Oxygen-Free Csp3-H Oxidation of Pyridin-2-yl-methanes to Pyridin-2-yl-methanones with Water by Copper Catalysis
Abstract
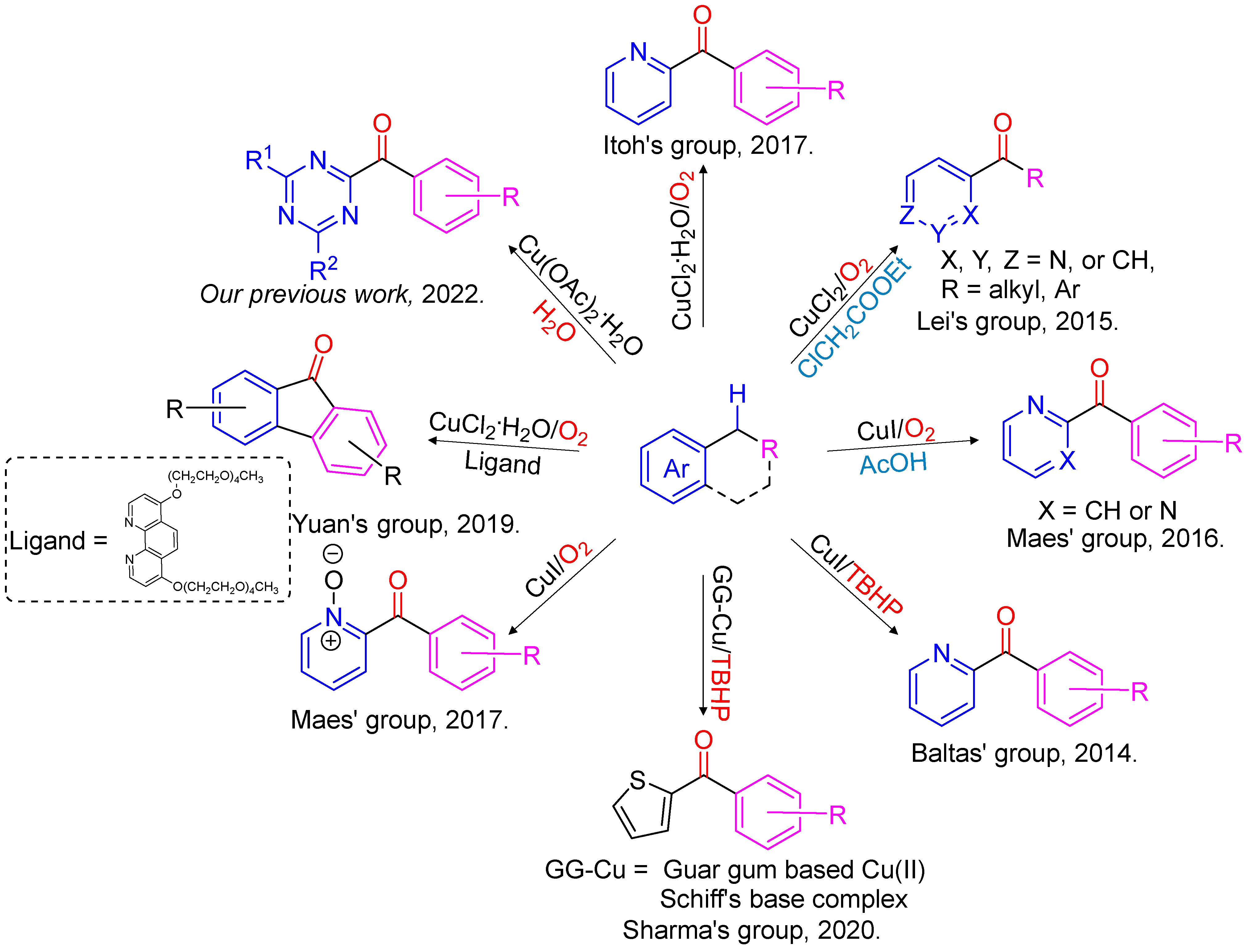
:1. Introduction
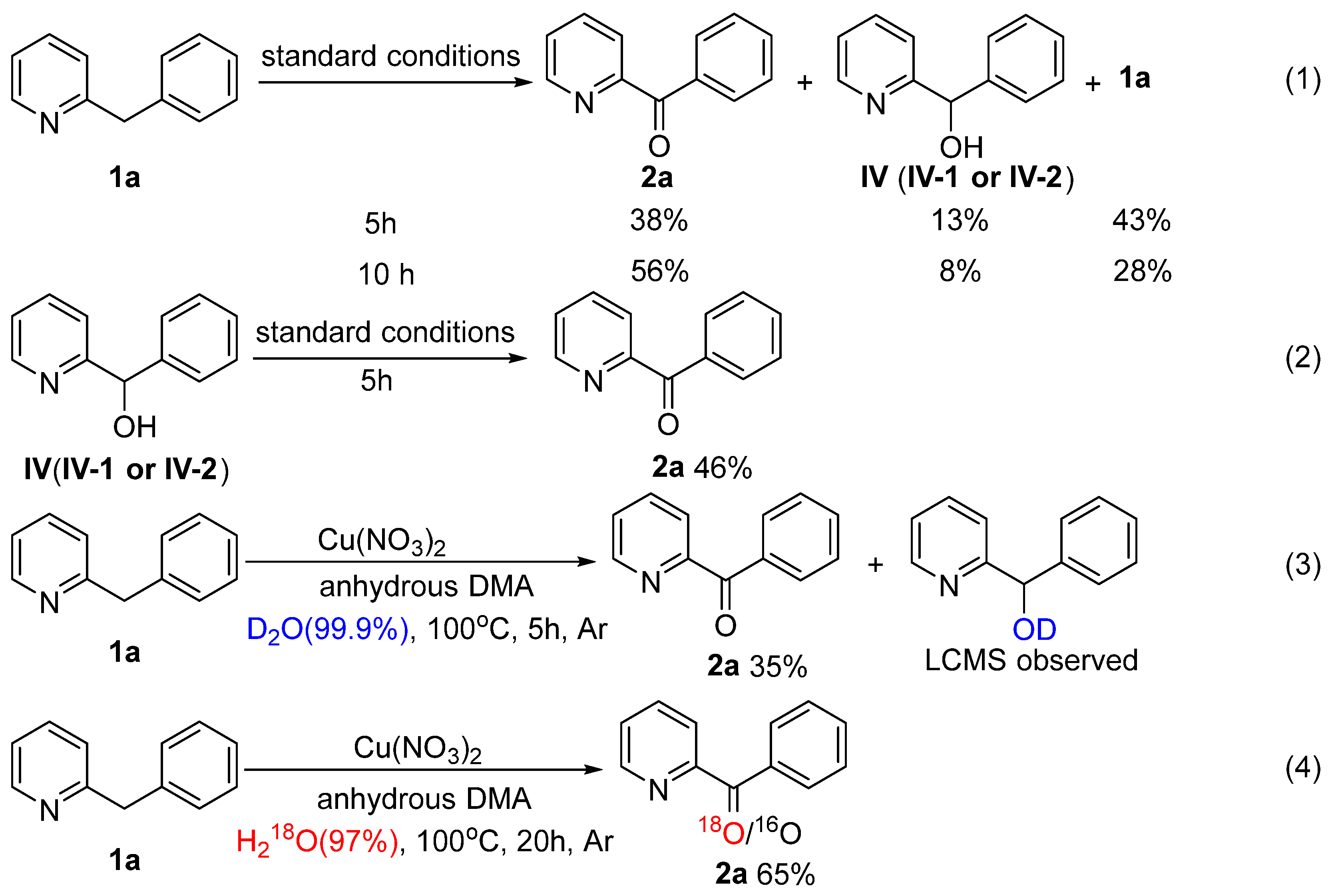
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntress, E.H.; Walter, H.C. Beckmann Rearrangement of the Oximes of Phenyl 2-Pyridyl Ketone (2-Benzoylpyridine). J. Am. Chem. Soc. 1948, 70, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hughes, C.O.; Toste, F.D. Synthesis of Aromatic Ketones by a Transition Metal-Catalyzed Tandem Sequence. J. Am. Chem. Soc. 2006, 128, 7436–7437. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, I.; Bastienne, M.; Gosmini, C.; Paris, J.-M.; Périchon, J. Convenient Processes for the Synthesis of Aromatic Ketones from Aryl Bromides and Carboxylic Anhydrides Using a Cobalt Catalysis. J. Org. Chem. 2004, 69, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Kim, H.-K. Facile one-pot synthesis of ketones from primary alcohols under mild conditions. New. J. Chem. 2021, 45, 13323–13328. [Google Scholar] [CrossRef]
- Das, K.; Waiba, S.; Jana, A.; Maji, B. Manganese-catalyzed hydrogenation, dehydrogenation, and hydroelementation reactions. Chem. Soc. Rev. 2022, 51, 4386–4464. [Google Scholar] [CrossRef]
- Yang, L.; Huang, H. Transition-Metal-Catalyzed Direct Addition of Unactivated C–H Bonds to Polar Unsaturated Bonds. Chem. Rev. 2015, 115, 3468–3517. [Google Scholar] [CrossRef]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem., Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
- Liu, L.A.; Liu, W.B.; Cui, D.M.; Zeng, M. Progress in the Synthesis of Aroyl Compounds. Chin. J. Org. Chem. 2021, 41, 4289–4305. [Google Scholar] [CrossRef]
- Pieber, B.; Kappe, C.O. Direct aerobic oxidation of 2-benzylpyridines in a gas–liquid continuous-flow regime using propylene carbonate as a solvent. Green Chem. 2013, 15, 320–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, Y.; Wang, X.; Wang, K.; Lou, Y.; Yao, M.; Zhuo, K.; Lv, Q.; Liu, J. DMSO-Promoted Metal-Free Aerobic Oxidation of Heterobenzylic Methylene to Prepare N-Heterocyclic Ketones. Asian J. Org. Chem. 2018, 7, 2459–2463. [Google Scholar] [CrossRef]
