Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening and Compound Identification
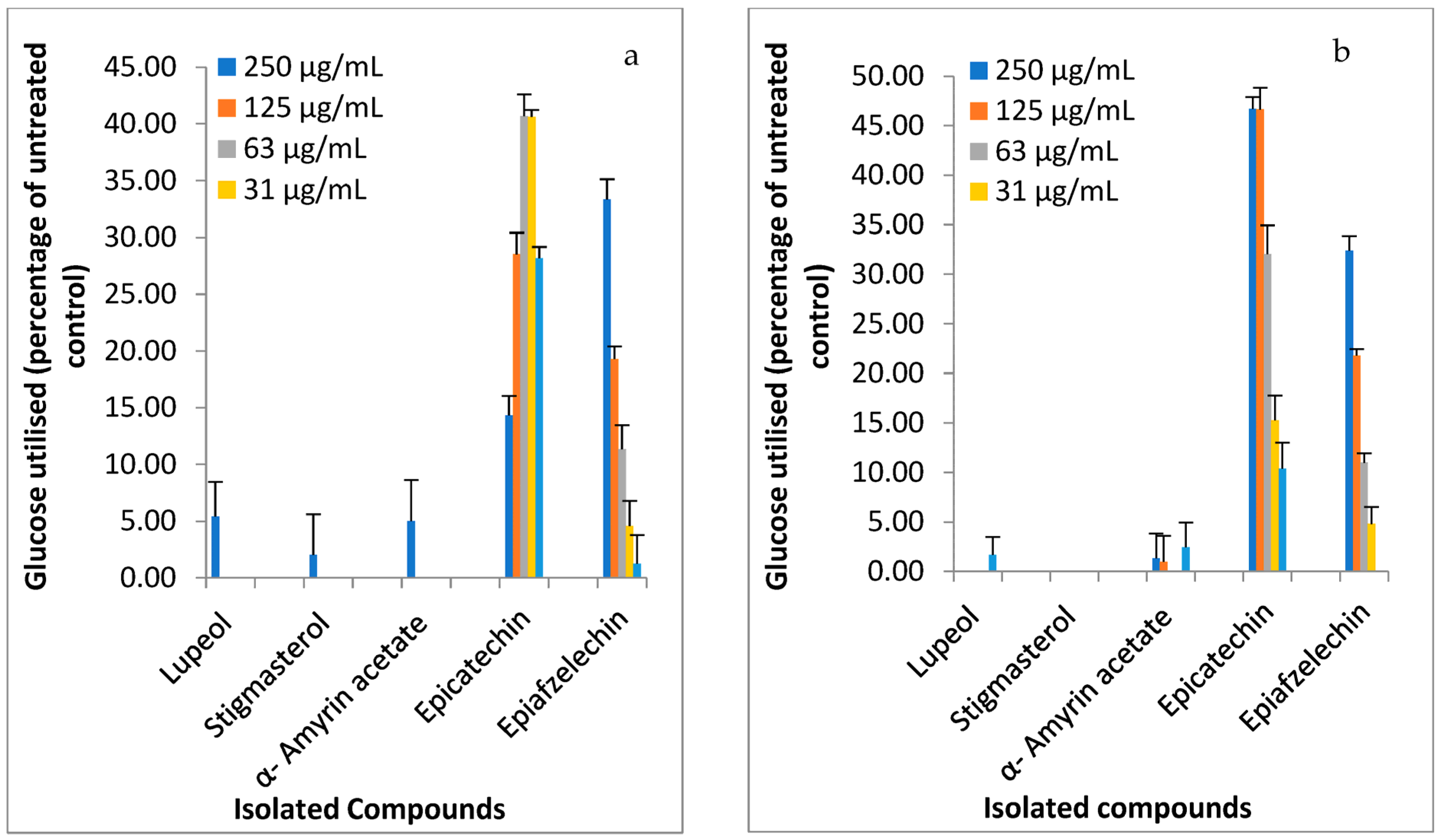
2.2. Inhibition of α-Glucosidase Activity and Glucose Utilization Enhanced by the Compounds
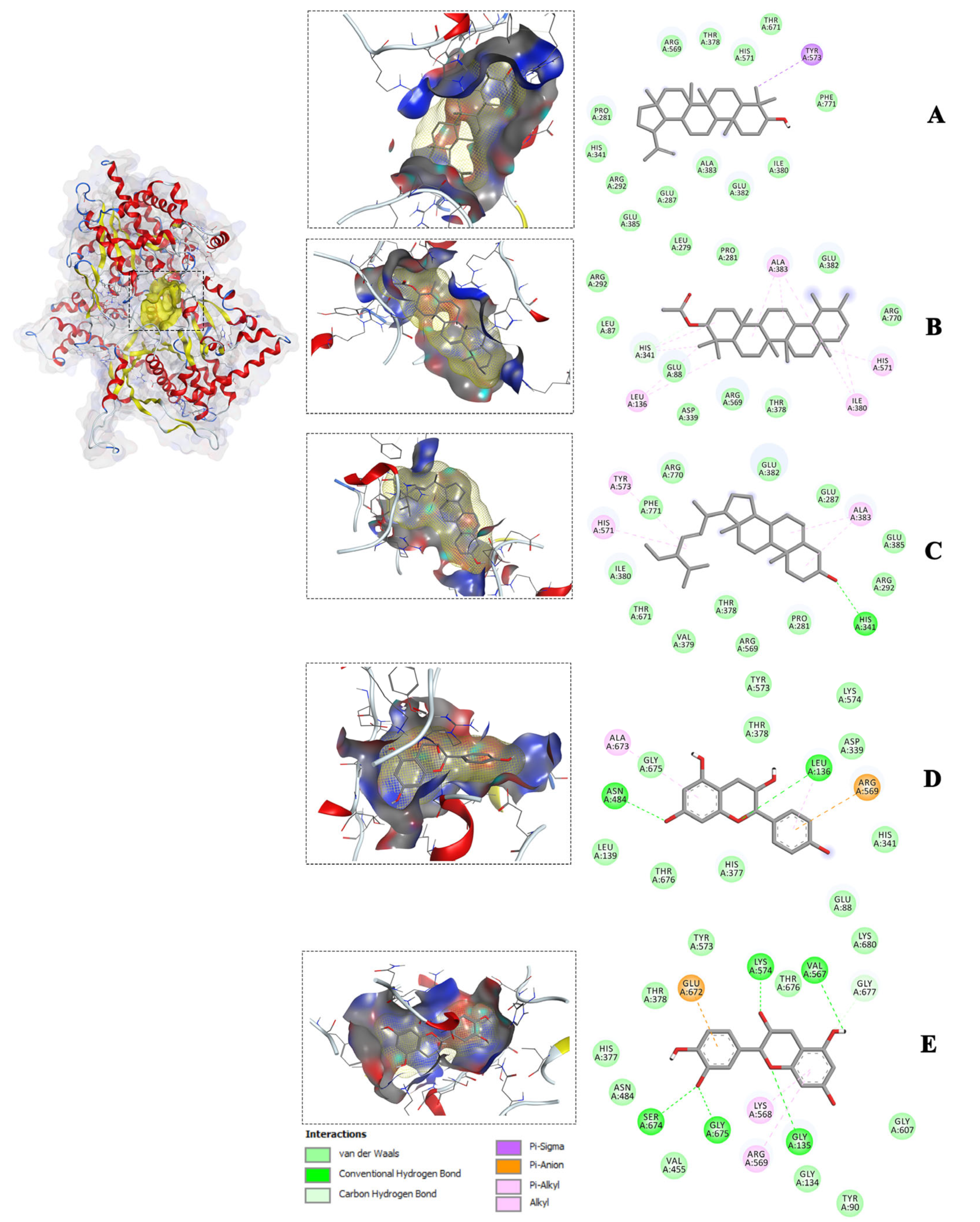
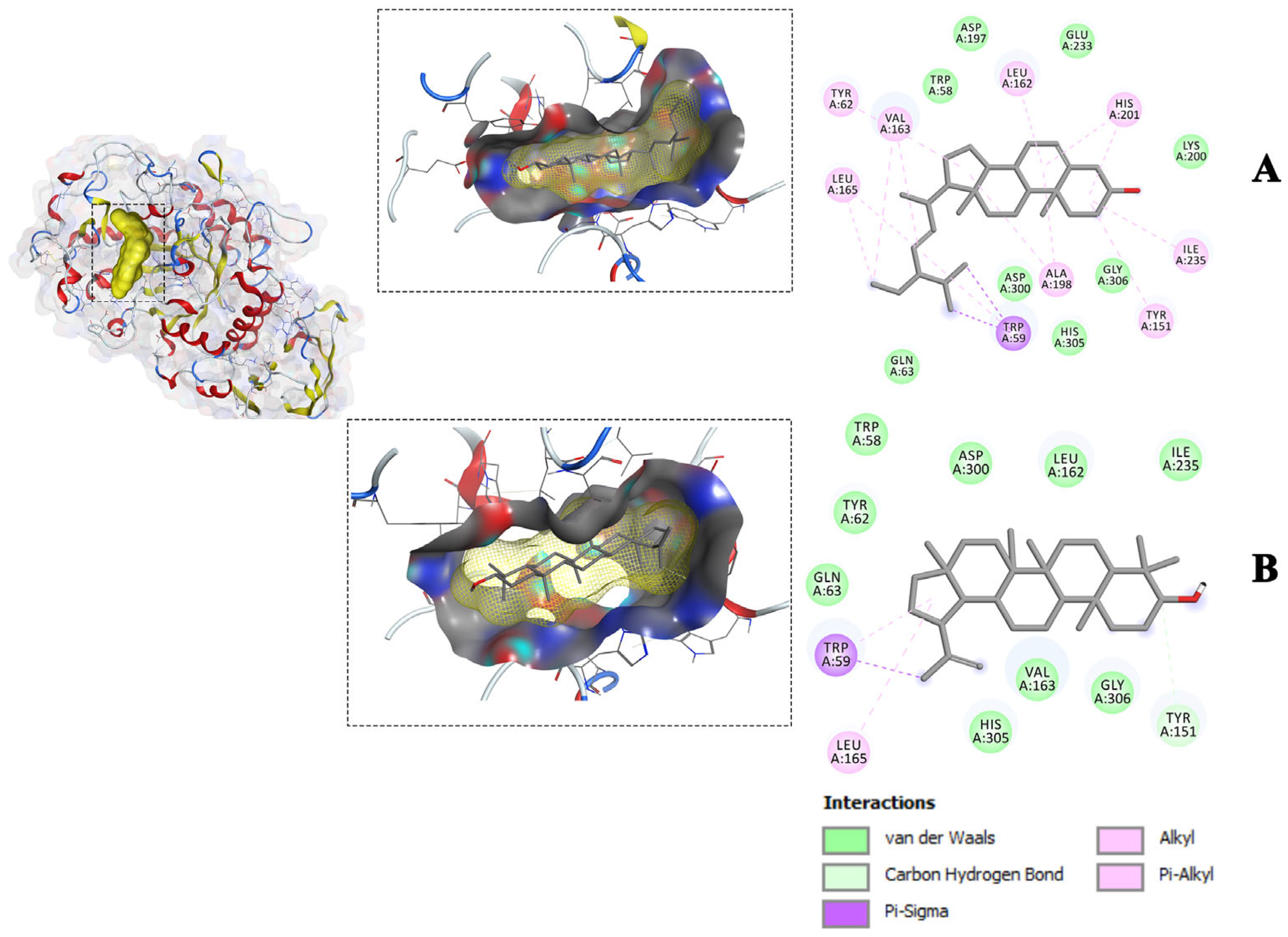
2.3. Molecular Docking of Isolated Compounds against Glucose-Metabolizing Receptors
2.4. Drug-Likeness and ADMET Properties
3. Discussion
4. Materials and Methods
4.1. General
4.2. Extraction and Isolation
4.3. Preparation of Samples
4.4. Phytochemical Screening
4.5. α-Glucosidase Inhibition Assay
4.6. Glucose Utilization Activity
4.7. Statistical Analysis
4.8. In Silico Analyses of the Isolated Compounds
4.8.1. Ligand and Target Protein Preparation, and Molecular Docking
4.8.2. ADMET Profiling of the Isolated Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutyambizi, C.; Booysen, F.; Stokes, A.; Pavlova, M.; Groot, W. Lifestyle and Socio-Economic Inequalities in Diabetes Prevalence in South Africa: A Decomposition Analysis. PLoS ONE 2019, 14, e0211208. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Consultation, Definition, diagnosis and classification of diabetes mellitus and its complications. In Part 1: Diagnosis and Classification of Diabetes Mellitus; Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Amos, A.F.; McCarty, D.J.; Zimmet, P. The Rising Global Burden of Diabetes and Its Complications: Estimates and Projections to the Year 2010. Diabet. Med. 1997, 14, S7–S85. [Google Scholar] [CrossRef]
- Statistics South Africa. Mortality and Causes of Death in South Africa, Findings from Death Notification; Statistics South Africa: Pretoria, South Africa, 2015. [Google Scholar]
- IDF Diabetes Atlas, 8th ed. 2017. Available online: http://www.diabetesatlas.org/resources/2017-atlas.html (accessed on 15 March 2021).
