From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents
Abstract
:1. Introduction
2. Methodology
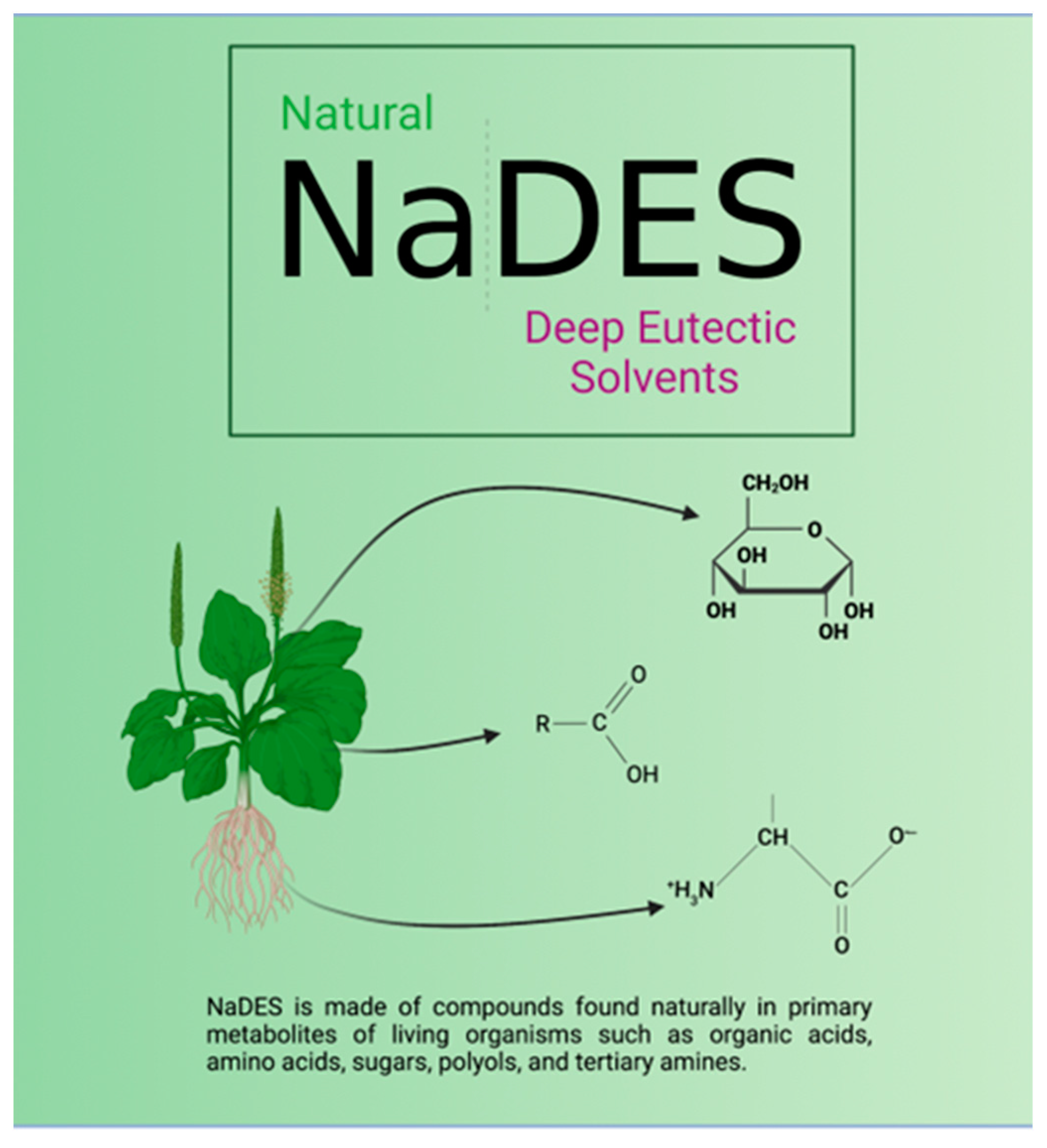
3. NaDESs as They Are
4. NaDESs: Learning from Nature
5. The Solvent Solution: New Solutions to All Your Problems
6. Small but Mighty: How Nanocarriers Are Revolutionizing Drug Delivery and the Role That NaDESs Play in This Story
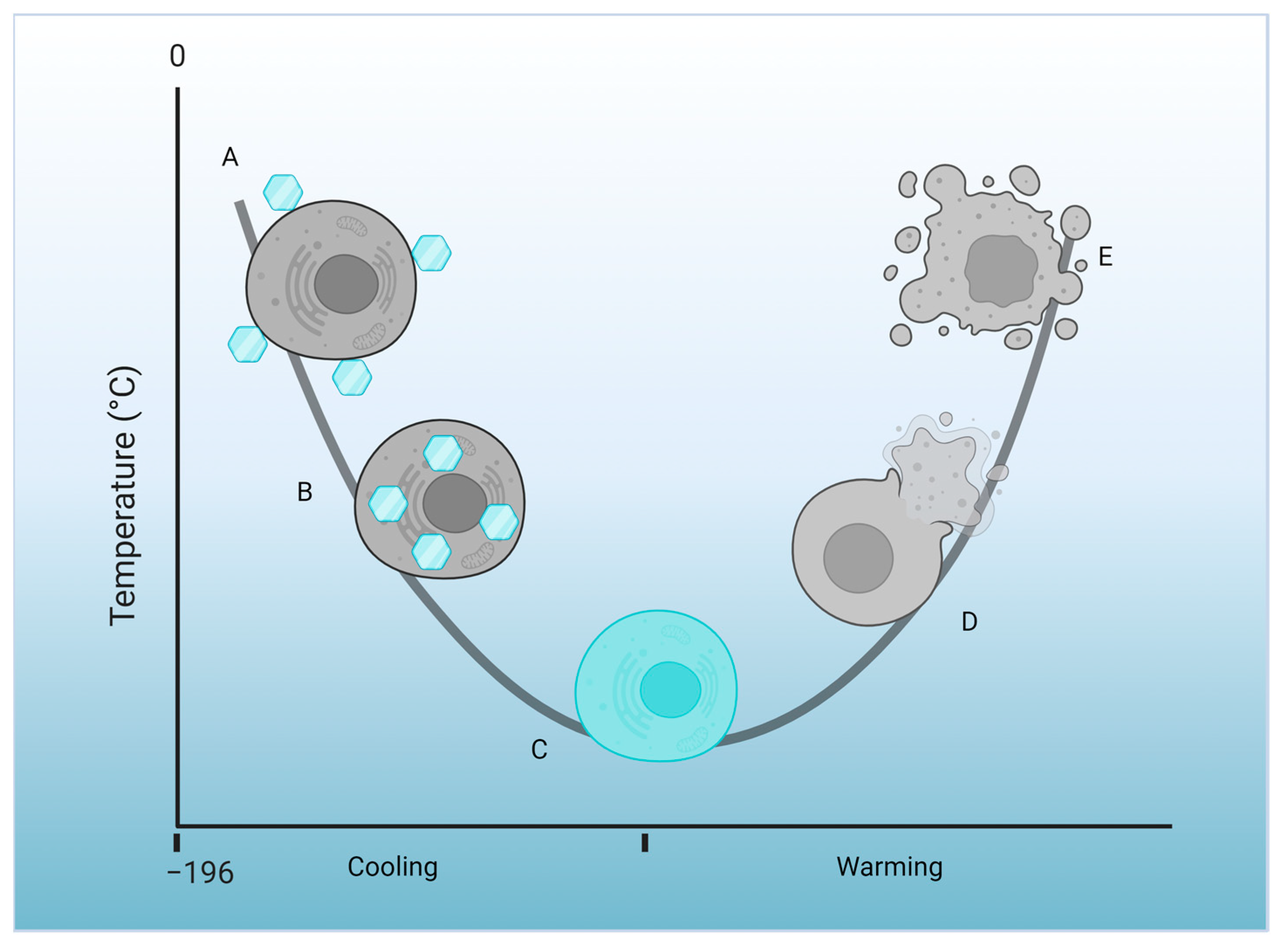
7. NaDESs in Cryopreservation: Advancing Preservation Techniques
8. The Beauty of Science: The Fascinating Intersection of NaDESs, Cosmetics, Food, and Pharmaceuticals
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkandu, M.I.; Alibaba, H.Z. Biomimicry as an alternative approach to sustainability. Archit. Res. 2018, 8, 1–11. [Google Scholar]
- Pawlyn, M. Biomimicry in Architecture, 2nd ed.; RIBA Publishing: London, UK, 2019. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539–105545. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Mariano, A.M.; Rocha, M.S. Revisão da literatura: Apresentação de uma abordagem integradora. AEDEM Int. Conf. 2017, 18, 427–442. [Google Scholar]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Gill, I.; Vulfson, E. Enzymic catalysis in heterogeneous eutectic mixtures of substrates. Trends Biotechnol. 1994, 12, 118–122. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R.; Gill, I.; Vulfson, E.N. Protease-catalyzed synthesis of oligopeptides in heterogenous substrate mixtures. Biotechnol. Bioeng. 1994, 43, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.; Garside, J.; Hilton, A.; McEwan, D.; Morrison, J. Purification of molecular mixtures below the eutectic by emulsion crystallization. Nature 1995, 375, 664–666. [Google Scholar] [CrossRef]
