The Synthesis, Structure, and Dielectric Properties of a One-Dimensional Hydrogen-Bonded DL-α-Phenylglycine Supramolecular Crown-Ether-Based Inclusion Compound
Abstract
:1. Introduction
2. Results and Discussion
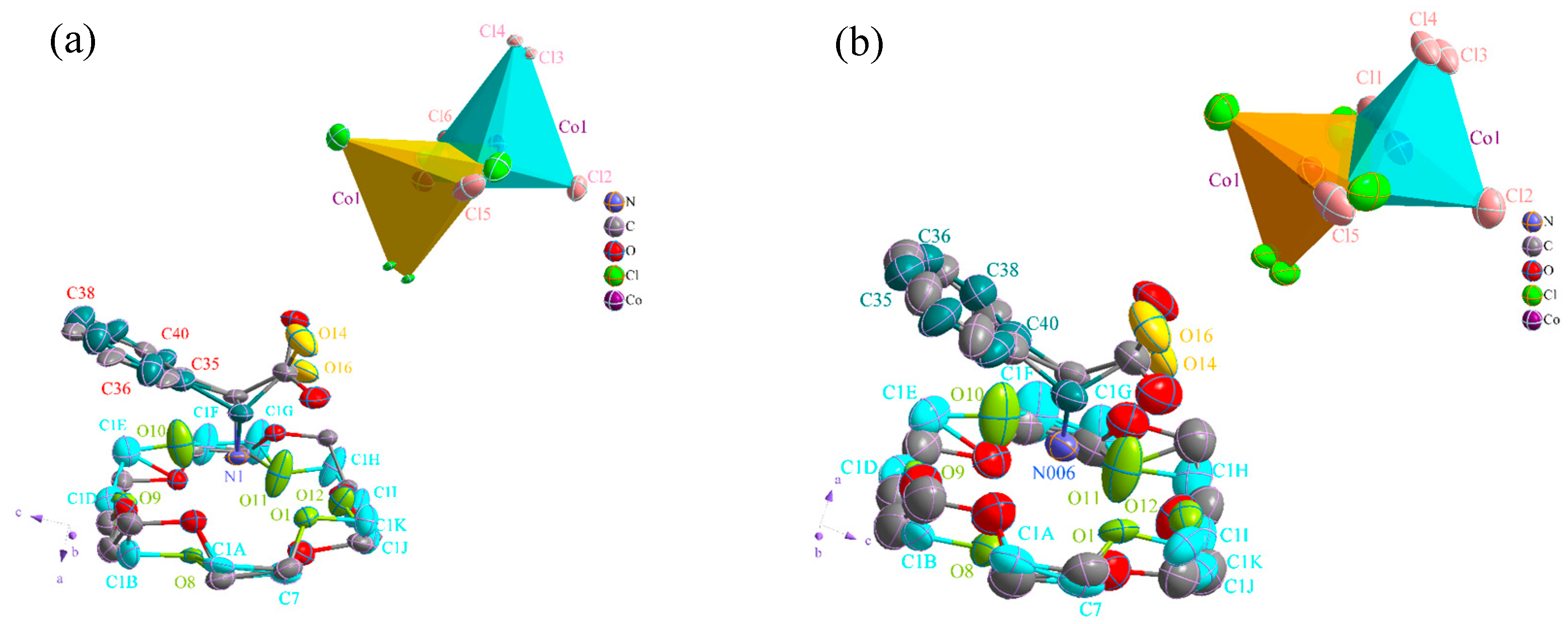
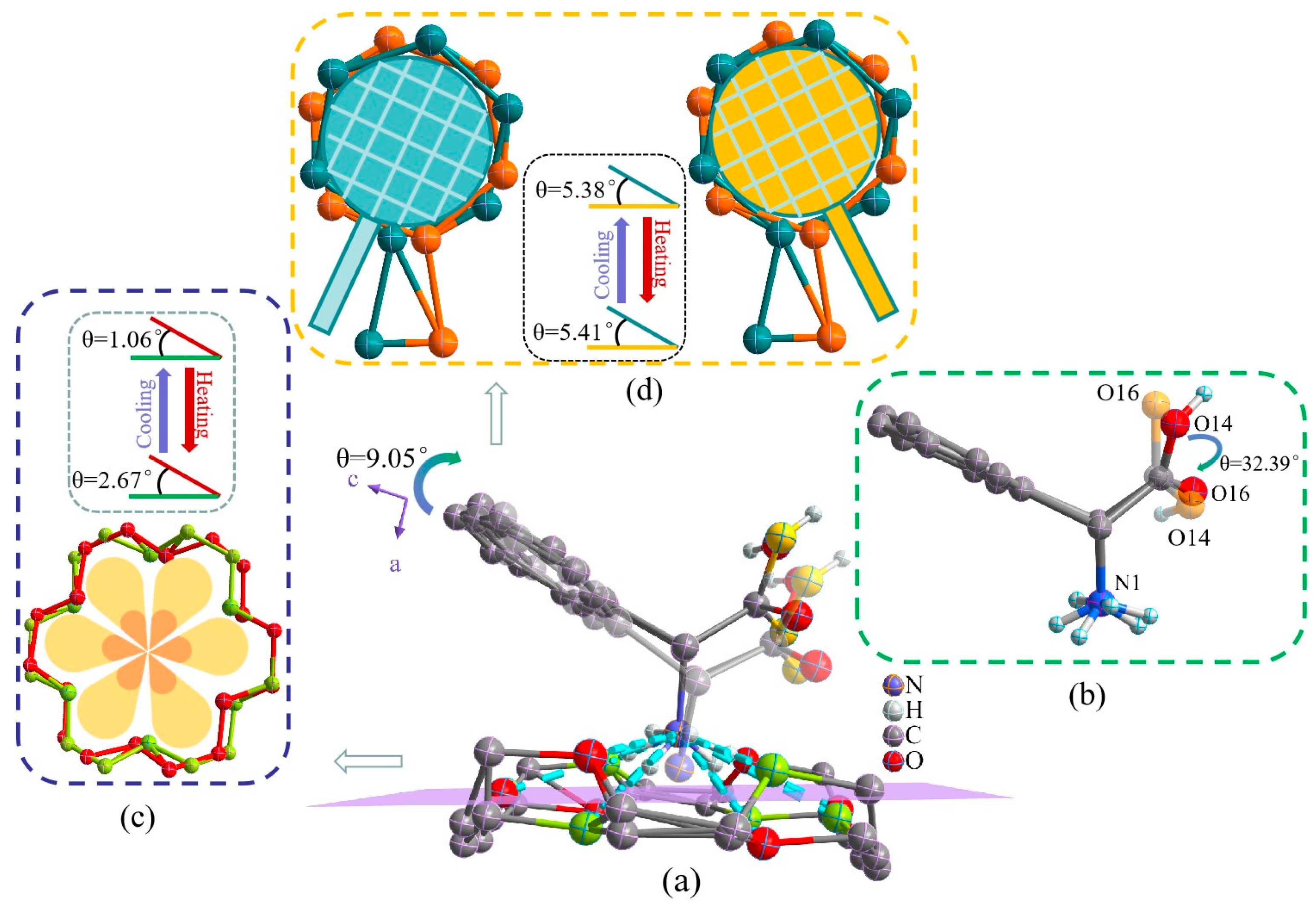
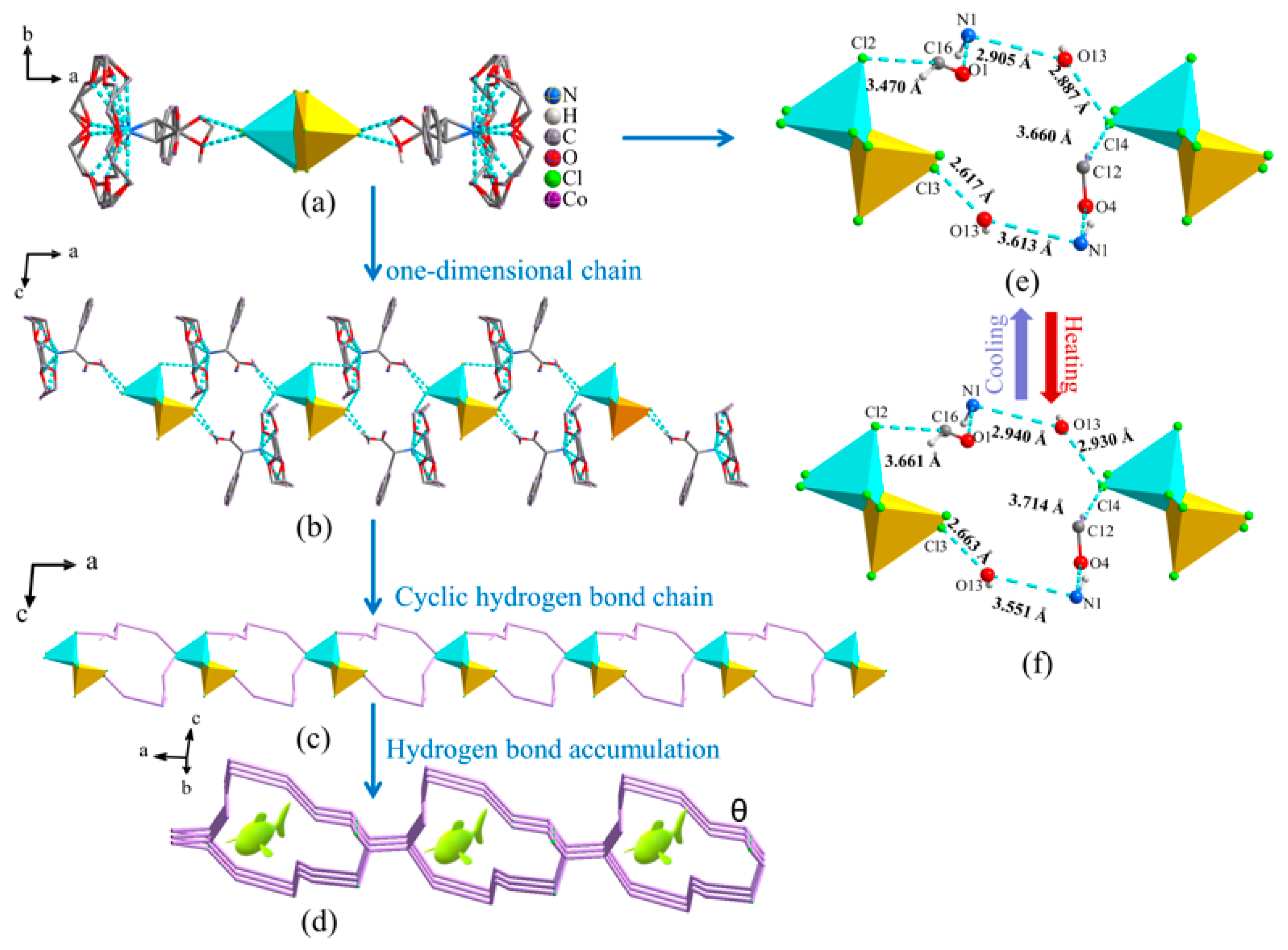
2.1. Description of the Single-Crystal Structure of Compound 1
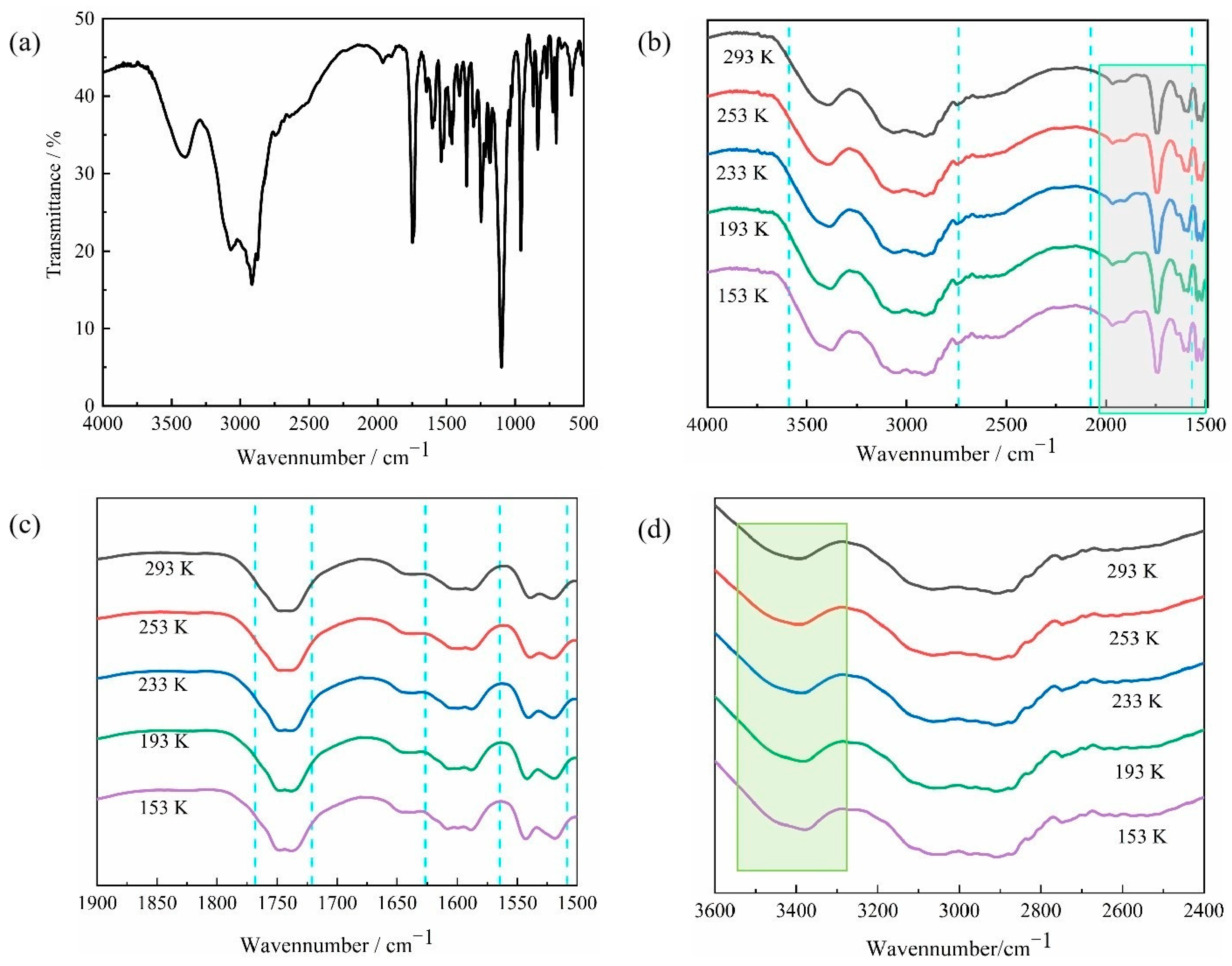
2.2. Infrared and Variable-Temperature Infrared Spectroscopy of Compound 1
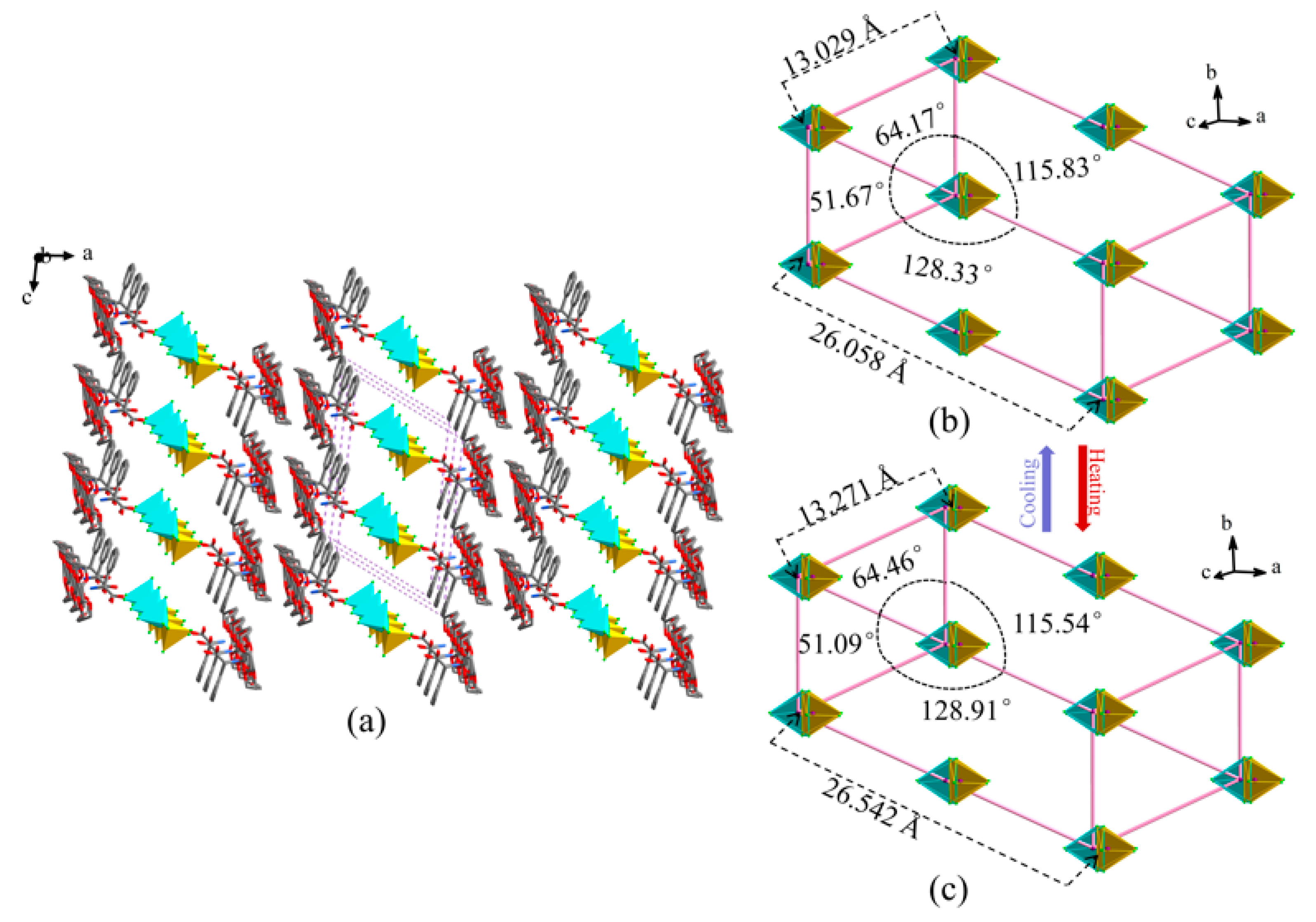
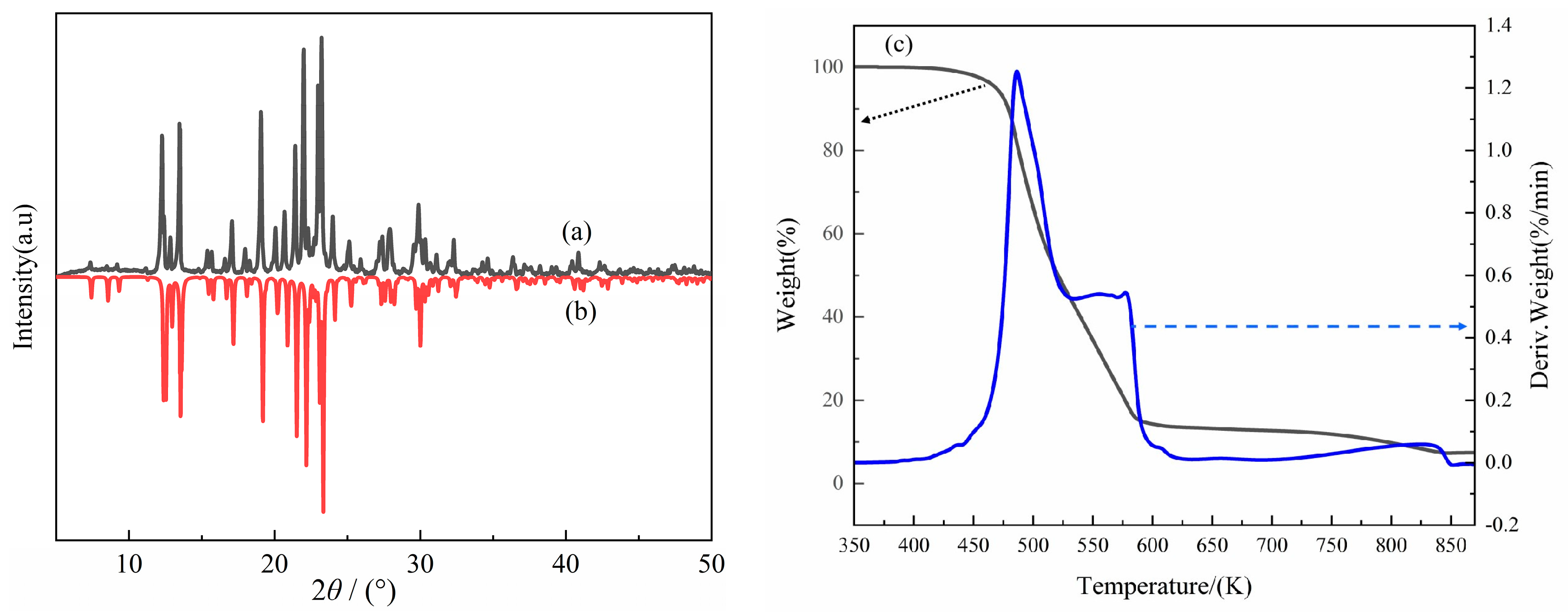
2.3. XRD and TG Analysis of Compound 1
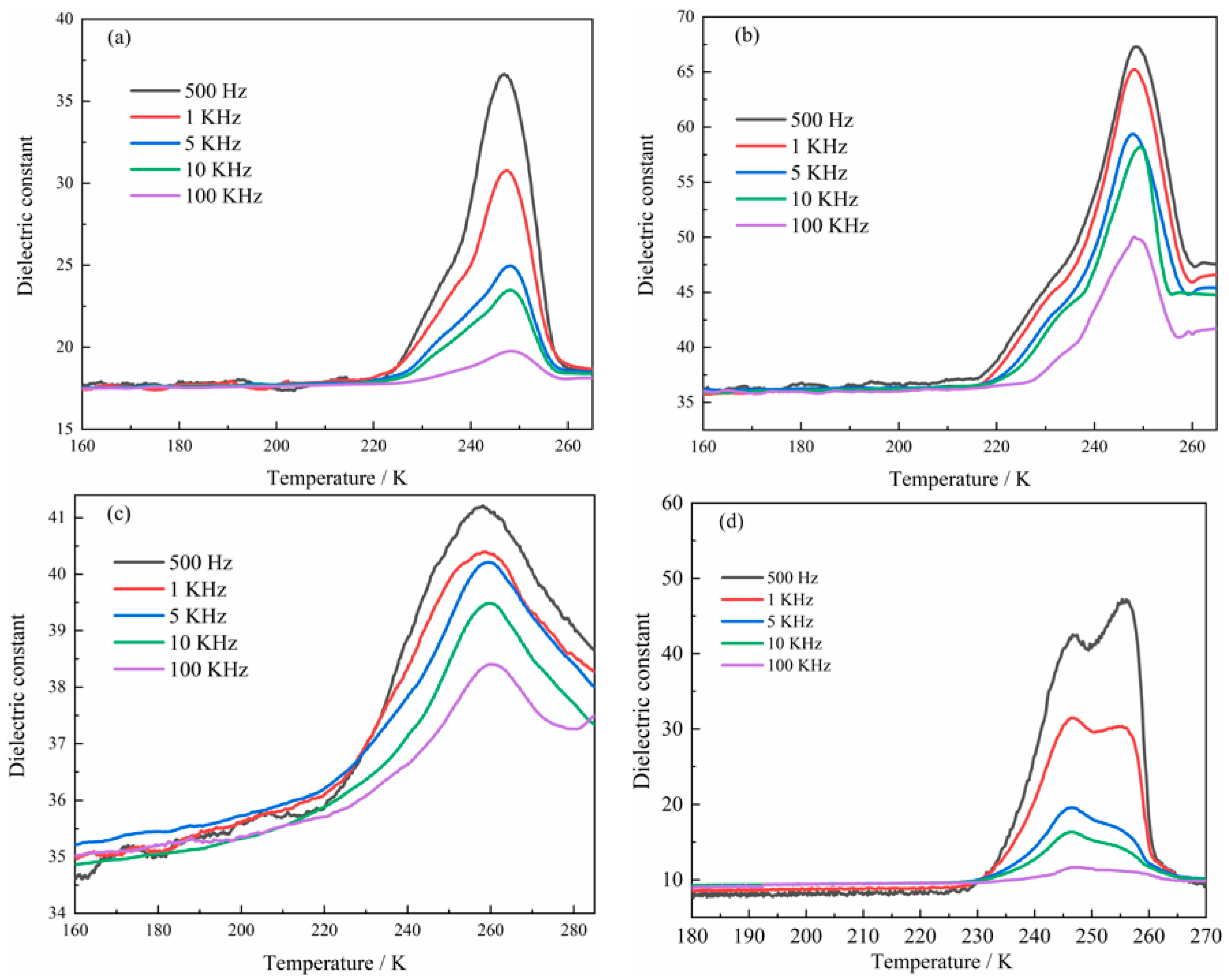
2.4. Determination of Dielectric Properties
3. Experimental Section
3.1. Experimental Materials and Instruments
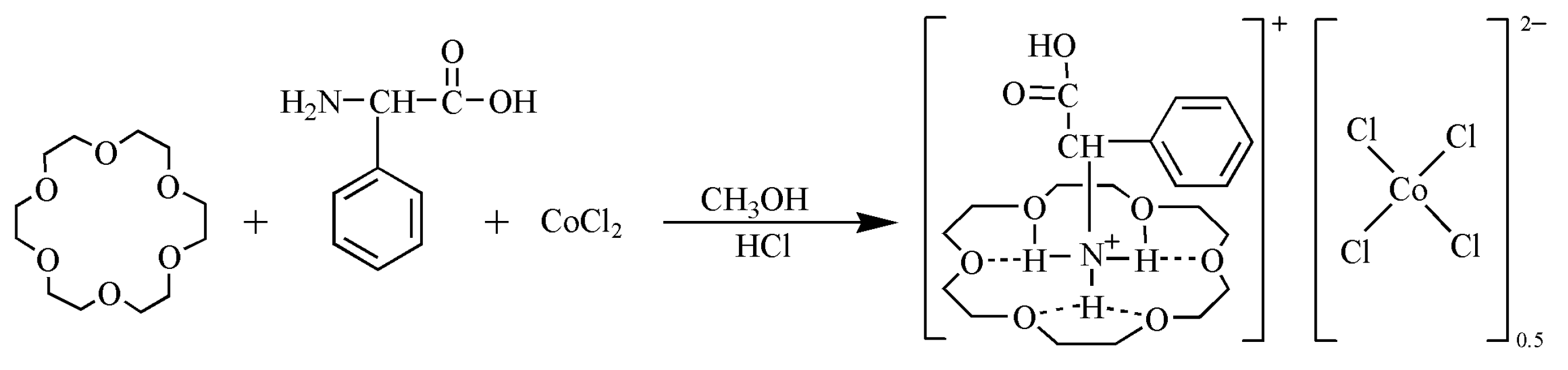
3.2. Synthesis of the Compound
3.3. Crystal Structure of the Compound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Bin, X.; Arnon, Z.A.; Hui, Y.; Santu, B.; Qi, L.; Zaguri, D.; Nicholas, P.R.; Hui, L.; Yu, C.; et al. Rigid Tightly-Packed Amino Acid Crystals as Functional Supramolecular. Mater. J. Am. Chem. Soc. 2019, 13, 14477–14485. [Google Scholar]
- Ji, X.; Wang, F.; Yan, X.; Dong, S.Y.; Huang, F.H. Construction of Supramolecular Polymers Based on Host-Guest Recognition. Chin. J. Chem. 2020, 38, 1473–1479. [Google Scholar] [CrossRef]
- Xiong, J.; Luo, T.; Zhang, J.; Li, X.X.; Lv, S.F.; Peng, J.J.; Li, M.; Li, W.; Nakamura, T. Two Supramolecular Inorganic-Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers. Crystals 2018, 8, 17. [Google Scholar] [CrossRef]
- Hasuo, N.; Takahashi, K.; Hisaki, I.; Kokado, K.; Nakamura, T. Molecular Motion of Halogenated Ethylammonium[18]-crown-6 Supramolecular Ions In Nickel Dithiolate Magnetic Crystals. CrystEngComm 2021, 23, 2756–2763. [Google Scholar] [CrossRef]