- Odeyemi, S.; Bradley, G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef] [PubMed]
- Olaokun, O.O. The Value of Extracts of Ficus lutea (Moraceae) in the Management of Type II Diabetes in a Mouse Obesity Model. Ph.D. Dissertation, University of Pretoria, Pretoria, South Africa, 2012. [Google Scholar]
- Tabasum, S.; Khare, S. Safety of medicinal plants: An important concern. Int. J. Pharma Bio. Sci. 2016, 7, 237–243. [Google Scholar]
- Nyakudya, T.T.; Tshabalala, T.; Dangarembizi, R.; Erlwanger, K.H.; Ndhlala, A.R. The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders. Molecules 2020, 25, 2669. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.M.; Breyer-Brandwijk, M.G. Medicinal and Poisonous Plants of Southern and Eastern Africa; Livingstone Ltd.: London, UK, 1962; pp. 773–780. [Google Scholar]
- Ramadan, M.; Ahmad, A.S.; Nafady, A.M.; Mansour, A. Chemical Composition of the Stem Bark and Leaves of Ficus Pandurata Hance. Nat. Prod. Res. 2009, 23, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Olaokun, O.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Evaluation of the Inhibition of Carbohydrate Hydrolysing Enzymes, Antioxidant Activity and Polyphenolic Content of Extracts of Ten African Ficus Species (Moraceae) Used Traditionally to Treat Diabetes. BMC Complement. Altern. Med. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Coates-Palgrave, K. Moraceae (The fig and mulberry family). In Keith Coates-Palgrave Trees of Southern Africa Cape Town: 2nd imp, 3rd ed.; Struik Publishers: Cape Town, South Africa, 2002; pp. 129–153. [Google Scholar]
- Olaokun, O.O.; McGaw, L.J.; Awouafack, M.D.; Eloff, J.N.; Naidoo, V. The Potential Role of GLUT4 Transporters and Insulin Receptors in the Hypoglycaemic Activity of Ficus Lutea Acetone Leaf Extract. BMC Complement. Altern. Med. 2014, 14, 269. [Google Scholar] [CrossRef]
- Olaokun, O.O.; McGaw, L.J.; Janse van Rensburg, I.; Eloff, J.N.; Naidoo, V. Antidiabetic Activity of the Ethyl Acetate Fraction of Ficus lutea (Moraceae) Leaf Extract: Comparison of an in Vitro Assay with an In Vivo Obese Mouse Model. BMC Complement. Altern. Med. 2016, 16, 110. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C NMR Spectra of Pentacyclic Triterpenoids—A Compilation and Some Salient Features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Poumale, H.M.P.; Songfack Djoumessi, A.V.B.; Ngameni, B.; Sandjo, L.P.; Ngadjui, B.T.; Shiono, Y. A New Ceramide Isolated from Ficus lutea Vahl (moraceae). Acta Chim. Slov. 2011, 58, 81–86. [Google Scholar] [PubMed]
