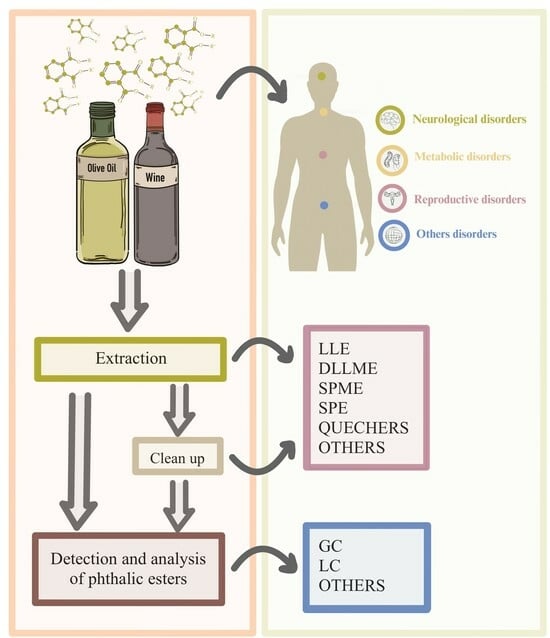
A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine
Abstract
:1. Phthalates Esters in Olive Oil and Wine
1.1. Toxicity
1.2. Health Risks
1.3. Regulation
2. Identification and Quantification of Phthalate Esters in Olive Oil and Wine
| PAEs | Sample Preparation | Analytical Technique Column | LOD | LOQ | Recovery % | Concentration of PAEs | R * |
|---|---|---|---|---|---|---|---|
| DMP, DEP, DIBP, DBP, BBP, DCHP, DEHP, DOP, DINP, DIDP | LLE Isohexane | GC/MS Agilent DB5- MS (30 m × 0.25 mm × 0.25 μm) | 0.004–0.020 mg/L | 0.01–0.05 mg/L | 98–100 | 0.008–0.273 mg/kg DBP, BBP, DEHP | [46] |
| DEP | LLE 1,1,2-trichlorotrifluoroethane | GC/MS Varian VF-Xms column (29.3 m × 0.25 mm × 0.25 μm) | 0.7 mg/L | 2.6 mg/L | 103.9–110.4 | <LOD | [47] |
| DMP, DEP, DIPrP, DAP, DPrP, DIBP, DBP, DMEP, DIPP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DHP, DPhP, DNOP, DINP, DIDP, DNP | LLE Acetonitrile | LC/MS/MS Agilent Poroshell 120 EC-C18 (100 × 4.6 mm × 2.7 µm) | 0.8–15 µg/kg | 10–100 µg/kg | 75.5–113.3 | ns | [48] |
| DBP | LLE Hexane | FI–CL | 0.03 pg/mL | 96–103.3 | 0.09–0.22 µg/mL | [49] | |
| DBP | LLE Hexane | icELISA | 64.5 ng/mL | 83.1–101.7 | ns | [50] | |
| DEP | LLE Hexane | GNP-rt-IPCR | 1.06 pg/L | 96.65–110.02 | 41.88 µg/kg | [51] | |
| DMP | LLE Hexane | BA-rt-IPCR | 1.98 pg/L | 88.18–108.99 | 86.96–182.85 µg/L | [52] | |
| DMP, DEP, DIBP, DBP, BBP, DEHP, DOP, DINP, DIDP | Filter 0.2 µm | HPLC/MS/MS Phenomenex 75 mm Synergi Hydro-RP (2 mm × 4 µm × 4 mm) | 0.5–8.8 µg/L | 1.6–26.6 µg/L | 94.6–105.7 | 1.8–10.7 µg/L DIBP, DBP, BBP | [53] |
| DMP, DEP, DBP, BMEP, DPP | MA-LLME | GC/MS Teknokroma TRB-624 (30 m × 0.25 mm × 1.40 μm) | 0.1–0.4 µg/L | 0.3–1 µg/L | 4.2–25 µg/L DBP, DPP, DEP | [54] | |
| DMP, DEP, DBP, BBP, BBP, DEHP | USVADLLME | GC/MS Lab-made SE-54 (30 m × 250 μm × 0.25 μm) | 0.022–0.1 µg/L | 0.075–0.335 µg/L | 85–100.5 | 11.5–312.4 pg/µL DBP, BBP, DEHP | [55] |
| DBP, BBP, DEP, DIOP | DLLME | GC-FID Lab-made SE-54 (15 m × 0.25 mm × 0.33 μm) | 0.34–0.78 µg/L | 70–120 | 1.2–5.8 µg/L BBP, DBP | [56] | |
| DBP, BBP, DCHP, DEHP, DOP | UA-DLLME-SFOD | GC-FID Agilent HP-5 (30 m × 0.250 mm × 0.25 μm) | 0.64–2.82 µg/L | 1.93–8.47 µg/L | 75–98 | ns | [57] |
| DIBP, DBP, BBP, DEHP | IL-DLLME [C8MIM] [PF6] | HPLC/DAD Waters Xterra C18 (15 cm × 4.6 mm × 5 μm) | 1.5–2.2 ng/mL | 5–7.3 ng/mL | 91.6–10.6 | 0.018–0.122 µg/mL DIBP DBP | [58] |
| DMP, DEP, DBP, DEHP, BBP, DOP | HS-SPME PDMS | GC/MS Varian CP-WAX 52 CB (30 m × 0.32 mm × 0.25 µm) | 16–35 ng/L | 72–121 | 0.3–7.40 µg/L DMP, DEP, DBP, DEHP, BBP, DOP | [36] | |
| DMP, DEP, DBP, DEHP, BBP, DOP | HS-SPME CW-DVB; PDMS-DVB with sodium chloride | GC/MS Varian CP-WAX 52 CB (30 m × 0.32 mm × 0.25 µm) | 0.06–2.9 µg/L | 0.1–4.2 µg/L | 64–135 | Total ranging from 7–12 ng/mL | [59] |
| DBP, BBP, BDE, DOP | HS-SPME PDMS-DVB with sodium chloride | GC/MS SGE HP-5 (60 m × 0.25 mm × 0.25 μm) | 0.03–0.11 µg/L | 0.09–0.36 µg/L | 80.3–107.6 | 0.71–20.8 µg/L DBP, DOP | [60] |
| DEP, DBP, DEHP | DI-HF-SPME MWCNTs/SiO2 reinforced hollow fibre | GC/MS Thermo TR-5 MS (30 m × 0.25 mm × 0.25 μm) | 0.006–0.03 ng/mL | 0.02–0.1 ng/mL | <LOD | [61] | |
| DBP | DI-SPME Graphene oxide | GC/MS HP-5 MS (30 m × 0.25 mm × 0.25 μm) | 0.3 ng/L | 98 | <LOD | [62] | |
| DMP, DEP, DBP, DIBP, BBP, DEHP | SPE Amberlite XAD-2 | GC-FID Lab-made SE-54 (15 m × 0.25 mm × 0.24 μm) | 1.21–2.51 pg/µL | 2.42–5.03 pg/µL | 94–103 | 4.9–12.3 pg/µLDBP, DEHP | [63] |
| DMP, DEP, DBP, BCEP, BBP, DEHP | SPE Carbograph 1 | GC/MS Lab-made SE-54 (30 m × 250 μm × 0.23 μm) | 0.2–14 ng/mL | 0.5–25 ng/mL | 78–105 | 0.1–23 ng/mL DBP, BBP, DEHP | [64] |
| DMP, DEP, DEHP, DIBP, DBP, BBP | SPE C18 | GC/MS Restek RTX-5MS (30 m × 0.25 mm × 0.25 µm) | 0.015–0.018 µg/mL | 0.024–0.029 µg/mL | 33–109 | 0.025–0.276 µg/mL DIBP, DBP, BBP, DEHP | [65] |
| DBP, DEHP, DEP | Filter; SPE 0.45 µm; C18 | LC/DAD Poroshell 120 EC C18 (4.6 mm × 50 mm × 2.7 µm) | 0.25–0.38 ng/mL | 0.75–1.10 ng/mL | 23.6–334 ng/mL DBP, DEHP, DEP | [66] | |
| BBP, DEP, DBP, DMP | MIP-SPE | HPLC/MS Agilent ZORBAX Eclipse XDB-C8 (50 mm × 2.1 mm × 3.5 µm) | 0.03–0.20 µg/L | 0.09–0.68 µg/L | 74–98 | 0.3–5 µg/L BBP, DEP, DBP, DMP | [67] |
| DBP, BBP, DEHP | QuEChERS | GC/MS J&W DB-5MS (30 m × 0.25 mm × 0.25 μm) | 0.08–2.25 ng/mL | 104–123 | 1.69–9.72 ng/mL DBP, BBP | [68] | |
| DMP, DEP, DIBP, DBP, DHP, BBP, DCHP, DEHP, DNOP, DINP, DIDP | LLE Acetonitrile | GC/MS/MS Restek Rxi-5Sil MS (30 m × 0.25 mm × 0.25 μm) | 0.004–0.130 mg/L | 0.012–2.600 mg/L | 90.1–108.2 | 0.03–7.52mg/kg DIBP, DBP, BBP, DEHP, DINP | [69] |
| DMP, DEP, DIBP, DBP, BBP, DHP, DEHP, DOP, DINP, DIDP | LLE Acetonitrile | GC/MS Supelco SPB-5MS (30 m × 0.25 mm × 0.25 mm) | 0.003–1.2 mg/kg | 0.010–4.0 mg/kg | 93.5–99.4 | 0.060–6.249mg/kg DMP, DEP, DIBP, DBP, BBP, DEHP, DINP, DIDP | [70] |
| DMP, DEP, DBP, DEHP | LLE by means of the carbon nanotube Pseudophase Distilled water; MWCNTs | GC/MS Supelco SLB-5 ms (30 m × 0.25 mm × 0.25 μm) | 25–50 μg/L | 92–104 | 0.15–5.1 mg/L DMP, DEP, DBP, DEHP | [71] | |
| DMP, DEP, DPP, DIBP, DBP, BBP, BMPP, DEHP, DOP | LLE Acetonitrile | GC/MS/MS Restek Rxi-5ms (30 m × 0.25 mm × 0.25 µm) | 0.43–1.67 μg/L | 1.48–5.75 μg/L | 89–114 | 0.17 mg/kg DEHP | [72] |
| DMP, DEP, DIBP, DBP, DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DPhP, DOP | LLE hexane saturated acetonitrile and hexane | GC/MS/MS Agilent HP-5MS (30 m × 0.25 mm × 0.25 μm) | 0.1–4.0 μg/kg | 70.0–110.8 | ns | [73] | |
| DEHP, DBP, DIBP, DINP | Dilution Hexane | GC/MS Lab-made pre-column OV-1701-OH (0.5 m × 0.25 mm × 0.05 mm) in series w/2 lab-made columns OV-61-OH (2.5 m × 0.32 mm × 0.20 µm) OV-225-OH (15–20 m × 0.25 mm × 0.20 µm) | 10 μg/kg - 1 mg/kg | 40 μg/kg - 3 mg/kg | 82–106 | 90–6480 μg/kg DEHP, DINP, DBP | [74] |
