishery Statistical Yearbook; China Agriculture Publishing House: Beijing, China, 2021; Chapter 2. [Google Scholar]
- Özden, Ö.; Ìnugur, M.; Erkn, N. Effect of different dose gamma radiation and refrigeration on the chemical and sensory properties and microbiological status of aqua cultured sea bass (Dicentrarchus labrax). Radiat. Phys. Chem. 2007, 76, 1169–1178. [Google Scholar] [CrossRef]
- Lian, F.; Måge, I.; Lorentzen, G.; Siikavuopio, S.I.; Øverbø, K.; Vang, B.; Lindberg, D. Exploring the effect of inhibitors, cooking and freezing on melanosis in snow crab (Chionoecetes opilio) clusters. Food Control 2018, 92, 255–266. [Google Scholar] [CrossRef]
- Gottsauner-Wolf, F.; Grabowski, J.J.; Chao, E.Y.; An, K.N. Effects of freeze/thaw conditioning on the tensile properties and failure mode of bone-muscle-bone units: A biomechanical and histological study in dogs. J. Orthop. Res. 2010, 13, 90. [Google Scholar] [CrossRef]
- Lorentzen, G.; Rotabakk, B.T.; Olsen, S.H.; Skuland, A.V.; Siikavuopio, S.I. Shelf life of snow crab clusters (Chionoecetes opilio) stored at 0 and 4 °C. Food Control 2016, 59, 454–460. [Google Scholar] [CrossRef]
- Huang, T.; Lin, J.; Fang, Z.X.; Yu, W.W.; Li, Z.H.; Xu, D.L.; Yang, W.G.; Zhang, J.J. Preparation and characterization of irradiated kafirin-quercetin film for packaging cod (Gadus morhua) during cold storage at 4 °C. Food Bioprocess Technol. 2020, 13, 522–532. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Fatty acid composition, lipid oxidation, and fishy odour development in seabass (Lates calcarifer) skin during iced storage. Eur. J. Lipid Sci. Technol. 2014, 116, 885–894. [Google Scholar] [CrossRef]
- Anupama, T.K.; Laly, S.J.; Kumar, K.N.A.; Sankar, T.V.; Ninan, G. Biochemical and microbiological assessment of Crucifix crab (Charybdis feriatus) stored at 4 °C. J. Aquat. Food Prod. Technol. 2018, 27, 531–541. [Google Scholar] [CrossRef]
- Hernández, M.; López, M.; Álvarez, A.; Ferrandini, E.; García, B.G.; Garrido, M. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- G.B. 10136-2016; Animal Based Aquatic Products. Standards Press of China: Beijing, China, 2016.
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Control 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Kilinc, B.; Cakli, S.; Tolasa, S.; Dincer, T. Chemical, microbiological and sensory changes associated with fish sauce processing. Eur. Food Res. Technol. 2006, 222, 604–613. [Google Scholar] [CrossRef]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433–445. [Google Scholar]
