Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease
Abstract
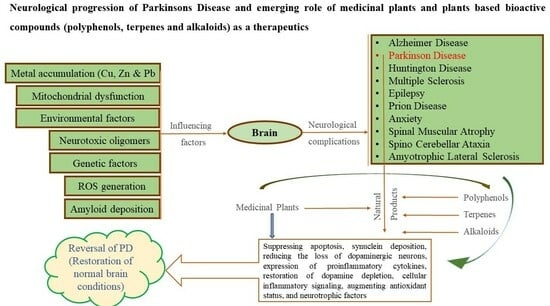
:1. Introduction
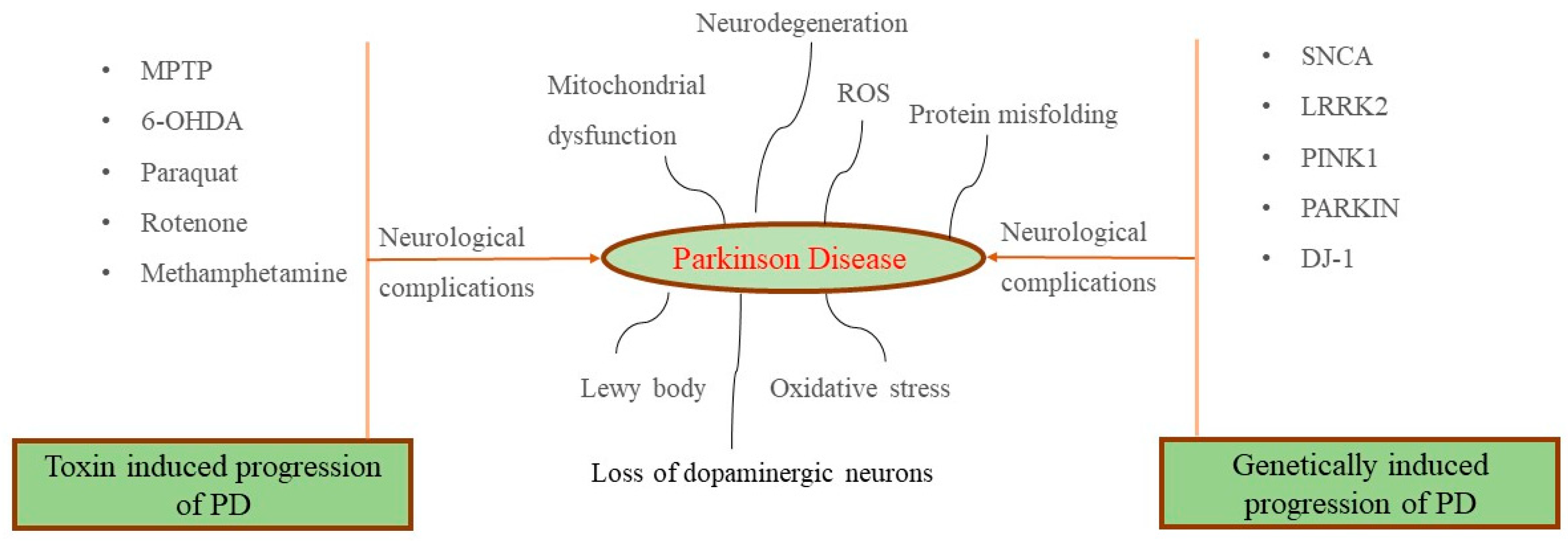
2. Pathophysiology of Parkinson’s Disease (PD)
3. Medicinal Plants, Herbal Formulations and Plant-Based Bioactives (Polyphenols, Terpenes, and Alkaloids) as a Potent Therapeutics for PD Management
3.1. Polyphenolic Compounds
- Structure activity relationship
- In vitro and in vivo studies
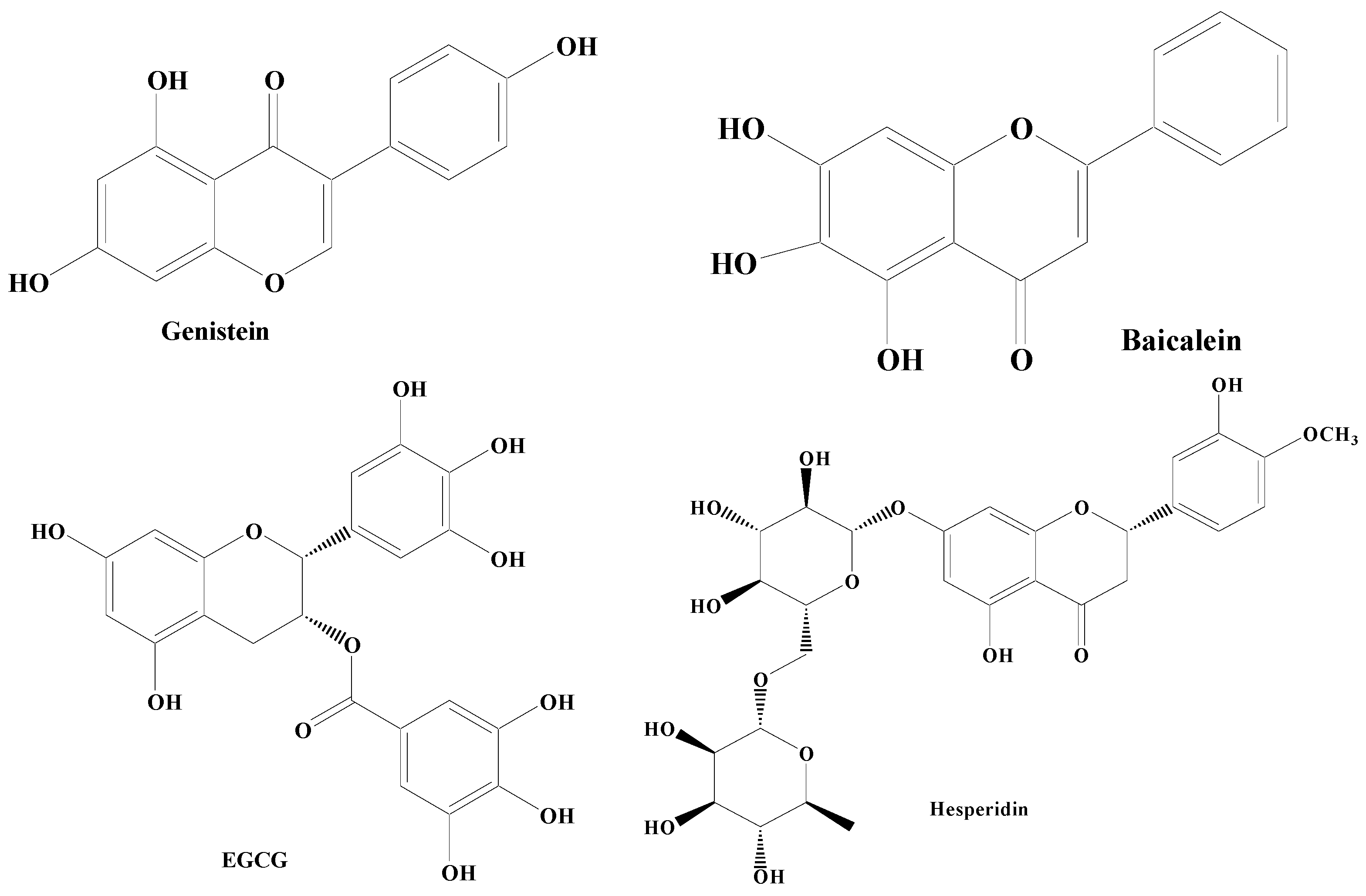
3.1.1. Flavonoids
3.1.2. Non-Flavonoids
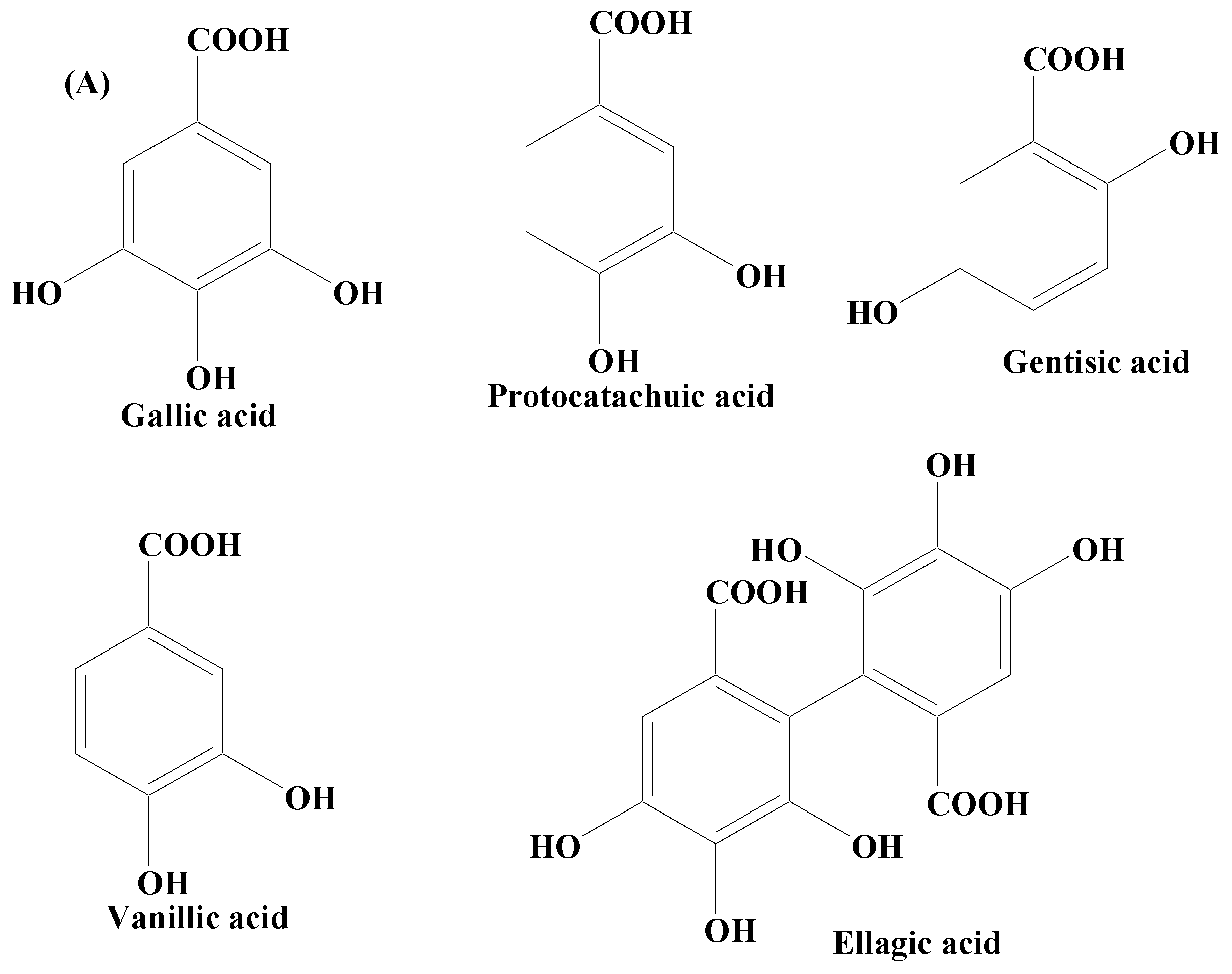
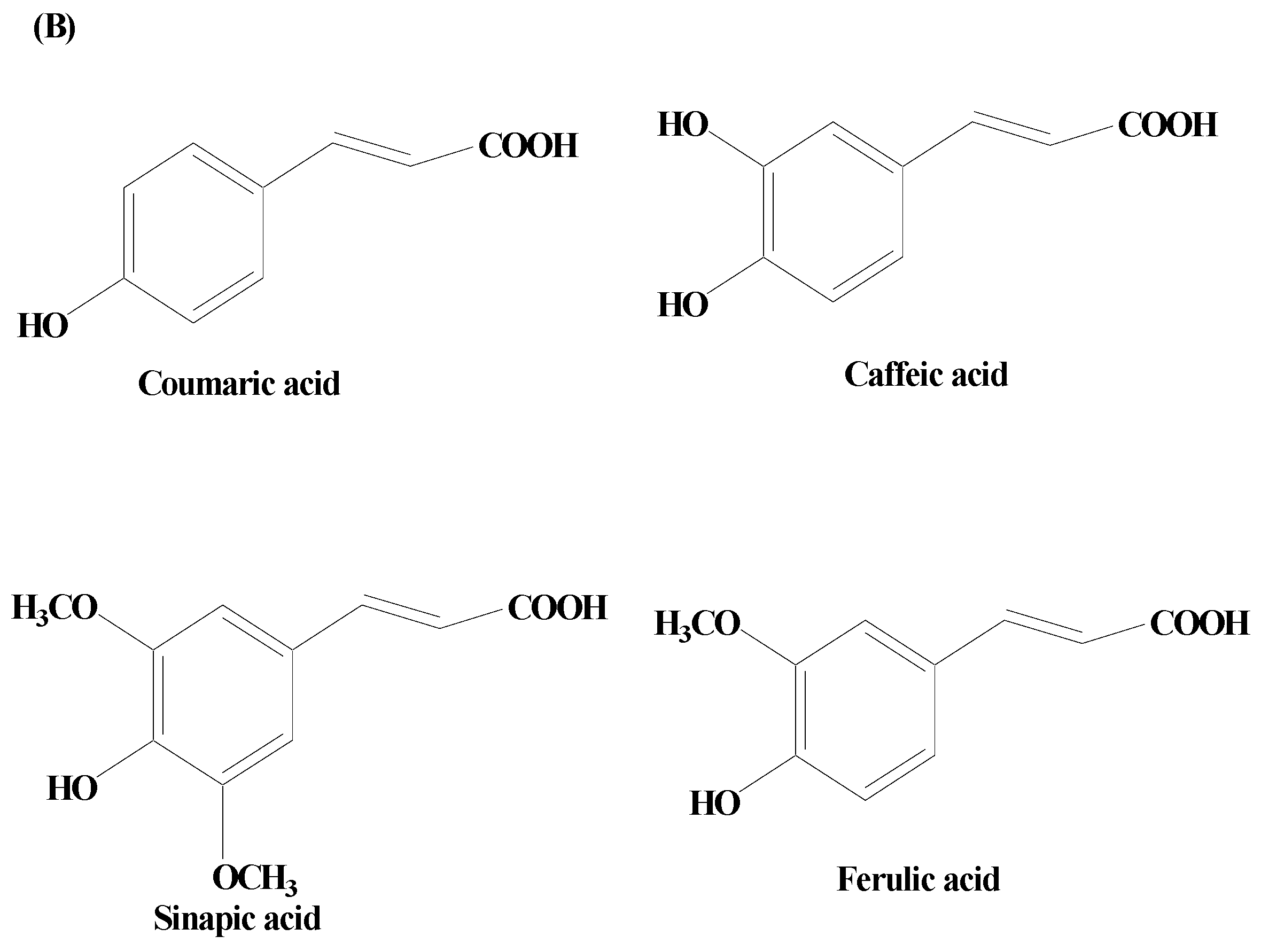
Phenolic Acids
Phenolic Alcohols
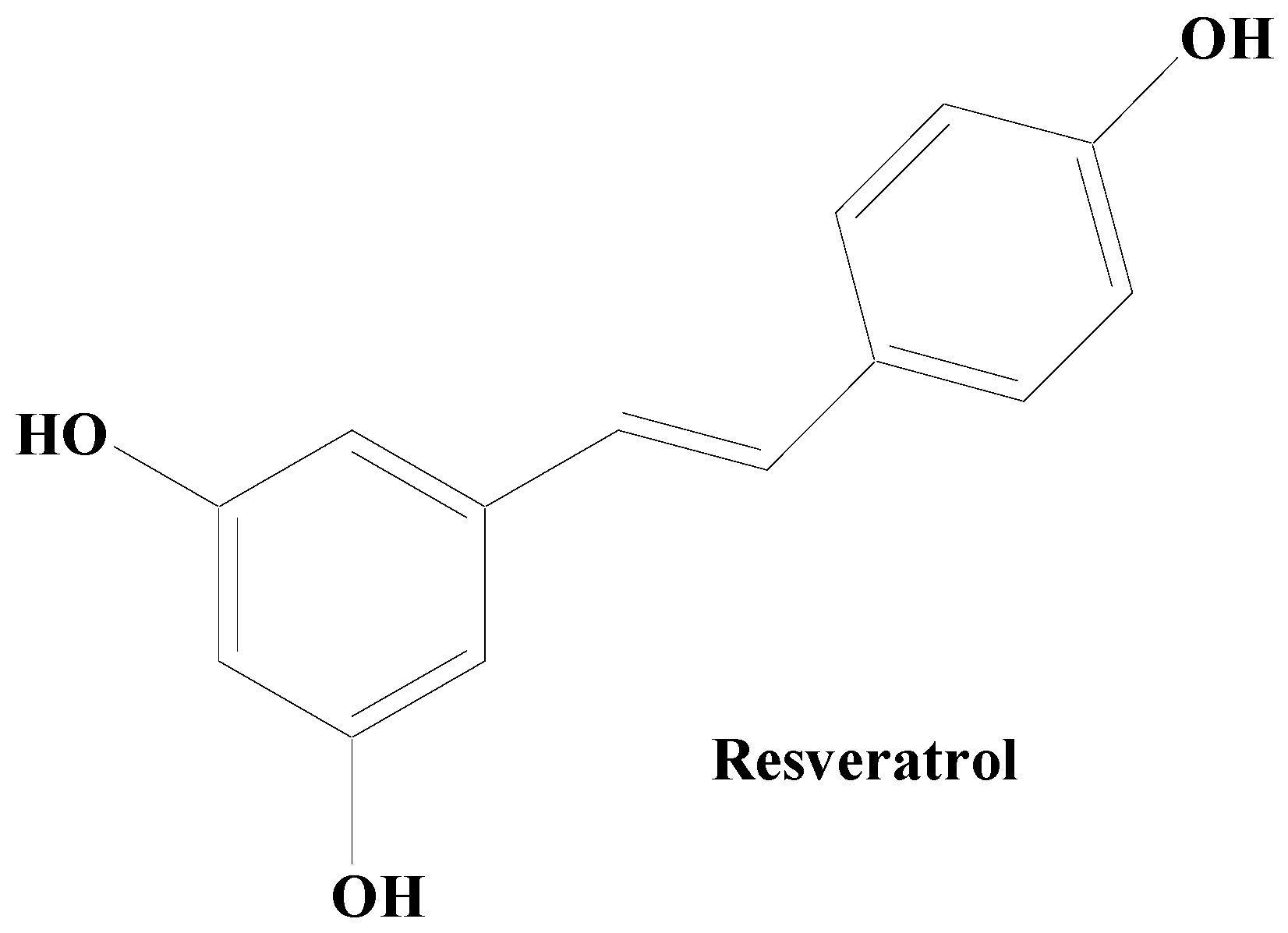
Stilbenes
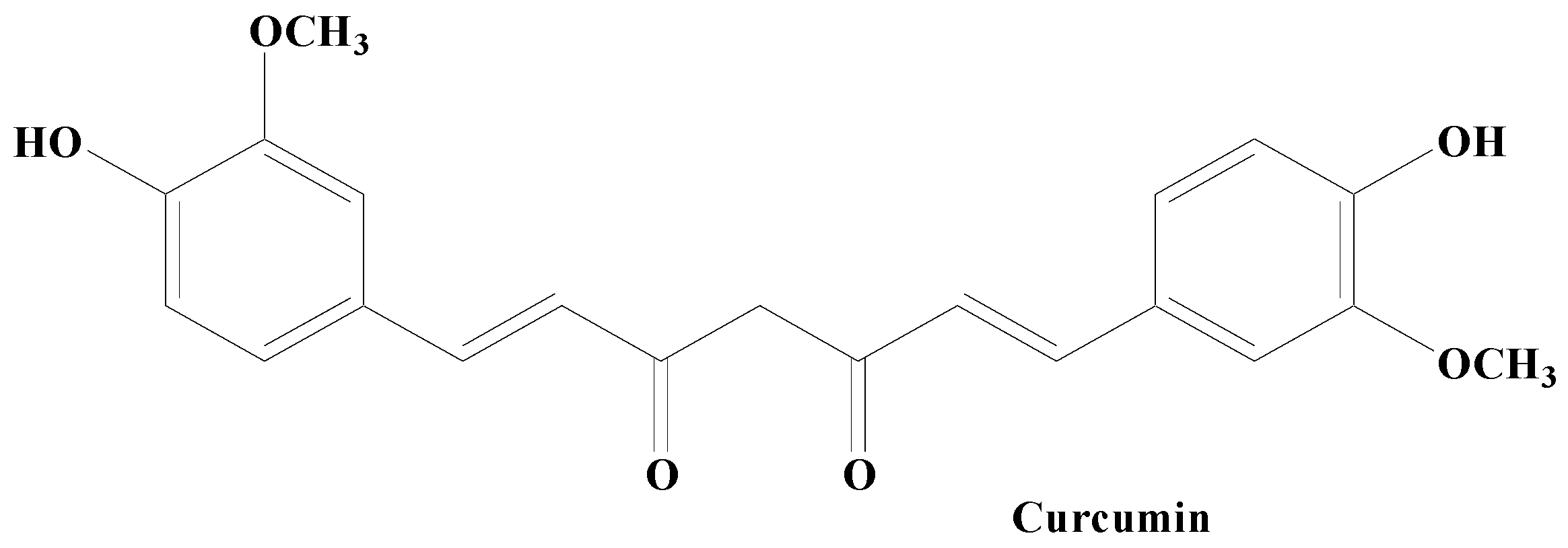
Curcumin