- Vaganova, T.A.; Gatilov, Y.V.; Pishchur, D.P.; Malykhin, E.V. Polyfluorinated Hydroxy and Carboxy Benzenes as a New Type of H-Donors for Self-Assembly with 18-crown-6 Ether: Synthesis Supramolecular Structure and Stability Of Co-Crystals. J. Fluor. Chem. 2020, 236, 109577. [Google Scholar] [CrossRef]
- Luo, J.H.; Ji, C.M.; Liu, S.J.; Han, S.G.; Tao, K.W.; Sun, Z.H. Toward Spectrally Customized Photoresponse from an Organic-Inorganic Hybrid Ferroelectric. Angew. Chem. Int. Ed. 2018, 130, 17006–17009. [Google Scholar]
- Ohshima, Y.; Kubo, K.; Matsumoto, T.; Ye, H.Y.; Noro, S.; Akutagawa, T.; Nakamura, T. One-Dimensional Supramolecular Columnar Structure of Trans-Syn-Trans-Dicyclohexano 18-crown-6 And Organic Ammonium Cations. CrystEngComm 2016, 18, 7959–7964. [Google Scholar] [CrossRef]
- Tan, Y.H.; Zhou, H.T.; Wang, C.F.; Liu, Y.; Fan, X.W.; Yang, K.; Wei, W.J.; Tang, Y.Z. Designing and Constructing a High Temperature Molecular Ferroelectric by Double Regulation of Anion and Cation in a Simple Crown Ether Clathrate. Chem. Asian J. 2019, 14, 3946–3952. [Google Scholar]
- Gao, Y.F.; Zhang, Z.X.; Zhang, T.; Su, C.Y.; Zhang, W.Y.; Fu, D.W. Regulated Molecular Rotor in Phase Transition Materials with Switchable Dielectric and SHG Effect. Mater. Chem. Front. 2020, 4, 3003–3012. [Google Scholar] [CrossRef]
- Jia, Z.H.; Liu, J.Y.; Liu, D.X.; Zhang, S.Y.; Du, Z.Y.; He, C.T.; Chen, X.M.; Zhang, W.X. Four-Step Thermosensitive Dielectric Response Arising from Motionable Low-Symmetry Ammonium Confined in Deformable Supramolecular Cages. J. Mater. Chem. A 2021, 9, 8076–8082. [Google Scholar] [CrossRef]
