Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Photocatalytic CO2 Reduction Activity of a New Dinuclear Gd(III) Complex with 6-Phenylpyridine-2-Carboxylic Acid and 1,10-Phenanthroline Ligands
Abstract
:1. Introduction
2. Results and Discussion
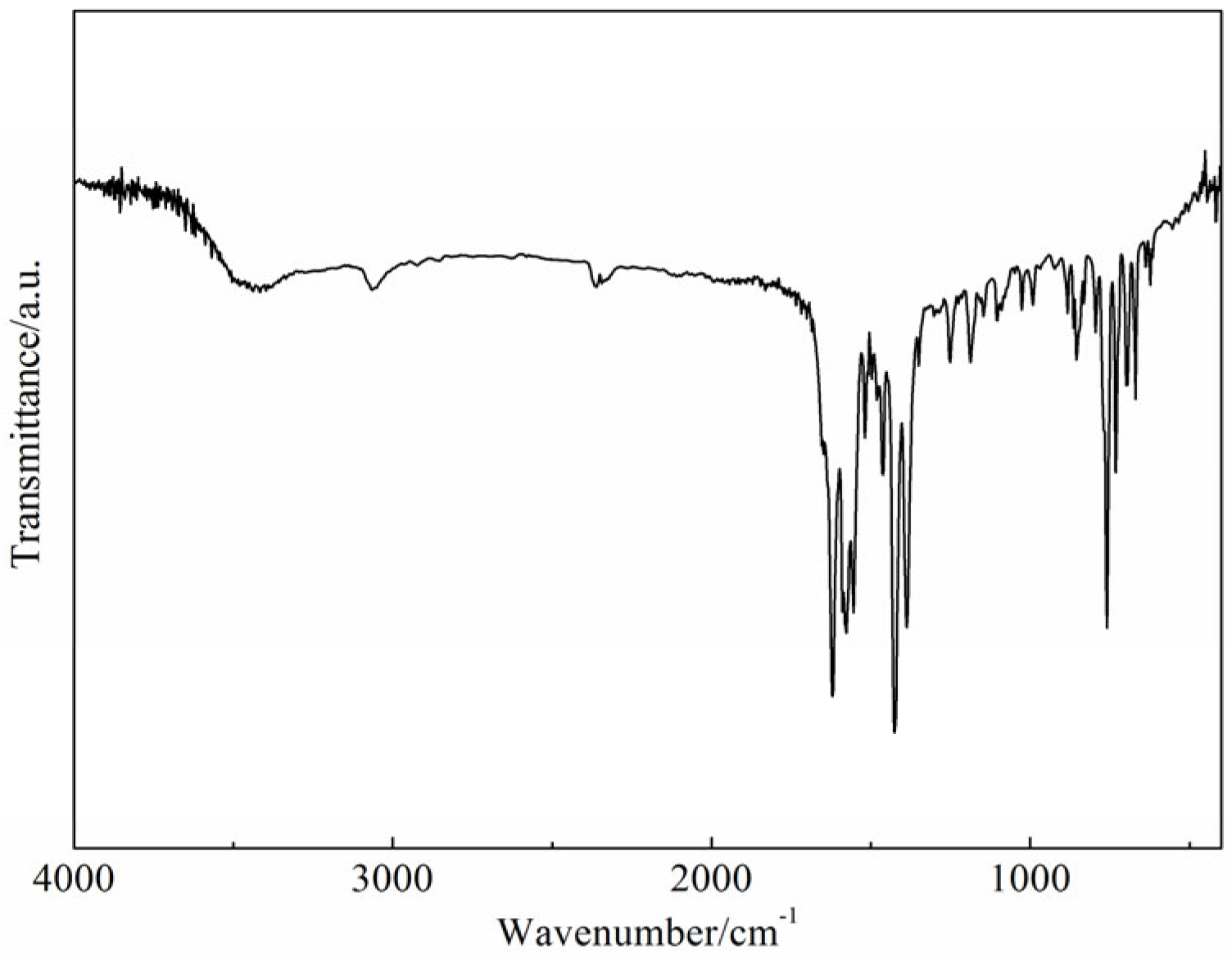
2.1. Infrared Spectra
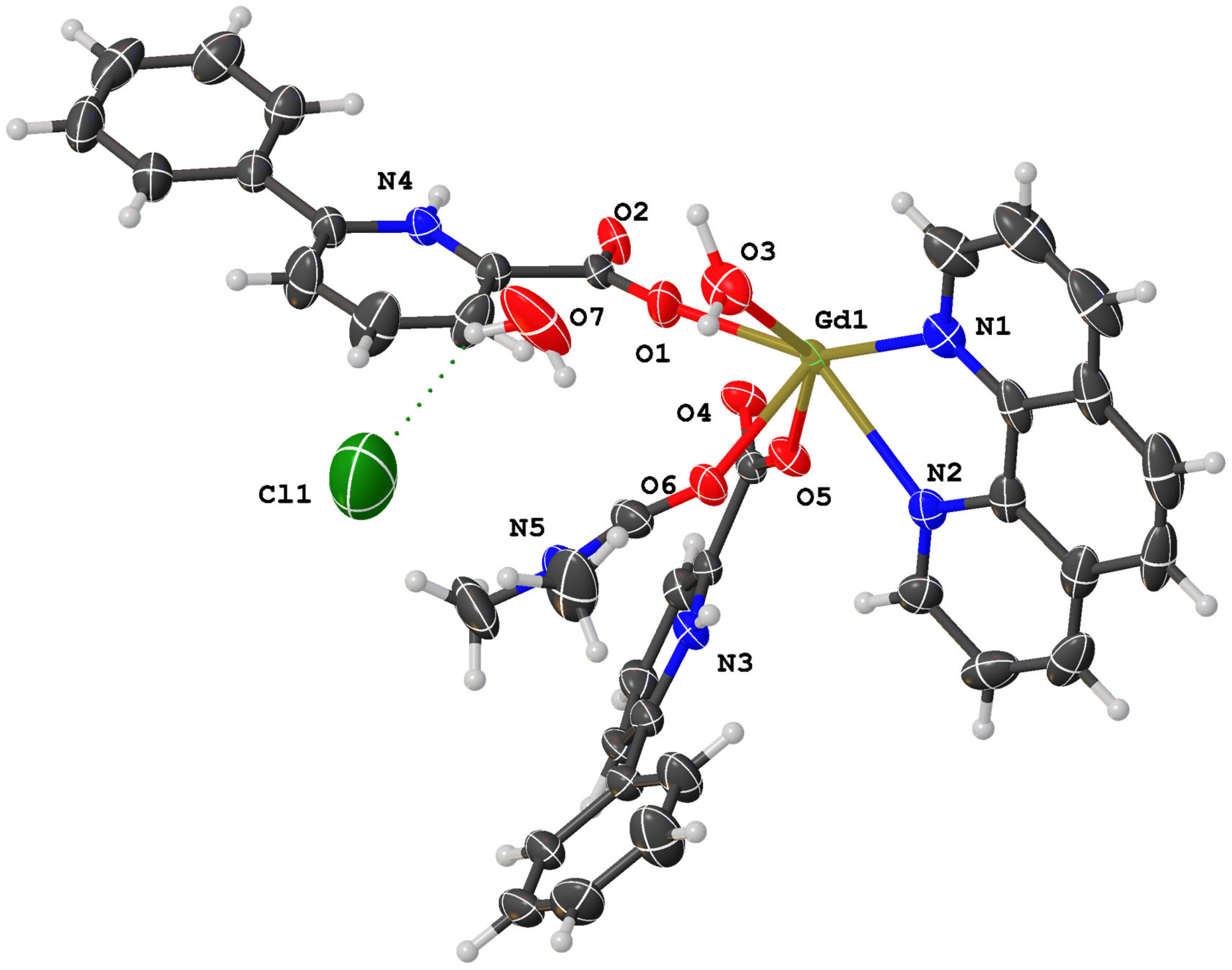
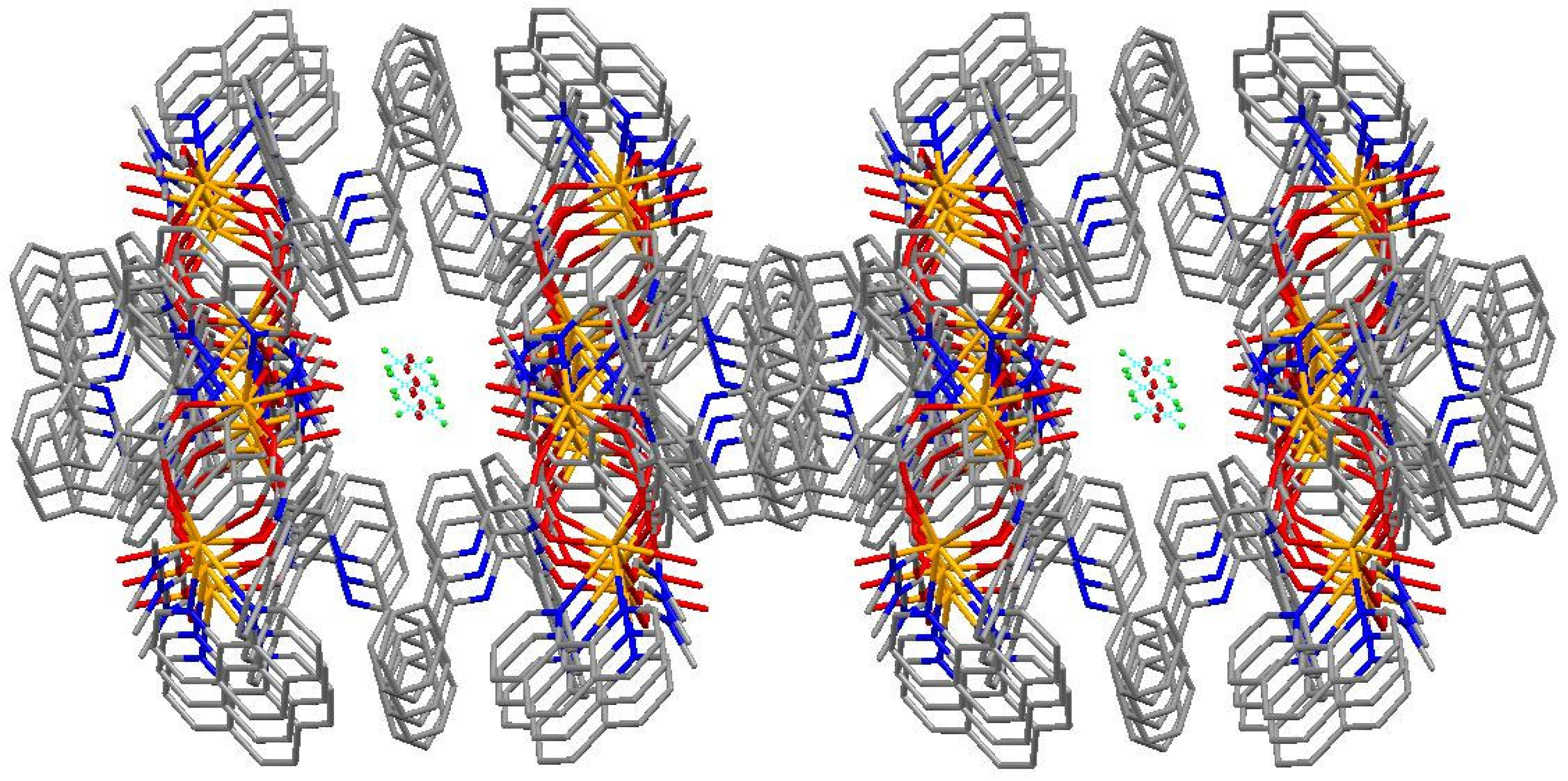
2.2. Structural Description of Complex (1)
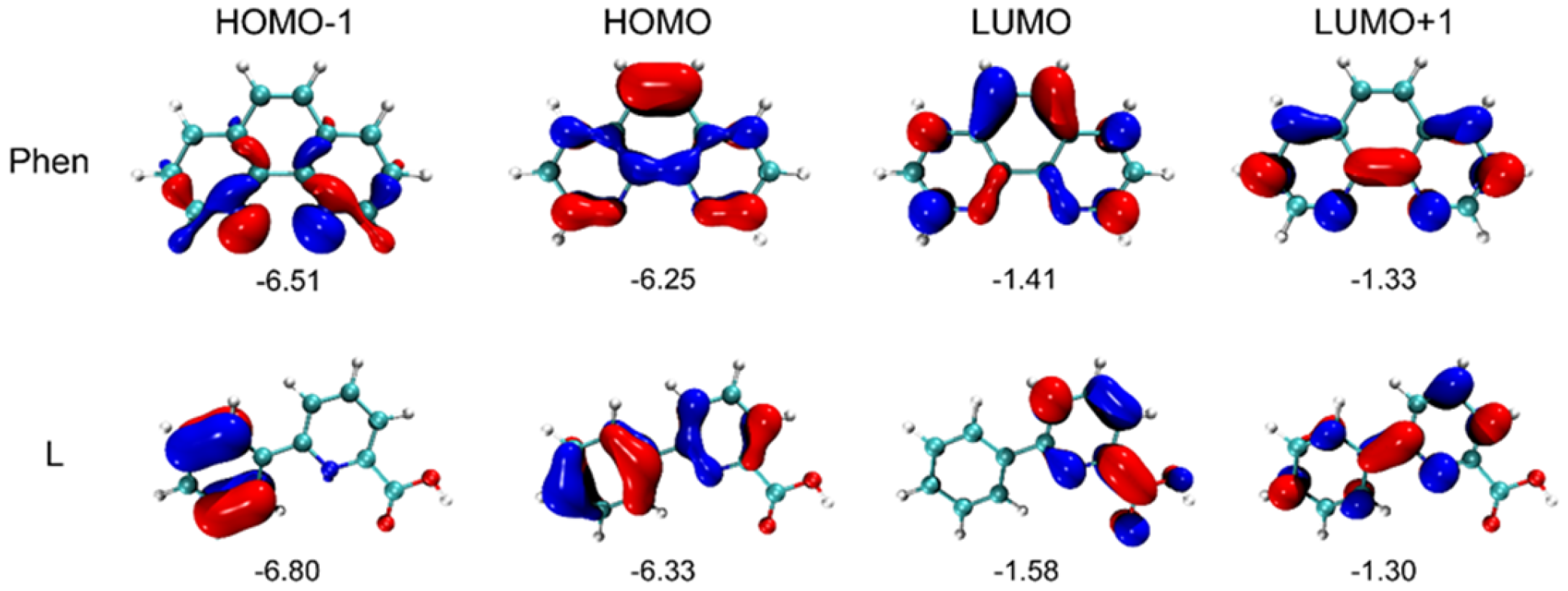
2.3. DFT Computation
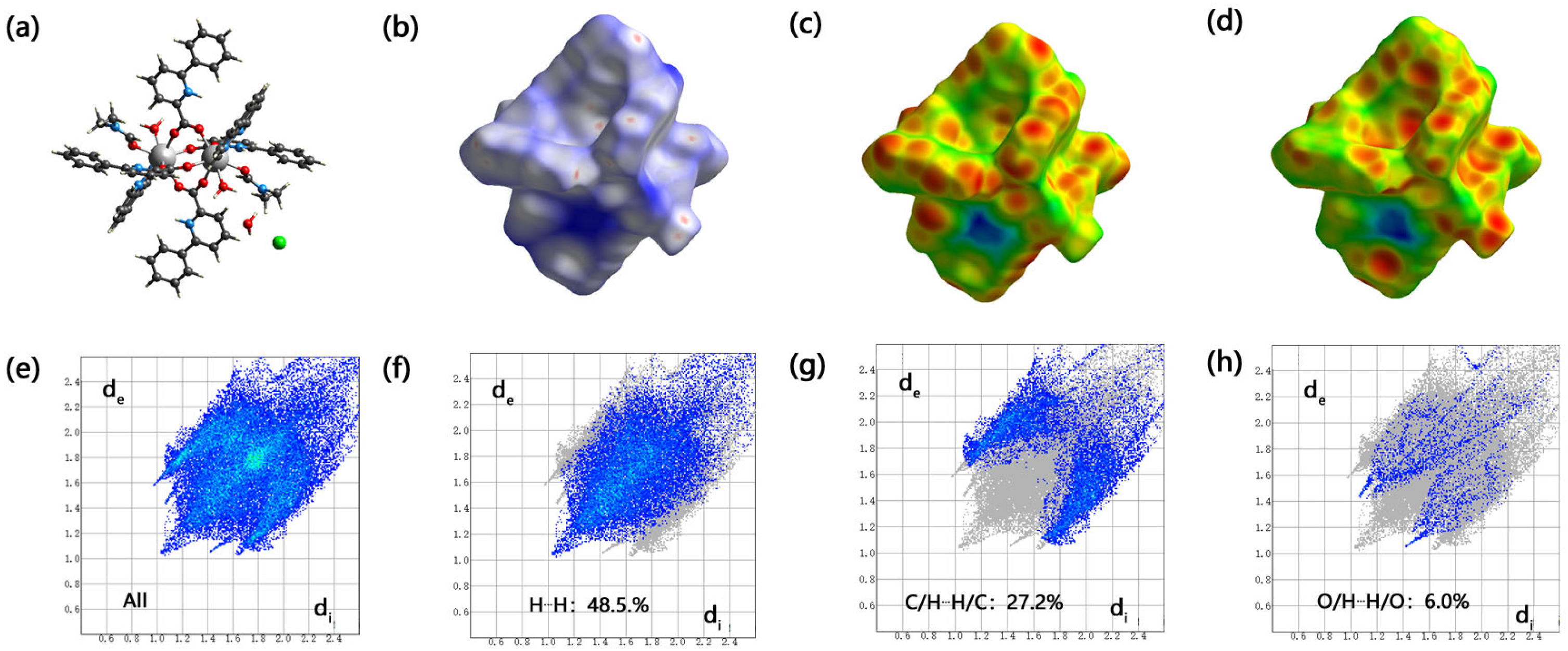
2.4. Hirschfeld Surface Analysis of Complex (1)
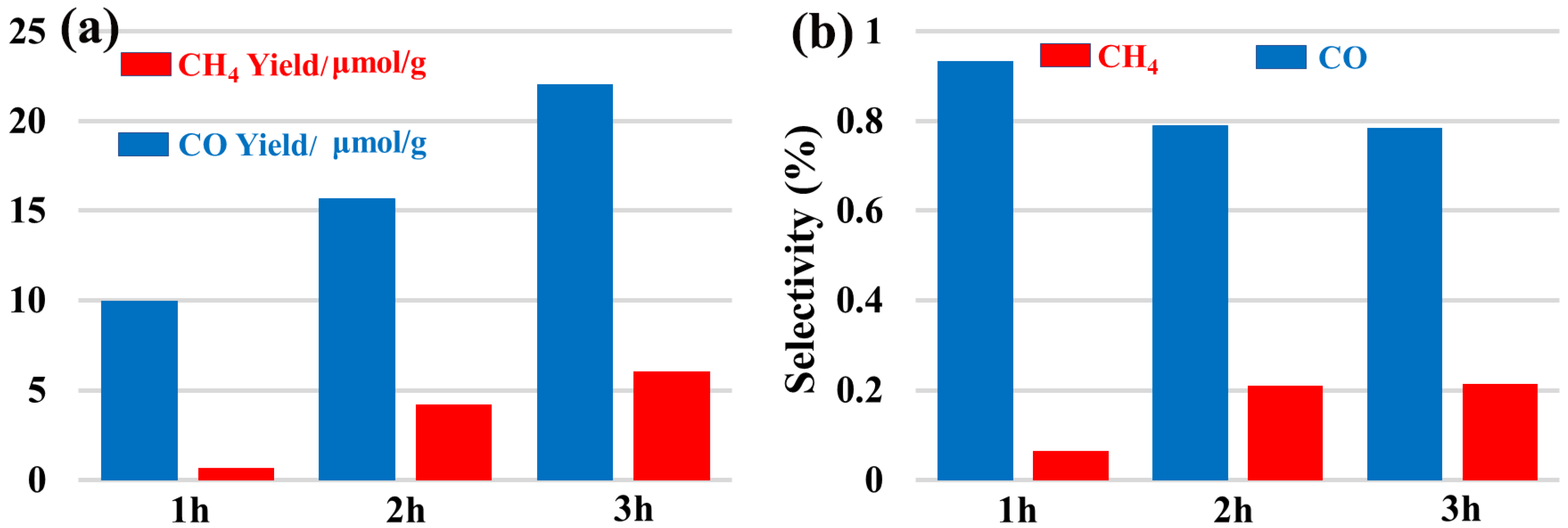
2.5. Photocatalytic CO2 Reduction Activity Assessment of the Complex (1)
3. Experimental
3.1. Materials and Measurements
3.2. Synthesis of Complex (1)
3.3. Crystal Structure Determination
3.4. Photocatalytic CO2 Reduction Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.J.; Chen, T.Q.; Xue, Y.H.; Fan, J.C.; Shen, S.L.; Hossain, M.S.A.; Amin, M.A.; Pan, L.K.; Xu, X.T.; Yamauchi, Y. Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Tang, L.Q.; Jia, Y.; Zhu, Z.S.; Wu, C.P.; Zhou, Y.; Zou, Z.G. Development of functional materials for photocatalytic reduction of CO2. Prog. Phys. 2021, 41, 254–263. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhang, X.H.; Yuan, Z.M.; Chen, J.C.; Huang, B.B.; Dionysiou, D.D.; Yang, G.H. Enhanced photocatalytic CO2 reduction via the synergistic effect between Ag and activated carbon in TiO2/AC-Ag ternary composite. Chem. Eng. J. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Gao, X.Q.; Cao, L.L.; Chang, Y.; Yuan, Z.Y.; Zhang, S.X.; Liu, S.J.; Zhang, M.T.; Fan, H.; Jiang, Z.Y. Improving the CO2 Hydrogenation Activity of Photocatalysts via the Synergy between Surface Frustrated Lewis Pairs and the CuPt Alloy. ACS Sustain. Chem. Eng. 2023, 11, 5597–5607. [Google Scholar] [CrossRef]
- Yin, H.B.; Li, J.H. New insight into photocatalytic CO2 conversion with nearly 100% CO selectivity by CuO-Pd/HxMoO3−y hybrids. Appl. Catal. B Environ. 2023, 320, 121927. [Google Scholar] [CrossRef]
- Heng, Q.Q.; Ma, Y.B.; Wang, X.; Wu, Y.F.; Li, Y.Z.; Chen, W. Role of Ag, Pd cocatalysts on layered SrBi2Ta2O9 in enhancing the activity and selectivity of photocatalytic CO2 reaction. Appl. Surf. Sci. 2023, 632, 1257564. [Google Scholar] [CrossRef]
- Shang, X.F.; Li, G.J.; Wang, R.N.; Xie, T.; Ding, J.; Zhong, Q. Precision loading of Pd on Cu species for highly selective CO2 photoreduction to methanol. Chem. Eng. J. 2023, 456, 140805. [Google Scholar] [CrossRef]