| DMP, DEP, DPP, DBP, BBP, DCHP, DEHP, DINP, DIDP | Dilution Hexane | GC×GC/MS/MS 1D Merck SLB-5 ms (10 m × 0.25 mm × 0.10 μm) 2D Merck SLB-35 ms (1.5 m × 0.10 mm × 0.10 μm) | 0.02–0.63 mg/kg | 0.06–2.10 mg/kg | 0.22–8.0 mg/kgDPP, DEHP, DINP, DIDP | [75] | |
| DMP, DEP, DPP, DBP, BBP, DCHP, DEHP, DINP, DIDP, | Dilution Hexane | GC/MS/MS Equity-5 (5 m × 0.53 mm × 0.53 μm) | 0.004–0.341 mg/kg | 0.013–1.136 mg/kg | 0.018–55.9 mg/kgDEHP, DIDP DBP, DPP, DINP, DEP | [76] | |
| DMP, DEP, DIPrP, DAP, DPrP, DIBP, DBP, DMEP, DIPP, BMPP, DEEP, DPP, DHXP, BBP, DBEP DCHP, DEHP, DHP, DPhP, DNOP, DINP, DIDP, DNP, | LLE Acetonitrile | LC/MS/MS Agilent Poroshell 120 EC-C18 (100 × 4.6 mm × 2.7 µm) | 0.8–15 μg/kg | 10–100 μg/kg | 82.2–112.6 | ns | [48] |
| BBP, DBP, DEHP, DEP, DIBP, DIDP, DINP, DMP, DHXP, DOP, DAP, DPP | LLE Hexane saturated acetonitrile | UHPLC/MS Thermo Accucore aQ C18 (2.6 μm × 2.1 × 100 mm) | 0.02–0.35 mg/kg | 0.07–1.17 mg/kg | 79–109 | 0.3–256.2 mg/kg DEHP, DIDP, DINP, DMP, DNOP, BBP, DEP, DIBP | [77] |
| DMP, DEP, DAP, DPrP, DIBP, BBP, DBP, DCHP, DHXP, DEHP | LLE Acetonitrile | UHPLC/MS/MS Thermo Syncronis C18 (100 × 2.1 mm, 1.7 µm) | 0.1–1 μg/kg | 0.3–3.3 μg/kg | 85.1–95.5 | 3.0–309 μg/kg DMP, DEP, DIBP, BBP, DBP, DEHP | [78] |
| PAEs hydrolyzed in Phthalic Acid | LPME Tributyl phosphate | HPLC/MS/MS GL Sciences Inertsil ODS-3 (250 mm × 4.6 mm × 5 m) | 1 μmol/kg | 1.3 μmol/kg | 86–107 | 4.82 μmol/kg | [79] |
| DMP, DEP, DPP, DIBP, DBP, BBP, DCHP, DEHP, DINP, DIDP, | LLE and DI-SPME Acetonitrile; PDMS | GC/MS/MS Supelco SLB-5ms (10 m × 0.1 mm × 0.1 mm) | 0.015–0.144 mg/kg | 0.228–7.207 mg/kg DEP, DIBP, DBP, DEHP, DINP, DIDP, | [80] | ||
| DMP, DEP, DAP, DIBP, DBP, BBP, DCHP, DEHP | LLE and SPME Acetonitrile; MIL-88(Fe)/Go | GC-FID Agilent HP-5 (30 m × 0.32 mm × 0.25 μm) | 0.5–2 ng/g | 1.7–6.7 ng/g | 83.1–104.1 | <LOD | [81] |
| DPP, DBP, DEHP | HS-SPME G/PVC nanocomposite | GC-FID Varian CP-Sil 8 CB (30 m × 0.32 mm × 0.25 µm) | 0.06–0.08 μg/L | 0.2–0.3 μg/L | 87–112 | <LOD | [82] |
| DMP, DEP, DIBP, DBP, DMEP, 1,2MPP, 1,3MPP, DEEP, DAP, DHP, BBP, BBEP, DCHP, DEHP, DOP, DNP | SPME DVB/CAR/PDMS | GC/MS/MS Phenomenex Zebron ZB-5ms (30 m × 0.25 mm × 0.25 m) | 0.02–0.05 mg/kg | 87–840 μg/kg DIBP, DBP, BBP, DEHP | [83] | ||
| DMP, DEP, DBP, BBP, DEHP | LLE and SPE Acetonitrile; PSA | GC/MS Thermo TG- 5MS column (30 m × 0.25 mm × 0.25 µm) | 0.10–0.79 μg/kg | 0.33–2.6 μg/kg | 72,4–103 | 0.05–1.28 mg/kg DMP, DBP, BBP, DEHP | [84] |
| DBP, BBP, DEHP | LLE and SPE Acetonitrile; Florisil | GC/TOFMS Agilent DB-5MS column (30 m × 0.25 mm × 0.25 μm) | 4.70–10 μg/kg | 14.2–30.4 μg/kg | 83.9–97.8 | 13.2–729 μg/kgDBP, DEHP | [85] |
| DMP, DEP, DBP, DIBP, DEHP, BBP, DINP, DIDP | LLE and SPE Acetonitrile and tetrahydrofuran; Alumina | GC/MS HP-5MS (30 m × 0.25 mm × 0.25 μm) | 2–170 ng/mL | 6–500 ng/g | 62–110 | <LOD | [86] |
| DMP, DEP, DIPrP, DPrP, DIBP, DBP, DMEP, DIPP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DHP, DPhP, DEHP, DNOP, DNP | MAE-GPC-SPE C18 | GC/MS/MS Agilent HP-5MS (30 m × 0.32 mm × 0.25 μm) | 0.218–1.367 μg/kg | 0.72–4.51 μg/kg | 93.04–104.7 | 0.42–0.70 mg/kg DBP, DEHP | [87] |
| DMP, DEP, DPP, DBP, BBP, DOP | LLE and SPE Hexane; Florisil | GC/MS Santa Clara HP-5MS (30 m × 0.25 mm × 0.25 μm) | 0.002–0.004 mg/L | 0.006–0.012 mg/L | 87–102 | 0.049–2.295 mg/L DMP, DEP, DBP, DPP, BBP, DOP | [88] |
| DMP, DEP, DIBP, DBP, BMPP, DEEP, DPP, DHP, BBP, DBEP, DCHP, DHP, DPhP, DOP, DNP | LLE and SPE (QuEChERS modified) Methanol; GCB and PSA | GC/MS/MS DB-5MS (30 m × 0,25 mm × 0,25 μm) | 0.02–8 μg/kg | 0.07–26.68 μg/kg | 70.11–115.33 | 0.10–1.85 mg/kg DIBP, DHP | [89] |
| DMP, DEP, DPrP, DAP, DIBP, DBP, DPP, DHXP, BBP, DHP, DEHP, DPhP, DNP, DDP | LLE and dSPE Acetonitrile; Q-sep QuEChERS | GC/MS Shimadzu SHRXI-5MS (30 m × 0.25 mm × 0.25 μm) | 1.4–7.5 μg/kg | 4.8–25.1 μg/kg | 60.9–101.3 | 14–6166 μg/kg DMP, DEP, DAP, DPP DIBP, DBP, DPP, DHXP, BBP, DEHP, DNP, DDP | [37] |
| DEP, DIBP, DBP, BBP, DEHP, DOP, DINP, DIDP | LLE and SPE Acetone: methanol; DSC-18 | HPLC-MS/MS Phenomenex Kinetex C18 (50 mm × 2.1 mm × 5.0 µm) | 5.5–110 μg/kg | 42–100 | 0.014–4.7 mg/kg DIBP, DBP, BBP, DEHP, DOP, DINP, DIDP | [90] | |
| DEP, DIBP, DBP, BBP | LLE and SPE (QuEChERS) Acetonitrile; PSA | HPLC/DAD | 6–9 ng/g | 18–29 ng/g | <LOD | [91] | |
| DEP, DBP, BMPP, DEEP, DNPP, DHXP, BBP, DBEP, DCHP, DEHP, DNOP, DMP, DMEP, DPP, DINP, DIDP | SPE Florisil | LC-MS/MS Agilent ZORBAX SB-C18 (10 cm × 3.5 μm × 2.1mm) | 0.5–25 μg/kg | 1.4–65 μg/kg | 50.94–140.83 | ns | [92] |
| BBP, DEHP | LLE and SPE (QuEChERS) Acetonitrile; PSA | SFC-UV Thermo Acclaim 120 C18 (5 μm, 4.6 mm × 250 mm | 0.09–0.12 μg/mL | 0.30–0.39 μg/mL | 80.3–106.4 | <LOD | [93] |
| DMP, DEP, DBP, BBP, DEHP, DOP | GPC Cyclohexane: dichloromethane | GC/MS/MS Varian Factor Four 5-ms (30 m × 0.25 mm × 0.25µm) | 0.1–148 μg/kg | 0.2–182 μg/kg | 0.029–4.70 mg/kg DBP, BBP, DEHP | [41] | |
| DBP, DEHP | Raman spectroscopy with SERS | At a concentration of 0.2 mg/kg, the peaks for both plasticizers were still clearly detectable | ns | [94] |
2.1. Sample Preparation
2.1.1. Liquid–Liquid Extraction
2.1.2. Dispersive Liquid-Liquid Microextraction
2.1.3. Solid-Phase Microextraction Extraction
2.1.4. Solid-Phase Extraction
2.1.5. QuEChERS
2.1.6. Other Extraction/Clean-Up Methods
2.2. Separation and Detection of Phthalates in Wine and Olive Oil
2.2.1. Gas Chromatography
2.2.2. Liquid Chromatography
2.2.3. Other Analytical Techniques
2.3. The Major Challenge in the Laboratory Analysis of Phthalates
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Global Consumption of Olive Oil 2022/23|Statista. Available online: https://www.statista.com/statistics/940491/olive-oil-consumption-worldwide/ (accessed on 11 October 2023).
