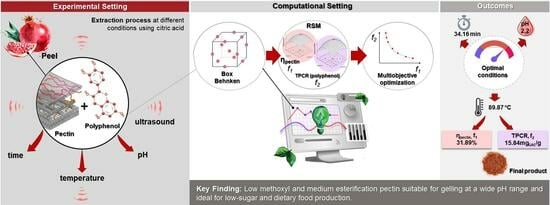
Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bio-Waste Characterization
2.2. Pectin Characterization
2.3. Fitting Model and Optimization
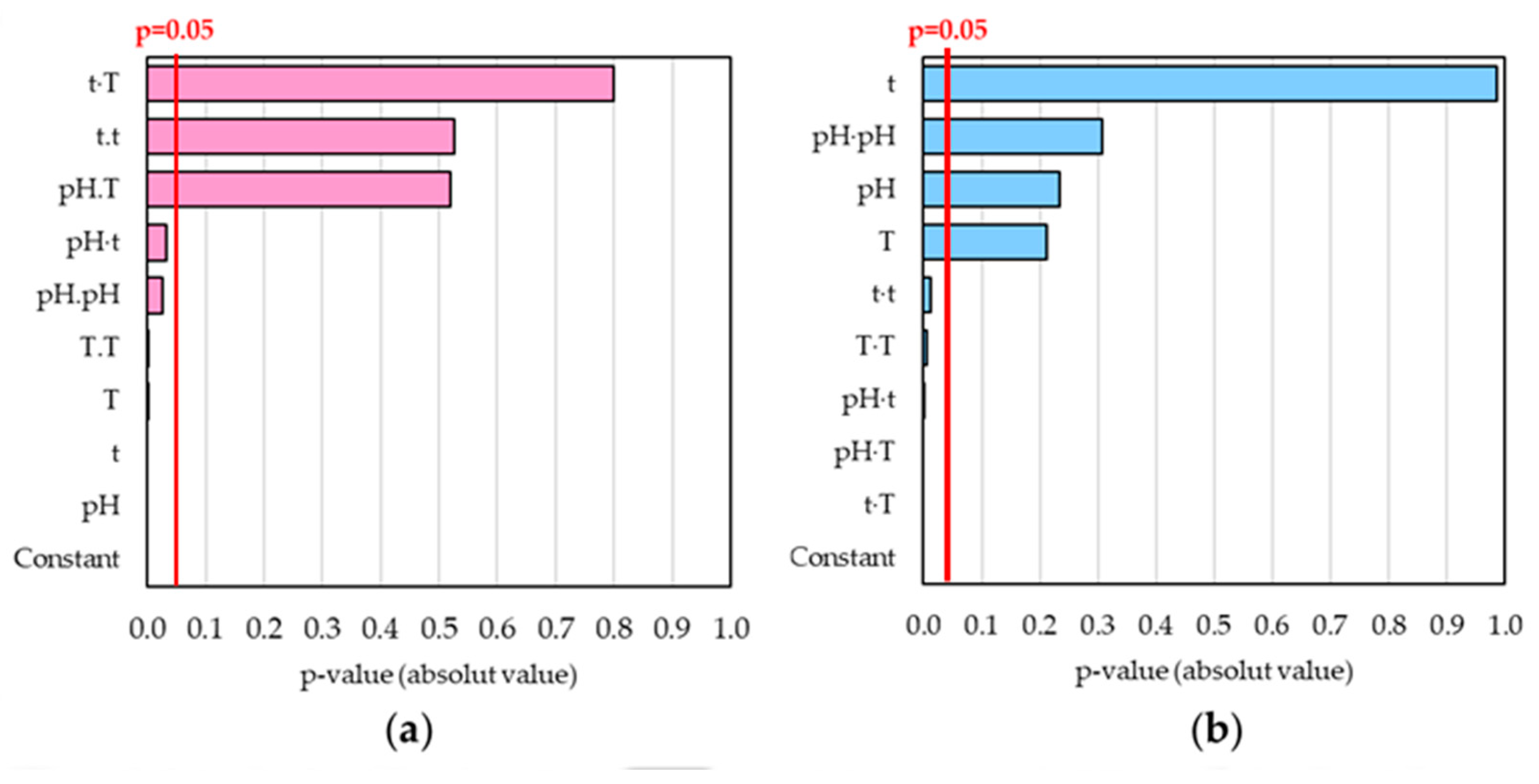
2.3.1. Development of Second-Order Polynomial Mathematical Models
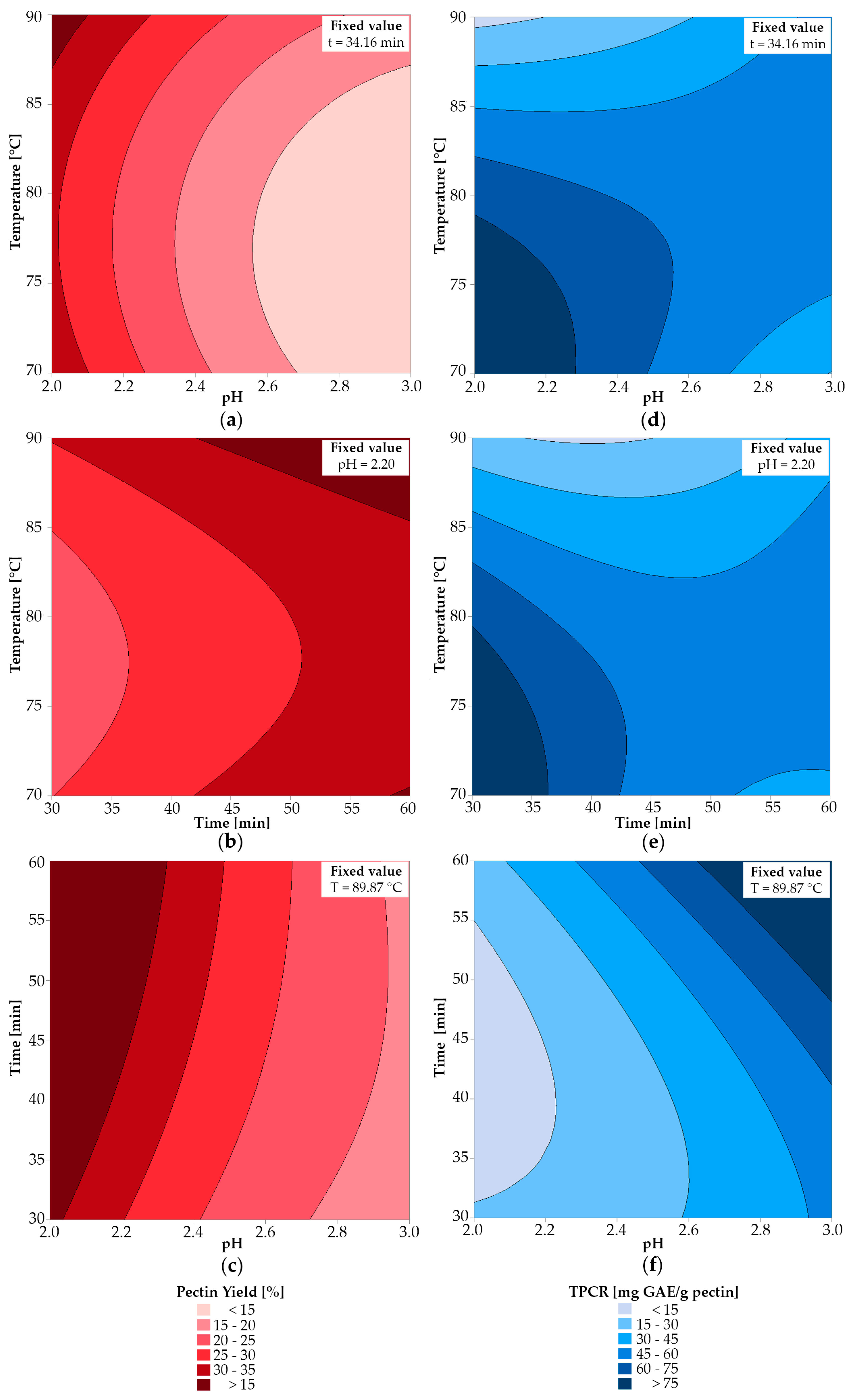
2.3.2. Multi-Objective Optimization
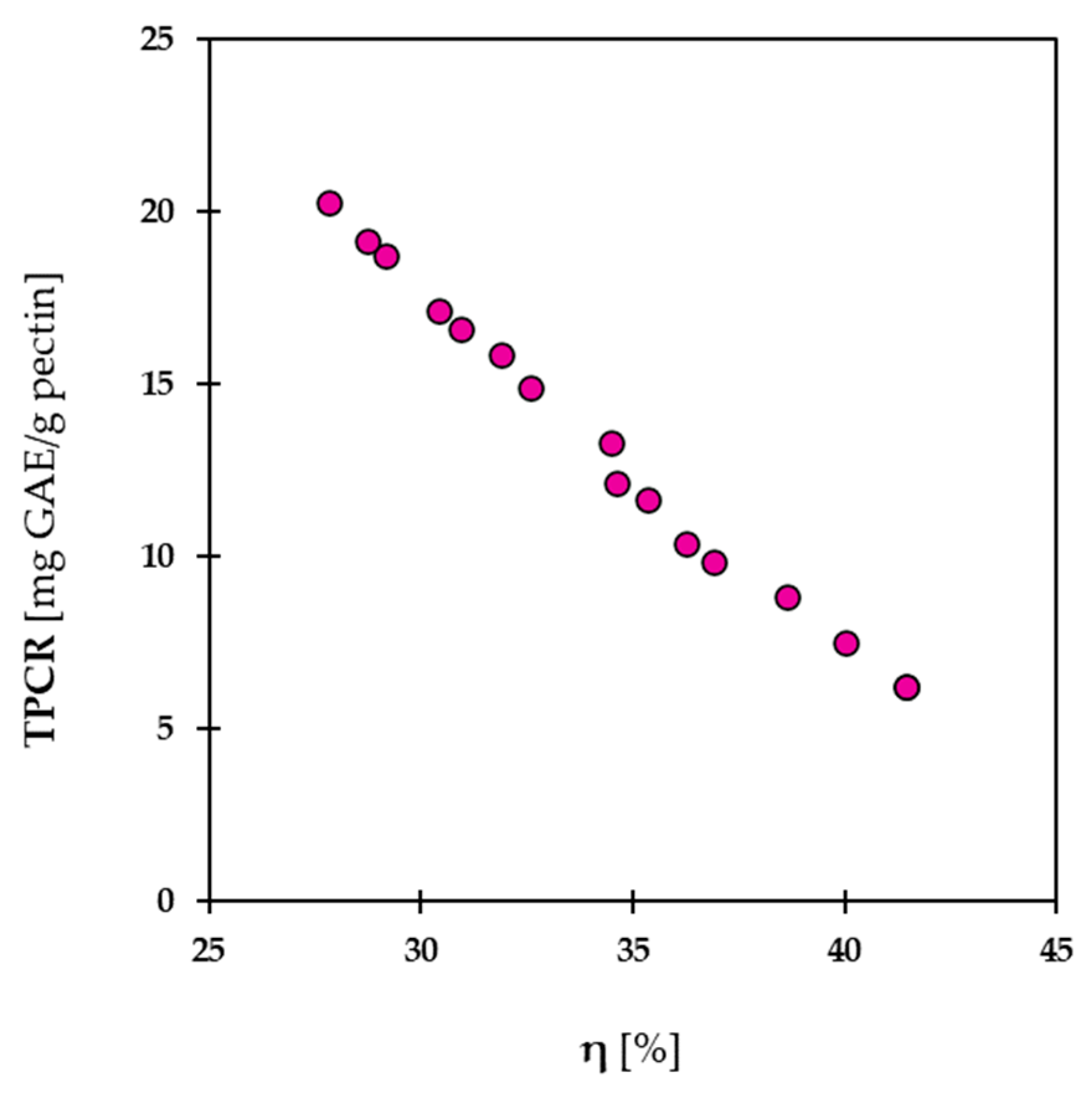
2.3.3. Effects of Simultaneous Variables on η and TPCR
2.4. Identification and Quantification of Phenolic Compounds
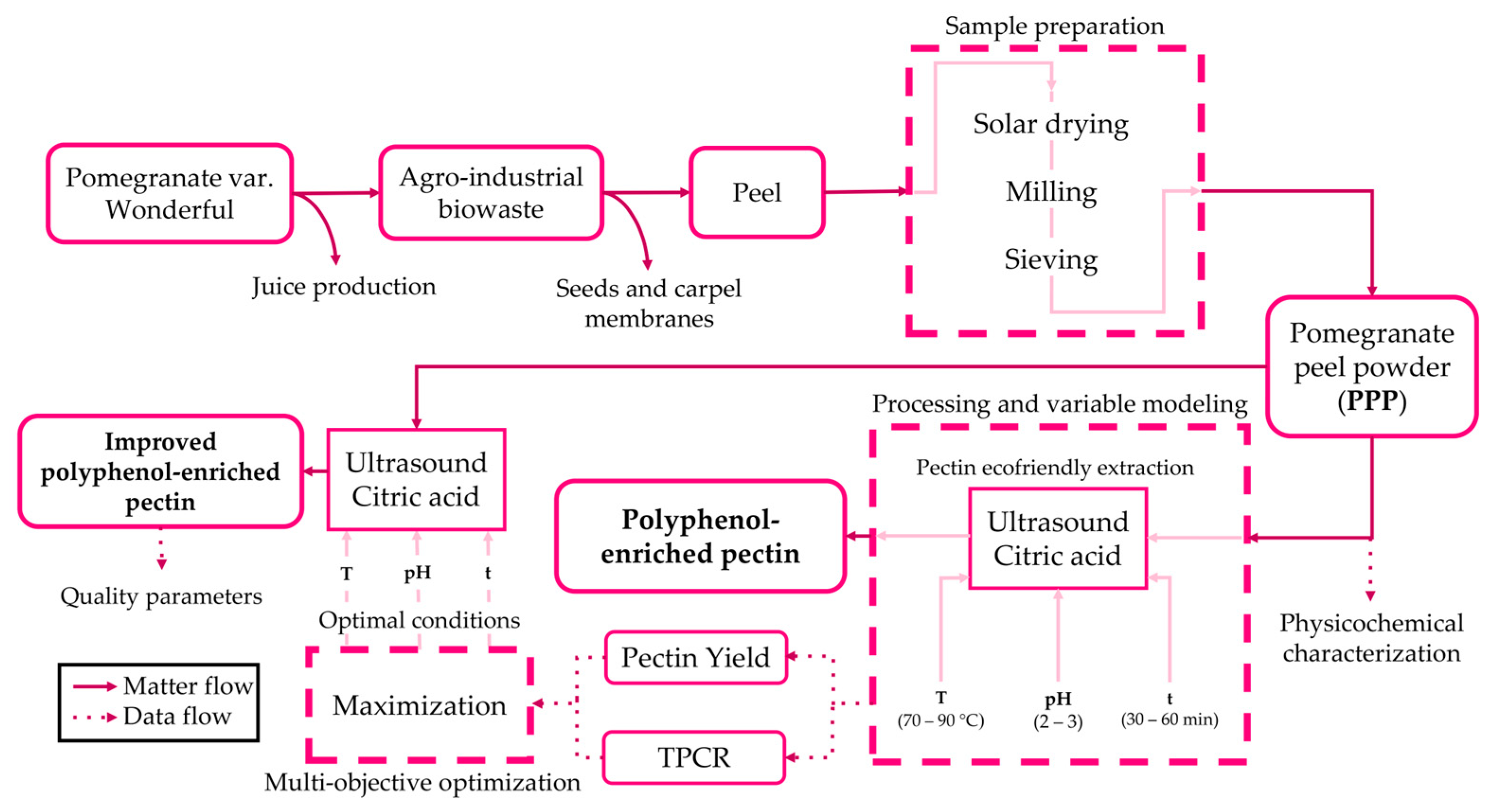
3. Materials and Methods
3.1. Raw Material and Its Pretreatment
3.2. Bio-Waste Physicochemical Characterization
3.3. Pectin Extraction: Experimental Design
3.4. Determination of Total Polyphenol Content (TPC)
3.5. Multi-Objective Optimization
3.6. Pectin Characterization
3.7. Identification and Quantification of Phenolics by HPLC-PDA-ESI-QTOF MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Independent Variables | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| t [min] | T [°C] | pH | Pectin Yield [%] | TPCR [mg GAE/g pectin] | |||||
| Experimental | Adjusted | Residue | Experimental | Adjusted | Residue | ||||
| 1 | 30 | 80 | 2.0 | 24.17 ± 1.05 | 28.03 | 3.86 | 93.82 ± 1.05 | 80.64 | 13.18 |
| 2 | 30 | 80 | 3.0 | 12.12 ± 0.19 | 8.18 | 3.94 | 65.49 ± 0.53 | 57.52 | 7.97 |
| 3 | 60 | 80 | 2.0 | 37.55 ± 1.49 | 39.97 | 2.42 | 44.19 ± 0.95 | 51.24 | 7.05 |
| 4 | 60 | 80 | 3.0 | 15.13 ± 0.17 | 9.74 | 5.39 | 74.95 ± 0.17 | 87.22 | 12.27 |
| 5 | 45 | 70 | 2.0 | 42.47 ± 3.02 | 38.05 | 4.42 | 54.73 ± 0.45 | 67.40 | 12.67 |
| 6 | 45 | 70 | 3.0 | 8.12 ± 0.68 | 11.50 | 3.38 | 11.92 ± 1.04 | 18.92 | 6.99 |
| 7 | 45 | 90 | 2.0 | 47.03 ± 1.38 | 42.11 | 4.92 | 13.48 ± 2.40 | 5.57 | 7.91 |
| 8 | 45 | 90 | 3.0 | 15.69 ± 2.16 | 18.58 | 2.89 | 80.49 ± 1.54 | 66.91 | 13.58 |
| 9 | 30 | 70 | 2.5 | 18.34 ± 1.46 | 16.55 | 1.79 | 70.89 ± 1.48 | 69.93 | 0.96 |
| 10 | 60 | 70 | 2.5 | 24.06 ± 2.64 | 23.89 | 0.17 | 53.89 ± 2.72 | 33.18 | 20.71 |
| 11 | 30 | 90 | 2.5 | 24.09 ± 1.76 | 22.72 | 1.37 | 6.31 ± 0.22 | 26.12 | 19.81 |
| 12 | 60 | 90 | 2.5 | 28.86 ± 2.61 | 28.88 | 0.02 | 63.10 ± 2.42 | 63.17 | 0.07 |
| 13 | 45 | 80 | 2.5 | 19.66 ± 2.28 | 18.89 | 0.76 | 50.56 ± 5.20 | 50.10 | 0.45 |