- Moriyama, K.; Takemura, M.; Togo, H. Direct and Selective Benzylic Oxidation of Alkylarenes via C–H Abstraction Using Alkali Metal Bromides. Org. Lett. 2012, 14, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.T. Preparation of Substituted Cyclopropanes Containing Aldehyde and Ketone Groups. J. Org. Chem. 1959, 24, 1536–1539. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, K. Recent advances in the copper-catalyzed aerobic Csp3–H oxidation strategy. Org. Biomol. Chem. 2021, 19, 3569–3583. [Google Scholar] [CrossRef] [PubMed]
- Sterckx, H.; De Houwer, J.; Mensch, C.; Caretti, I.; Tehrani, K.A.; Herrebout, W.A.; Van Doorslaer, S.; Maes, B.U.W. Mechanism of the CuII-catalyzed benzylic oxygenation of (aryl)(heteroaryl)methanes with oxygen. Chem. Sci. 2016, 7, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Bolm, C. Iron-Catalyzed Benzylic Oxidation with Aqueous tert-Butyl Hydroperoxide. Adv. Syn. Catal. 2007, 349, 861–864. [Google Scholar] [CrossRef]
- Dong, J.J.; Unjaroen, D.; Mecozzi, F.; Harvey, E.C.; Saisaha, P.; Pijper, D.; de Boer, J.W.; Alsters, P.; Feringa, B.L.; Browne, W.R. Manganese-Catalyzed Selective Oxidation of Aliphatic C—H groups and Secondary Alcohols to Ketones with Hydrogen Peroxide. ChemSusChem 2013, 6, 1774–1778. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Q.; Li, H.; Guo, H.; He, W. Ag/C nanoparticles catalysed aerobic oxidation of diaryl and aryl(hetero) methylenes into ketones. Nano Res. 2017, 10, 3261–3267. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Dehghani, A. Visible-light-driven photochemical activity of ternary Ag/AgBr/TiO2 nanotubes for oxidation C(sp3)–H and C(sp2)–H bonds. New. J. Chem. 2020, 44, 16776–16785. [Google Scholar] [CrossRef]
- Napoly, F.; Kieffer, R.; Jean-Gérard, L.; Goux-Henry, C.; Draye, M.; Andrioletti, B. Fe(TAML)Li/tert-butyl hydroperoxide as a new combination for benzylic C–H oxidation. Tetrahedron Lett. 2015, 56, 2517–2520. [Google Scholar] [CrossRef]
- Nawratil, S.; Grypioti, M.; Menendez, C.; Mallet-Ladeira, S.; Lherbet, C.; Baltas, M. Synthesis of α-Keto-1,2,3-triazoles Through Copper Iodide Catalyzed Oxygenation. Eur. J. Org. Chem. 2014, 2014, 654–659. [Google Scholar] [CrossRef]