- Wine Consumption Worldwide 2022|Statista. Available online: https://www.statista.com/statistics/232937/volume-of-global-wine-consumption/ (accessed on 11 October 2023).
- Economic Affairs & Promotion Unit—International Olive Council. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 18 October 2023).
- State of the World Vine and Wine Sector in 2022—International Organisation of Vine and Wine. Available online: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2.pdf (accessed on 18 October 2023).
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.J.; McDowell, D.; Coles, R. Food Packaging Technology; Blackwell: Oxford, UK, 2003; ISBN 978-1-405-14771-2. [Google Scholar]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Hoppe, M.; de Voogt, P.; Franz, R. Identification and Quantification of Oligomers as Potential Migrants in Plastics Food Contact Materials with a Focus in Polycondensates—A Review. Trends Food Sci. Technol. 2016, 50, 118–130. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food Packaging’s Materials: A Food Safety Perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Craver, C.; Carraher, C. Applied Polymer Science: 21st Century; Elsevier Science: Kidlington/Oxford, UK, 2000; ISBN 0080434177. [Google Scholar]
- Seyoum, A.; Pradhan, A. Effect of Phthalates on Development, Reproduction, Fat Metabolism and Lifespan in Daphnia Magna. Sci. Total Environ. 2019, 654, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and Other Additives in Plastics: Human Exposure and Associated Health Outcomes. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2097. [Google Scholar] [CrossRef]
- Staples, C. Phthalate Esters (Handbook of Environmental Chemistry); Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540009922. [Google Scholar]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Envrion. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T.; Skakkebæk, N.E.; De Kretser, D.; Leffers, H. Human Exposure to Phthalates via Consumer Products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and Diet: A Review of the Food Monitoring and Epidemiology Data. Env. Health 2014, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Calafat, A.M.; Hauser, A.R. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of Substances from Food Packaging Materials to Foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef] [PubMed]
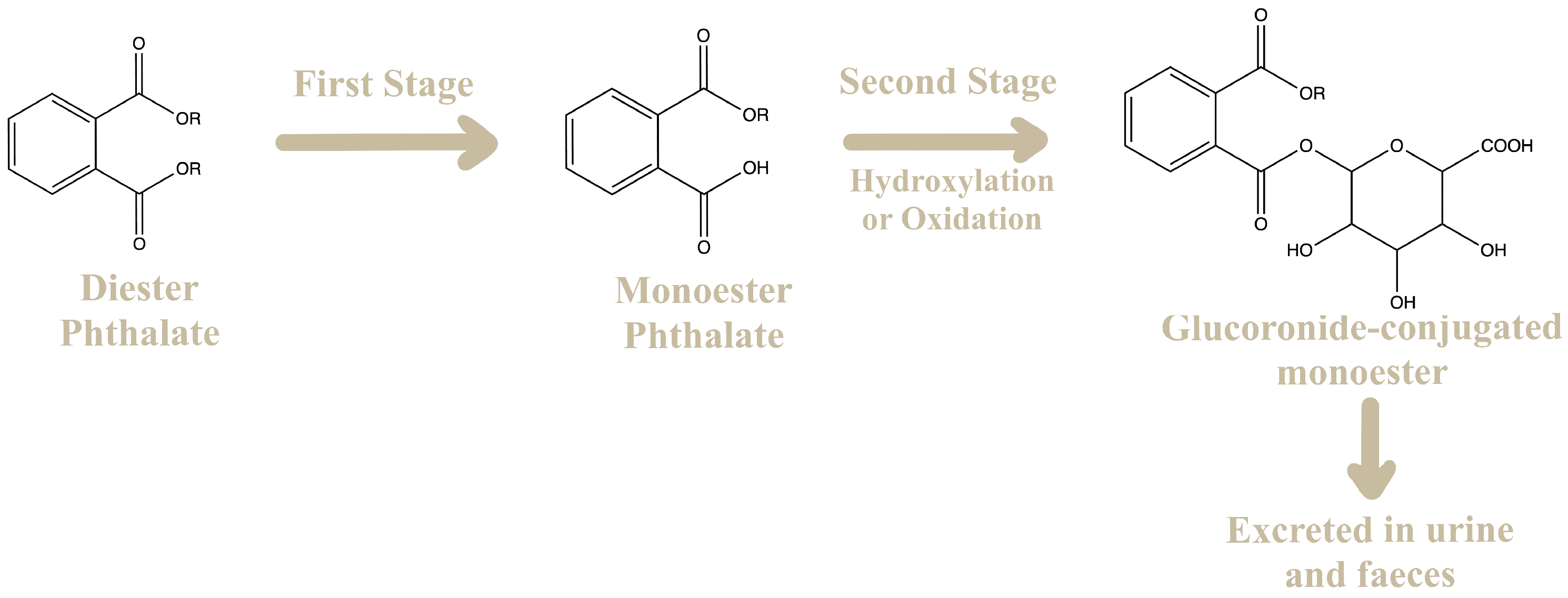
- Frederiksen, H.; Skakkebæk, N.E.; Andersson, A.M. Metabolism of Phthalates in Humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Silva, M.; Samandar, E.; Reidy, J.; Hauser, R.; Needham, L.; Calafat, A. Metabolite Profiles of Di-n-Butyl Phthalate in Humans and Rats. Environ. Sci. Technol. 2007, 41, 7576–7580. [Google Scholar] [CrossRef]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and Exposure. Int. J. Androl. 2008, 31, 131–138. [Google Scholar] [CrossRef]
- Ramesh Kumar, A.; Sivaperumal, P. Analytical Methods for the Determination of Biomarkers of Exposure to Phthalates in Human Urine Samples. TrAC Trends Anal. Chem. 2016, 75, 151–161. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New Metabolites of Di(2-Ethylhexyl)Phthalate (DEHP) in Human Urine and Serum after Single Oral Doses of Deuterium-Labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef]
- Knudsen, L.; Franco, D. Biomarkers and Human Biomonitoring: Volume 1; Royal Society of Chemistry: London, UK, 2012; Volume 1, ISBN 978-1-84973-241-3. [Google Scholar]
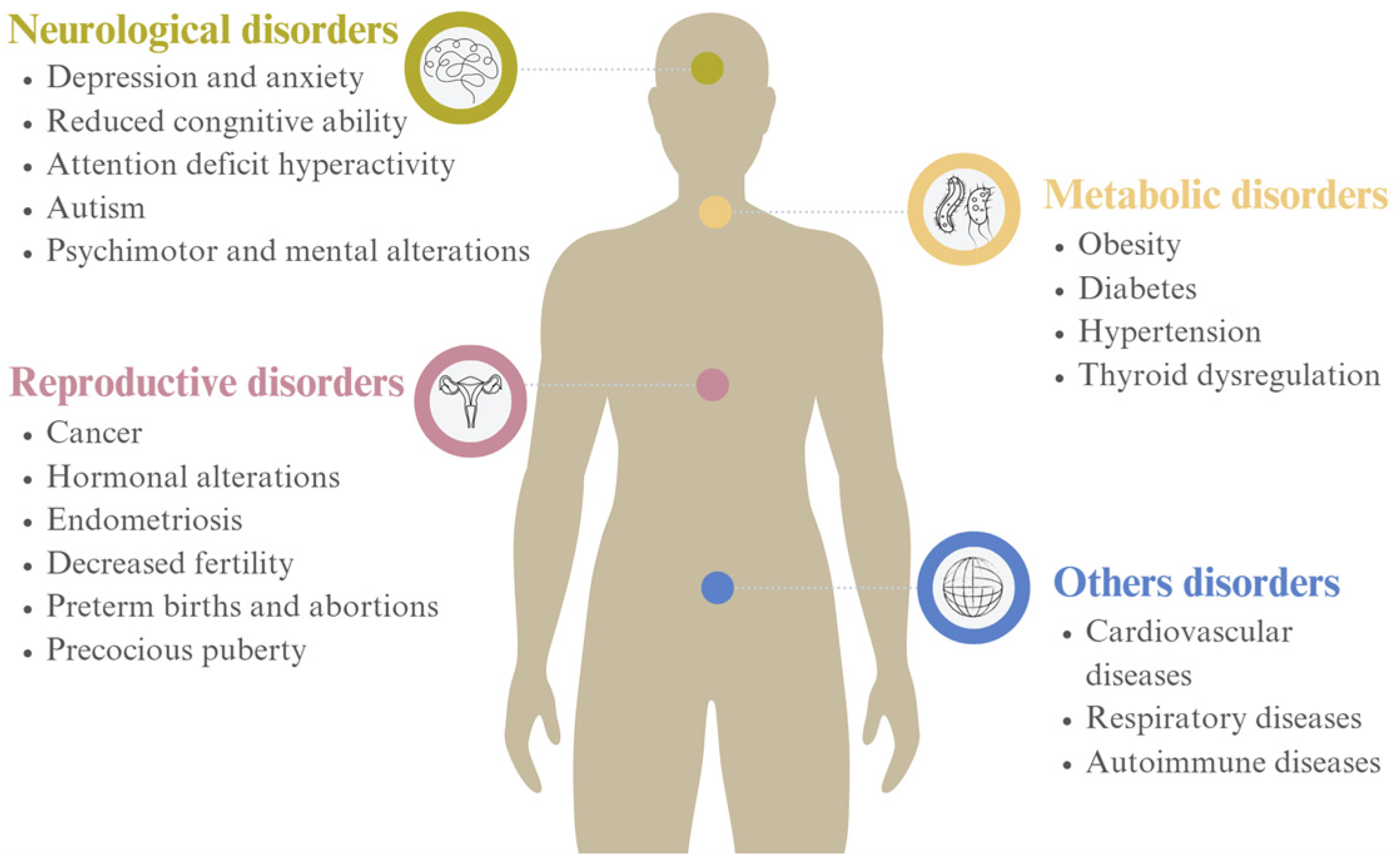
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Neurological Processes and Neural Health: A Literature Review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Guo, J.L.; Xue, J.C.; Bai, C.L.; Guo, Y. Phthalate Metabolites: Characterization, Toxicities, Global Distribution, and Exposure Assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef] [PubMed]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Mini Review Phthalates: European Regulation, Chemistry, Pharmacokinetic and Related Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935 (accessed on 15 October 2023).