| Response Variables | Variation Source | Sum of Squares | Degrees of Freedom | Mean Square | Calculated F Value | Tabulated F Value, F (9,29, 0.05) |
|---|---|---|---|---|---|---|
| Pectin yield | regression | 4635.88 | 9 | 515.10 | 32.18 | 2.223“significant” |
| residues | 464.22 | 29 | 16.01 | |||
| total | 5100.10 | 38 | ||||
| R2 | 0.909 | |||||
| adjusted R2 | 0.881 | |||||
| TPCR | regression | 21,848.22 | 9 | 2427.58 | 13.42 | 2.223“significant” |
| residues | 5247.62 | 29 | 180.95 | |||
| total | 27,095.84 | 38 | ||||
| R2 | 0.806 | |||||
| adjusted R2 | 0.746 |
| Term | η [%] | TPCR [mg GAE/g pectin] | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | T-Value | p-Value | VIF | Coefficient | T-Value | p-Value | VIF | |
| Constant | 440.000 | 8.51 | 0.000 | 1074.000 | 0.000 | |||
| pH | −92.900 | −15.33 | 0.000 | 1.00 | −628.000 | 1.21 | 0.235 | 1.00 |
| t | 1.640 | 4.15 | 0.000 | 1.00 | −20.250 | −0.02 | 0.987 | 1.00 |
| T | −8.170 | 3.51 | 0.001 | 1.00 | 5.560 | −1.28 | 0.212 | 1.00 |
| pH pH | 14.270 | 2.33 | 0.027 | 1.35 | 21.300 | 1.04 | 0.308 | 1.35 |
| t t | −0.004 | −0.64 | 0.526 | 1.35 | 0.061 | 2.67 | 0.012 | 1.35 |
| T T | 0.051 | 3.34 | 0.002 | 1.35 | −0.157 | −3.06 | 0.005 | 1.35 |
| pH t | −0.346 | −2.24 | 0.033 | 1.00 | 1.970 | 3.80 | 0.001 | 1.00 |
| pH T | 0.151 | 0.65 | 0.519 | 1.00 | 5.491 | 7.07 | 0.000 | 1.00 |
| t T | −0.002 | −0.26 | 0.799 | 1.00 | 0.123 | 4.75 | 0.000 | 1.00 |
References
- Kahramanoglu, I. Trends in Pomegranate Sector: Production, Postharvest Handling and Marketing. Int. J. Agric. For. Life Sci. 2019, 3, 239–246. [Google Scholar]
- Aguilera-Arango, G.A.; Lombo-Ortiz, D.F.; Burbano-Erazo, E.; Orduz-Rodriguez, J.O. Pomegranate (Punica granatum L.), a Crop with Productive Potential: Review and Situation in Colombia. Trop. Subtrop. Agroecosyst. 2020, 23. [Google Scholar] [CrossRef]
- Gimenez, L.; Varela, B.; Vignale, N.D.; Gurni, A.A. Caracterización Micrográfica Del Fruto de Punica granatum y Su Importancia En El Control de Calidad Botánica. Dominguezia 2020, 36, 11–15. [Google Scholar]