- Beldjoudi, Y.; Atilgan, A.; Weber, J.A.; Roy, I.; Young, R.M.; Yu, J.; Deria, P.; Enciso, A.E.; Wasielewski, M.R.; Hupp, J.T.; et al. Supramolecular Porous Organic Nanocomposites for Heterogeneous Photocatalysis of a Sulfur Mustard Simulant. Adv. Mater. 2020, 32, e2001592. [Google Scholar] [CrossRef]
- Li, X.B.; Fei, J.B.; Xu, Y.; Li, D.X.; Yuan, T.T.; Li, G.; Li, J.B.; Wang, C.L. A Photoinduced Reversible Phase Transition in a Dipeptide Supramolecular Assembly. Angew. Chem. Int. Ed. Engl. 2018, 57, 1903–1907. [Google Scholar] [CrossRef]
- Wu, S.G.; Cai, C.Y.; Li, F.F.; Tan, Z.G.; Dong, S.Y. Deep Eutectic Supramolecular Polymers: Bulk Supramolecular Materials. Angew. Chem. Int. Ed. Engl. 2020, 59, 11871–11875. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.J.; Webber, M.J. Temperature-Responsive Supramolecular Hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef] [PubMed]
- Vaganova, T.A.; Gatilov, Y.V.; Malykhin, S.E.; Pishchur, D.P.; Larichev, Y.V.; Rodionov, V.I.; Malykhin, E.V. Design and Supramolecular Structure of Crystal Associates of Polyflfluoroarylenediamines and 18-crown-6 (2:1). J. Mol. Struct. 2017, 1133, 122–134. [Google Scholar] [CrossRef]
- Yokoya, M.; Kimura, S.; Yamanaka, M. Urea Derivatives as Functional Molecules: Supramolecular Capsules Supramolecular Polymers Supramolecular Gels Artificial Hosts and Catalysts. Chemistry 2021, 27, 5601–5614. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, K.; Shi, P.P.; Ye, Q.; Zhang, W. An Order-Disorder Type High-Temperature Multiaxial Supramolecular Ferroelectric. Adv. Electron. Mater. 2021, 8, 2100635. [Google Scholar] [CrossRef]