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010 (accessed on 15 October 2023).
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mortensen, A.; et al. Update of the Risk Assessment of Di-Butylphthalate (DBP), Butyl-Benzyl-Phthalate (BBP), Bis(2-Ethylhexyl)Phthalate (DEHP), Di-Isononylphthalate (DINP) and Di-Isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA J. 2019, 17, e05838. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/1442 of 11 July 2023 amending Annex I to Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food, as Regards Changes to Substance Authorisations and Addition of New Substances. Off. J. Eur. Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R1442 (accessed on 15 October 2023).
- Carrillo, J.D.; Martínez, M.P.; Tena, M.T. Determination of Phthalates in Wine by Headspace Solid-Phase Microextraction Followed by Gas Chromatography-Mass Spectrometry. Use of Deuterated Phthalates as Internal Standards. J. Chromatogr. A 2008, 1181, 125–130. [Google Scholar] [CrossRef]
- Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer Contamination in Edible Vegetable Oil in a U.S. Retail Market. J. Agric. Food Chem. 2013, 61, 9502–9509. [Google Scholar] [CrossRef]
- Wang, C.; Huang, P.; Qiu, C.; Li, J.; Hu, S.; Sun, L.; Bai, Y.; Gao, F.; Li, C.; Liu, N.; et al. Occurrence, Migration and Health Risk of Phthalates in Tap Water, Barreled Water and Bottled Water in Tianjin, China. J. Hazard. Mater. 2021, 408, 124891. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Liu, Y.; Wang, S.; Wang, L. Development of Rapid Determination of 18 Phthalate Esters in Edible Vegetable Oils by Gas Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2013, 61, 1160–1164. [Google Scholar] [CrossRef]
- Cavaliere, B.; Macchione, B.; Sindona, G.; Tagarelli, A. Tandem Mass Spectrometry in Food Safety Assessment: The Determination of Phthalates in Olive Oil. J. Chromatogr. A 2008, 1205, 137–143. [Google Scholar] [CrossRef]
- Lu, J.Y. Plasticizer Event in Taiwan. J. Formos. Med. Assoc. 2011, 110, 553–554. [Google Scholar] [CrossRef]
- Sanchis, Y.; Yusà, V.; Coscollà, C. Analytical Strategies for Organic Food Packaging Contaminants. J. Chromatogr. A 2017, 1490, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Haji Harunarashid, N.Z.I.; Lim, L.H.; Harunsani, M.H. Phthalate Sample Preparation Methods and Analysis in Food and Food Packaging: A Review. Food Anal. Methods 2017, 10, 3790–3814. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent Advances in Analysis of Phthalate Esters in Foods. TrAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of Wines and Spirits by Phthalates: Types of Contaminants Present, Contamination Sources and Means of Prevention. Food Addit. Contam. Part A 2014, 31, 1605–1615. [Google Scholar] [CrossRef]
- Leitz, J.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Chemical Analysis and Risk Assessment of Diethyl Phthalate in Alcoholic Beverages with Special Regard to Unrecorded Alcohol. PLoS ONE 2009, 4, e8127. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Deng, X.; Fang, E.; Zheng, X.; Zhou, Y.; Lin, L.; Chen, L.; Wu, M.; Huang, Z. Determination of 23 Phthalic Acid Esters in Food by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1324, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Luo, K.; Chen, D.; Tan, X.; Song, Z. A Rapid and Sensitive Method for the Determination of Dibutyl Phthalate in Wine by Flow-Injection Chemiluminescence Analysis. J. Food Compos. Anal. 2013, 31, 226–231. [Google Scholar] [CrossRef]
- Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Wang, Z.; Guo, P.; Sun, S.; Ding, S. Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Detection of Dibutyl Phthalate in White Wine, Compared With GC-MS. Food Anal. Methods 2014, 7, 1619–1626. [Google Scholar] [CrossRef]
- Sun, R.; Zhuang, H. An Ultrasensitive Gold Nanoparticles Improved Real-Time Immuno-PCR Assay for Detecting Diethyl Phthalate in Foodstuff Samples. Anal. Biochem. 2015, 480, 49–57. [Google Scholar] [CrossRef]
- Sun, R.; Zhuang, H. Biotin-Streptavidin-Amplified Real-Time Immune-PCR Assay for Detecting Dimethyl Phthalate in Beverage and Drinking Water Samples. Anal. Bioanal. Chem. 2015, 407, 1261–1265. [Google Scholar] [CrossRef]
- Hayasaka, Y. Analysis of Phthalates in Wine Using Liquid Chromatography Tandem Mass Spectrometry Combined with a Hold-Back Column: Chromatographic Strategy to Avoid the Influence of Pre-Existing Phthalate Contamination in a Liquid Chromatography System. J. Chromatogr. A 2014, 1372, 120–127. [Google Scholar] [CrossRef]
- March, J.G.; Cerdà, V. An Innovative Arrangement for In-Vial Membrane-Assisted Liquid-Liquid Microextraction: Application to the Determination of Esters of Phthalic Acid in Alcoholic Beverages by Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 4213–4217. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Rapid Analysis of Six Phthalate Esters in Wine by Ultrasound-Vortex-Assisted Dispersive Liquid-Liquid Micro-Extraction Coupled with Gas Chromatography-Flame Ionization Detector or Gas Chromatography-Ion Trap Mass Spectrometry. Anal. Chim. Acta 2013, 769, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cui, S.; Wang, W.; Miao, J.; Feng, J.; Chen, J. Determination of Phthalate Esters in Wine Using Dispersive Liquid-Liquid Microextraction and Gas Chromatography. Anal. Lett. 2014, 47, 1874–1887. [Google Scholar] [CrossRef]
- Pérez-Outeiral, J.; Millán, E.; Garcia-Arrona, R. Determination of Phthalates in Food Simulants and Liquid Samples Using Ultrasound-Assisted Dispersive Liquid-Liquid Microextraction Followed by Solidification of Floating Organic Drop. Food Control 2016, 62, 171–177. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, S.; Xie, Q. Rapid Determination of Phthalate Esters in Alcoholic Beverages by Conventional Ionic Liquid Dispersive Liquid-Liquid Microextraction Coupled with High Performance Liquid Chromatography. Talanta 2014, 119, 291–298. [Google Scholar] [CrossRef]
- Carrillo, J.D.; Salazar, C.; Moreta, C.; Tena, M.T. Determination of Phthalates in Wine by Headspace Solid-Phase Microextraction Followed by Gas Chromatography-Mass Spectrometry: Fibre Comparison and Selection. J. Chromatogr. A 2007, 1164, 248–261. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Monitoring Phthalates in Table and Fortified Wines by Headspace Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry Analysis. J. Agric. Food Chem. 2020, 68, 8431–8437. [Google Scholar] [CrossRef]
- Li, J.; Su, Q.; Li, K.Y.; Sun, C.F.; Zhang, W.B. Rapid Analysis of Phthalates in Beverage and Alcoholic Samples by Multi-Walled Carbon Nanotubes/Silica Reinforced Hollow Fibre-Solid Phase Microextraction. Food Chem. 2013, 141, 3714–3720. [Google Scholar] [CrossRef]
- Liu, M.; Peng, Q.Q.; Chen, Y.F.; Tang, Q.; Feng, Q. A Rapid Space-Resolved Solid-Phase Microextraction Method as a Powerful Tool to Determine Contaminants in Wine Based on Their Volatility. Food Chem. 2015, 176, 12–16. [Google Scholar] [CrossRef]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Study of XAD-2 Adsorbent for the Enrichment of Trace Levels of Phthalate Esters in Hydroalcoholic Food Beverages and Analysis by Gas Chromatography Coupled with Flame Ionization and Ion-Trap Mass Spectrometry Detectors. Food Chem. 2014, 146, 181–187. [Google Scholar] [CrossRef]