- Tozzi, F.; Núñez-Gómez, D.; Legua, P.; Del Bubba, M.; Giordani, E.; Melgarejo, P. Qualitative and Varietal Characterization of Pomegranate Peel: High-Value Co-Product or Waste of Production? Sci. Hortic. 2022, 291, 110601. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of Pectins Extracted from Pomegranate Peel and Their Gelling Properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of Phenolic Compounds from Pomegranate (Punica granatum L.) by-Product with Significant Antioxidant and Therapeutic Effects: A Narrative Review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef] [PubMed]
- Chezanoglou, E.; Kenanidou, N.; Spyropoulos, C.; Xenitopoulou, D.; Zlati, E.; Goula, A.M. Encapsulation of Pomegranate Peel Extract in Sucrose Matrix by Co-Crystallization. Sustain. Chem. Pharm. 2023, 31, 100949. [Google Scholar] [CrossRef]
- Zalazar-Garcia, D.; Fernandez, A.; Rodriguez-Ortiz, L.; Torres, E.; Reyes-Urrutia, A.; Echegaray, M.; Rodriguez, R.; Mazza, G. Exergo-Ecological Analysis and Life Cycle Assessment of Agro-Wastes Using a Combined Simulation Approach Based on Cape-Open to Cape-Open (COCO) and SimaPro Free-Software. Renew. Energy 2022, 201, 60–71. [Google Scholar] [CrossRef]
- Parascanu, M.M.; Kaltschmitt, M.; Rödl, A.; Soreanu, G.; Sánchez-Silva, L. Life Cycle Assessment of Electricity Generation from Combustion and Gasification of Biomass in Mexico. Sustain. Prod. Consum. 2021, 27, 72–85. [Google Scholar] [CrossRef]
- Awan, U.; Sroufe, R. Sustainability in the Circular Economy: Insights and Dynamics of Designing Circular Business Models. Appl. Sci. 2022, 12, 1521. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Cuvelier, G.; Leverrier, C.; Renard, C.M.G.C.; Attia, H.; Ayadi, M.A. Rheological Properties of Pomegranate Peel Suspensions: The Effect of Fibrous Material and Low-Methoxyl Pectin at Acidic PH. Food Hydrocoll. 2017, 62, 174–181. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Anoopkumar, A.N.; Surendhar, A.; Liz Kuriakose, L.; Tiwari, A.; Sirohi, R.; Kuddus, M.; Rebello, S.; Kumar Awasthi, M.; et al. Integrated Biorefinery Development for Pomegranate Peel: Prospects for the Production of Fuel, Chemicals and Bioactive Molecules. Bioresour. Technol. 2022, 362, 127833. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, S.S.; Kumar, A.; Mandavgane, S.A.; Rahagude, R.; Gokhale, S.; Yadav, K.; Borua, A.P. Valorization of Punica granatum (Pomegranate) Peels: A Case Study of Circular Bioeconomy. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate Peel Pectin Can Be Used as an Effective Emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Fırat, E.; Koca, N.; Kaymak-Ertekin, F. Extraction of Pectin from Watermelon and Pomegranate Peels with Different Methods and Its Application in Ice Cream as an Emulsifier. J. Food Sci. 2023, 88, 4353–4374. [Google Scholar] [CrossRef]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-Based Injectable and Biodegradable Self-Healing Hydrogels for Enhanced Synergistic Anticancer Therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef]
- Bin Emran, T.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C.M.G.; et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules 2022, 27, 7405. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Bin Emran, T.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A First Evidence in the Synergism and Bioactivities. Food Rev. Int. 2022, 39, 4419–4441. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.; Li, C.; He, F.; Jiang, L.; Aisa, H.A. Integrating Chemical and Biological Catalysis for Simultaneous Production of Polyphenolics and Butyric Acid from Waste Pomegranate Peels. Sci. Total Environ. 2021, 778, 146095. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Pomegranate Peel Using Response Surface Methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Niveditha, A.; Chidanand, D.V.; Sunil, C.K. Effect of Ultrasound-Assisted Drying on Drying Kinetics, Color, Total Phenols Content and Antioxidant Activity of Pomegranate Peel. Meas. Food 2023, 12, 100114. [Google Scholar] [CrossRef]
- Rifna, E.J.; Dwivedi, M. Effect of Pulsed Ultrasound Assisted Extraction and Aqueous Acetone Mixture on Total Hydrolysable Tannins from Pomegranate Peel. Food Biosci. 2022, 45, 101496. [Google Scholar] [CrossRef]
- Riveros-Gomez, M.; Zalazar-García, D.; Mut, I.; Torres-Sciancalepore, R.; Fabani, M.P.; Rodriguez, R.; Mazza, G. Multiobjective Optimization and Implementation of a Biorefinery Production Scheme for Sustainable Extraction of Pectin from Quince Biowaste. ACS Eng. Au 2022, 2, 496–506. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, S.; Liu, M.; Tian, S. Simultaneous Process Optimization of Ultrasound-Assisted Extraction of Polyphenols and Ellagic Acid from Pomegranate (Punica granatum L.) Flowers and Its Biological Activities. Ultrason. Sonochem. 2021, 80, 105833. [Google Scholar] [CrossRef]