- Hsu, S.-F.; Plietker, B. PNNP-Ligated RuII Complexes as Efficient Catalysts for Mild Benzylic C H Oxidation. ChemCatChem 2013, 5, 126–129. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, X.-H.; Xu, N.; An, Z.-X.; Chu, Y.; Wang, X.-L. A new metal–organic complex with coordination unsaturated Co(II) as high-efficiency heterogeneous catalyst for selective oxidation of alkylbenzenes. Molecular Catal. 2023, 548, 113428. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Zhang, Y.; Wang, J. An efficient approach for enhancing the catalytic activity of Ni-MOF-74 via a relay catalyst system for the selective oxidation of benzylic C–H bonds under mild conditions. Chem. Commun. 2018, 54, 3701–3704. [Google Scholar] [CrossRef] [PubMed]
- De Houwer, J.; Abbaspour Tehrani, K.; Maes, B.U.W. Synthesis of Aryl(di)azinyl Ketones through Copper- and Iron-catalyzed Oxidation of the Methylene Group of Aryl(di)azinylmethanes. Angew. Chem. Int. Ed. 2012, 51, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tanaka, S.; Ogawa, A.; Tamura, M.; Sato, K.; Itoh, S. Copper-catalyzed Selective Oxygenation of Methyl and Benzyl Substituents in Pyridine with O2. Chem. Lett. 2017, 46, 348–350. [Google Scholar] [CrossRef]
- Sterckx, H.; De Houwer, J.; Mensch, C.; Herrebout, W.; Tehrani, K.A.; Maes, B.U.W. Base metal-catalyzed benzylic oxidation of (aryl)(heteroaryl)methanes with molecular oxygen. Beilstein J. Org. Chem. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Al-hunaiti, A.; Räisänen, M.; Repo, T. From DNA to catalysis: A thymine-acetate ligated non-heme iron(iii) catalyst for oxidative activation of aliphatic C–H bonds. Chem. Commun. 2016, 52, 2043–2046. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Yi, H.; Liu, C.; Liu, R.; Zhang, H.; Zhuo, K.; Lei, A. Chloroacetate-Promoted Selective Oxidation of Heterobenzylic Methylenes under Copper Catalysis. Angew. Chem. Int. Ed. 2015, 54, 1261–1265. [Google Scholar] [CrossRef]
- Hruszkewycz, D.P.; Miles, K.C.; Thiel, O.R.; Stahl, S.S. Co/NHPI-mediated aerobic oxygenation of benzylic C–H bonds in pharmaceutically relevant molecules. Chem. Sci. 2017, 8, 1282–1287. [Google Scholar] [CrossRef]
- Nakai, S.; Uematsu, T.; Ogasawara, Y.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. Aerobic Oxygenation of Alkylarenes over Ultrafine Transition-Metal-Containing Manganese-Based Oxides. ChemCatChem 2018, 10, 1096–1106. [Google Scholar] [CrossRef]