- Erbeldinger, M.; Ni, X.; Halling, P.J. Enzymatic synthesis with mainly undissolved substrates at very high concentrations. Enzym. Microb. Technol. 1998, 23, 141–148. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Freemantle, M.; Welton, T.; Rogers, R.D. An Introduction to Ionic Liquids; The Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.M.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids—Challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215–125240. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. SpringerPlus 2016, 5, 913–924. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; Khan, M.Y.; He, D.; Peng, H.; Chen, D.; Xie, X.; Xue, Z. Deep eutectic solvents for green and efficient iron-mediated ligand-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1616–1627. [Google Scholar] [CrossRef]
- Gómez, A.V.; Biswas, A.; Tadini, C.C.; Furtado, R.F.; Alves, C.R.; Cheng, H.N. Use of natural deep eutectic solvents for polymerization and polymer reactions. J. Braz. Chem. Soc. 2019, 30, 717–726. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Villeneuve, P.; Bourlieu-lacanal, C.; Carrière, F. Chapter Six—Natural Deep Eutectic Solvents: Hypothesis for Their Possible Roles in Cellular Functions and Interaction with Membranes and Other Organized Biological Systems. In Advances in Botanical Research; Verpoorte, R., Witkamp, G.J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 133–158. Available online: https://www.sciencedirect.com/science/article/pii/S0065229620300483 (accessed on 10 November 2022).