- Zalazar-García, D.; Feresin, G.E.; Rodriguez, R. Optimal Operational Variables of Phenolic Compound Extractions from Pistachio Industry Waste (Pistacia Vera Var. Kerman) Using the Response Surface Method. Biomass Convers. Biorefinery 2022, 12, 3761–3770. [Google Scholar] [CrossRef]
- Man, G.; Ma, Y.; Xu, L.; Liao, X.; Zhao, L. Comparison of Thermal and Non-Thermal Extraction Methods on Free and Bound Phenolics in Pomegranate Peel. Innov. Food Sci. Emerg. Technol. 2023, 84, 103291. [Google Scholar] [CrossRef]
- Rashid, R.; Masoodi, F.A.; Wani, S.M.; Manzoor, S.; Gull, A. Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate Peel, Their Nanoencapsulation and Application for Improvement in Shelf Life Extension of Edible Oils. Food Chem. 2022, 385, 132608. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, L.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Chao, Z. A Review on Phytochemicals, Metabolic Profiles and Pharmacokinetics Studies of the Different Parts (Juice, Seeds, Peel, Flowers, Leaves and Bark) of Pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel. LWT 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Al-Rawahi, A.S.; Rahman, M.S.; Guizani, N.; Essa, M.M. Chemical Composition, Water Sorption Isotherm, and Phenolic Contents in Fresh and Dried Pomegranate Peels. Dry Technol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Mirsaeedghazi, H.; Azarikia, F.; Abedinia, A. Investigation of Antimicrobial Activity of Orange and Pomegranate Peels Extracts and Their Use as a Natural Preservative in a Functional Beverage. J. Food Meas. Charact. 2021, 15, 5683–5694. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.A.; Pillay, Y. Microbial Safety of Low Water Activity Foods: Study of Simulated and Durban Household Samples. J. Food Qual. 2017, 2017, 4931521. [Google Scholar] [CrossRef]
- Akuru, E.A.; Oyeagu, C.E.; Mpendulo, T.C.; Rautenbach, F.; Oguntibeju, O.O. Effect of Pomegranate (Punica granatum L.) Peel Powder Meal Dietary Supplementation on Antioxidant Status and Quality of Breast Meat in Broilers. Heliyon 2020, 6, e05709. [Google Scholar] [CrossRef]