- Russo, M.V.; Notardonato, I.; Cinelli, G.; Avino, P. Evaluation of an Analytical Method for Determining Phthalate Esters in Wine Samples by Solid-Phase Extraction and Gas Chromatography Coupled with Ion-Trap Mass Spectrometer Detector. Anal. Bioanal. Chem. 2012, 402, 1373–1381. [Google Scholar] [CrossRef]
- Del Carlo, M.; Pepe, A.; Sacchetti, G.; Compagnone, D.; Mastrocola, D.; Cichelli, A. Determination of Phthalate Esters in Wine Using Solid-Phase Extraction and Gas Chromatography-Mass Spectrometry. Food Chem. 2008, 111, 771–777. [Google Scholar] [CrossRef]
- Vidal, R.B.P.; Ibañez, G.A.; Escandar, G.M. A Green Method for the Quantification of Plastics-Derived Endocrine Disruptors in Beverages by Chemometrics-Assisted Liquid Chromatography with Simultaneous Diode Array and Fluorescent Detection. Talanta 2016, 159, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Barciela-Alonso, M.C.; Otero-Lavandeira, N.; Bermejo-Barrera, P. Solid Phase Extraction Using Molecular Imprinted Polymers for Phthalate Determination in Water and Wine Samples by HPLC-ESI-MS. Microchem. J. 2017, 132, 233–237. [Google Scholar] [CrossRef]
- Fasano, E.; Cirillo, T.; Esposito, F.; Lacorte, S. Migration of Monomers and Plasticizers from Packed Foods and Heated Microwave Foods Using QuEChERS Sample Preparation and Gas Chromatography/Mass Spectrometry. LWT 2015, 64, 1015–1021. [Google Scholar] [CrossRef]
- Pereira, J.; do Céu Selbourne, M.; Poças, F. Determination of Phthalates in Olive Oil from European Market. Food Control 2019, 98, 54–60. [Google Scholar] [CrossRef]
- Mo Dugo, G.; Fotia, V.; Lo Turco, V.; Maisano, R.; Potortì, A.G.; Salvo, A.; Di Bella, G. Phthalate, Adipate and Sebacate Residues by HRGC-MS in Olive Oils from Sicily and Molise (Italy). Food Control 2011, 22, 982–988. [Google Scholar] [CrossRef]
- López-Feria, S.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Surfactant-Coated Carbon Nanotubes for the Liquid-Liquid Extraction of Phthalates and Other Migrants in Virgin Olive Oils. Anal. Bioanal. Chem. 2009, 395, 737–746. [Google Scholar] [CrossRef]
- Xiao, Y.; Wong, W.Y.; Chan, L.Y.; Yong, C.K.; Abe, K.; Hancock, P.; Hird, S. Simultaneous Determination of Nine Phthalates in Vegetable Oil by Atmospheric Pressure Gas Chromatography with Tandem Mass Spectrometry (APGC-MS/MS). Toxics 2023, 11, 200. [Google Scholar] [CrossRef]
- Zhou, R.Z.; Jiang, J.; Mao, T.; Zhao, Y.S.; Lu, Y. Multiresidue Analysis of Environmental Pollutants in Edible Vegetable Oils by Gas Chromatography-Tandem Mass Spectrometry. Food Chem. 2016, 207, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Nanni, N.; Fiselier, K.; Grob, K.; Di Pasquale, M.; Fabrizi, L.; Aureli, P.; Coni, E. Contamination of Vegetable Oils Marketed in Italy by Phthalic Acid Esters. Food Control 2011, 22, 209–214. [Google Scholar] [CrossRef]
- Arena, A.; Zoccali, M.; Mondello, L.; Tranchida, P.Q. Direct Analysis of Phthalate Esters in Vegetable Oils by Means of Comprehensive Two-Dimensional Gas Chromatography Combined with Triple Quadrupole Mass Spectrometry. Food Chem. 2022, 396, 133721. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, A.; Zoccali, M.; Arena, A.; Mondello, M.; Tranchida, P.Q.; Mondello, L. A Dilute-and-Inject Low-Pressure Gas Chromatography-Tandem Mass Spectrometry Method for Phthalate Determination in Extra Virgin Olive Oil. J. Sep. Sci. 2023, 46, 2300529. [Google Scholar] [CrossRef] [PubMed]
- Amelio, M.; Gandalini, M. Proposal for a Fast Method to Determine Routinely 15 Plasticisers in Olive Oil by Liquid Extraction and Ultra High-Performance Liquid Chromatography Heated Electro Spray Ionisation High Resolution Mass Spectrometry (Orbitrap) Analysis. Riv. Ital. Delle Sostanze Grasse 2021, 98, 169–175. [Google Scholar]
- Pardo-Mates, N.; Serrano, F.; Núñez, O. Determination of Phthalic Acid Esters in Drinking Water and Olive Oil by Ultra-High Performance Liquid Chromatography-Electrospray-Tandem Mass Spectrometry: Study of Phthalate Migration from Plastic Bottles to Drinking Water at Different Domestic Exposure Conditions. Trends Chromatogr. 2017, 11, 27–48. [Google Scholar]
- Liu, S.; Liu, L.; Han, Y.; Sun, J.; Feng, J.; Wang, J.; Zhong, C. Rapid Screening of Edible Oils for Phthalates Using Phase-Transfer Catalyst-Assisted Hydrolysis and Liquid Phase Microextraction Coupled to High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2015, 1420, 26–34. [Google Scholar] [CrossRef]
- Barp, L.; Purcaro, G.; Franchina, F.A.; Zoccali, M.; Sciarrone, D.; Tranchida, P.Q.; Mondello, L. Determination of Phthalate Esters in Vegetable Oils Using Direct Immersion Solid-Phase Microextraction and Fast Gas Chromatography Coupled with Triple Quadrupole Mass Spectrometry. Anal. Chim. Acta 2015, 887, 237–244. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Li, Z.; Wang, W.; Zang, X.; Wang, C.; Wang, Z. Solid Phase Microextraction of Phthalic Acid Esters from Vegetable Oils Using Iron (III)-Based Metal-Organic Framework/Graphene Oxide Coating. Food Chem. 2018, 263, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, H.; Yamini, Y.; Moradi, M.; Asl, Y.A. Determination of Phthalate Esters in Drinking Water and Edible Vegetable Oil Samples by Headspace Solid Phase Microextraction Using Graphene/Polyvinylchloride Nanocomposite Coated Fiber Coupled to Gas Chromatography-Flame Ionization Detector. J. Chromatogr. A 2016, 1465, 38–46. [Google Scholar] [CrossRef]
- Rios, J.J.; Morales, A.; Márquez-Ruiz, G. Headspace Solid-Phase Microextraction of Oil Matrices Heated at High Temperature and Phthalate Esters Determination by Gas Chromatography Multistage Mass Spectrometry. Talanta 2010, 80, 2076–2082. [Google Scholar] [CrossRef]
- Shi, L.K.; Zhang, M.M.; Liu, Y.L. Concentration and Survey of Phthalic Acid Esters in Edible Vegetable Oils and Oilseeds by Gas Chromatography-Mass Spectrometry in China. Food Control 2016, 68, 118–123. [Google Scholar] [CrossRef]
- Oh, M.S.; Lee, S.H.; Moon, M.H.; Lee, D.S.; Park, H.M. Simultaneous Analysis of Phthalates, Adipate and Polycyclic Aromatic Hydrocarbons in Edible Oils Using Isotope Dilution-Gas Chromatography-Mass Spectrometry. Food Addit. Contam. Part. B Surveill. 2014, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ierapetritis, I.; Lioupis, A.; Lampi, E. Determination of Phthalates into Vegetable Oils by Isotopic Dilution Gas Chromatography Mass Spectrometry. Food Anal. Methods 2014, 7, 1451–1457. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Li, H.; Zhang, J.; Sun, N. Development of Multiresidue Analysis for Twenty Phthalate Esters in Edible Vegetable Oils by Microwave-Assisted Extraction-Gel Permeation Chromatography-Solid Phase Extraction-Gas Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Sungur, S.; Okur, R.; Turgut, F.H.; Ustun, I.; Gokce, C. Migrated Phthalate Levels into Edible Oils. Food Addit. Contam. Part B Surveill. 2015, 8, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Wang, X.; Qi, X.; Wang, D.; Jiang, J.; Mao, J.; Ma, F.; Yu, L.; Zhang, L.; et al. Determination of 15 Phthalic Acid Esters Based on GC–MS/MS Coupled with Modified QuEChERS in Edible Oils. Food Chem. X 2022, 16, 100520. [Google Scholar] [CrossRef] [PubMed]
- Vavrouš, A.; Pavloušková, J.; Ševčík, V.; Vrbík, K.; Čabala, R. Solution for Blank and Matrix Difficulties Encountered during Phthalate Analysis of Edible Oils by High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1456, 196–204. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, S.; Fan, Y.; Sun, J.; Zhang, X. Determination of Phthalate Esters in Edible Oils by Use of QuEChERS Coupled with Ionic-Liquid-Based Dispersive Liquid-Liquid Microextraction before High-Performance Liquid Chromatography. Anal. Bioanal. Chem. 2014, 406, 4563–4569. [Google Scholar] [CrossRef]