- Chen, J.Y.; Li, M.; Liao, R.Z. Mechanistic insights into photochemical CO2 reduction to CH4 by a molecular iron-porphyrin catalyst. Inorg. Chem. 2023, 62, 9400–9417. [Google Scholar] [CrossRef]
- Cometto, C.; Kuriki, R.; Chen, L.J.; Maeda, K.; Lau, T.C.; Ishitani, O.; Robert, M. A Carbon nitride/Fe quaterpyridine catalytic system for photostimulated CO2-to-CO conversion with visible light. J. Am. Chem. Soc. 2018, 140, 7437–7440. [Google Scholar] [CrossRef]
- Arikawa, Y.; Tabata, I.; Miura, Y.; Tajiri, H.; Seto, Y.; Horiuchi, S.; Sakuda, E.; Umakoshi, K. Photocatalytic CO2 reduction under visible-light irradiation by ruthenium CNC pincer complexes. Chem.-A Eur. J. 2020, 26, 5603–5606. [Google Scholar] [CrossRef]
- Yasuomi, Y.; Takayuki, O.; Jun, I.; Shota, F.; Chinatsu, T.; Tomoya, U.; Taro, T. Photocatalytic CO2 reduction using various heteroleptic diimine-diphosphine Cu(I) complexes as photosensitizers. Front. Chem. 2019, 7, 288. [Google Scholar] [CrossRef]
- Liu, D.C.; Wang, H.J.; Ouyang, T.; Wang, J.W.; Jiang, L.; Zhong, D.C.; Lu, T.B. Conjugation effect contributes to the CO2-to-CO conversion driven by visible-light. ACS Appl. Energy Mater. 2018, 1, 2452–2459. [Google Scholar] [CrossRef]