- Muhammad, A.; Dayisoylu, K.S.; Pei, J.; Khan, M.R.; Salman, M.; Ahmad, R.; Ullah, H.; Noor, G.R. Compositional Analysis of Natural Pomegranate Peel Powder Dried by Different Methods and Nutritional and Sensory Evaluation of Cookies Fortified with Pomegranate Peel Powder. Front. Nutr. 2023, 10, 1118156. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Hussain, Z.; Aihetasham, A.; El-Sharnouby, M.; Abdul Rehman, R.; Azmat Ullah Khan, M.; Zahra, S.; Saleem, A.; Azhar, S.; Alhazmi, A.; et al. Pomegranate Peels Waste Hydrolyzate Optimization by Response Surface Methodology for Bioethanol Production. Saudi J. Biol. Sci. 2021, 28, 4867–4875. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Ortega-Rivas, E.; Zamudio-Flores, P.B.; Perez-Vega, S.; Sepulveda, D.R. Water Activity, Not Moisture Content, Explains the Influence of Water on Powder Flowability. LWT 2019, 100, 35–39. [Google Scholar] [CrossRef]
- Karimi, M.; Sadeghi, R.; Kokini, J. Pomegranate as a Promising Opportunity in Medicine and Nanotechnology. Trends Food Sci. Technol. 2017, 69, 59–73. [Google Scholar] [CrossRef]
- Zaidel, D.N.A.; Rashid, J.M.; Hamidon, N.H.; Salleh, L.M.; Kassim, A.S.M. Extraction and Characterisation of Pectin from Dragon Fruit (Hylocereus polyrhizus) Peels. Chem. Eng. Trans. 2017, 56, 805–810. [Google Scholar] [CrossRef]
- Ebrahim, S.A.; Othman, H.A.; Mosaad, M.M.; Hassabo, A.G. Eco-Friendly Natural Thickener (Pectin) Extracted from Fruit Peels for Valuable Utilization in Textile Printing as a Thickening Agent. Textiles 2023, 3, 26–49. [Google Scholar] [CrossRef]
- Siddiqui, A.; Chand, K.; Shahi, N.C. Effect of Process Parameters on Extraction of Pectin from Sweet Lime Peels. J. Inst. Eng. Ser. A 2021, 102, 469–478. [Google Scholar] [CrossRef]
- Nguyen, B.M.N.; Pirak, T. Physicochemical Properties and Antioxidant Activities of White Dragon Fruit Peel Pectin Extracted with Conventional and Ultrasound-Assisted Extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Kareem, A.H.; Naji, E.Z. Study of the Chemical Properties of Pectin Extracted from Residues of Some Plant Sources. Ann. Biol. 2022, 38, 252–257. [Google Scholar]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, Purification and Characterization of Pectin from Alternative Sources with Potential Technological Applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Balli, D.; Khatib, M.; Cecchi, L.; Adessi, A.; Melgarejo, P.; Nunes, C.; Coimbra, M.A.; Mulinacci, N. Pomegranate Peel as a Promising Source of Pectic Polysaccharides: A Multi-Methodological Analytical Investigation. Food Chem. 2022, 397, 133550. [Google Scholar] [CrossRef]