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Cambridge Dictionary. Meaning of Biomimicry in English; Cambridge Dictionary: Cambridge, UK, 2023; Available online: https://dictionary.cambridge.org/us/dictionary/english/biomimicry (accessed on 7 February 2023).
- Hornberger, K.; Li, R.; Duarte, A.R.C.; Hubel, A. Natural deep eutectic systems for nature-inspired cryopreservation of cells. AIChE J. 2021, 67, e17085. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.W.; Zong, M.H.; Li, N.; Lou, W.Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.R., Jr.; Sformo, T.; Barnes, B.M.; Duman, J.G. Freeze tolerance in an arctic Alaska stonefly. J. Exp. Biol. 2009, 212, 305–312. [Google Scholar] [CrossRef]
- Mamajanov, I.; Engelhart, A.E.; Bean, H.D.; Hud, N.V. DNA and RNA in anhydrous media: Duplex, triplex, and G-quadruplex secondary structures in a deep eutectic solvent. Angew. Chem. Int. Ed. 2010, 49, 6310–6314. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633–132645. [Google Scholar] [CrossRef]
- Zahrina, I.; Nasikin, M.; Krisanti, E.; Mulia, K. Deacidification of palm oil using betaine monohydrate-based natural deep eutectic solvents. Food Chem. 2018, 240, 490–495. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Wikene, K.O.; Rukke, H.V.; Bruzell, E.; Tønnesen, H.H. Investigation of the antimicrobial effect of natural deep eutectic solvents (NADES) as solvents in antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2017, 171, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, S.N.; Friesen, J.B.; Nikolić, D.; Choules, M.P.; McAlpine, J.B.; Lankin, D.C.; Gemeinhart, R.A.; Pauli, G.F. The influence of natural deep eutectic solvents on bioactive natural products: Studying interactions between a hydrogel model and Schisandra chinensis metabolites. Fitoterapia 2018, 127, 212–219. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S. Natural deep eutectic solvent (NADES) as a greener alternative for the extraction of hydrophilic (polar) and lipophilic (non-polar) phytonutrients. Key Eng. Mater. 2019, 797, 20–28. [Google Scholar] [CrossRef]
- Savi, L.K. Desenvolvimento de Solventes Eutéticos Naturais Profundos (NADES) e o Estudo de Suas Propriedades Físico-Químicas, Térmicas e Reológicas. [Dissertation on the Internet]. Paraná (BR): Universidade Federal do Paraná. Setor de Tecnologia. Programa de Pós-Graduação em Engenharia de Alimentos; 2019. Available online: https://hdl.handle.net/1884/61414 (accessed on 2 February 2023).
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Chemical Safety Facts. Solvents; American Chemistry Council: Washington, DC, USA, 2022; Available online: https://www.chemicalsafetyfacts.org/chemicals/solvents/ (accessed on 7 February 2023).