- Morsy, M.K.; Zor, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Khalaf, H. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Azari, A.; Ahari, H.; Anvar, A.A. Increased shelf life of Oncorhynchus mykiss (Rainbow trout) through Cu-Clay nanocomposites. Food Sci. Biotechnol. 2022, 31, 295–309. [Google Scholar] [CrossRef]
- Chu, C.H.; Bjeldanes, L.F. Effect of diamines, polyamines and tuna fish extracts on the binding of histamine to mucin in vitro. J. Food Sci. 1982, 47, 79–80. [Google Scholar] [CrossRef]
- Mackie, I.; Pirie, L.; Ritchie, A.; Yamanaka, H. The formation of non-volatile amines in relation to concentrations of free basic amino acids during postmortem storage of the muscle of scallop (Pecten maximus), herring (Clupea harengus) and mackerel (Scomber scombrus). Food Chem. 1997, 60, 291–295. [Google Scholar] [CrossRef]
- Veciana-Nogues, M.; Albala-Hurtado, S.; Marine-Font, A.; Vidal-Carou, M. Changes in biogenic amines during the manufacture and storage of semipreserved anchovies. J. Food Prot. 1996, 59, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.M.A.; Khanzadi, S.; Fazlara, A.; Najafzadehvarzi, H.; Azizzadeh, M. Effect of lactic acid and ajwain (Carum copticum) on the biogenic amines and quality of refrigerated common carp (Cyprinus carpio). LWT 2018, 97, 434–439. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, X.; Hadiatullah, H.; Zhang, J.; Zhao, G.Z. Determination of microbial diversities and aroma characteristics of beitang shrimp paste. Food Chem. 2020, 344, 128695. [Google Scholar] [CrossRef]
- Inturri, R.; Molinaro, A.; Lorenzo, F.D.; Blandino, G.; Tomasello, B.; Hidalgo-Cantabrana, C.; Castro, C.D.; Ruas-Madiedo, P. Chemical and biological properties of the novel exopolysaccharide produced by a probiotic strain of Bifidobacterium longum. Carbohyd. Polym. 2017, 174, 1172–1180. [Google Scholar] [CrossRef]
- Deetae, P.; Spinnler, H.E.; Bonnarme, P.; Helinck, S. Growth and aroma contribution of Microbacterium foliorum, Proteus vulgaris and Psychrobacter sp. during ripening in a cheese model medium. Appl. Microbiol. Biot. 2009, 82, 169–177. [Google Scholar] [CrossRef]