- Forgo, P.; Kövér, K.E. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, B.; Wang, J.; Li, B.; Jiang, Y. Identification of the Major Flavonoids from Pericarp Tissues of Lychee Fruit in Relation to Their Antioxidant Activities. Food Chem. 2006, 98, 539–544. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug. Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In Vitro and In Vivo Antidiabetic Activity, Isolation of Flavonoids, and in Silico Molecular Docking of Stem Extract of Merremia Tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Sona, A.N.; Bekono, B.D.; Ntie-Kang, F. Fundamental Physical and Chemical Concepts behind “Drug-Likeness” and “Natural Product-Likeness”. Phys. Sci. Rev. 2019, 4, 20180101. [Google Scholar] [CrossRef]
- Johnson, D.E.; Wolfgang, G.H.I. Predicting Human Safety: Screening and Computational Approaches. Drug. Discov. Today 2000, 5, 445–454. [Google Scholar] [CrossRef]
- Pathak, M.; Ojha, H.; Tiwari, A.K.; Sharma, D.; Saini, M.; Kakkar, R. Design, Synthesis and Biological Evaluation of Antimalarial Activity of New Derivatives of 2,4,6-s-Triazine. Chem. Cent. J. 2017, 11, 132. [Google Scholar] [CrossRef]
- Naspiah, N.; Rizki Fadhil Pratama, M.; Sukardiman, S.S. Xanthine Oxidase Inhibition Activity and ADMET Properties of Terap (Artocarpus Odoratissimus Blanco) Leaves Metabolites: Phytochemical Screening and in Silico Studies. Pharmacogn. J. 2021, 13, 1150–1160. [Google Scholar] [CrossRef]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Ngbolua, J.P.K.T.N.; Kilembe, J.T.; Matondo, A.; Ashande, C.M.; Mukiza, J.; Nzanzu, C.M.; Ruphin, F.P.; Baholy, R.; Mpiana, P.T.; Mudogo, V. In Silico Studies on the Interaction of Four Cytotoxic Compounds with Angiogenesis Target Protein HIF-1α and Human Androgen Receptor and Their ADMET Properties. Bull. Natl. Res. Cent. 2022, 46, 101. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.; Frearson, J.; Wyatt, P. Lessons Learnt from Assembling Screening Libraries for Drug Discovery for Neglected Diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Trends in Virtual Combinatorial Library Design. Curr. Med. Chem. 2002, 9, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Bucao, X.E.; Solidum, J. In Silico Evaluation of Antidiabetic Activity and ADMET Prediction of Compounds from Musa Acuminata Colla Peel. Philipp. J. Sci. 2021, 151, 171–192. [Google Scholar] [CrossRef]
- Ottaviani, G.; Gosling, D.J.; Patissier, C.; Rodde, S.; Zhou, L.; Faller, B. What Is Modulating Solubility in Simulated Intestinal Fluids? Euro. J. Pharm. Sci. 2010, 41, 452–457. [Google Scholar] [CrossRef]
- Artursson, P.; Neuhoff, S.; Matsson, P.; Tavelin, S. Passive permeability and active transport models for the prediction of oral absorption. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 259–278. [Google Scholar]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Yeni, Y.; Rachmania, R.A. The Prediction of Pharmacokinetic Properties of Compounds in Hemigraphis Alternata (Burm.F.) T. Ander Leaves Using PkCSM. Indones. J. Chem. 2022, 22, 1081. [Google Scholar] [CrossRef]