- Li, X.; Xiong, W.; Lin, H.; Zhuo, L.; Lv, S.; Tang, X.; Chen, M.; Zou, Z.; Lin, Z.; Qiu, B.; et al. Analysis of 16 Phthalic Acid Esters in Food Simulants from Plastic Food Contact Materials by LC-ESI-MS/MS. J. Sep. Sci. 2013, 36, 477–484. [Google Scholar] [CrossRef]
- Gan, Y.; Zhu, Y. Multi-Residue Analysis of Chemical Additives in Edible Vegetable Oils Using QuEChERS Extraction Method Followed by Supercritical Fluid Chromatography. Molecules 2022, 27, 1681. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, R.; Xu, B.; Wang, Z.; Du, Z.; Zhang, X.; Niu, Y.; Gao, S.; Liu, H.; Zhang, Y. Qualitative and Quantitative Studies of Plasticizers in Extra Virgin Olive Oil by Surface-Enhanced Raman Spectroscopy Combined with Chemometrics. Vib. Spectrosc. 2023, 126, 103527. [Google Scholar] [CrossRef]
- Rotzche, H. Stationary Phases in Gas Chromatography—Journal of Chromatography Library; Elsevier: Amsterdam, The Netherlands, 1991; Volume 48, ISBN 0-444-98733-9. [Google Scholar]
- Stauffer, M. Ideas and Applications Toward Sample Preparation for Food and Beverage Analysis; IntechOpen: Rijeka, Croatia, 2017; ISBN 9789535136859. [Google Scholar]
- Brinco, J.; Guedes, P.; Gomes da Silva, M.; Mateus, E.P.; Ribeiro, A.B. Analysis of Pesticide Residues in Soil: A Review and Comparison of Methodologies. Microchem. J. 2023, 195, 109465. [Google Scholar] [CrossRef]
- Müller, E.; Berger, R.; Blass, E.; Sluyts, D.; Pfennig, A. Liquid–Liquid Extraction. Ullmann’s Encycl. Ind. Chem. 2008, 21, 249–307. [Google Scholar] [CrossRef]
- Othmer, D.F.; White, R.E.; Trueger, E. Liquid-Liquid Extraction Data. Ind. Eng. Chem. 2002, 33, 1240–1248. [Google Scholar] [CrossRef]
- Handbook for the Montreal Protocol on Substances That Deplete the Ozone Layer; United Nations Environment Programme: Nairobi, Kenya, 2020.
- Frankhauser-Noti, A.; Grob, K. Injector-Internal Thermal Desorption from Edible Oils Performed by Programmed Temperature Vaporizing (PTV) Injection. J. Sep. Sci. 2006, 29, 2365–2374. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of Organic Compounds in Water Using Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T. Dispersive Liquid-Liquid Microextraction. TrAC Trends Anal. Chem. 2011, 30, 1382–1399. [Google Scholar] [CrossRef]
- Khalili Zanjani, M.R.; Yamini, Y.; Shariati, S.; Jönsson, J.Å. A New Liquid-Phase Microextraction Method Based on Solidification of Floating Organic Drop. Anal. Chim. Acta 2007, 585, 286–293. [Google Scholar] [CrossRef]
- Pena, M.T.; Casais, M.C.; Mejuto, M.C.; Cela, R. Development of an Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Method for the Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples. J. Chromatogr. A 2009, 1216, 6356–6364. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Wang, J. Ionic Liquids in the Assay of Proteins. Anal. Methods 2010, 2, 1222–1226. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Kislik, V.S. Chapter 14 -Recent Advances in Solvent Extraction Processes and Techniques. Solvent Extr. 2012, 483–524. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Mechlińska, A.; Zygmunt, B.; Namieśnik, J. Green Analytical Chemistry in Sample Preparation for Determination of Trace Organic Pollutants. TrAC Trends Anal. Chem. 2009, 28, 943–951. [Google Scholar] [CrossRef]
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding Solid-Phase Microextraction: Key Factors Influencing the Extraction Process and Trends in Improving the Technique. Chem. Rev. 2013, 113, 1667–1685. [Google Scholar] [CrossRef]
- Liu, W. Determination of Sub-Ppb Level of Phthalates in Water by Auto-SPME and GC-MS. Agil. Technol. 2008. Available online: https://gcms.cz/labrulez-bucket-strapi-h3hsga3/application::paper.paper/5989-7726EN.pdf (accessed on 18 October 2023).
- Shirey, R.E. 4 - SPME Commercial Devices and Fibre Coatings. Handb. Solid. Phase Microextr. 2012, 99–133. [Google Scholar] [CrossRef]
- Rascón, A.J.; Rocío-Bautista, P.; Moreno-González, D.; García-Reyes, J.F.; Ballesteros, E. Fiber Coating Based on a Green Metal-Organic Framework to Determine Phthalates in Bottled Waters by Direct-Immersion Micro Solid-Phase Extraction. Microchem. J. 2023, 191, 108767. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS Analysis of Phthalate Esters in Food Matrices: A Review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Holadová, K.; Prokůpková, G.; Hajšlová, J.; Poustka, J. Headspace Solid-Phase Microextraction of Phthalic Acid Esters from Vegetable Oil Employing Solvent Based Matrix Modification. Anal. Chim. Acta 2007, 582, 24–33. [Google Scholar] [CrossRef]
- Hennion, M.C. Solid-Phase Extraction: Method Development, Sorbents, and Coupling with Liquid Chromatography. J. Chromatogr. A 1999, 856, 3–54. [Google Scholar] [CrossRef]
- Thurman, M.; Mills, S. Solid-Phase Extraction: Principles and Practice; Wiley: Cornwall, UK, 1998; ISBN 047161422. [Google Scholar]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent Advances and Trends in Applications of Solid-Phase Extraction Techniques in Food and Environmental Analysis. Chromatographia 2019, 82, 1207–1249. [Google Scholar] [CrossRef]
- Tienpont, B. Determination of Phthalates in Environmental, Food and Biomatrices-An Analytical Challenge; Ghent University: Ghent, Belgium, 2004. [Google Scholar]
- Wolska, J.; Bryjak, M. Sorption of Phthalates on Molecularly Imprinted Polymers. Sep. Sci. Technol. 2012, 47, 1316–1321. [Google Scholar] [CrossRef]
- Anastassiades, M. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Y.; Wang, L.; Peng, Y.; Wang, W.; Liu, X. Determination of 255 Pesticides in Edible Vegetable Oils Using QuEChERS Method and Gas Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2017, 409, 1017–1030. [Google Scholar] [CrossRef]
- Polgár, L.; Kmellár, B.; García-Reyes, J.F.; Fodor, P. Comprehensive Evaluation of the Clean-up Step in QuEChERS Procedure for the Multi-Residue Determination of Pesticides in Different Vegetable Oils Using LC-MS/MS. Anal. Methods 2012, 4, 1142–1148. [Google Scholar] [CrossRef]
- Cunha, S.C.; Lehotay, S.J.; Mastovska, K.; Fernandes, J.O.; Beatriz, M.; Oliveira, P.P. Evaluation of the QuECHERS Sample Preparation Approach for the Analysis of Pesticide Residues in Olives. J. Sep. Sci. 2007, 30, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C. Gel Permeation Chromatography. I. A New Method for Molecular Weight Distribution of High Polymers. J. Polym. Sci. A 1964, 2, 835–843. [Google Scholar] [CrossRef]
- Stalling, D.L.; Tindle, R.C.; Johnson, J.L. Cleanup of Pesticide and Polychlorinated Biphenyl Residues in Fish Extracts by Gel Permeation Chromatography. J. AOAC Int. 1972, 55, 32–38. [Google Scholar] [CrossRef]
- Sparr Eskilsson, C.; Björklund, E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- McNair, H.; Miller, J.; Snow, N. Basic Gas Chromatography, 3rd ed.; Wiley: Cornwall, UK, 2019; ISBN 978-1-119-45075-7. [Google Scholar]
- Zhou, X.; Shao, X.; Shu, J.J.; Liu, M.M.; Liu, H.L.; Feng, X.H.; Liu, F. Thermally Stable Ionic Liquid-Based Sol–Gel Coating for Ultrasonic Extraction–Solid-Phase Microextraction–Gas Chromatography Determination of Phthalate Esters in Agricultural Plastic Films. Talanta 2012, 89, 129–135. [Google Scholar] [CrossRef]
- Poole, C.F. GAS CHROMATOGRAPHY|Instrumentation. Encycl. Anal. Sci. Second. Ed. 2005, 65–74. [Google Scholar] [CrossRef]