- Häckl, K.; Kunz, W. Some aspects of green solvents. Comptes Rendus Chimi. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Gurak, P.D.; De Bona, G.S.; Tessaro, I.C.; Marczak, L.D.F. Jaboticaba Pomace Powder Obtained as a Co-product of Juice Extraction: A Comparative Study of Powder Obtained from Peel and Whole Fruit. Food Res. Int. 2014, 62, 786–792. [Google Scholar] [CrossRef]
- Benvenutti, L.; del Pilar Sanchez-Camargo, A.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761–113770. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhong, F.Y.; Huang, J.Y.; Peng, H.L.; Huang, K. Sugar-based natural deep eutectic solvents as potential absorbents for NH3 capture at elevated temperatures and reduced pressures. J. Mol. Liq. 2020, 317, 113992–113999. [Google Scholar] [CrossRef]
- Fu, H.; Luo, Z.; Hu, S. A temporal-spatial analysis and future trends of ammonia emissions in China. Sci. Total Environ. 2020, 731, 138897–138905. [Google Scholar] [CrossRef]
- Liu, B.; Tian, J. Investigation of glycolic acid natural deep eutectic solvents with strong proton donors for ammonia capture and separation. Ind. Eng. Chem. Res. 2021, 60, 11600–11610. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. Comptes Rendus Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef]
- Sun, X.; Pradeepkumar, P.; Rajendran, N.K.; Shakila, H.; Houreld, N.N.; Al Farraj, D.A.; Elnahas, Y.M.; Elumalai, N.; Rajan, M. Natural deep eutectic solvent supported targeted solid–liquid polymer carrier for breast cancer therapy. RSC Adv. 2020, 10, 36989–37004. [Google Scholar] [CrossRef]
- Pradeepkumar, P.; Elgorban, A.M.; Bahkali, A.H.; Rajan, M. Natural solvent-assisted synthesis of amphiphilic co-polymeric nanomicelles for prolonged release of camptothecin delivery. New J. Chem. 2018, 42, 10366–10375. [Google Scholar] [CrossRef]
- Gheybi, H.; Entezami, A.A. Nanosized micelles self-assembled from amphiphilic poly(citric acid)–poly(ε-caprolactone)–poly(citric acid) copolymers. Polym. Bull. 2013, 70, 1875–1894. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17-71. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Wojcieszek, J.; Asztemborska, M.; Zhou, Y.; Ruzik, L. Analysis of cerium oxide and copper oxide nanoparticles bioaccessibility from radish using SP-ICP-MS. J. Sci. Food Agric. 2020, 100, 4950–4958. [Google Scholar] [CrossRef]
- Jakubowska, M.; Ruzik, L. Application of Natural Deep Eutectic Solvents for the metal nanoparticles extraction from plant tissue. Anal. Biochem. 2021, 617, 114117–114122. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Bolea, E.; Jiménez-Lamana, J. Single Particle Inductively Coupled Plasma Mass Spectrometry: A Powerful Tool for Nanoanalysis. Anal. Chem. 2014, 86, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.H.; Kostarelos, K. Prospects and Challenges of Graphene in Biomedical Applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Diogo, D.; Costa, E.C.; Alves, C.G.; Lima-Sousa, R.; Ferreira, P.; Louro, R.O.; Correia, I.J. POxylated graphene oxide nanomaterials for combination chemo-phototherapy of breast cancer cells. Eur. J. Pharm. Biopharm. 2018, 131, 162–169. [Google Scholar] [CrossRef]
- Chen, K.; Ling, Y.; Cao, C.; Li, X.; Chen, X.; Wang, X. Chitosan derivatives/reduced graphene oxide/alginate beads for small-molecule drug delivery. Mater. Sci. Eng. C 2016, 69, 1222–1228. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. Doxorubicin Loading on Functional Graphene as a Promising Nanocarrier Using Ternary Deep Eutectic Solvent Systems. ACS Omega 2020, 5, 1656–1668. [Google Scholar] [CrossRef]
- Jacob Rani, B.S.; Venkatachalam, S. Cleaner approach for the cascade production of nanocellulose, nanohemicellulose and nanolignin from Prosopis juliflora. Carbohydr. Polym. 2022, 294, 119807–119818. [Google Scholar] [CrossRef]
- Douard, L.; Belgacem, M.N.; Bras, J. Extraction of Carboxylated Nanocellulose by Combining Mechanochemistry and NADES. ACS Sustain. Chem. Eng. 2022, 10, 13017–13025. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J.; Chin, S.F.; Ho, B.K. Formulation of choline chloride/ascorbic acid natural deep eutectic solvent: Characterization, solubilization capacity and antioxidant property. LWT 2020, 133, 110096–110105. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer: Cham, Switzerland, 2015; Volume 1. [Google Scholar] [CrossRef]
- Nystedt, H.L.; Grønlien, K.G.; Tønnesen, H.H. Interactions of natural deep eutectic solvents (NADES) with artificial and natural membranes. J. Mol. Liq. 2021, 328, 115452–115463. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Tong, X.; Yang, J.; Zhao, Y.; Wan, H.; He, Y.; Zhang, L.; Wan, H.; Li, C. Greener extraction process and enhanced in vivo bioavailability of bioactive components from Carthamus tinctorius L. by natural deep eutectic solvents. Food Chem. 2021, 348, 129090–129096. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116–125. [Google Scholar] [CrossRef]