3.2. Terpenes
- Structure Activity Relationship
- In vitro and in vivo studies
3.3. Alkaloids
- Structure Activity relationship
- In vitro and in vivo studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bassett, D.S.; Gazzaniga, M.S. Understanding complexity in the human brain. Trends Cogn. Sci. 2011, 15, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Colom, R.; Karama, S.; Jung, R.E.; Haier, R.J. Human intelligence and brain networks. Dialogues Clin. Neurosci. 2010, 12, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Vasic, V.; Barth, K.; Schmidt, M.H.H. Neurodegeneration and Neuro-Regeneration-Alzheimer’s Disease and Stem Cell Therapy. Int. J. Mol. Sci. 2019, 20, 4272. [Google Scholar] [CrossRef]
- Gao, H.; Hong, J. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T. Saeed Samarghandian Molecular and cellular pathways contributing to brain aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Choonara, Y.E.; Pillay, V.; Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K.; Sibambo1, S.R. Trends in the Molecular Pathogenesis and Clinical Therapeutics of Common Neurodegenerative Disorders. Int. J. Mol. Sci. 2009, 10, 2510–2557. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbulla, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Pang, S.Y.Y.; Ho, P.W.L.; Liu, H.F.; Leung, C.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 21 December 2018; Chapter 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536722/ (accessed on 13 November 2023).