- Ghannay, S.; Kadri, A.; Aouadi, K. Synthesis, in Vitro Antimicrobial Assessment, and Computational Investigation of Pharmacokinetic and Bioactivity Properties of Novel Trifluoromethylated Compounds Using in Silico ADME and Toxicity Prediction Tools. Monatsh. Chem.-Chem. Mon. 2020, 151, 267–280. [Google Scholar] [CrossRef]
- Awortwe, C.; Fasinu, P.S.; Rosenkranz, B. Application of Caco-2 Cell Line in Herb-Drug Interaction Studies: Current Approaches and Challenges. J. Pharm. Pharm. Sci. 2014, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.; Schmidt, S.; Derendorf, H. When Is Protein Binding Important? J. Pharm. Sci. 2013, 102, 3458–3467. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, V.K.; Altit, G.; Autmizguine, J.; Chemtob, S. “Basic Pharmacologic Principles” in Fetal and Neonatal Physiology, 5th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 187–201. [Google Scholar]
- Sadeghi, M.; Moradi, M.; Madanchi, H.; Johari, B. In Silico Study of Garlic (Allium sativum L.)-Derived Compounds Molecular Interactions with α-Glucosidase. Silico Pharmacol. 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- [OECD] Organisation for Economic Co-operation and Development. Test no. 452: Chronic toxicity studies. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Ferrari, I.V.; Di Mario, M. Prediction of physicochemical property/Biological Activity and ADMET (absorption, distribution, mechanism, excretion, and toxicity) parameters of approved HIV Medications. Int. J. Sci. Res. Biol. Sci. 2022, 9, 76–83. [Google Scholar]
- Wadanambi, P.M.; Seneviratne, K.N.; Jayathilaka, N. In Silico Evaluation of Coconut Milk Phenolic Antioxidants and Their Inhibition of Oxidative Stress in Intestinal lactobacillus spp. In Vitro Chem. Pap. 2023, 77, 2611–2624. [Google Scholar] [CrossRef]
- Rashied, R.M.H.; Abdelfattah, M.A.O.; El-Beshbishy, H.A.; El-Shazly, A.M.; Mahmoud, M.F.; Sobeh, M. Syzygium Samarangense Leaf Extract Exhibits Distinct Antidiabetic Activities: Evidences from in Silico and in Vivo Studies. Arab. J. Chem. 2022, 15, 103822. [Google Scholar] [CrossRef]
- Das, G.; Nath, R.; Talukdar, A.D.; Ağagündüz, D.; Yılmaz, B.; Capasso, R.; Shin, H.; Patra, J.K. Major Bioactive Compounds from Java Plum Seeds: An Investigation of Its Extraction Procedures and Clinical Effects. Plants 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Sen, S.; Chakraborti, A.S. Action of Pelargonidin on Hyperglycemia and Oxidative Damage in Diabetic Rats: Implication for Glycation-Induced Hemoglobin Modification. Life Sci. 2008, 82, 1102–1110. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic Properties of Dietary Flavonoids: A Cellular Mechanism Review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2004, 53, 28–31. [Google Scholar] [CrossRef]