- Ren, R.; Jin, Q.; He, H.L.; Bian, T.-b.; Wang, S.-t.; Fan, J.-c. Determination of 17 Phthalate Esters in Infant Milk Powder and Dairy Products by GC–MS with 16 Internal Standards. Chromatographia 2016, 79, 903–910. [Google Scholar] [CrossRef]
- Fiselier, K.; Biedermann, M.; Grob, K. Injector-Internal Thermal Desorption from Edible Oils. Part 2: Chromatographic Optimization for the Analysis of Migrants from Food Packaging Material. J. Sep. Sci. 2005, 28, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Górecki, T.; Panić, O.; Oldridge, N. Recent Advances in Comprehensive Two-Dimensional Gas Chromatography (GC×GC). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1077–1104. [Google Scholar] [CrossRef]
- Marriott, P.; Shellie, R. Principles and Applications of Comprehensive Two-Dimensional Gas Chromatography. TrAC Trends Anal. Chem. 2002, 21, 573–583. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Dugo, P.; Dugo, G.; Mondello, L. Comprehensive Two-Dimensional Chromatography in Food Analysis. J. Chromatogr. A 2004, 1054, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Branco, S.; Paiva, M.R.; Schütz, S.; Mateus, E.P.; da Silva, M.G. Unveiling Chemical Cues of Insect-Tree and Insect-Insect Interactions for the Eucalyptus Weevil and Its Egg Parasitoid by Multidimensional Gas Chromatographic Methods. Molecules 2022, 27, 4042. [Google Scholar] [CrossRef]
- David, F.; Sandra, P.; Tienpont, B.; Vanwalleghem, F.; Ikonomou, M. Analytical Methods Review. Handb. Environ. Chem. 2003, 3, 9–56. [Google Scholar]
- Niessen, W. Liquid Chromatography-Mass Spectrometry, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 97, ISBN 0-8247-4082-3. [Google Scholar]
- Purcaro, G.; Moret, S.; Conte, L. Hyphenated Liquid Chromatography–Gas Chromatography Technique: Recent Evolution and Applications. J. Chromatogr. A 2012, 1255, 100–111. [Google Scholar] [CrossRef]
- Hyötyläinen, T.; Riekkola, M.L. On-Line Coupled Liquid Chromatography–Gas Chromatography. J. Chromatogr. A 2003, 1000, 357–384. [Google Scholar] [CrossRef]
- Weber, S.; Schrag, K.; Mildau, G.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. Analytical Methods for the Determination of Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH)—A Short Review. Anal. Chem. Insights 2018, 13, 1177390118777757. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Jauho, K.; Riekkola, M.L. Analysis of Pesticides in Red Wines by On-Line Coupled Reversed-Phase Liquid Chromatography–Gas Chromatography with Vaporiser/Precolumn Solvent Split/Gas Discharge Interface. J. Chromatogr. A 1998, 813, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, F.J.; Toledano, R.M.; Andini, J.C.; Cortés, J.M.; Vázquez, A.M. New Analytical Method for Determination of Phthalates in Wastewater by on Line LC-GC-MS Using the TOTAD Interface and Fraction Collector. Processes 2021, 9, 920. [Google Scholar] [CrossRef]
- Hyötyläinen, T.; Grob, K.; Biedermann, M.; Riekkola, M.L. Reversed Phase HPLC Coupled On-Line to GC by the Vaporizer/Precolumn Solvent Split/Gas Discharge Interface; Analysis of Phthalates in Water. J. High. Resolut. Chromatogr. 1997, 20, 410–416. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Pulkrabova, J.; Hajslova, J. Optical Screening Methods for Pesticide Residue Detection in Food Matrices: Advances and Emerging Analytical Trends. Foods 2021, 10, 88. [Google Scholar] [CrossRef]
- Fankhauser-Noti, A.; Grob, K. Blank Problems in Trace Analysis of Diethylhexyl and Dibutyl Phthalate: Investigation of the Sources, Tips and Tricks. Anal. Chim. Acta 2007, 582, 353–360. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Challenges Encountered in the Analysis of Phthalate Esters in Foodstuffs and Other Biological Matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef]
- Liu, H.C.; Den, W.; Chan, S.F.; Kin, K.T. Analysis of Trace Contamination of Phthalate Esters in Ultrapure Water Using a Modified Solid-Phase Extraction Procedure and Automated Thermal Desorption–Gas Chromatography/Mass Spectrometry. J. Chromatogr. A 2008, 1188, 286–294. [Google Scholar] [CrossRef]
- Marega, M.; Grob, K.; Moret, S.; Conte, L. Phthalate Analysis by Gas Chromatography–Mass Spectrometry: Blank Problems Related to the Syringe Needle. J. Chromatogr. A 2013, 1273, 105–110. [Google Scholar] [CrossRef]
- Berset, J.D.; Etter-Holzer, R. Determination of Phthalates in Crude Extracts of Sewage Sludges by High-Resolution Capillary Gas Chromatography with Mass Spectrometric Detection. J. AOAC Int. 2001, 84, 383–391. [Google Scholar] [CrossRef]
- Gómez-Hens, A.; Aguilar-Caballos, M.P. Social and Economic Interest in the Control of Phthalic Acid Esters. TrAC Trends Anal. Chem. 2003, 22, 847–857. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Determination of Phthalic Acid Esters in Water Samples Using Core-Shell Poly(Dopamine) Magnetic Nanoparticles and Gas Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1530, 35–44. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Montes, R.; Quintana, J.B.; Rodil, R. Plasticizers|Environmental Analysis. Encycl. Anal. Sci. 2019, 309–317. [Google Scholar] [CrossRef]
- Net, S.; Delmont, A.; Sempéré, R.; Paluselli, A.; Ouddane, B. Reliable Quantification of Phthalates in Environmental Matrices (Air, Water, Sludge, Sediment and Soil): A Review. Sci. Total Environ. 2015, 515–516, 162–180. [Google Scholar] [CrossRef] [PubMed]

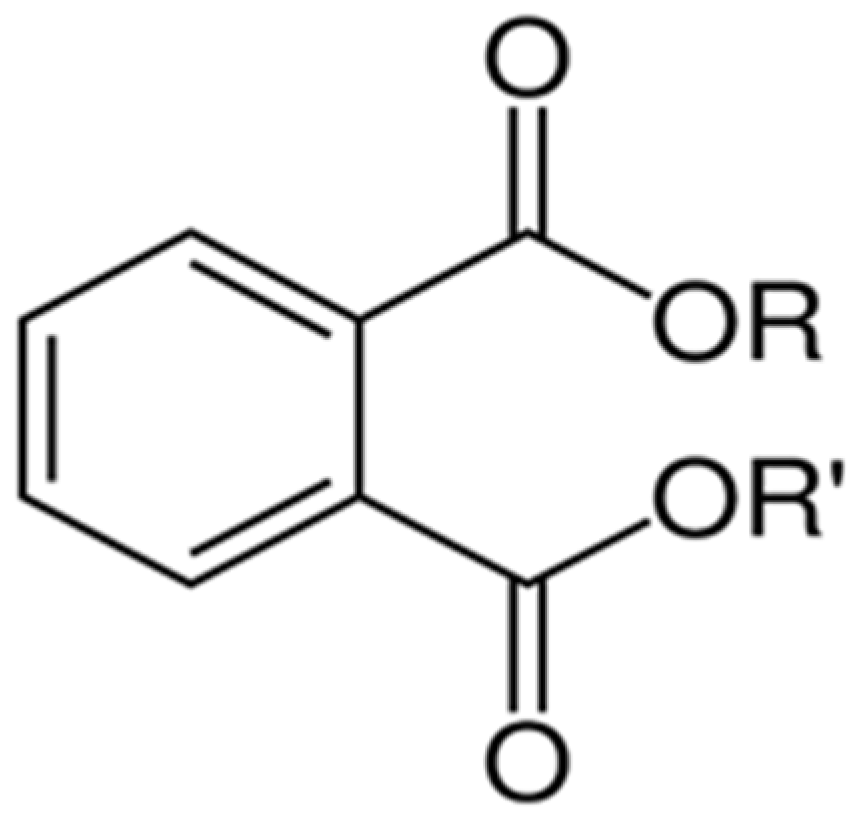
| Name | Molecule | CAS | Molecular Structure | Molecular Weight (g/mol) | Density (g/cm3) | Melting Point (°C) | Boiling Point (°C) | Solubility (mg/L in Water) | Applications |
|---|---|---|---|---|---|---|---|---|---|
| Bis(2-ethylhexyl) phthalate DEHP | 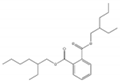 | 117-81-7 | C24H38O4 | 390.6 | 0.981 (25 °C) | −50 | 384 | 0.27 (25 °C) | Used as a plasticizer; also used in pesticides (an inert ingredient), dielectric fluids, erasable inks, and vacuum pump oils; |
| Dimethyl phthalate DMP | 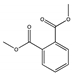 | 131-11-3 | C10H10O4 | 194.2 | 1.194 (20 °C) | 5.5 | 284 | 4 (25 °C) | Used as a plasticizer in solid rocket propellants, lacquers, plastics, safety glasses, rubber coating agents, molding powders, insect repellents, and pesticides. |
| Diisodecyl phthalate DIDP | 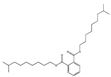 | 26761-40-0 | C26H46O4 | 446.7 | 0.966 (20 °C) | −58 | 53 | 0.28 (25 °C) | Used as a plasticizer for polyvinyl chloride in calendered film, coated fabrics, building wire jackets, wire, and cable extrusion. |
| Benzyl butyl phthalate BBP | 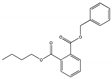 | 85-68-7 | C19H20O4 | 312.4 | 1.119 (25 °C) | −35 | 370 | 2.69 (25 °C) | Used as an organic intermediate and a plasticizer for PVC-based flooring products, polyvinyl acetate emulsion adhesives, polyvinyl and cellulose resins, vinyl foams, and other plastics. |
| Dibutyl phthalate DBP | 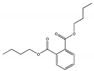 | 84-74-2 | C16H22O4 | 278.3 | 1.049 (20 °C) | −35 | 340 | 11,2 (25 °C) | Used as a plasticizer to help make plastics soft and flexible; also used in shower curtains, raincoats, food wraps, bowls, car interiors, vinyl fabrics, and floor tiles. |
| Dioctyl phthalate DOP | 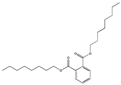 | 117-84-0 | C24H38O4 | 390.4 | 0.978 (20 °C) | −25 | 220 | 0.022 (25 °C) | Used as a plasticizer in carpet backing, packaging films, medical tubing, blood storage bags, floor tile, wire, cables, adhesives, cosmetics, and pesticides. |