- Castro, V.I.B.; Craveiro, R.; Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology 2018, 83, 15–26. [Google Scholar] [CrossRef]
- Jesus, A.R.; Meneses, L.; Duarte, A.R.C.; Paiva, A. Natural deep eutectic systems, an emerging class of cryoprotectant agents. Cryobiology 2021, 101, 95–104. [Google Scholar] [CrossRef]
- Faustino, L.R.; Silva, C.M.G.; Rossetto, R.; Rodrigues, G.Q.; Figueiredo, J.R.; Rodrigues, A.P.R. Current status and challenges of cryopreservation of ovarian tissue in mammals. Rev. Bras. De Reprodução Anim. 2011, 35, 3–15. [Google Scholar]
- Picoli, T.; Barbosa, J.S.; Vargas, G.D.; de Oliveira Hübner, S.; Fischer, G. Toxicidade e eficiência do dimetilsulfóxido (dmso) no congelamento de células madin-darby bovine kidney (mdbk). Sci. Anim. Health 2015, 3, 159–168. [Google Scholar] [CrossRef]
- Bove, K.E. Ethylene glycol toxicity. Am. J. Clin. Pathol. 1966, 45, 46–50. [Google Scholar] [CrossRef]
- da Silva, G.P.; Contiero, J.; Ávila Neto, P.M.; de Lima, C.J.B. 1,3-Propanediol: Production, applications and biotechnological potential. Química Nova 2014, 37, 527–534. [Google Scholar] [CrossRef]
- Lewis, J.K.; Bischof, J.C.; Braslavsky, I.; Brockbank, K.G.M.; Fahy, G.M.; Fuller, B.J.; Rabin, Y.; Tocchio, A.; Woods, E.J.; Wowk, B.G.; et al. The Grand Challenges of Organ Banking: Proceedings from the first global summit on complex tissue cryopreservation. Cryobiology 2016, 72, 169–182. [Google Scholar] [CrossRef]
- Jesus, A.R.; Duarte, A.R.C.; Paiva, A. Use of natural deep eutectic systems as new cryoprotectant agents in the vitrification of mammalian cells. Sci. Rep. 2022, 12, 8095–8103. [Google Scholar] [CrossRef]
- Benoit, C.; Virginie, C.; Boris, V. Chapter Twelve—The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research; Verpoorte, R., Witkamp, G.J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97, pp. 309–332. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural Polyphenols: Biological Activity, Pharmacological Potential, Means of Metabolic Engineering (Review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Panić, M.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Radojčić Redovniković, I.; Radošević, K. Biological activity and sensory evaluation of cocoa by-products NADES extracts used in food fortification. Innov. Food Sci. Emerg. Technol. 2020, 66, 102514–102520. [Google Scholar]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M.S. Comparative assessment of various extraction approaches for the isolation of essential oil from polygonum minus using ionic liquids. J. King Saud Univ. Sci. 2019, 31, 230–239. [Google Scholar] [CrossRef]
- Boussetta, N.; Lanoisellé, J.L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochemistry 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Li, X.L.; Cai, Y.Q.; Qin, H.; Wu, Y.J. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can. J. Physiol. Pharmacol. 2008, 86, 841–849. [Google Scholar] [CrossRef]
- Cheah, K.Y.; Bastian, S.E.; Acott, T.M.; Abimosleh, S.M.; Lymn, K.A.; Howarth, G.S. Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Dig. Dis. Sci. 2013, 58, 970–977. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, X.L.; Wang, L.; Cui, M.X.; Cai, Y.Q.; Li, X.L.; Wu, Y.J. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2010, 88, 888–898. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638. [Google Scholar] [CrossRef]
- Vislocky, L.M.; Fernandez, M.L. Biomedical effects of grape products. Nutr. Rev. 2010, 68, 656–670. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy Isoflavones: A Safety Review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Soy Isoflavones and Cancer Prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef]
- Sang, J.; Li, B.; Huang, Y.; Ma, Q.; Liu, K.; Li, C.Q. Deep eutectic solvent-based extraction coupled with green two-dimensional HPLC-DAD-ESI-MS/MS for the determination of anthocyanins from Lycium ruthenicum Murr. fruit. Anal. Methods 2018, 10, 1247–1257. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Maheswari, V.; Babu, P.A.S. Phlorotannin and its derivatives, a potential antiviral molecule from brown seaweeds, an overview. Russ. J. Mar. Biol. 2022, 48, 309–324. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.S.; Quarta, A.; Ragusa, A.; Jena, M. Algal phlorotannins as novel antibacterial agents with reference to the antioxidant modulation: Current advances and future directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Jin, L.; Zhao, Y.; Zhu, G.; Shen, W. Dieckol inhibits non-small–cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.C.; Eun, S.Y. Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway. Korean J. Physiol. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef]
- Wang, L.; Je, J.G.; Yang, H.W.; Jeon, Y.J.; Lee, S. Dieckol, an algae-derived phenolic compound, suppresses UVB-induced skin damage in human dermal fibroblasts and its underlying mechanisms. Antioxidants 2021, 10, 352. [Google Scholar] [CrossRef]