- Jokar, F.; Rahaiee, S.; Zare, M.; Nasiri kenari, M.; Mirzakhani, N. Bioactive Wound Dressing Using Bacterial Cellulose/Dextran Biopolymers Loaded with Pomegranate Peel Extract: Preparation, Characterization and Biological Properties. J. Drug Deliv. Sci. Technol. 2023, 84, 104461. [Google Scholar] [CrossRef]
- Das, I.; Arora, A. Kinetics and Mechanistic Models of Solid-Liquid Extraction of Pectin Using Advance Green Techniques—A Review. Food Hydrocoll. 2021, 120, 106931. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and Functional Properties of Mango Peel Pectin Extracted by Ultrasound Assisted Citric Acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An Integrated Green Biorefinery Approach towards Simultaneous Recovery of Pectin and Polyphenols Coupled with Bioethanol Production from Waste Pomegranate Peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of Pomegranate Peel Extract as a Natural Additive in Foods. Trends Food Sci. Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Sabater, C.; Villamiel, M.; Montilla, A. Integral Use of Pectin-Rich by-Products in a Biorefinery Context: A Holistic Approach. Food Hydrocoll. 2022, 128, 107564. [Google Scholar] [CrossRef]
- Capossio, J.P.; Fabani, M.P.; Reyes-Urrutia, A.; Torres-Sciancalepore, R.; Deng, Y.; Baeyens, J.; Rodriguez, R.; Mazza, G. Sustainable Solar Drying of Brewer’s Spent Grains: A Comparison with Conventional Electric Convective Drying. Processes 2022, 10, 339. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2010. [Google Scholar]
- ASTM D1106-21; Standard Test Method for Acid-Insoluble Lignin in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D1103-60; Standard Test Methods for Alpha-Cellulose in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D1104-56; Standard Test Methods for Hollocellulose in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Fabani, M.P.; Román, M.C.; Rodriguez, R.; Mazza, G. Minimization of the Adverse Environmental Effects of Discarded Onions by Avoiding Disposal through Dehydration and Food-Use. J. Environ. Manag. 2020, 271, 110947. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Optimization of Ultrasound-Microwave Assisted Acid Extraction of Pectin from Potato Pulp by Response Surface Methodology and Its Characterization. Food Chem. 2019, 289, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Fabani, M.P.; Baroni, M.V.; Luna, L.; Lingua, M.S.; Monferran, M.V.; Paños, H.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Changes in the Phenolic Profile of Argentinean Fresh Grapes during Production of Sun-Dried Raisins; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 58. [Google Scholar] [CrossRef]

| Parameter | Value ♠ |
|---|---|
| Moisture content [%] | 60.62 ± 1.07 |
| pH [dimensionless] | 4.19 ± 0.03 |
| Titratable acidity [%] | 3.28 ± 0.12 |
| Soluble solids [°Brix] | 20.00 ± 0.01 |
| Proximal Composition | Value ♠ |
|---|---|
| Moisture [%] | 6.67 ± 1.53 |
| pH [dimensionless] | 3.27 ± 0.12 |
| aw [dimensionless] | 0.50 ± 0.02 |
| Titratable acidity [%] | 6.25 ± 0.44 |
| Protein [%] | 2.42 ± 0.07 |
| Crude fiber [%] | 8.16 ± 0.22 |
| Ash [%] | 2.69 ± 0.49 |
| Lignin [%] | 14.97 ± 1.03 |
| Cellulose [%] | 16.23 ± 0.19 |
| Hemicellulose [%] | 29.82 ± 0.30 |
| Parameter | Value 1 |
|---|---|
| aw [dimensionless] | 0.51 ± 0.02 |
| ash content [%] | 2.55 ± 0.11 |
| equivalent weight [g/mol] | 175.27 ± 3.95 |
| methoxyl content [%] | 6.79 ± 0.08 |
| Degree of esterification [%] | 41.60 ± 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podetti, C.; Riveros-Gomez, M.; Román, M.C.; Zalazar-García, D.; Fabani, M.P.; Mazza, G.; Rodríguez, R. Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process. Molecules 2023, 28, 7656. https://doi.org/10.3390/molecules28227656
Podetti C, Riveros-Gomez M, Román MC, Zalazar-García D, Fabani MP, Mazza G, Rodríguez R. Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process. Molecules. 2023; 28(22):7656. https://doi.org/10.3390/molecules28227656
Chicago/Turabian StylePodetti, Celina, Mathias Riveros-Gomez, María Celia Román, Daniela Zalazar-García, María Paula Fabani, Germán Mazza, and Rosa Rodríguez. 2023. "Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process" Molecules 28, no. 22: 7656. https://doi.org/10.3390/molecules28227656
APA StylePodetti, C., Riveros-Gomez, M., Román, M. C., Zalazar-García, D., Fabani, M. P., Mazza, G., & Rodríguez, R. (2023). Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process. Molecules, 28(22), 7656. https://doi.org/10.3390/molecules28227656