- Sterckx, H.; Sambiagio, C.; Médran-Navarrete, V.; Maes, B.U.W. Copper-Catalyzed Aerobic Oxygenation of Benzylpyridine N-Oxides and Subsequent Post-Functionalization. Adv. Syn. Catal. 2017, 359, 3226–3236. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Tang, S.; Li, J.; Ren, X.; Yang, G.; Li, H.; Yuan, B. Amphiphilic ligands for Cu-catalyzed aerobic oxidation to synthesize 9-fluorenones in water. Catal. Commun. 2019, 127, 34–38. [Google Scholar] [CrossRef]
- Krishna; Kumari, S.; Yadav, D.; Sharma, S.K. Cu (II) Schiff base complex grafted guar gum: Catalyst for benzophenone derivatives synthesis. Appl. Catal. A Gen. 2020, 601, 117529. [Google Scholar] [CrossRef]
- Citterio, A.; Sebastiano, R.; Caceres Carvayal, M. Oxidation of diethyl (pyridylmethyl)malonates with manganese(III) acetate, cerium(IV) ammonium nitrate, and iron(III) perchlorate in the presence of alkenes and alkynes. J. Org. Chem. 1991, 56, 5335–5341. [Google Scholar] [CrossRef]
- Zhang, J.; Du, J.; Zhang, C.; Liu, K.; Yu, F.; Yuan, Y.; Duan, B.; Liu, R. Selective Oxidation of Alkylarenes to the Aromatic Ketones or Benzaldehydes with Water. Org. Lett. 2022, 24, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, Y.-X.; Zheng, J.-H.; Zhao, L.; Zhu, Q.-H.; Jiang, D.; Ling, Y.; Liu, W.; Zeng, S.-X. Direct α-methylenation of triazines to terminal olefins with DMA. New. J. Chem. 2022, 46, 20065–20068. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, Z.-X.; Wen, L.-F.; Jiang, D.; Lu, H.; Liu, W.; Dai, J.; Zeng, S.-X. The copper-catalyzed oxidation of arylmethyl triazines with H2O toward the oxidant-free synthesis of aroyl triazines. Org. Biomol. Chem. 2022, 20, 5406–5411. [Google Scholar] [CrossRef]
- King, A.E.; Brunold, T.C.; Stahl, S.S. Mechanistic Study of Copper-Catalyzed Aerobic Oxidative Coupling of Arylboronic Esters and Methanol: Insights into an Organometallic Oxidase Reaction. J. Am. Chem. Soc. 2009, 131, 5044–5045. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; He, Y.; Liu, L.; Zhu, Q. Cu-Catalyzed Oxidative C(sp2)–H Cycloetherification of o-Arylphenols for the Preparation of Dibenzofurans. Org. Lett. 2012, 14, 1078–1081. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Ghafuri, H.; Karimi-Jaberi, Z. Room Temperature Catalytic Aromatization of Hantzsch 1,4-Dihydropyridines by Sodium Nitrite in the Presence of Acidic Silica Gel. Monatshefte für Chemie. 2006, 137, 197–200. [Google Scholar] [CrossRef]
- Loev, B.; Snader, K.M. The Hantzsch Reaction. I. Oxidative Dealkylation of Certain Dihydropyridines. J. Org. Chem. 1965, 30, 1914–1916. [Google Scholar] [CrossRef]