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-Like Mechanisms in Parkinson’s Disease. Front. Neurosci. 2019, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Lazzaro, G.D.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell. Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- González-Hernández, T.; Rodríguez, M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J. Comp. Neurol. 2000, 421, 107–135. [Google Scholar] [CrossRef]
- Gröger, A.; Kolb, R.; Schäfer, R.; Klose, U. Dopamine Reduction in the Substantia Nigra of Parkinson’s Disease Patients Confirmed by In Vivo Magnetic Resonance Spectroscopic Imaging. PLoS ONE 2014, 9, e84081. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and Therapeutic Strategies against Parkinson’s Disease: Recent Perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Azam, S.; Cho, D.; Su-Kim, I.; Choi, D. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, W. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef]
- Shahpiri, Z.; Bahramsoltani, R.; Farzaei, M.H.; Farzaei, F.; Rahimi, R. Phytochemicals as future drugs for Parkinson’s disease: A comprehensive review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Makeen, H.A.; Alshehri, B.M.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef]
- Varadi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, X.; Jiang, Z.; Ma, J.; Li, Y.; Qian, Z.; Li, H. The Mechanism of SNHG8/Microrna-421-3p/Sorting Nexin 8 Axis on Dopaminergic Neurons in Substantia Nigra in a Mouse Model of Parkinson’s Disease. Neurochem. Res. 2023, 48, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rabbani, S.A.; Agarwal, T.; Baboota, S.; Pottoo, F.H.; Kadian, R. Nanotechnology Driven Approaches for the Management of Parkinson’s Disease: Current Status and Future Perspectives. Curr. Drug Metab. 2021, 22, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.P.C.F.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Mallet, D.; Dufourd, T.; Decourt, M.; Carcenac, C.; Bossù, P.; Verlin, L.; Fernagut, P.; Benoit-Marand, M.; Spalletta, G.; Barbier, E.L.; et al. A metabolic biomarker predicts Parkinson’s disease at the early stages in patients and animal models. J. Clin. Investig. 2022, 132, e146400. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Pang, M.; Peng, R.; Wang, Y.; Zhu, Y.; Wang, P.; Moussian, B.; Su, Y.; Liu, X.; Ming, D. Molecular understanding of the translational models and the therapeutic potential natural products of Parkinson’s disease. Biomed. Pharmacother. 2022, 155, 113718. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Solati, K.; Amini-Khoei, H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019, 57, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, T.; Huh, Y.; Lee, J.; Lee, Y. Amelioration of Mitochondrial Quality Control and Proteostasis by Natural Compounds in Parkinson’s Disease Models. Int. J. Mol. Sci. 2019, 20, 5208. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Kianmehr, M.; Anaeigoudari, A. Effects of Medicinal Plants and Flavonoids on Parkinson’s Disease: A Review on Basic and Clinical Evidences. Adv. Pharm. Bull. 2021, 11, 224–232. [Google Scholar] [CrossRef]
- Carrera, I.; Cacabelos, R. Current Drugs and Potential Future Neuroprotective Compounds for Parkinson’s Disease. Curr. Neuropharmacol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Yin, R.; Xue, J.; Tan, Y.; Fang, C.; Hu, C.; Yang, Q.; Mei, X.; Qi, D. The Positive Role and Mechanism of Herbal Medicine in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 9923331. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Dube, B.; Mishra, S.H. Herbs in Metal Health; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2012; Volume 1, pp. 1–75. [Google Scholar]
- Li, J.; He, Y.; Fu, J.; Wang, Y.; Fan, X.; Zhong, T.; Zhou, H. Dietary supplementation of Acanthopanax senticosus extract alleviates motor deficits in MPTP-induced Parkinson’s disease mice and its underlying mechanism. Front. Nutr. 2023, 9, 1121789. [Google Scholar] [CrossRef]
- Han, C.; Guo, L.; Yang, Y.; Li, W.; Sheng, Y.; Wang, J.; Guan, Q.; Zhang, X. Study on antrodia camphorata polysaccharide in alleviating the neuroethology of PD mice by decreasing the expression of NLRP3 inflammasome. Phytother. Res. 2019, 33, 2288–2297. [Google Scholar] [CrossRef]
- Han, C.; Shen, H.; Yang, Y.; Sheng, Y.; Wang, J.; Li, W.; Zhou, X.; Guo, L.; Zhai, L.; Guan, Q. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. 2020, 10, 01824. [Google Scholar] [CrossRef]
- Wang, C.; Nguyen, T.; Yang, X.; Mellick, G.D.; Feng, Y. Phytochemical investigation of Asarum sieboldii var. seoulense and the phenotypic profiles of its constituents against a Parkinson’s Disease olfactory cell line. Bioorg. Med. Chem. Lett. 2023, 92, 129386. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Y.; Yang, M.; Wang, X.; Peng, Y. Velvet Antler Methanol Extracts Ameliorate Parkinson’s Disease by Inhibiting Oxidative Stress and Neuroinflammation: From, C. elegans to Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8864395. [Google Scholar] [CrossRef]
- Silva, J.; Martins, A.; Alves, C.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Natural Approaches for Neurological Disorders-The Neuroprotective Potential of Codium tomentosum. Molecules 2020, 25, 5478. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, S.I.; Ibrakaw, A.S.; Ekpo, O.E.; Boatwright, J.S.; Cupido, C.N.; Hussein, A.A. Neuroprotective Activities of Crossyne flava Bulbs and Amaryllidaceae Alkaloids: Implications for Parkinson’s Disease. Molecules 2021, 26, 3990. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, C.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.E.; Yılmaz, A. Neuroprotective effects of Geranium robertianum L. Aqueous extract on the cellular Parkinson’s disease model. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Paola, R.D.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-Oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, Y.; Tang, D.; Cai, Y. Study on the Mechanism of Liuwei Dihuang Pills in Treating Parkinson’s Disease Based on Network Pharmacology. BioMed Res. Int. 2021, 2021, 4490081. [Google Scholar] [CrossRef]
- Kabra, A.; Baghel, U.S.; Hano, C.; Martins, N.; Khalid, M.; Sharma, R. Neuroprotective potential of Myrica esulenta in Haloperidol induced Parkinson’s disease. J. Ayurveda Integr. Med. 2020, 11, 448–454. [Google Scholar] [CrossRef]
- Ly, H.T.; Nguyen, T.T.H.; Le, V.M.; Lam, B.T.; Mai, T.T.T.; Dang, T.P.T. Therapeutic Potential of Polyscias fruticosa (L.) Harms Leaf Extract for Parkinson’s Disease Treatment by Drosophila melanogaster Model. Oxid. Med. Cell. Longev. 2022, 2022, 5262677. [Google Scholar] [CrossRef]
- Nghi, N.B.T.; Uyen, T.T.; Anh, H.M.; Linh, D.M.; Thao, D.T.P. Rumdul (Sphaerocoryne affinis) Antioxidant Activity and Its Potential for Parkinson’s Disease Treatment. Oxid. Med. Cell. Longev. 2022, 2022, 8918966. [Google Scholar] [CrossRef]