- Villamil, S.M.I.; Huerlimann, R.; Maes, G.E.; Condon, K.; Jerry, D.R. Bacterial signatures of productivity decay in Penaeus monodon ponds infected with PirA toxin. Aquaculture 2019, 511, 734202. [Google Scholar] [CrossRef]
- Sun, Y.F.; Han, W.F.; Liu, J.; Liu, F.; Cheng, Y.X. Microbiota comparison in the intestine of juvenile Chinese mitten crab Eriocheir sinensis fed different diets. Aquaculture 2019, 515, 734518. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Xu, K.; Zhang, X.Y.; Yan, M.T. White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 84, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Qi, C.L.; Jia, Y.Y.; Gu, Z.M.; Li, E.C. Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 2020, 524, 735256. [Google Scholar] [CrossRef]
- Lorentzen, G.; Skuland, A.V.; Sone, I.; Johansen, J.O.; Rotabakk, B.T. Determination of the shelf life of cluster of the red king crab (Paralithodes camtschaticus) during chilled storage. Food Control 2014, 42, 207–213. [Google Scholar] [CrossRef]
- Parlapani, F.; Michailidou, S.; Anagnostopoulos, D.; Koromilas, S.; Kios, K.; Pasentsis, K.; Boziaris, I. Bacterial communities and potential spoilage markers of whole blue crab (Callinectes sapidus) stored under commercial simulated conditions. Food Microbiol. 2019, 82, 325–333. [Google Scholar] [CrossRef]
- Qian, L.; Li, D.; Na, Q.; Hui, H.; Luo, Y. Comparative studies of quality changes in white and dark muscles from common carp (Cyprinus carpio) during refrigerated (4 °C) storage. Int. J. Food Sci. Technol. 2016, 51, 1130–1139. [Google Scholar]
- Cen, S.J.; Fang, Q.; Tong, L.; Yang, W.G.; Huang, T. Effects of chitosansodium alginate-nisin preservatives on the quality and spoilage microbiota of penaeus vannamei shrimp during cold storage. Int. J. Food Microbiol. 2021, 349, 109227. [Google Scholar] [CrossRef]


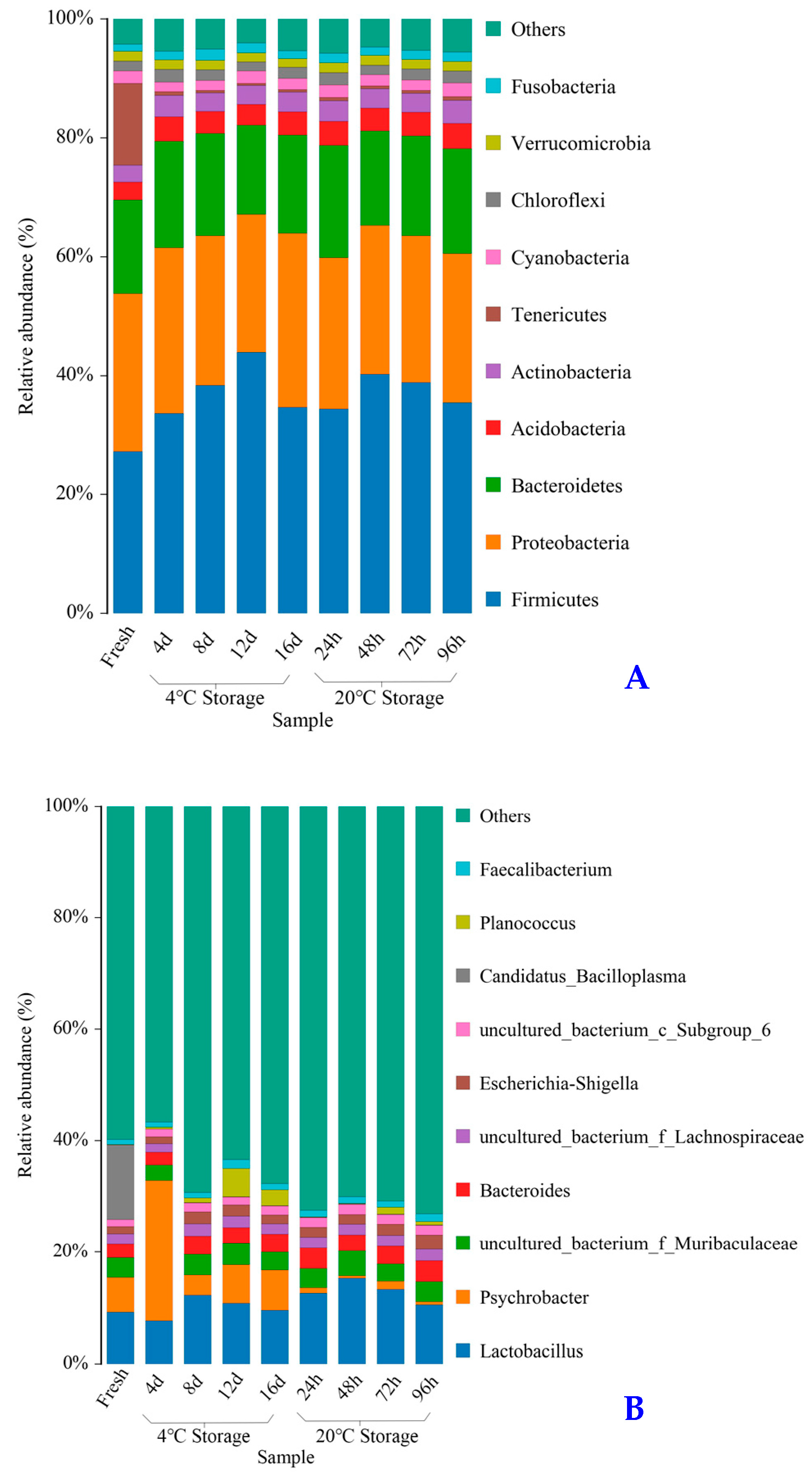

| Samples | Firmicutes | Proteobacteria | Bacteroidetes | Tenericutes | Acidobacteria | Actinobacteria | Cyanobacteria | Chloroflexi | Verrucomicrobia | Fusobacteria | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 27.19% | 26.63% | 15.73% | 13.71% | 3.02% | 2.86% | 2.11% | 1.66% | 1.65% | 1.21% | 4.22% | |
| 4 °C | 4 d | 25.09% | 44.82% | 14.65% | 0.41% | 3.05% | 2.91% | 1.45% | 1.48% | 1.10% | 1.10% | 3.92% |
| 8 d | 38.30% | 25.26% | 17.21% | 0.46% | 3.68% | 3.09% | 1.64% | 1.83% | 1.62% | 1.81% | 5.11% | |