- Kumar, G.S.; Jayaveera, K.N.; Kumar, C.K.; Sanjay, U.P.; Swamy, B.M.; Kumar, D.V. Antimicrobial Effects of Indian Medicinal Plants against Acne-Inducing Bacteria. Trop. J. Pharm. Res. 2007, 6, 717–723. [Google Scholar] [CrossRef]
- Olaokun, O.O.; Manonga, S.A.; Zubair, M.S.; Maulana, S.; Mkolo, N.M. Molecular Docking and Molecular Dynamics Studies of Antidiabetic Phenolic Compound Isolated from Leaf Extract of Englerophytum magalismontanum (Sond.) T.D.Penn. Molecules 2022, 27, 3175. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Meneses, L.; Cuesta, S. Determinación computacional de la afinidad y eficiencia de enlace de antinflamatorios no esteroideos inhibidores de la Ciclooxigenasa-2. Rev. Ecuat. Med. Y Cienc. BiolóGicas 2015, 36, 17–25. [Google Scholar] [CrossRef][Green Version]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
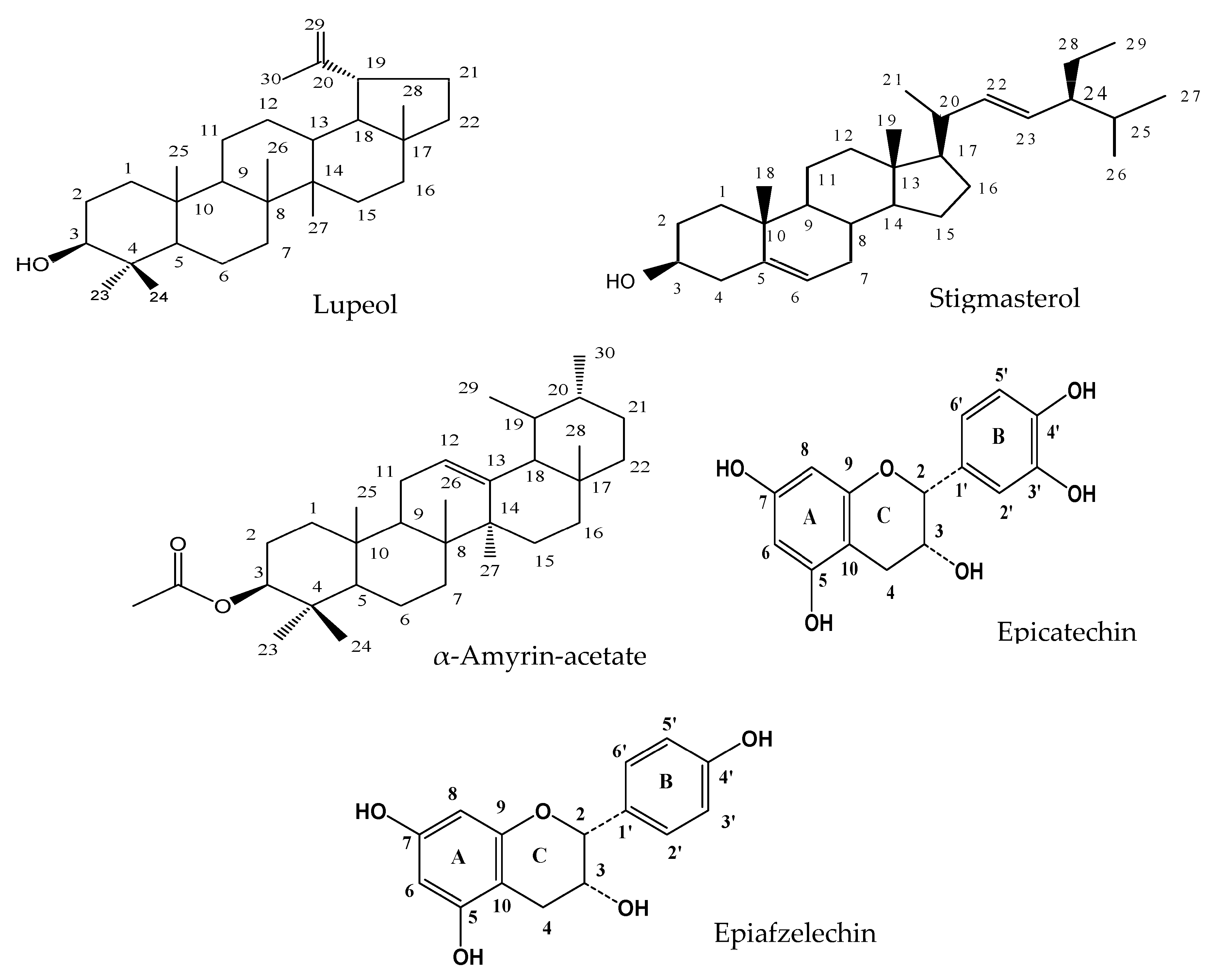

| Compounds | Terpenoids | Steroids | Flavonoids |
|---|---|---|---|
| Compound 1 | + | − | − |
| Compound 2 | − | + | − |
| Compound 3 | + | − | − |
| Compound 4 | − | − | + |
| Compound 5 | − | − | + |
| Compound | IC50 (µg/mL) |
|---|---|
| Lupeol | >1000 |
| Stigmasterol | 115.71 ± 11.6 a |
| α-Amyrin acetate | 335.82 ± 22.6 a |
| Epicatechin | 5.72 ± 2.6 b |
| Epiafzelechin | 7.64 ± 4.9 b |
| Compounds | Free Energy of Binding ΔG (kcal/mol) with Target Receptors | |||||||
|---|---|---|---|---|---|---|---|---|
| 1NOI | 3G9E | 2P8S | 5EQG | 4RCH | 5T19 | 1OSE | 2QMJ | |
| Lupeol | −9.3 | −8.1 | −9.5 | −10.8 | −8.1 | −8.3 | −10 | −7.8 |
| Stigmasterol | −8.3 | −8.6 | −9.0 | −11.2 | −9.2 | −7.8 | −10.6 | −9.1 |
| a-Amyrin-acetate | −8.5 | −7.9 | −9.4 | −10.1 | −7.7 | −7.6 | −9.2 | −7.5 |
| Epicatechin | −8.1 | −7.6 | −7.8 | −8.6 | −8.0 | −7.5 | −8.9 | −7.2 |
| Epiafzelechin | −8.2 | −7.6 | −7.6 | −8.7 | −8.6 | −7.7 | −8.8 | −7.0 |
| Native ligand | −7.2 | −9.4 | −9.4 | −9.9 | −8.5 | −9.5 | −9.8 | −7.5 |
| Parameters | Compounds | ||||
|---|---|---|---|---|---|
| Epiafzelechin | Epicatechin | Lupeol | Stigmasterol | α-Amyrin-Acetate | |
| Molecular weight (MW) (g/mol) | 274.3 | 290.3 | 426.7 | 412.7 | 468.8 |
| Fraction Csp3 | 0.2 | 0.2 | 0.93 | 0.86 | 0.91 |
| #Rotatable bonds | 1 | 1 | 1 | 5 | 2 |
| #H-bond acceptors | 5 | 6 | 1 | 1 | 2 |
| #H-bond donors | 4 | 5 | 1 | 1 | 0 |
| Molecular refractivity | 72.31 | 74.33 | 135.14 | 132.76 | 144.88 |
| Topological Polar Surface Area (Å2) | 90.15 | 110.38 | 20.23 | 20.23 | 26.3 |
| Lipophilicity Log Po/w | 1.84 | 1.55 | 8.02 | 7.8 | 8.6 |
| Water solubility Log S (Ali) | Soluble | Soluble | Insoluble | Poorly soluble | Insoluble |
| Drug likeness (Lipinski rule), #violations | Yes, 0 | Yes, 0 | Yes, 1 | Yes, 1 | Yes, 1 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Leadlikeness #violations | 0 | 0 | 2 | 2 | 2 |
| Parameters | Compounds | ||||
|---|---|---|---|---|---|