- Sanad, S.M.; Farouk, R.; Nassar, S.E.; Alshahrani, M.Y.; Suliman, M.; Ahmed, A.E.; Elesawi, I.E. The neuroprotective effect of quercetin nanoparticles in the therapy of neuronal damage stimulated by acrolein. Saudi J. Biol. Sci. 2023, 30, 103792. [Google Scholar] [CrossRef] [PubMed]
- Yarim, G.F.; Kazak, F.; Yarim, M.; Sozmen, M.; Genc, B.; Ertekin, A.; Gokceoglu, A. Apigenin alleviates neuroinflammation in a mouse model of Parkinson’s disease. Int. J. Neurosci. 2022, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022, 59, 6834–6856. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, B.; Zheng, Y.; Liu, X.; Rostyslav, P.; Finiuk, N.; Sik, A.; Stoika, R.; Liu, K.; Jin, M. Neuroprotective effect of chlorogenic acid on Parkinson’s disease like symptoms through boosting the autophagy in zebrafish. Eur. J. Pharmacol. 2023, 956, 175950. [Google Scholar] [CrossRef]
- Brunetti, G.; Rosa, G.D.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodríguez-Gómez, J.A.; Troncoso, A.M.; García-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; de Pablos, R.M. Hydroxytyrosol Decreases LPS- and α-Synuclein-Induced Microglial Activation in Vitro. Antioxidants 2020, 9, 36. [Google Scholar] [CrossRef]
- Pathania, A.; Kumar, R.; Sandhir, R. Hydroxytyrosol as anti-parkinsonian molecule: Assessment using in-silico and MPTP-induced Parkinson’s disease model. Biomed. Pharmacother. 2021, 139, 111525. [Google Scholar] [CrossRef]
- Perez-Barron, G.; Montes, S.; Aguirre-Vidal, Y.; Santiago, M.; Gallardo, E.; Espartero, J.L.; Ríos, C.; Monroy-Noyola, A. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP+ Model of Parkinson’s Disease. Neurochem. Res. 2021, 46, 2923–2935. [Google Scholar] [CrossRef]
- Mursaleen, L.; Noble, B.; Somavarapu, S.; Zariwala, M.G. Micellar Nanocarriers of Hydroxytyrosol Are Protective against Parkinson’s Related Oxidative Stress in an in Vitro hCMEC/D3-SH-SY5Y Co-Culture System. Antioxidants 2021, 10, 887. [Google Scholar] [CrossRef]
- Ba, Q.; Cui, C.; Wen, L.; Feng, S.; Zhou, J.; Yang, K. Schisandrin B shows neuroprotective effect in 6-OHDA-induced Parkinson’s disease via inhibiting the negative modulation of miR-34a on Nrf2 pathway. Biomed. Pharmacother. 2015, 75, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.C.; Fu, J.J.; Dang, Y.Y.; Zhang, Q.; Wang, X.F.; Chen, H.B.; Jia, X.J.; Lee, S.M.; Li, C.W. Pinocembrin-7-Methylether Protects SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity via Modulating Nrf2 Induction Through AKT and ERK Pathways. Neurotox. Res. 2021, 39, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, D.; Zhang, X.; Liu, D.; Xu, S.; Chen, G.; Huang, B.; Ren, W.; Wang, W.; Fu, S.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y.; He, C.; Chen, X.; Yu, L.; Yu, C.; Law, B.Y.; et al. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. elegans and Cellular Models of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef]
- Varshney, M.; Kumar, B.; Rana, V.S.; Sethiya, N.K. An Overview on Therapeutic and Medicinal Potential of Poly-hydroxy Flavone viz. Heptamethoxyflavone, Kaempferitrin, Vitexin and Amentoflavone for Management of Alzheimer’s and Parkinson’s Diseases: A critical analysis on mechanistic insight. Crit. Rev. Food Sci. Nutr. 2023, 63, 2749–2772. [Google Scholar] [CrossRef]
- Walia, V.; Chaudhary, S.K.; Sethiya, N.K. Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochem. Int. 2021, 143, 104939. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Ghiloria, N.; Srivastav, A.; Bisht, D.; Chaudhary, S.K.; Walia, V.; Alam, M.S. Therapeutic Potential of Myricetin in the Treatment of Neurological, Neuropsychiatric, and Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2023. online ahead of print. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Sun, J.; Cong, Q.; Chen, W.; Ahsan, H.M.; Gao, J.; Qian, J. Asiatic Acid Protects Dopaminergic Neurons from Neuroinflammation by Suppressing Mitochondrial ROS Production. Biomol. Ther. 2019, 27, 442–449. [Google Scholar] [CrossRef]
- He, Z.; Huan, P.; Wang, L.; He, J. Paeoniflorin ameliorates cognitive impairment in Parkinson’s disease via JNK/p53 signaling. Metab. Brain Dis. 2022, 37, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qu, R.; Zhang, J.; Li, L.; Ma, S. Neuroprotective effects of Madecassoside in early stage of Parkinson’s disease induced by MPTP in rats. Fitoterapia 2013, 90, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, C.; Sun, M.; Zhu, Y.; Chu, M.; Shi, Y.; Lin, S.L.; Yang, X.; Shen, Y. Neuroprotective Effects of Loganin on MPTP-Induced Parkinson’s Disease Mice: Neurochemistry, Glial Reaction and Autophagy Studies. J. Cell. Biochem. 2017, 118, 3495–3510. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Panda, S.R.; Kwatra, M.; Sahu, B.D.; Naidu, V. Perillyl Alcohol Attenuates NLRP3 Inflammasome Activation and Rescues Dopaminergic Neurons in Experimental In Vitro and In Vivo Models of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yang, W.; Cha, D.S.; Han, Y.T. Synthesis of a natural quinoline alkaloid isolated from the deep-sea-derived fungus and its potential as a therapeutic for Parkinson’s disease. J. Asian Nat. Prod. Res. 2023, 25, 446–455. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, J.; Xie, L.; Zhou, J.Y.; Li, Q.X.; Yang, R.Y.; Liu, Y.P.; Fu, Y.H. Monoterpenoids indole alkaloids with potential neuroprotective activities from the stems and leaves of Melodinus cochinchinensis. Nat. Prod. Res. 2022, 36, 5181–5188. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.; Pan, L.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Caffeine: An Overview of Its Beneficial Effects in Experimental Models and Clinical Trials of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 4766. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Meeran, M.F.N.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson’s Disease. Molecules 2019, 24, 2182. [Google Scholar] [CrossRef]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef] [PubMed]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Ostroumova, O.S. Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure-Activity Relationships, and Implications in Reconstituted Ion Channels. Membranes 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and Their Nanoformulations: Protective Effects against Human Diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms. Foods 2023, 12, 1309. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of Dietary Phenolics for Inhibition of Human Monoamine Oxidases A and B. BioMed Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. MONOAMINE OXIDASE: From Genes to Behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Carecho, R.; Carregosa, D.; Ratilal, B.O.; Figueira, I.; Ávila-Gálvez, M.A.; Santos, C.N.; Loncarevic-Vasiljkovic, N. Dietary (Poly)phenols in Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 8908. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Yan, L.; Guo, M.; Zhang, Y.; Yu, L.; Wu, J.; Tang, Y.; Ai, W.; Zhu, F.; Law, B.Y.; Chen, Q.; et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure–Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ju, M.S.; Ha, S.K.; Lee, H.; Lee, H.; Kim, S.Y.; Oh, M.S. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol. Pharm. Bull. 2012, 35, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, Y.J.; Shin, M.; Kim, H.; Kim, M.J.; Lee, S.H.; Yun, S.P.; Kwon, S. Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson’s disease model. Bioorg. Med. Chem. Lett. 2017, 27, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Jadhav, R.; Kulkarni, Y.A. Effects of baicalein with memantine on aluminium chloride-induced neurotoxicity in Wistar rats. Front. Pharmacol. 2023, 14, 1034620. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, N.; Li, X. Preclinical Evidence and Possible Mechanisms of Baicalein for Rats and Mice with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 277. [Google Scholar] [CrossRef]
- Chen, M.; Peng, L.; Gong, P.; Zheng, X.; Sun, T.; Zhang, X.; Huo, J. Baicalein Induces Mitochondrial Autophagy to Prevent Parkinson’s Disease in Rats via miR-30b and the SIRT1/AMPK/mTOR Pathway. Front. Neurol. 2021, 12, 646817. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef]
- Sergi, C.M. Epigallocatechin gallate for Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1029–1041. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, M.; Xue, J.; Xiang, L.; Li, Y.; Xiao, J.; Xiao, G.; Wang, H. EGCG ameliorates neuronal and behavioral defects by remodeling gut microbiota and TotM expression in Drosophila models of Parkinson’s disease. FASEB J. 2020, 34, 5931–5950. [Google Scholar] [CrossRef]
- Caruana, M.; Vassallo, N. Tea Polyphenols in Parkinson’s Disease. Adv. Exp. Med. Biol. 2015, 863, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Tamilselvam, K.; Radhiga, T.; Rao, S.; Essa, M.M.E.; Manivasagam, T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson’s disease. Brain Res. 2012, 1433, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Grelle, G.; Otto, A.; Lorenz, M.; Frank, R.F.; Wanker, E.E.; Bieschke, J. Black tea theaflavins inhibit formation of toxic amyloid-β and α-synuclein fibrils. Biochemistry 2011, 50, 10624–10636. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Lee, Y.S.; Park, H.; Kwak, M.; Yoo, B.K.; Kim, J.Y.; Kim, J. Protective effects of fustin, a flavonoid from Rhus verniciflua Stokes, on 6-hydroxydopamine-induced neuronal cell death. Exp. Mol. Med. 2007, 39, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Farzaei, M.H.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Hesperidin plays beneficial roles in disorders associated with the central nervous system: A review. Int. J. Food Prop. 2023, 26, 1867–1884. [Google Scholar] [CrossRef]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective Effects of Hesperidin, a Plant Flavanone, on Rotenone-Induced Oxidative Stress and Apoptosis in a Cellular Model for Parkinson’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef]
- Antunes, M.S.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef]
- Li, P.; Feng, D.; Yang, D.; Li, X.; Sun, J.; Wang, G.; Tian, L.; Jiang, X.; Bai, W. Protective effects of anthocyanins on neurodegenerative diseases. Trends Food Sci. Technol. 2021, 117, 205–217. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.; Simon, J.E.; Lila, M.A.; Rochet, J. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinsons disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Robinson, M.; Ding, X.; Geetha, T.; Al-Nakkash, L.; Broderick, T.L.; Babu, J.R. Genistein: A focus on several neurodegenerative diseases. J. Food Biochem. 2022, 46, e14155. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Ali, F.; Rahul. Effect of Genistein on the Transgenic Drosophila Model of Parkinson’s Disease. J. Diet. Suppl. 2019, 16, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, W.; Xie, J.; Wong, M. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci. Res. 2008, 60, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Chandrasekhar, Y.; Kumar, G.P.; Ramya, E.M.; Anilakumar, K.R. Gallic Acid Protects 6-OHDA Induced Neurotoxicity by Attenuating Oxidative Stress in Human Dopaminergic Cell Line. Neurochem. Res. 2018, 43, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, S.A.; Renu, K.; Zahra, H.A.; Abdallah, B.M.; Ali, E.M.; Veeraraghavan, V.P.; Sivalingam, K.; Ronsard, L.; Ammar, R.B.; Vidya, D.S.; et al. Polyphenols Mediate Neuroprotection in Cerebral Ischemic Stroke-An Update. Nutrients 2023, 15, 1107. [Google Scholar] [CrossRef]
- Pathan, A.S.; Jain, P.G.; Kumawat, V.S.; Katolkar, U.N.; Surana, S.J. Neuroprotective Effects of P-Coumaric Acid on Haloperidol-Induced Catalepsy Through Ameliorating Oxidative Stress and Brain Dopamine Level. J. Pharmacol. Pharmacother. 2022, 13, 364–374. [Google Scholar] [CrossRef]
- Dolrahman, N.; Mukkhaphrom, W.; Sutirek, J.; Thong-Asa, W. Benefits of p-coumaric acid in mice with rotenone-induced neurodegeneration. Metab. Brain Dis. 2023, 38, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Yuksel, Y.; Sehitoglu, M.H.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Goksel, F.; Karavelioglu, E.; Cosar, M. The Effect of Coumaric Acid on Ischemia-Reperfusion Injury of Sciatic Nerve in Rats. Inflammation 2015, 38, 2124–2132. [Google Scholar] [CrossRef]
- Pluta, R.; Miziak, B.; Czuczwar, S.J. Apitherapy in Post-Ischemic Brain Neurodegeneration of Alzheimer’s Disease Proteinopathy: Focus on Honey and Its Flavonoids and Phenolic Acids. Molecules 2023, 28, 5624. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; Masi, L.D.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Ardah, M.T.; Bharathan, G.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents Dopamine Neuron Degeneration from Oxidative Stress and Neuroinflammation in MPTP Model of Parkinson’s Disease. Biomolecules 2020, 10, 1519. [Google Scholar] [CrossRef]
- Sarkaki, A.; Farbood, Y.; Dolatshahi, M.; Mansouri, S.M.T.; Khodadadi, A. Neuroprotective Effects of Ellagic Acid in a Rat Model of Parkinson’s Disease. Acta Med. Iran. 2016, 54, 494–502. [Google Scholar]
- Tian, L.; Wang, X.; Sun, Y.; Li, C.; Xing, Y.; Zhao, H.; Duan, M.; Zhou, Z.; Wang, S. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int. J. Biochem. Cell Biol. 2008, 40, 409–422. [Google Scholar] [CrossRef]
- Zhou, J.; Qu, X.; Li, Z.; Ji, W.; Liu, Q.; Ma, Y.; He, J. Salvianolic Acid B Attenuates Toxin-Induced Neuronal Damage via Nrf2-Dependent Glial Cells-Mediated Protective Activity in Parkinson’s Disease Models. PLoS ONE 2014, 9, e101668. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, X.; Zhang, L.; Yang, H.; Zhang, Q. Current Progress of Research on Neurodegenerative Diseases of Salvianolic Acid B. Oxid. Med. Cell. Longev. 2019, 2019, 3281260. [Google Scholar] [CrossRef] [PubMed]