- Ai, Y.; Chen, X.G.; Shi, P.P.; Tang, Y.Y.; Li, P.F.; Liao, W.Q.; Xiong, R.G. Fluorine Substitution Induced High Tc of Enantiomeric Perovskite Ferroelectrics: (R)-And (S)-3-(Fluoropyrrolidinium)MnCl3. J. Am. Chem. Soc. 2019, 141, 4474–4479. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.; Luo, X.; Liao, W.Q.; Tang, Y.Y.; Xiong, R.G. An Above-Room-Temperature Molecular Ferroelectric: [Cyclopentylammonium]2CdBr4. Inorg. Chem. 2020, 59, 829–836. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, D.; Zhao, H.X.; Dong, X.W.; Long, L.S.; Zheng, L.S. Inorganic-Organic Hybrid Molecular Materials: From Multiferroic to Magnetoelectric. Adv. Mater. 2021, 33, 2004542. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. Host-Guest Chemistry in Supramolecular Theranostics. Theranostics 2019, 9, 3041–3074. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.P.; Omary, M.A. Crystallographic Observation of Dynamic Gas Adsorption Sites and Thermal Expansion in a Breathable Fluorous Metal-Organic Framework. Angew. Chem.-Int. Ed. 2009, 48, 2500–2505. [Google Scholar] [CrossRef]
- Shi, H.Y.; Lu, Y.C.; Liu, Y.H.; Song, J.; Deng, K.; Zeng, Q.D.; Wang, C. Nanotribological Study of Supramolecular Template Networks Induced by Hydrogen Bonds and van Der Waals Forces. ACS Nano 2018, 12, 8781–8790. [Google Scholar] [CrossRef]
- Kubo, K.; Takahashi, K.; Nakagawa, S.; Sakai, K.I.; Noro, S.; Akutagawa, T.; Nakamura, T. Substituent Effect On Molecular Motions Of M-Halogenated Anilinium/Dibenzo[18]crown-6 Supramolecular Cations in [Ni(dmit)2]-Crystals. Cryst. Growth Des. 2021, 21, 2340–2347. [Google Scholar] [CrossRef]
- Han, D.C.; Tan, Y.H.; Li, Y.K.; Wen, J.H.; Tang, Y.Z.; Wei, W.J.; Du, P.K.; Zhang, H. High-Temperature and Large-Polarization Ferroelectric with Second Harmonic Generation Response in a Novel Crown Ether Clathrate. Chem. Eur. J. 2021, 27, 13575–13581. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Fan, X.W.; Liu, Y.; Tang, Y.Z.; Wei, W.J.; Zhang, J.C.; Wang, C.F. High-Temperature Reversible Phase-Transition Behavior, Switchable Dielectric and Second Harmonic Generation Response of Two New Homochiral Crown Ether Clathrates. Chem. Asian J. 2019, 14, 2203–2209. [Google Scholar]