- Powell, M.F.; Wu, J.C.; Bruice, T.C. Ferricyanide oxidation of dihydropyridines and analogs. J. Am. Chem. Soc. 1984, 106, 3850–3856. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Conrad, J.; Beifuss, U. Laccase-catalyzed oxidation of Hantzsch 1,4-dihydropyridines to pyridines and a new one pot synthesis of pyridines. Green Chem. 2012, 14, 2686–2690. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Shirini, F.; Choghamarani, A.G.; Mohammadpoor-Baltork, I. Silica modified sulfuric acid/NaNO2 as a novel heterogeneous system for the oxidation of 1,4-dihydropyridines under mild conditions. Green Chem. 2002, 4, 562–564. [Google Scholar] [CrossRef]
- Vessally, E.; Hosseinian, A.; Edjlali, L.; Bekhradnia, A.; Esrafili, M.D. New page to access pyridine derivatives: Synthesis from N-propargylamines. RSC Adv. 2016, 6, 71662–71675. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, T.; Cui, D.-M.; Zhang, C. Ruthenium-catalyzed synthesis of tri-substituted 1,3,5-triazines from alcohols and biguanides. New. J. Chem. 2016, 40, 8225–8228. [Google Scholar] [CrossRef]
- Zeng, M.; Xie, Z.P.; Cui, D.-M.; Zhang, C. Ruthenium-catalyzed synthesis of arylethyl 1,3,5-triazines from arylallyl alcohols and biguanides. Org. Biomol. Chem. 2018, 16, 6140–6145. [Google Scholar] [CrossRef]
- Zeng, M.; Xie, Z.P.; Cui, D.-M.; Zhang, C. Ruthenium-catalyzed synthesis of 1,3,5-triazin-2(1H)-ones and dihydro[1,3,5]triazino[1,2-a]benzimidazoles from alcohols and guanides. New. J. Chem. 2018, 42, 11905–11907. [Google Scholar] [CrossRef]
- Peters, E.; Von Hahn, H.E.A. Kinetics of copper(II)- and copper(I)-catalyzed deuterium exchange in sulfuric and perchloric acid solutions. J. Phys. Chem. 1971, 75, 571–579. [Google Scholar] [CrossRef]
- Karthikeyan, I.; Alamsetti, S.K.; Sekar, G. Isolation and characterization of a trinuclear cobalt complex containing trigonal-prismatic cobalt in secondary alcohol aerobic oxidation. Organometallics 2014, 33, 1665–1671. [Google Scholar] [CrossRef]
- Mikami, S.; Nakamura, S.; Ashizawa, T.; Sasaki, S.; Taniguchi, T.; Nomura, I.; Kawasaki, M. Preparation of 2-oxo-2,3-dihydropyrido[2,3-b]pyrazine-4(1H)-carboxamide Derivatives as PDE2A Inhibitors. WO2013161913 A1, 31 October 2013. [Google Scholar]