- Jing, H.W.; Zhao, L.; Song, G.Y.; Li, J.Y.; Wang, Z.Y.; Han, Y.; Wang, Z.X. Application of a mixed-ligand metal-organic framework in photocatalytic CO2 reduction, antibacterial activity and dye adsorption. Molecules 2023, 28, 5204. [Google Scholar] [CrossRef]
- Meng, S.Y.; Li, G.; Wang, P.; He, M.; Sun, X.H.; Li, Z.X. Rare earth-based MOFs for photo/electrocatalysis. Mater. Chem. Front. 2023, 5, 806–827. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, Y.F.; Wang, L.H.; Yan, X.H. Synthesis, structural characterization, hirschfeld surface analysis and photocatalytic CO2 reduction of Yb(III) complex with 4-aacetylphenoxyacetic acid and 1,10-phenanthroline ligands. Bull. Chem. React. Eng. Catal. 2023, 18, 285–293. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, Y.F.; Wang, L.H.; Yan, X.H. Synthesis, structural characterization, hirschfeld surface analysis and photocatalytic CO2 reduction activity of a new Gd(III) coordination polymer with 6-phenylpyridine-2-carboxylic acid and 4,4’-bipyridine ligands. Bull. Chem. React. Eng. Catal. 2023, 18, 353–361. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Chen, W.Z.; Huang, M.L. Synthesis and crystal structure of the mixed complex [Gd(Ts-p-aba)3(phen)]2·2DMF·4.4H2O. J. Synth. Cryst. 2016, 45, 2113–2117. [Google Scholar] [CrossRef]