- Li, K.; Hu, S.; Zou, Q.; Zhang, H.; Zhao, Y.; Wang, Y. Synthesis and Characterizations of a Plutonium(III) Crown Ether Inclusion Complex. Inorg. Chem. 2021, 60, 8984–8989. [Google Scholar] [CrossRef]
- Wang, L.; Qin, L.L.; Liu, Y.; Wang, P.; Xu, H.T.; Liu, Z.Q. Synthesis Structure and Dielectric Properties of (3-Nitroanilinium) (18-Crown-6) (PF6). Crystals 2020, 10, 1028. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.L.; Qin, L.L.; Zheng, X.Y.; Liu, Z.Q. Anisotropic Dielectric Phase Transition Triggered by Pendulum-Like Motion Coupled with Proton Transfer in a Layered Hybrid Crystalline Material (4-nitroanilinium+) (18-crown-6) (H2PO4−)(H3PO4)2. J. Mol. Struct. 2018, 1164, 556–562. [Google Scholar] [CrossRef]
- Pal, P.; Das, K.; Hossain, A.; Frontera, A.; Mukhopadhyay, S. Supramolecular and Theoretical Perspective of 2,2’:6’,2”-Terpyridine Based Ni(II) and Cu(II) Complexes: On the Importance Of C-H⋯Cl and π⋯π Interactions. New J. Chem. 2020, 44, 7310–7318. [Google Scholar] [CrossRef]
- Yang, C.K.; Chen, W.N.; Ding, Y.T.; Wang, J.; Rao, Y.; Liao, W.Q.; Xiong, R.G. The First 2D Homochiral Lead Iodide Perovskite Ferroelectrics: [R-and S-1-(4-Chlorophenyl)Ethylammonium]2PbI4. Ad. Mater. 2019, 31, 1808088. [Google Scholar] [CrossRef]
- Wang, L.; Qin, L.L.; Liu, Y.; Ren, L.; Xu, H.T.; Liu, Z.Q. Synthesis, structure and dielectric properties of a one-dimensional chain hydrogen glycine supramolecular compound [(Gly)2+(18-crown-6)2(MnCl4)2−]. Chem. J. Chin. Univ. 2021, 42, 691–699. [Google Scholar]