- Wang, B.; Zhou, H.; Lu, G.; Liu, Q.; Jiang, X. Bifunctional Oxo-Tethered Ruthenium Complex Catalyzed Asymmetric Transfer Hydrogenation of Aryl N-Heteroaryl Ketones. Org. Lett. 2017, 19, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Meisenbach, M.; Schenkel, B.; Sedelmeier, J. Continuous flow as an enabling technology: A fast and versatile entry to functionalized glyoxal derivatives. Org. Biomol. Chem. 2021, 19, 2420–2424. [Google Scholar] [CrossRef]
- Dou, L.; Wang, J.; Yan, J.; Wu, H.; Wang, Y.; Sun, B. Synthesis, Characterization and Fluorescence of a New β-Diketone Ligand and Its Europium(III) Complexes. Zhongguo Xitu Xuebao 2009, 27, 587–591. [Google Scholar]
- Lebedev, Y.; Polishchuk, I.; Maity, B.; Dinis Veloso Guerreiro, M.; Cavallo, L.; Rueping, M. Asymmetric Hydroboration of Heteroaryl Ketones by Aluminum Catalysis. J. Am. Chem. Soc. 2019, 141, 19415–19423. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A.; Fantin, G.; Fogagnolo, M.; Medici, A.; Pedrini, P. Synthesis of (trimethylsilyl)thiazoles and reactions with carbonyl compounds. Selectivity aspects and synthetic utility. J. Org. Chem. 1988, 53, 1748–1761. [Google Scholar] [CrossRef]

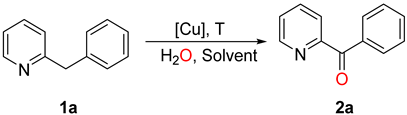
| Entry | Cu Salt (mol %) | Water | Solvent | Time/h | Yield/% |
|---|---|---|---|---|---|
| 1 | Cu(NO3)2·3H2O (10 mol%) | 5.0 equiv. | DMA | 20 | 69 |
| 2 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMA | 20 | 68 |
| 3 | Cu(NO3)2·3H2O (10 mol%) | - | DMA | 20 | 36 |
| 4 | Cu(NO3)2 (10 mol%) | - | DMA | 20 | 8 |
| 5 | Cu(NO3)2 (10 mol%) | 2.5 equiv. | DMA | 20 | 59 |
| 6 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMA | 20 | 70 b |
| 7 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMA | 20 | 43 c |
| 8 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMA | 12 | 41 |
| 9 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMA | 30 | 61 |
| 10 | Cu(NO3)2·3H2O (5 mol%) | 2.5 equiv. | DMA | 20 | 40 |
| 11 | Cu(NO3)2·3H2O (20 mol%) | 2.5 equiv. | DMA | 20 | 68 |
| 12 | Cu(OAc)·H2O (10 mol%) | 2.5 equiv. | DMA | 20 | 42 |
| 13 | CuCl2·2H2O (10 mol%) | 2.5 equiv. | DMA | 20 | 45 |
| 14 | CuSO4 (10 mol%) | 2.5 equiv. | DMA | 20 | 36 |
| 15 | CuBr2 (10 mol%) | 2.5 equiv. | DMA | 20 | 40 |
| 16 | Cu(CF3SO3)2 (10 mol%) | 2.5 equiv. | DMA | 20 | 22 |
| 17 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMF | 20 | 45 |