- Coban, M.B. Hydrothermal synthesis, crystal structure, luminescent and magnetic properties of a new mononuclear GdIII coordination complex. J. Mol. Struct. 2018, 1162, 109–116. [Google Scholar] [CrossRef]
- Chen, L.Z.; Huang, D.D.; Cao, X.X. Synthesis, structure and dielectric properties of a novel 3D Gd(III) complex {[Gd(HPIDC)·(μ4-C2O4)0.5·H2O]·2H2O}n. Chin. J. Struct. Chem. 2013, 32, 1553–1559. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Calmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–593. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer:a program for Hirshfeld surface analysis, vis-ualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2, A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallographica 2015, C71, 3–8. [Google Scholar] [CrossRef]
| Bond | d | Angle | (°) |
|---|---|---|---|
| Gd1-N1 | 2.560(3) | N1-Gd1-N2 | 63.79(11) |
| Gd1-N2 | 2.585(3) | N1-Gd1-O1 | 147.23(12) |
| Gd1-O1 | 2.313(3) | O1-Gd1-N2 | 145.07(11) |
| Gd1-O2a | 2.379(3) | O1-Gd1-O2a | 123.07(11) |
| Gd1-O3 | 2.436(3) | O1-Gd1-O3 | 77.17(12) |
| Gd1-O4a | 2.372(3) | O1-Gd1-O4a | 79.20(10) |
| Gd1-O5 | 2.331(3) | O1-Gd1-O5 | 74.45(11) |
| Gd1-O6 | 2.406(3) | O1-Gd1-O6 | 81.61(11) |
| N1-Gd1-O2a | 74.76(11) | ||
| O2a-Gd1-N2 | 69.35(10) | ||
| O2a-Gd1-O3 | 139.53(10) | ||
| O2a-Gd1-O6 | 139.90(10) | ||
| N1-Gd1-O3 | 73.26(12) | ||
| O3-Gd1-N2 | 115.87(12) | ||
| N1-Gd1-O4a | 79.48(11) | ||
| O4a-Gd1-N2 | 134.52(10) | ||
| O4a-Gd1-O2a | 76.06(10) | ||
| O4a-Gd1-O3 | 74.29(11) | ||
| O4a-Gd1-O6 | 143.58(10) | ||
| N1-Gd1-O5 | 138.32(11) | ||
| N2-Gd1-O5 | 77.15(11) | ||
| O5-Gd1-O2a | 78.50(10) | ||
| O5-Gd1-O3 | 141.59(11) | ||
| O4a-Gd1-O5 | 123.93(10) | ||
| O5-Gd1-O6 | 79.25(10) | ||
| O6-Gd1-N1 | 101.73(11) | ||
| N2-Gd1-O6 | 73.42(10) | ||
| O6-Gd1-O3 | 71.41(10) |
| Donor-H | Acceptor | D-H (Å) | H…A (Å) | D…A (Å) | D-H…A (°) |
|---|---|---|---|---|---|
| O3-H3A | O7 | 1.07 | 2.46 | 3.125(6) | 120 |
| O3-H3B | O7 #1 | 1.07 | 2.59 | 3.294(6) | 123 |
| O7-H7B | Cl1 | 0.85 | 2.50 | 3.143(5) | 133 |
| Ring1 | Ring2 | Symmetry | Distance between Ring Centroids | Slippage |
|---|---|---|---|---|
| Cg2 | Cg8 | 1/2 − x, 1/2 − y, 3/2 − z | 3.713(2) | 1.412 |
| Cg5 | Cg5 | 3.465(3) | 0.620 | |
| Cg5 | Cg8 | 3.746(3) | 1.563 | |
| Cg5 | Cg9 | 3.432(3) | 0.583 | |