- Hua, X.N.; Liao, W.Q.; Tang, Y.Y.; Li, P.F.; Shi, P.P.; Zhao, D.W.; Xiong, R.G. A Room-Temperature Hybrid Lead Iodide Perovskite Ferroelectric. J. Am. Chem. Soc. 2018, 140, 12296–12302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.Y.; Chen, X.G.; Xiong, R.G. Molecular Design Principles for Ferroelectrics: Ferroelectrochemistry. J. Am. Chem. Soc. 2020, 142, 15205–15218. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.H.; Chen, S. Theoretical Estimation of Important Ferroelectricity-Related Parameters for Molecular Design of Host-Guest Compounds, Substituted Anilinium Tetrafluoroborate 18-Crown-6. J. Phys. Chem. 2019, 123, 10996–11003. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Liu, Y.; Qiu, L.L.; Yu, F.F.; Zhu, C.L.; Liu, Z.Q. Synthesis, Structure and Dielectric Properties of Novel Cobalticyanide (III) Hydrogen-Bonding Cage-like Supermolecular Crystal. Chin. J. Inorg. Chem. 2019, 35, 277–284. [Google Scholar]
- Manikandana, A.; Dhanalakshmi, M.; Guganathana, L.; Kokila, T.; Santhamoorthyc, M.; Markkandana, R.; Kimc, S.C.; Balakrishnana, C. Synthesis Structural Characterization and Hirshfeld Surface Analysis of C-H···O Hydrogen-Bonded Supramolecular Complexes of 18-crown-6 With Imidazolinium Derivatives. J. Mol. Struc. 2022, 1254, 132395. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Di, F.F.; Li, P.F.; Xiong, R.G. Crown Ether Host-Guest Molecular Ferroelectrics. Chem. Eur. J. 2022, 28, e202102990. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Liu, Y.; Liu, Y.; Qin, L.L.; Wang, L.; Liu, Z.Q. Synthesis, Phase Transition and Dielectric Properties of Ferrate Cyanogen (III) Hydrogen-Bonding Supramolecular Crystal. Chin. J. Inorg. Chem. 2020, 36, 406–414. [Google Scholar]