| 12 d | 40.95% | 28.04% | 13.77% | 0.33% | 3.44% | 3.47% | 2.13% | 1.32% | 1.37% | 1.53% | 3.66% | |
| 16 d | 34.66% | 29.31% | 16.54% | 0.44% | 3.83% | 3.35% | 1.85% | 1.89% | 1.44% | 1.35% | 5.34% | |
| 20 °C | 24 h | 34.39% | 25.47% | 18.87% | 0.56% | 4.01% | 3.46% | 2.12% | 2.06% | 1.71% | 1.60% | 5.75% |
| 48 h | 37.50% | 26.63% | 16.07% | 0.46% | 4.21% | 3.78% | 2.33% | 1.41% | 1.64% | 1.54% | 4.43% | |
| 72 h | 38.80% | 24.72% | 16.81% | 0.47% | 3.98% | 3.20% | 1.74% | 1.88% | 1.59% | 1.55% | 5.27% | |
| 96 h | 35.43% | 25.10% | 17.61% | 0.61% | 4.29% | 3.89% | 2.28% | 1.97% | 1.65% | 1.60% | 5.54% | |
| Samples | Coverage | Alpha Diversity | ||||
|---|---|---|---|---|---|---|
| ACE | Chao1 | Simpson | Shannon | |||
| Fresh | 0.9973 | 2282.4731 | 2285.9071 | 0.9604 | 8.5563 | |
| 4 d | 0.9980 | 2259.5870 | 2275.9723 | 0.9953 | 9.5851 | |
| 4 °C | 8 d | 0.9980 | 2256.2301 | 2267.0133 | 0.9938 | 9.4586 |
| 12 d | 0.9975 | 2252.7042 | 2259.9469 | 0.9924 | 9.1744 | |
| 16 d | 0.9980 | 2265.2872 | 2276.1444 | 0.9895 | 9.2712 | |
| 24 h | 0.9982 | 2267.7451 | 2278.8716 | 0.9960 | 9.6734 | |
| 20 °C | 48 h | 0.9979 | 2258.9893 | 2263.6768 | 0.9938 | 9.3706 |
| 72 h | 0.9981 | 2267.6745 | 2280.7749 | 0.9942 | 9.4571 | |
| 96 h | 0.9979 | 2264.1723 | 2281.5283 | 0.9955 | 9.6389 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, P.; Xu, D.; Zhang, X.; Huang, T. Quality and Microbiome Analysis of Pickled Swimming Crabs (Portunus trituberculatus) during Storage at Two Alternative Temperatures. Molecules 2023, 28, 7744. https://doi.org/10.3390/molecules28237744
Chen Y, Li P, Xu D, Zhang X, Huang T. Quality and Microbiome Analysis of Pickled Swimming Crabs (Portunus trituberculatus) during Storage at Two Alternative Temperatures. Molecules. 2023; 28(23):7744. https://doi.org/10.3390/molecules28237744
Chicago/Turabian StyleChen, Yu, Peipei Li, Dan Xu, Xiaojun Zhang, and Tao Huang. 2023. "Quality and Microbiome Analysis of Pickled Swimming Crabs (Portunus trituberculatus) during Storage at Two Alternative Temperatures" Molecules 28, no. 23: 7744. https://doi.org/10.3390/molecules28237744
APA StyleChen, Y., Li, P., Xu, D., Zhang, X., & Huang, T. (2023). Quality and Microbiome Analysis of Pickled Swimming Crabs (Portunus trituberculatus) during Storage at Two Alternative Temperatures. Molecules, 28(23), 7744. https://doi.org/10.3390/molecules28237744