| Cg5 | Cg10 | 3.464(2) | 0.731 | |
| Cg8 | Cg2 | 3.713(2) | 1.577 | |
| Cg8 | Cg5 | 3.746(3) | 1.554 | |
| Cg8 | Cg9 | 3.526(2) | 1.002 | |
| Cg9 | Cg5 | 3.432(3) | 0.527 | |
| Cg9 | Cg8 | 3.526(2) | 0.963 | |
| Cg9 | Cg9 | 3.800(2) | 1.722 | |
| Cg9 | Cg10 | 3.586(2) | 1.172 | |
| Cg10 | Cg5 | 3.465(2) | 0.711 | |
| Cg10 | Cg9 | 3.586(2) | 1.186 | |
| Cg10 | Cg10 | 3.6798(19) | 1.445 |
| Empirical formula | C78H74Cl2Gd2N10O14 |
| Formula weight | 1760.87 |
| Temperature/K | 219.98(10) |
| Crystal system | monoclinic |
| Space group | P-1 |
| a/Å | 25.5838(6) |
| b/Å | 13.6998(4) |
| c/Å | 21.8202(6) |
| α/° | 90 |
| β/° | 90.824(3) |
| γ/° | 90 |
| Volume/Å3 | 7647.0(4) |
| Z | 4 |
| ρcalc, mg/mm3 | 1.529 |
| μ/mm−1 | 1.860 |
| S | 1.060 |
| F(000) | 3544 |
| Index ranges | −23 ≤ h ≤ 30, −13 ≤ k ≤ 16, −25 ≤ l ≤ 25 |
| Reflections collected | 17406 |
| 2θ/° | 4.722–49.999 |
| Independent reflections | 6746 [R(int) = 0.0252] |
| Data/restraints/parameters | 6746/2/484 |
| Goodness-of-fit on F2 | 1.044 |
| Refinement method | Full-matrix least-squares on F2 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0328, wR2 = 0.0792 |