- Güzelad, O.; Özkan, A.; Parlak, H.; Sinen, O.; Afşar, E.; Öğüt, E.; Yıldırım, F.B.; Bülbül, M.; Ağar, A.; Aslan, M. Protective mechanism of Syringic acid in an experimental model of Parkinson’s disease. Metab. Brain Dis. 2021, 36, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Ogut, E.; Armagan, K.; Gül, Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab. Brain Dis. 2022, 37, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Cheemanapalli, S.; Mopuri, R.; Golla, R.; Anuradha, C.M.; Chitta, S.K. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Presti-Silva, S.M.; Herlinger, A.L.; Martins-Silva, C.; Pires, R.G.W. Biochemical and behavioral effects of rosmarinic acid treatment in an animal model of Parkinson’s disease induced by MPTP. Behav. Brain Res. 2023, 440, 114257. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Liu, X.; Zhou, F.; Zhang, Z.; Zhang, L. Rosmarinic acid attenuates inflammatory responses through inhibiting HMGB1/TLR4/NF-κB signaling pathway in a mouse model of Parkinson’s disease. Life Sci. 2019, 223, 158–165. [Google Scholar] [CrossRef]
- Cai, G.; Lin, F.; Wu, D.; Lin, C.; Chen, H.; Wei, Y.; Weng, H.; Chen, Z.; Wu, M.; Huang, E.; et al. Rosmarinic Acid Inhibits Mitochondrial Damage by Alleviating Unfolded Protein Response. Front. Pharmacol. 2022, 13, 859978. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Giuliano, C.; Cerri, S.; Blandini, F. Potential therapeutic effects of polyphenols in Parkinson’s disease: In vivo and in vitro pre-clinical studies. Neural Regen. Res. 2021, 16, 234–241. [Google Scholar] [CrossRef]
- Huh, E.; Choi, J.G.; Choi, Y.; Ju, I.G.; Noh, D.; Shin, D.; Kim, D.H.; Park, H.; Oh, M.S. 6-Shogaol, an Active Ingredient of Ginger, Improves Intestinal and Brain Abnormalities in Proteus Mirabilis-Induced Parkinson’s Disease Mouse Model. Biomol. Ther. 2023, 31, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.G.; Gu, H.; Leem, J.; Park, K.K. Protective Effects of 6-Shogaol, an Active Compound of Ginger, in a Murine Model of Cisplatin-Induced Acute Kidney Injury. Molecules 2021, 26, 5931. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Avila, S.; Diaz, N.F.; Gómez-Pinedo, U.; Canales-Aguirre, A.A.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; Marquez-Aguirre, A.L.; Díaz-Martínez, N.E. Neuroprotective effects of phytochemicals on dopaminergic neuron cultures. Neurologia 2019, 34, 114–124. [Google Scholar] [CrossRef]
- Angeline, M.S.; Sarkar, A.; Anand, K.; Ambasta, R.K.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef]
- Singh, N.; Vishwas, S.; Kaur, A.; Kaur, H.; Kakoty, V.; Khursheed, R.; Chaitanya, M.V.N.L.; Babu, M.R.; Awasthi, A.; Corrie, L.; et al. Harnessing role of sesamol and its nanoformulations against neurodegenerative diseases. Biomed. Pharmacother. 2023, 167, 115512. [Google Scholar] [CrossRef]
- Bosebabu, B.; Cheruku, S.P.; Chamallamudi, M.R.; Nampoothiri, M.; Shenoy, R.R.; Nandakumar, K.; Parihar, V.K.; Kumar, N. An Appraisal of Current Pharmacological Perspectives of Sesamol: A Review. Mini Rev. Med. Chem. 2020, 20, 988–1000. [Google Scholar] [CrossRef]
- Shah, A.; Lobo, R.; Krishnadas, N.; Surubhotla, R. Sesamol and Health—A Comprehensive Review. Indian J. Pharm. Educ. 2019, 53, S28–S42. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application—A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Huang, K.S.; Lin, M. Oligostilbenes from the roots of Vitis amurensis. J. Asian Nat. Prod. Res. 1999, 2, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Oh, W.K.; Jang, I.; Park, J. Amurensin G induces autophagy and attenuates cellular toxicities in a rotenone model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2013, 433, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, M.; Deng, J.; Zhang, C.; Liu, M. Resveratrol exhibits neuroprotection against paraquat-induced PC12 cells via heme oxygenase 1 upregulation by decreasing MiR-136-5p expression. Bioengineered 2022, 13, 7065–7081. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.G.D.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In vivo and In vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Patel, A.; Olang, C.A.; Lewis, G.; Mandalaneni, K.; Anand, N.; Gorantla, V.R. An Overview of Parkinson’s Disease: Curcumin as a Possible Alternative Treatment. Cureus 2022, 14, e25032. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, Y.; Botchway, B.O.A.; Zhang, J.; Fan, R.; Zhang, Y.; Liu, X. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol. 2022, 164, 113091. [Google Scholar] [CrossRef]
- Mogharbel, B.F.; Cardoso, M.A.; Irioda, A.C.; Stricker, P.E.F.; Slompo, R.C.; Appel, J.M.; Oliveira, N.B.; Perussolo, M.C.; Saçaki, C.S.; Rosa, N.N.; et al. Biodegradable Nanoparticles Loaded with Levodopa and Curcumin for Treatment of Parkinson’s Disease. Molecules 2022, 27, 2811. [Google Scholar] [CrossRef]
- Fikry, H.; Saleh, L.A.; Gawad, S.A. Neuroprotective effects of curcumin on the cerebellum in a rotenone- induced Parkinson’s Disease Model. CNS Neurosci. Ther. 2022, 28, 732–748. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, H.; Gao, J. Natural Terpenoids as Neuroinflammatory Inhibitors in LPS-stimulated BV-2 Microglia. Mini Rev. Med. Chem. 2021, 21, 520–534. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandaraf, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tsai, C.; Chang, S.; Lin, C.; Huang, L.; Tsai, C. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem. Biol. Interact. 2015, 225, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Lin, C.Y.; Wu, C.R.; Tsai, C.H.; Tsai, C.W. Carnosic Acid Alleviates Levodopa-Induced Dyskinesia and Cell Death in 6-Hydroxydopamine-lesioned Rats and in SH-SY5Y Cells. Front. Pharmacol. 2021, 12, 703894. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S.; Holsinger, R.M.D. Neuroprotective Effects of Carnosic Acid: Insight into Its Mechanisms of Action. Molecules 2023, 28, 2306. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Ding, C.; Feng, X. Protective Effect of Ginkgolide B on Dopaminergic Neurons and Expression of Neuroinflammatory Factors in 6-OHDA-Induced Parkinson’s Disease Mice. J. Biomater. Tissue Eng. 2020, 10, 1312–1317. [Google Scholar] [CrossRef]
- Yin, J.; Miao, Q.; Wang, Q.; Huang, J.; Xiao, B.; Ma, C. The protective effect of Ginkgolide B on MPTP-induced Parkinson mice. J. Neurol. Sci. 2021, 429, 119481. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, S.; Liu, P.; Liu, W.; Wang, Q.; Liu, Y.; Tan, H.; Chen, X.; Shi, X.; Wang, Q.; et al. Polymeric Nanoparticles-Based Brain Delivery with Improved Therapeutic Efficacy of Ginkgolide B in Parkinson’s Disease. Int. J. Nanomed. 2020, 15, 10453–10467. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Habtemariam, S.; Daglia, M.; Braidy, N.; Loizzo, M.R.; Tundis, R.; Nabavi, S.F. Neuroprotective Effects of Ginkgolide B Against Ischemic Stroke: A Review of Current Literature. Curr. Top. Med. Chem. 2015, 15, 2222–2232. [Google Scholar] [CrossRef]