- Catarino, M.D.; Pires, S.M.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.; Silva, A.M.S.; Cardoso, S.M. Overview of phlorotannins’ constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidans: Downstream processes and recent applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A bioactive substance derived from brown seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep eutectic solvents for extraction of hydrophilic and lipophilic compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Solubility and stability of some pharmaceuticals in natural deep eutectic solvents-based formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Pimentel-Mora, S.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646–109655. [Google Scholar] [CrossRef]
- Fu, J.J.; Sun, C.; Xu, X.B.; Zhou, D.Y.; Song, L.; Zhu, B.W. Improving the functional properties of bovine serum albumin-glucose conjugates in natural deep eutectic solvents. Food Chem. 2020, 328, 127122–127129. [Google Scholar] [CrossRef]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef] [PubMed]
- Chandra Roy, V.; Ho, T.C.; Lee, H.J.; Park, J.S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021, 284, 125417–125428. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Grønlien, K.G.; Pedersen, M.E.; Tønnesen, H.H. A natural deep eutectic solvent (NADES) as potential excipient in collagen-based products. Int. J. Biol. Macromol. 2020, 156, 394–402. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Kua, Y.L.; Chew, Z.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Insights on the potential of natural deep eutectic solvents (NADES) to fine-tune durian seed gum for use as edible food coating. Food Hydrocoll. 2022, 132, 107861–107876. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, D.W.; Zhu, Z. Development of natural deep eutectic solvents (NADESs) as anti-freezing agents for the frozen food industry: Water-tailoring effects, anti-freezing mechanisms and applications. Food Chem. 2022, 371, 131150–131158. [Google Scholar] [CrossRef]
- da Silva, D.T.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; da Veiga, M.L.; Vizzoto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718–109729. [Google Scholar] [CrossRef] [PubMed]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337–129347. [Google Scholar] [CrossRef]
- Bener, M.; Özyürek, M.; Güçlü, K.; Apak, R. Optimization of Microwave-Assisted Extraction of Curcumin from Curcuma longa L. (Turmeric) and Evaluation of Antioxidant Activity in Multi-Test Systems. Rec. Nat. Prod. 2016, 10, 542–554. [Google Scholar]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Naeini, M.B.; Momtazi, A.A.; Jaafari, M.R.; Johnston, T.P.; Barreto, G.; Banach, M.; Sahebkar, A. Antitumor effects of curcumin: A lipid perspective. J. Cell. Physiol. 2019, 234, 14743–14758. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Galambosi, B.; Galambosi, Z.; Hethelyi, E.; Szöke, E.; Volodin, V.; Poletaeva, I.; Iljina, I. Importance and quality of roseroot (Rhodiola rosea L.) growing in the European North. Z. Für Arznei-Gewürzpflanzen 2010, 15, 160–169. [Google Scholar]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea . Phytomedicinal Overv. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Khanum, F.; Bawa, A.S.; Singh, B. Rhodiola rosea: A versatile adaptogen. Compr. Rev. Food Sci. Food Saf. 2005, 4, 55–62. [Google Scholar] [CrossRef]
- Rohloff, J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry 2002, 59, 655–661. [Google Scholar] [CrossRef]
- Ioset, K.N.; Nyberg, N.T.; Van Diermen, D.; Malnoe, P.; Hostettmann, K.; Shikov, A.N.; Jaroszewski, J.W. Metabolic profiling of Rhodiola rosea rhizomes by 1H NMR spectroscopy. Phytochem. Anal. 2011, 22, 158–165. [Google Scholar] [CrossRef]
- Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef]
- Shikov, A.N.; Kosman, V.M.; Flissyuk, E.V.; Smekhova, I.E.; Elameen, A.; Pozharitskaya, O.N. Natural deep eutectic solvents for the extraction of phenyletanes and phenylpropanoids of Rhodiola rosea L. Molecules 2020, 25, 1826. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, J.; Yang, L.; Shen, S.; Li, P.; Yao, S.; Qu, H.; Li, J.; Yao, C.; Wei, W.; et al. Recent advances in chemical analysis of licorice (Gan-Cao). Fitoterapia 2021, 149, 104803–104814. [Google Scholar] [CrossRef]
- Gravel, I. The content of trace elements in biologically active additives and their aqueous extracts. Farmatsiia-Moskva 2005, 3, 43. [Google Scholar]
- Gravel, I.; Tsoi, Y.; Denisova, T.; Khotimchenko, S. Heavy metals in the raw materials and infusions of peppermint (Mentha piperita). Pharm. (Farmatsiya) 2010, 3, 27–30. [Google Scholar]
- Tsvetov, N.; Drogobuzhskaya, S. Recovery of Some Elements from Empetrum nigrum L. Growing in the Kola Peninsula Using Acid-Based Deep Eutectic Solvents; IOP Publishing: Bristol, UK, 2021; Volume 1, p. 012107. [Google Scholar]