- Wang, L.; He, C.T.; Zeng, Y.; Ji, C.M.; Du, Z.Y.; Zhang, W.X.; Chen, X.M. Crystalline Supramolecular Gyroscope with a Water Molecule as an Ultra-small Polar Rotator Modulated by Charge-Assisted Hydrogen Bonds. J. Am. Chem. Soc. 2017, 139, 8086–8089. [Google Scholar]
- Di, F.F.; Zhou, L.; Chen, W.J.; Liu, J.C.; Peng, H.; Tang, S.Y.; Yu, H.; Liao, W.Q.; Wang, Z.X. Room-Temperature Dielectric Switching in a Host-Guest Crown Ether Inclusion Complex. Inorg. Chem. Front. 2021, 8, 4896–4902. [Google Scholar] [CrossRef]
- Vaganova, T.A.; Gatilov, Y.V.; Benassi, E.; Chuikov, I.P.; Pishchur, D.; Malykhin, E.V. Impact of Molecular Packing Rearrangement on Solid-State Fluorescence: Polyhalogenated N-Hetarylamines vs their Co-Crystals with 18-crown-6. CrystEngComm 2019, 21, 5931–5946. [Google Scholar] [CrossRef]
- Wei, Y.L.; Jing, J.; Shi, C.; Ye, H.Y.; Wang, Z.X.; Zhang, Y. Unusual High-Temperature Reversible Phase Transition Containing Dielectric and Nonlinear Optical Switches in Host-Guest Supramolecular Crown Ether Clathrates. Chem. Commun. 2018, 54, 8076–8079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Zhou, L.; Shi, P.P.; Zheng, X.; Chen, X.G.; Gao, J.X.; Fu, D.W. Halogen Substitution Effects on Optical and Electrical Properties in 3D Molecular Perovskites. Chem. Commun. 2018, 54, 13275–13278. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Zhang, T.; Gu, Z.X.; Zhang, Z.X.; Fu, D.W.; Chen, X.G.; Zhang, H.Y.; Xiong, R.G. Record Enhancement of Curie Temperature in Host-Guest Inclusion Ferroelectrics. J. Am. Chem. Soc. 2021, 143, 5091–5098. [Google Scholar] [CrossRef]
| Temperature | 100 K | 293 K |
|---|---|---|
| Chemical formula | C39H68Cl4CoNO16 | C39H68Cl4CoNO16 |
| Formula weight | 1033.69 | 1033.69 |
| Crystal size (mm3) | 0.14 × 0.13 × 0.12 | 0.14 × 0.13 × 0.12 |