| Diisononyl phthalate DINP |  | 28553-12-0 | C26H42O4 | 418.6 | 0.972 (20 °C) | −48 | 78 | 0.2 (20 °C) | Used to impart softness and flexibility to PVC products. Used in perfumes and cosmetics, vinyl swimming pools, plasticized vinyl seats, and clothing. |
| Diisobutyl phthalate DIBP | 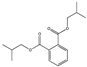 | 84-69-5 | C16H22O4 | 278.3 | 1.05 (15 °C) | −64 | 296 | 6.2 (25 °C) | Used as a plasticizer; used in paints, lacquers, and varnishes, in the paper and pulp industry, and to make boards, chemicals, polymers, adhesives, softeners, and viscosity adjusters. |
| Diethyl Phthalate DEP | 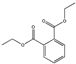 | 84-66-2 | C12H14O4 | 222.2 | 1.12 (20 °C) | −41 | 295 | 1.08 (25 °C) | Used as a plasticizer, insect repellent, and solvent; as a solvent in cellulose acetate, fragrances, and cosmetics; |
| Dipropyl phthalate DPrP | 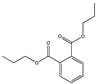 | 131-16-8 | C14H18O4 | 250.3 | 1.07 (25 °C) | −31 | 317.5 | 108.1 (20 °C) | Used to make plasticizers and polymer additives. It is also used in chemical reagents and organic intermediates. |
| Diphenyl phthalate DPhP | 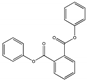 | 84-62-8 | C20H14O4 | 318.3 | 1.28 (TNS) | 75 | 402.5 | 0.082 (24 °C) | Used as a plasticizer in nitrocellulose lacquers. |
| Bis(2-butoxyethyl) phthalate DBEP | 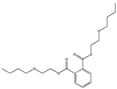 | 117-83-9 | C20H30O6 | 366.4 | 1.06 (20 °C) | −55 | 270 | 1.675 (25 °C) | Used as a plasticizer for resins, and as a softener and processing aid for chloroprene rubber, nitrile-butadiene rubber, and styrene-butadiene rubber. |
| Diisopentyl phthalate DIPP | 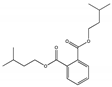 | 605-50-5 | C14H18O6 | 306.4 | 1.02 (TNS) | <−25 °C | 339 | 1.1 (20 °C) | Used as plasticizer of cellulose resin, polymethyl methacrylate, polystyrene, and chlorinated rubber. |
| Bis(4-methyl-2-pentyl) phthalate BMPP | 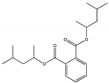 | 84-63-9 | C20H30O4 | 334.4 | 0.995 (TNS) | 341 | <0.1% | Used as a plasticizer and found in cosmetics and baby skin care products. | |
| Diallyl phthalate DAP | 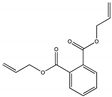 | 131-17-9 | C14H14O4 | 246.3 | 1.12 (20 °C) | −70 | 290 | 182 (25 °C) | Used to make insulators, potentiometers, and circuit boards in communication, computer, and aerospace systems, and a monomer in thermosetting plastics, a diluent in polyester spray systems, a dye carrier, and an impregnant for jewelry. |
| Dihexyl phthalate DHXP | 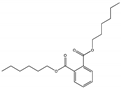 | 84-75-3 | C20H30O4 | 334.4 | 1.01 (20 °C) | −59 | 350 | 0.05 (25 °C) | Used as a plasticizer; used to make plastisols for automobile parts and dip-molded products. |
| Diheptyl phthalate DHP | 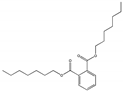 | 3648-21-3 | C24H34O4 | 362.5 | 1 (20 °C) | <−40 | 360 | 0.0018 (25 °C) | Used as a plasticizer for vinyl resins. |
| Dipentyl phthalate DPP | 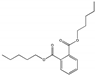 | 131-18-0 | C18H26O4 | 306.4 | 1.12 (20 °C) | <−55 | 342 | 0.8 (25 °C) | Used as plasticizers to soften polyvinyl chloride in shower curtains, vinyl upholstery, adhesives, floor tiles, food containers and wrappers, cleaning materials, and cosmetics. |
| Dicyclohexyl phthalate DCHP | 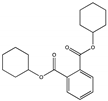 | 84-61-7 | C20H26O4 | 330.4 | 1.383 (20 °C) | 66 | 224 | 4.0 (24 °C) | Used as a plasticizer for nitrocellulose, ethyl cellulose, chlorinated rubber, polyvinyl acetate, polyvinyl chloride, and other polymers; And as a heat sealer for cellulose, in paper finishes, and to make printers ink water-resistant; |
| Bis(2-ethoxyethyl) phthalate DEEP | 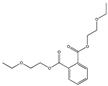 | 605-54-9 | C16H22O6 | 310.3 | 1.121 (20℃) | 34 | 345 | 1946 (TNS) | Used as a plasticizer, an apoptosis inhibitor, and an androstane receptor agonist. |
| Dinonyl phthalate DNP | 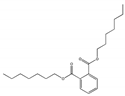 | 84-76-4 | C26H42O4 | 418.6 | 0.972 (20 °C) | −33.15 | 413 | 1.73 × 10−5 (25 °C) | Used in plastisols and coating pastes, as a low-volatility plasticizer for vinyl resins, as a stationary liquid phase in chromatography, and to make vinyl mixes resistant to heat and detergents; |
| Bis(2-methoxyethyl) phthalate DMEP | 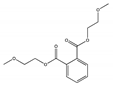 | 117-82-8 | C14H18O6 | 282.3 | 1.1596 (15 °C) | −45 | 340 | 8500 (25 °C) | Used in plastisols and coating pastes, as a plasticizer for vinyl resins, as a stationary liquid phase in chromatography, and to make vinyl mixes resistant to heat and detergents. |
| Bis(2-propylheptyl) phthalate DPHP | 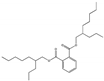 | 53306-54-0 | C28H46O4 | 446.7 | 0.964 (TNS) | −48 | 254 | 2 × 10−7 (25 °C) | Used as an adhesion/cohesion promoter, adhesives and sealant chemicals, intermediate, paint additives, and coating additives. |
| Substance | Regulation (EU) 2023/1442 Amending Annex I to Regulation (EU) 10/2011 * | Annex I to Regulation (EU) 10/2011 | Only to Be Used as: |
|---|---|---|---|
| DBP | SML: 0.12 mg/kg Total SML group restriction no.32: 60 mg/kg Total SML group restriction no.36: 0.6 mg/kg | SML: 0.3 mg/kg Total SML group restriction no.32: 60 mg/kg | (a) Plasticizer in repeated use materials and articles contacting non-fatty foods; (b) Technical support agent in polyolefins in concentrations up to 0.05% (w/w) in the final product. |
| BBP | SML: 6.0 mg/kg Total SML group restriction no.32: 60 mg/kg Total SML group restriction no.36: 0.6 mg/kg | SML: 30 mg/kg Total SML group restriction no.32: 60 mg/kg | (a) Plasticizer in repeated use materials and articles; (b) Plasticizer in single-use materials and articles contacting non-fatty foods except for infant formula and follow-on formula; (c) Technical support agent in concentrations up to 0.1% (w/w) in the final product. |
| DEHP | SML: 0.6 mg/kg Total SML group restriction no.32: 60 mg/kg Total SML group restriction no.36: 0.6 mg/kg | SML: 1.5 mg/kg Total SML group restriction no.32: 60 mg/kg | (a) Plasticizer in repeated use materials and articles contacting non-fatty foods; (b) Technical support agent in concentrations up to 0.1% (w/w) in the final product. |
| DINP and DIDP | Total SML group restriction no.26: 1.8 mg/kg (sum of DINP and DIDP) Total SML group restriction no.32: 60 mg/kg Not to be used in combination with FCM substances DBP, BBP, DEHP, and DIBP. | Total SML: 9 mg/kg (sum of DINP and DIDP) Total SML group restriction no.32: 60 mg/kg | (a) Plasticizer in repeated use materials and articles; (b) Plasticizer in single-use materials and articles contacting non-fatty foods except for infant formula and follow-on formula; (c) technical support agent in concentrations up to 0.1% (w/w) in the final product. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, F.; Cabrita, M.J.; da Silva, M.G. A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine. Molecules 2023, 28, 7628. https://doi.org/10.3390/molecules28227628
Freitas F, Cabrita MJ, da Silva MG. A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine. Molecules. 2023; 28(22):7628. https://doi.org/10.3390/molecules28227628
Chicago/Turabian StyleFreitas, Flávia, Maria João Cabrita, and Marco Gomes da Silva. 2023. "A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine" Molecules 28, no. 22: 7628. https://doi.org/10.3390/molecules28227628
APA StyleFreitas, F., Cabrita, M. J., & da Silva, M. G. (2023). A Critical Review of Analytical Methods for the Quantification of Phthalates Esters in Two Important European Food Products: Olive Oil and Wine. Molecules, 28(22), 7628. https://doi.org/10.3390/molecules28227628