- Shikov, A.N.; Obluchinskaya, E.D.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Pozharitskaya, O.N. The impact of natural deep eutectic solvents and extraction method on the co-extraction of trace metals from Fucus vesiculosus. Mar. Drugs 2022, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The ability of acid-based natural deep eutectic solvents to co-extract elements from the roots of Glycyrrhiza glabra L. and associated health risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef] [PubMed]
| Step | Procedure | Details and Actions |
|---|---|---|
| 1 | Keyword identification | Search for articles using the keywords: “Natural Deep Eutectic Solvents”, “Natural Deep Eutectic Solvent”, or “NaDES”. |
| 2 | Time range specification | Define the study’s time frame: 2011–2023. |
| 3 | Database selection | Choose databases for article retrieval: Web of Science (1036 articles); Scopus (1055 articles). |
| 4 | Research data analysis in databases | Analyze articles using various criteria: Assess journals based on impact factor, h-index, and cite score; Identify journals with the most publications on the subject; Examine the topic’s evolution over time; Identify the most-cited documents; Identify the most-published authors; Determine the most-cited authors; Identify the countries with the highest publication output; Determine the universities with the highest publication output; Identify the agencies that fund the most research; Determine the areas with the most publications. |
| 5 | Article selection and validation | Conduct the following analyses for article selection: Examine keyword frequency; Perform co-citation analysis; Implement bibliographic coupling analysis; Investigate co-authorship patterns. |
| 6 | Finalize article selection and review | Read the abstract of each article and conduct a review for selection. |
| Type of Bibliometric Filter | Law/Principle of Bibliometrics |
|---|---|
| Analysis of the most relevant magazines (Table S1a,b) | Bradford law, impact factor, and 80/20 |
| Analysis of the journals that publish the most on the subject (Table S2) | Bradford law |
| Evolution of the theme year by year (Table S5) | Literature obsolescence and Goffman’s epidemic theory |
| Most-cited documents (Table S6a,b) | Law of elitism, 80/20 law, and quotes |
| Most-published authors (Table S3) vs. most-cited authors (Table S4) | Law of Lokta and law of elitism |
| Countries that published the most (Table S7) | 80/20 law |
| Universities that published the most (Table S8) | 80/20 law |
| Agencies that fund the most research (Table S9) | 80/20 law |
| Areas that publish the most (Table S10) | 80/20 law |
| Keyword frequency | 80/20 law |
| IL | DES | NaDES | |
|---|---|---|---|
| Formed by | Organic cation and organic/inorganic anion | Hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) (amide compounds, inorganic salts, and quaternary ammonium salts) | HBA and HBD from natural sources |
| Solubilization Ability | Good solubilizing capacity of a number of organic compounds | High | High solubilization for a wide range of metabolites with low to medium polarity, as well as macromolecules such as DNA, proteins, and polysaccharides |
| Extraction Ability | High | High | High |
| Formulation/Synthesis Facility | Low, demand solvent use | High, but higher melting points of many DES, however, can hamper their application as a green solvent at room temperature | High |
| Can be Recycled | Yes | Yes | Yes |
| Biodegradability | Mean | High | High |
| Toxicity | Toxicity towards diverse organisms and ecosystems | Some formulations may contain metallic salts, which are known for their innate toxicity | Low, but the inclusion of organic acids in NaDESs can increase their overall toxicity |
| ECO Friendly | Potential environmental pollution through release via wastewater effluents | Lack of waste generation, but not entirely sustainable due to the presence of metal salts in some formulations | Yes |
| Cost | High | Low cost of their starting materials | Low |
| NaDES | Molar Ratio | Possibility of Use |
|---|---|---|
| Chlorine of chloride:proline | 1:2 1:1 (in water solution) | Anthocyanin extraction [54] |
| Citric acid:glucose:water | 1:1:3 1:9 (in water solution) | Pectin extraction [54] |
| Chlorine of chloride:fructose:water | 1:5:1 | Ammonia absorption [55] |
| Chlorine of chloride:xylose:water | 1:5:1 | |
| Glycolic acid:xylitol | 3:1 | Ammonia absorption [57] |
| NaDES | Molar Ratio | Possibility of Use |
|---|---|---|
| Chlorine of chloride:glucose | Not showed | Extracting metal oxide nanoparticles from plants without causing their transformation [67] |
| Chlorine of chloride:glycerol | Not showed | |
| Choline chloride:malonic acid | 1:1 | Reducing the cytotoxicity levels of graphene, while also demonstrating higher tamoxifen entrapment efficiency and loading capacity [74] |
| Choline chloride:lactic acid | 2:1 | Selective hemicellulose solubilization [75] |
| Choline chloride:folic acid | 3:1 | Lignin solubilization [75] |
| Choline chloride:oxalic acid dihydrate | 1:1 | Extraction of cellulose nanocrystals with 65% yield, high thermal stability and high crystallinity index [76] |
| Choline chloride:citric acid monohydrate | 1:1 | |