| 18 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | DMSO | 20 | 30 |
| 19 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | NMP | 20 | 21 |
| 20 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | PhCl | 20 | 23 |
| 21 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | Et3N | 20 | trace |
| 22 | Cu(NO3)2·3H2O (10 mol%) | 2.5 equiv. | H2O | 20 | trace |

| Entry | 1 | Ar | Time/h | 2 | Yield/% |
|---|---|---|---|---|---|
| 1 |  1a–1o | Ph | 20 | 2a | 68 |
| 2 | 4-t-BuC6H4 | 38 | 2b | 76 | |
| 3 | 2-naphthyl | 30 | 2c | 85 | |
| 4 | 4-PhC6H4 | 30 | 2d | 92 | |
| 5 | 4-OCF3C6H4 | 25 | 2e | 65 | |
| 6 | 4-ClC6H4 | 20 | 2f | 48 | |
| 7 | 3-ClC6H4 | 20 | 2g | 63 | |
| 8 | 3-BrC6H4 | 30 | 2h | 62 | |
| 9 | 2-CH3COC6H4 | 50 | 2i | 63 | |
| 10 | 4-CH3OOCC6H4 | 23 | 2j | 60 | |
| 11 | 3-CNC6H4 | 39 | 2k | 68 | |
| 12 | 3-NO2C6H4 | 30 | 2l | 54 | |
| 13 | 2-thiophenyl | 25 | 2m | 65 | |
| 14 | 2-thiazolyl | 24 | 2n | 51 | |
| 15 | 3-pyridyl | 20 | 2o | 60 | |
| 16 | 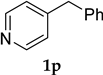 1p | - | 30 | 2p | 62 |
| 17 | 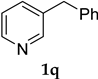 1q | - | 40 | 2q | trace |
| 18 |  1r | - | 10 | 2r | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Chen, J.-L.; Luo, X.; Zou, Y.-J.; Liu, Z.-N.; Dai, J.; Jiang, D.-Z.; Li, J.-J. Oxygen-Free Csp3-H Oxidation of Pyridin-2-yl-methanes to Pyridin-2-yl-methanones with Water by Copper Catalysis. Molecules 2023, 28, 7587. https://doi.org/10.3390/molecules28227587
Zeng M, Chen J-L, Luo X, Zou Y-J, Liu Z-N, Dai J, Jiang D-Z, Li J-J. Oxygen-Free Csp3-H Oxidation of Pyridin-2-yl-methanes to Pyridin-2-yl-methanones with Water by Copper Catalysis. Molecules. 2023; 28(22):7587. https://doi.org/10.3390/molecules28227587
Chicago/Turabian StyleZeng, Ming, Jia-Le Chen, Xue Luo, Yan-Jiao Zou, Zhao-Ning Liu, Jun Dai, Deng-Zhao Jiang, and Jin-Jing Li. 2023. "Oxygen-Free Csp3-H Oxidation of Pyridin-2-yl-methanes to Pyridin-2-yl-methanones with Water by Copper Catalysis" Molecules 28, no. 22: 7587. https://doi.org/10.3390/molecules28227587
APA StyleZeng, M., Chen, J.-L., Luo, X., Zou, Y.-J., Liu, Z.-N., Dai, J., Jiang, D.-Z., & Li, J.-J. (2023). Oxygen-Free Csp3-H Oxidation of Pyridin-2-yl-methanes to Pyridin-2-yl-methanones with Water by Copper Catalysis. Molecules, 28(22), 7587. https://doi.org/10.3390/molecules28227587