| Crystal system | monoclinic | monoclinic |
| Space group | C2 | C2 |
| a/Å | 23.454(3) | 23.948(4) |
| b/Å | 11.3549(15) | 11.4452(17) |
| c/Å | 9.4708(12) | 9.5311(15) |
| α/(°) | 90.00 | 90.00 |
| β/(°) | 95.087(13) | 96.123(14) |
| γ/(°) | 90.00 | 90.00 |
| V/Å3 | 2512.3(5) | 2597.5(7) |
| Z | 2 | 2 |
| Dc/(g·cm−1) | 1.366 | 1.322 |
| F(000) | 1090 | 1090 |
| μ/(mm−1) | 0.620 | 0.599 |
| 2θ range/(°) | 0.998–25.025 | 0.995–25.027 |
| Rint | 0.0830 | 0.0674 |
| R1 [I > 2σ(I)] a | 0.1248 | 0.0805 |
| wR2 (all data) b | 0.2520 | 0.2098 |
| GOF | 1.044 | 1.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, H.; Qi, H.; Lv, M.; Liu, Z. The Synthesis, Structure, and Dielectric Properties of a One-Dimensional Hydrogen-Bonded DL-α-Phenylglycine Supramolecular Crown-Ether-Based Inclusion Compound. Molecules 2023, 28, 7586. https://doi.org/10.3390/molecules28227586
Liu Y, Hu H, Qi H, Lv M, Liu Z. The Synthesis, Structure, and Dielectric Properties of a One-Dimensional Hydrogen-Bonded DL-α-Phenylglycine Supramolecular Crown-Ether-Based Inclusion Compound. Molecules. 2023; 28(22):7586. https://doi.org/10.3390/molecules28227586
Chicago/Turabian StyleLiu, Yang, Hongzhi Hu, Huanhuan Qi, Meixia Lv, and Zunqi Liu. 2023. "The Synthesis, Structure, and Dielectric Properties of a One-Dimensional Hydrogen-Bonded DL-α-Phenylglycine Supramolecular Crown-Ether-Based Inclusion Compound" Molecules 28, no. 22: 7586. https://doi.org/10.3390/molecules28227586
APA StyleLiu, Y., Hu, H., Qi, H., Lv, M., & Liu, Z. (2023). The Synthesis, Structure, and Dielectric Properties of a One-Dimensional Hydrogen-Bonded DL-α-Phenylglycine Supramolecular Crown-Ether-Based Inclusion Compound. Molecules, 28(22), 7586. https://doi.org/10.3390/molecules28227586