| Epiafzelechin | Epicatechin | Lupeol | Stigmasterol | α-Amyrin-Acetate | |
| Absorption | |||||
| Water solubility (log mol/L) | −3.254 | −3.117 | −5.861 | −6.682 | −6.67 |
| Caco2 permeability (log Papp in 10−6 cm/s) | 1.077 | −0.283 | 1.226 | 1.213 | 1.222 |
| GI absorption | High | High | Low | Low | Low |
| Skin Permeability log Kp (cm/s) | −2.735 | −2.735 | −2.744 | −2.783 | −2.82 |
| P-gp substrate (Yes/No) | Yes | Yes | No | No | No |
| P-gp I inhibitor (Yes/No) | No | No | Yes | Yes | Yes |
| P-gp II inhibitor (Yes/No) | No | No | Yes | Yes | Yes |
| Distribution | |||||
| VDss (human) (log L/kg) | 0.562 | 1.027 | 0 | 0.178 | 0.148 |
| Fraction unbound (human) | 0.194 | 0.235 | 0 | 0 | 0 |
| BBB permeant (log BB) | −0.818 | −1.054 | 0.726 | 0.771 | 0.599 |
| CNS permeability (log PS) | −2.473 | −3.298 | −1.714 | −1.652 | −1.963 |
| Metabolism | |||||
| CYP2D6 substrate | No | No | No | No | No |
| CYP3A4 substrate | No | No | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No |
| Excretion | |||||
| Total renal clearance (log mL/min/kg) | 0.255 | 0.183 | 0.153 | 0.618 | 0.025 |
| Renal OCT2 substrate | No | No | No | No | No |
| Parameters | Compounds | ||||
|---|---|---|---|---|---|
| Epiafzelechin | Epicatechin | Lupeol | Stigmasterol | α-Amyrin-Acetate | |
| Max. tolerated dose (human) (log mg/kg/day) | 0.136 | 0.438 | −0.502 | −0.664 | −0.485 |
| hERG I inhibitor | No | No | No | No | No |
| hERG II inhibitor | No | No | Yes | Yes | Yes |
| Oral Rat Acute Toxicity (LD50) (mol/kg) | 2.365 | 2.428 | 2.563 | 2.54 | 2.25 |
| Oral Rat Chronic Toxicity (LOAEL) (log mg/kg bw/day) | 2.215 | 2.5 | 0.89 | 0.872 | 2.039 |
| AMES toxicity | No | No | No | No | No |
| Hepatotoxicity | No | No | No | No | No |
| Skin Sensitization | No | No | No | No | No |
| Immunotoxicity | No | No | Yes | Yes | Yes |
| T. pyriformis toxicity (log µg/L) | 0.519 | 0.347 | 0.316 | 0.433 | 0.359 |
| Minnow toxicity (log mM) | 2.75 | 3.585 | −1.696 | −1.675 | −1.996 |
| Predicted LD50 (mg/kg) | 2500 | 10,000 | 2000 | 890 | 3460 |
| Predicted Toxicity Class | 5 | 6 | 4 | 4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaokun, O.O.; Zubair, M.S. Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract. Molecules 2023, 28, 7717. https://doi.org/10.3390/molecules28237717
Olaokun OO, Zubair MS. Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract. Molecules. 2023; 28(23):7717. https://doi.org/10.3390/molecules28237717
Chicago/Turabian StyleOlaokun, Oyinlola O., and Muhammad S. Zubair. 2023. "Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract" Molecules 28, no. 23: 7717. https://doi.org/10.3390/molecules28237717
APA StyleOlaokun, O. O., & Zubair, M. S. (2023). Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract. Molecules, 28(23), 7717. https://doi.org/10.3390/molecules28237717