| Final R indexes [all data] | R1 = 0.0397, wR2 = 0.0747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Tai, X.-S. Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Photocatalytic CO2 Reduction Activity of a New Dinuclear Gd(III) Complex with 6-Phenylpyridine-2-Carboxylic Acid and 1,10-Phenanthroline Ligands. Molecules 2023, 28, 7595. https://doi.org/10.3390/molecules28227595
Wang L-H, Tai X-S. Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Photocatalytic CO2 Reduction Activity of a New Dinuclear Gd(III) Complex with 6-Phenylpyridine-2-Carboxylic Acid and 1,10-Phenanthroline Ligands. Molecules. 2023; 28(22):7595. https://doi.org/10.3390/molecules28227595
Chicago/Turabian StyleWang, Li-Hua, and Xi-Shi Tai. 2023. "Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Photocatalytic CO2 Reduction Activity of a New Dinuclear Gd(III) Complex with 6-Phenylpyridine-2-Carboxylic Acid and 1,10-Phenanthroline Ligands" Molecules 28, no. 22: 7595. https://doi.org/10.3390/molecules28227595
APA StyleWang, L.-H., & Tai, X.-S. (2023). Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Photocatalytic CO2 Reduction Activity of a New Dinuclear Gd(III) Complex with 6-Phenylpyridine-2-Carboxylic Acid and 1,10-Phenanthroline Ligands. Molecules, 28(22), 7595. https://doi.org/10.3390/molecules28227595