| Choline chloride:ascorbic acid | 1:1 1:2 2:1 | Increasing the solubility and antioxidant properties of antioxidant extracts from Mangifera pajang fruit residues [77] |
| NaDES | Molar Ratio | Possibility of Use |
|---|---|---|
| Choline chloride:malic acid | 1:1 | Anti-freezing properties and resistance to high osmotic pressure [29] |
| Trehalose:glycerol | 1:30 | Cryopreservation of Jurkat cells [33] |
| Trehalose:glucose:sorbitol:water | 1:2:1:10 | A significant cryoprotective effect on L929 cells compared to DMSO or in the absence of a CPA, and for HaCaT cells, demonstrated a slight improvement in cell survival, while DMSO caused complete cell death [92] |
| Glucose:proline:glycerol:water | 3:5:3:21 | |
| Betaine:trehalose:glycerol:water | 2:1:3:7 | |
| Betaine:trehalose:water | 4:1:12 | |
| Betaine:sucrose:proline:water | 5:2:2:21 |
| NaDES | Molar Ratio | Possibility of Use |
|---|---|---|
| Lactic acid:glucose | 5:1 | Anthocyanin extraction [94] |
| Choline chloride:1,2-Propanediol | 1:1 1:1.5 1:2 1:3 | |
| Glucose:fructose:sucrose | 1:1:1 | |
| Choline chloride:citric acid | 2:1 | Polyphenol extraction [96] |
| Choline chloride:glycerol | 1:2 | |
| Choline chloride:glucose | 1:1 | |
| Betaine:citric acid | 1:1 | |
| Betaine:glycerol | 1:2 | |
| Betaine:glucose | 1:1 | |
| Choline chloride:fructose | 1.9:1 | Extracting phenolics in grape skin [39] |
| Choline chloride:citric acid | 1:1 | Extraction of isoflavones from soy products [112] |
| Choline chloride:propylene glycol | 1:4 | Extraction of flavonoids [115], anthocyanins [116], and phenolic compounds [117] |
| Choline chloride:glycerol | 1:1 | Extraction of phenolic compounds [118] |
| Choline chloride:ethylene glycol | 1:1 | |
| Choline chloride:lactic acid | 1:1 | |
| Lactic acid:glucose:water | 5:1:3 | Extraction of phenyletanes and phenylpropanoids [131] |
| Choline chloride:lactic acid Lactic acid:glucose:water | 1:3 5:1:3 | An alternative to the conventional techniques for the effective extraction of phlorotannins from F. vesiculosus with high antioxidant potential [131] |
| Choline chloride:oxalic acid | 1:1 | Selectivity for anthocyanins and higher extraction yields of the bioactive compound [134] |
| Choline chloride:glycerol | 1:1 | Anthocyanin extraction [134] |
| Choline chloride:glucose | 1:1 | A promising solvent to increase the glycation extents and property changes of BSA, also promoting its functional activities, making it applicable to the food industry [43,135,136,137,138,139,140] |
| Choline chloride:glycerol | 1:2 | Extraction of astaxanthin (ASX) using an ultrasound assisted (UAE) NaDES [137] |
| Choline chloride:oxalic acid | 1:2 | |
| Choline chloride:lactic acid | 1:2 | |
| Choline chloride:tartaric acid | 1:2 | |
| Choline chloride:malic acid | 1:2 | |
| Choline chloride:glycerol:citric acid | 0.5:2:0.5 | NaDESs can obtain biocompatible extracts of this fruit without needing to remove them for use. In conclusion, the NaDES vehicle contributed to some protective effects, and it has been seen as a potential medium for obtaining extracts of blueberry that exhibit gastroprotective effects [142] |
| Choline chloride:glucose | 5:2 | Potential extractor for the isolation of natural products from medicinal plants [143] |
| Choline chloride:sucrose:water | 4:1:4 | Curcumin and antioxidant extraction [145] |
| Choline chloride:fructose:water | 5:1:5 | |
| Sucrose:lactic acid:water | 1:5:7 | |
| Choline chloride:lactic acid:water | 1:1:2 | |
| Choline chloride:malic acid Choline chloride:lactic acid | 1:1 1:3 | Potential extractor for the isolation of natural products from medicinal plants [148] |
| Choline chloride:malonic acid Choline chloride:malic acid Choline chloride:tartaric acid Choline chloride:citric acid | 1:1 1:1 2:1 1:1 (All DESs with 30% water addition) | Alternative for co-extracting trace elements from the roots of Glycyrrhiza glabra L. [160,161,162] |
| Lactic acid:glucose:water Choline chloride:lactic acid Choline chloride:malic acid | 5:3:1 1:3 1:1 | |
| Sucrose:citric acid Sorbitol:citric acid Sucrose:Lactic Acid Sorbitol:Lactic Acid Choline Chloride:Lactic Acid | 3:1 3:1 3:1 3:1 1:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. https://doi.org/10.3390/molecules28227653
Schuh L, Reginato M, Florêncio I, Falcao L, Boron L, Gris EF, Mello V, Báo SN. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules. 2023; 28(22):7653. https://doi.org/10.3390/molecules28227653
Chicago/Turabian StyleSchuh, Luísa, Marcella Reginato, Isadora Florêncio, Leila Falcao, Luana Boron, Eliana Fortes Gris, Victor Mello, and Sônia Nair Báo. 2023. "From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents" Molecules 28, no. 22: 7653. https://doi.org/10.3390/molecules28227653
APA StyleSchuh, L., Reginato, M., Florêncio, I., Falcao, L., Boron, L., Gris, E. F., Mello, V., & Báo, S. N. (2023). From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules, 28(22), 7653. https://doi.org/10.3390/molecules28227653