- Gachowska, M.; Szlasa, W.; Saczko, J.; Kulbacka, J. Neuroregulatory role of ginkgolides. Mol. Biol. Rep. 2021, 48, 5689–5697. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Fang, D. Pharmacological action and mechanisms of ginkgolide B. Chin. Med. J. 2007, 120, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lin, C.C.; Chen, Y.; Yang, H.; Hung, S. Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson’s Disease through Activating Mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jiang, X.; Feng, J. The therapeutic potential of triptolide and celastrol in neurological diseases. Front. Pharmacol. 2022, 13, 1024955. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Zhang, S.; Yue, S.; Chen, Y.; Xu, D.; Tang, Y. Potential medicinal value of celastrol and its synthesized analogues for central nervous system diseases. Biomed. Pharmacother. 2021, 139, 111551. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Souza, M.T.S.; Almeida, J.R.G.S.; Araujo, A.A.S.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; Santos, M.R.V.; Quintans-Júnior, L.J. Structure–activity relationship of terpenes with anti-inflammatory profile- a systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; Yan, R.; Li, L.; Xiao, Z.; Zhou, W.; Wang, Z.; Xiao, W.; Du, G. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Wang, L.; Lei, Q.; Zhao, S.; Xu, W.J.; Dong, W.; Ran, J.H.; Shi, Q.H.; Fu, J.F. Ginkgolide B Maintains Calcium Homeostasis in Hypoxic Hippocampal Neurons by Inhibiting Calcium Influx and Intracellular Calcium Release. Front. Cell. Neurosci. 2021, 14, 627846. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Çankaya, I.T.; Karatoprak, G.S.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural Compounds as Medical Strategies in the Prevention and Treatment of Psychiatric Disorders Seen in Neurological Diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Kempste, P.; Ma, A. Parkinson’s disease, dopaminergic drugs and the plant world. Front. Pharmacol. 2022, 13, 970714. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential Therapeutic Applications of Plant-Derived Alkaloids against Inflammatory and Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
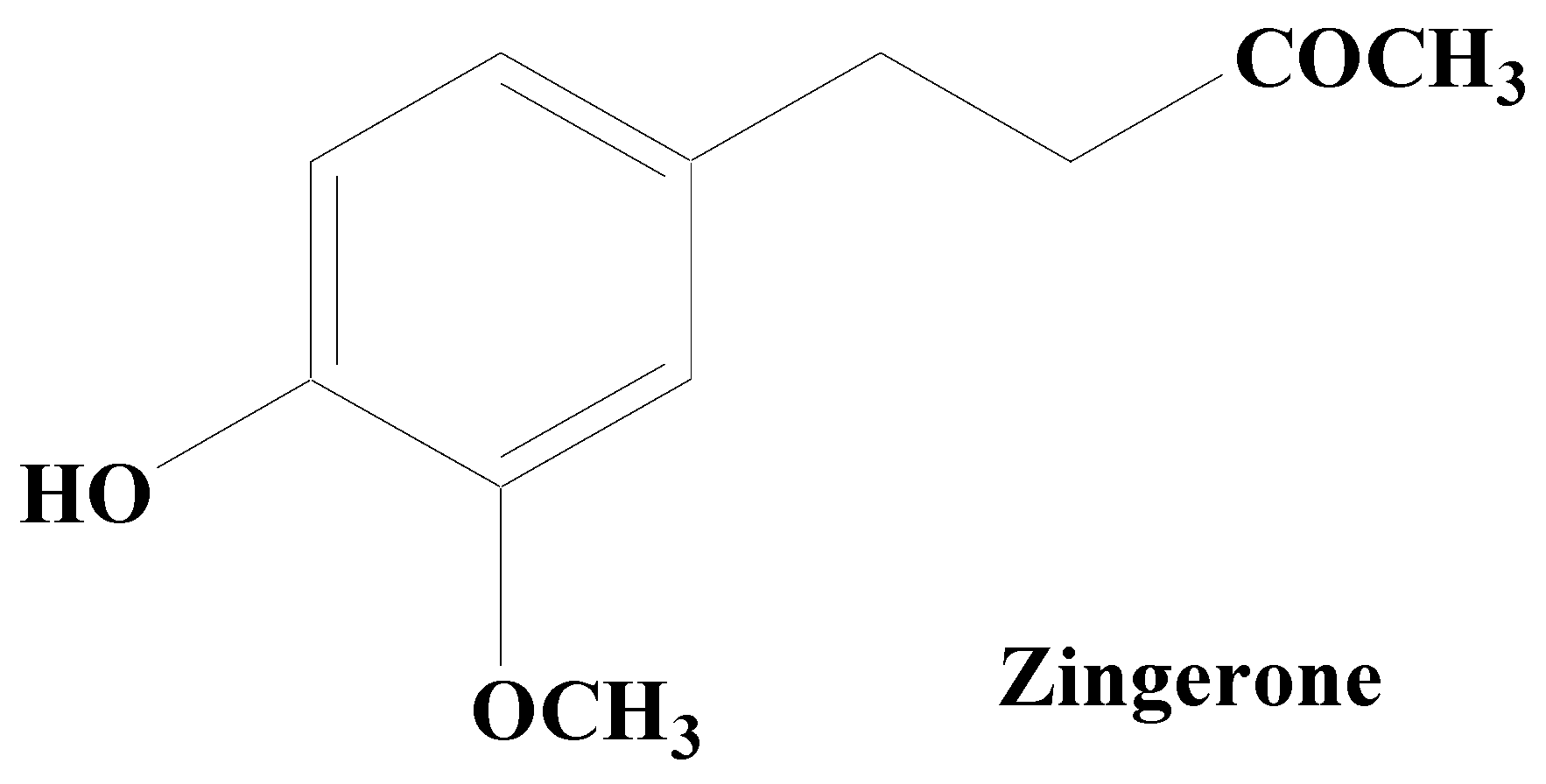
- Kabuto, H.; Yamanushi, T.T. Effects of zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] and eugenol [2-methoxy-4-(2-propenyl) phenol] on the pathological progress in the 6-hydroxydopamine-induced Parkinson’s disease mouse model. Neurochem. Res. 2011, 36, 2244–2249. [Google Scholar] [CrossRef]
- Kabuto, H.; Nishizawa, M.; Tada, M.; Higashio, C.; Shishibori, T.; Kohno, M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005, 30, 325–332. [Google Scholar] [CrossRef]
- Saraiva, A.A.; Silva, J.P.O.D.; Sousa, J.V.M.; Brandim, A.D. Molecular Docking of Zingerone and Gamma-Mangostin to Inhibit MAO-B and Catechol-O-Methyltransferase (COMT) in the Treatment of Parkinson’s Disease. Res. Soc. Dev. 2022, 11, e189111637853. [Google Scholar] [CrossRef]
- Rashid, S.; Wali, A.F.; Rashid, S.M.; Alsaffar, R.M.; Ahmad, A.; Jan, B.L.; Paray, B.A.; Alqahtani, S.M.A.; Arafah, A.; Rehman, M.U. Zingerone Targets Status Epilepticus by Blocking Hippocampal Neurodegeneration via Regulation of Redox Imbalance, Inflammation and Apoptosis. Pharmaceuticals 2021, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.R. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Y.; Chen, C.; Tsai, R.; Liu, S.; Chang, W.; Lin, H.; Lu, C.; Wei, J.; Wang, Z.; et al. Acetylcorynoline attenuates dopaminergic neuron degeneration and α-synuclein aggregation in animal models of Parkinson’s disease. Neuropharmacology 2014, 82, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research Progress on Natural Benzophenanthridine Alkaloids and their Pharmacological Functions: A Review. Nat. Prod. Commun. 2016, 11, 1181–1188. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yang, X. Blood-brain barrier permeability and neuroprotective effects of three main alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR cell monolayer and PC12 cell line. Biomed. Pharmacother. 2018, 98, 82–87. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Dong, S.; Yu, Y.; Wu, Y.; Xiang, B.; Li, Q. Research Progress on Neuroprotective Effects of Isoquinoline Alkaloids. Molecules 2023, 28, 4797. [Google Scholar] [CrossRef]
- Xiao, X.; Tong, Z.; Zhang, Y.; Zhou, H.; Luo, M.; Hu, T.; Hu, P.; Kong, L.; Liu, Z.; Yu, C.; et al. Novel Prenylated Indole Alkaloids with Neuroprotection on SH-SY5Y Cells against Oxidative Stress Targeting Keap1–Nrf2. Mar. Drugs 2022, 20, 191. [Google Scholar] [CrossRef]
- Kongkiatpaiboon, S.; Duangdee, N.; Prateeptongkum, S.; Chaijaroenkul, W. Acetylcholinesterase Inhibitory Activity of Alkaloids Isolated from Stephania venosa. Nat. Prod. Commun. 2016, 11, 1805–1806. [Google Scholar] [CrossRef]
- Nishal, S.; Phaugat, P.; Bazaad, J.; Dhaka, R.; Khatkar, S.; Khatkar, A.; Khayatkashani, M.; Alizadeh, P.; Haghighi, S.M.; Mehri, M.; et al. A Concise Review of Common Plant-derived Compounds as a Potential Therapy for Alzheimer’s Disease and Parkinson’s Disease: Insight into Structure-Activity-Relationship. CNS Neurol. Disord. Drug Targets 2023, 22, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, C.K.; Uti, D.E.; Mukherjee, D.; Alqahtani, T.; Alqahtani, S.; Bhattacharya, A.; Akash, S. Unveiling Nature’s potential: Promising natural compounds in Parkinson’s disease management. Park. Relat. Disord. 2023, 10, 105799. [Google Scholar] [CrossRef] [PubMed]
- Baburaj, R.; Kuntal, D.A. Neuroprotective role of a protoberberine alkaloid against aluminium-induced neuroinflammation and excitotoxicity. Not. Sci. Biol. 2023, 15, 11488. [Google Scholar] [CrossRef]


| Name of Extract or Formulation | Dose | Study Model | Mechanism of Action |
|---|---|---|---|
| Acanthopanax senticosus extract | 4.5 g/kg | MPTP-induced mice | Regulated multiple targets to improve motor deficits [40]. |
| Antrodia comphorata | 10–50 mmol/L (in vitro); 10, 50, and 100 mg/kg (in vivo) | 6-OHDA induced MES23.5 cells and C57BL/6 mice | Downregulate NLRP3, ASC, IL-1β, caspase-1, and ROS and upregulate dopaminergic neuron protection [41,42]. |
| Asarum sieboldii | 5 μM | Olfactory cell line (hONS) | Induced significant perturbation on biological organelles [43]. |
| Cervus nippon (Velvet antler from sika deer) | 20–40 μg/mL and 30 mg/kg | In vitro (BV2 cells), Caenorhabditis elegans, and MPTP-treated mice | Decreased aggregation of α-synuclein and protect from oxidative stress-induced DAergic neuron degeneration [44]. |
| Codium tomentosum enriched fractions | 100 µg/mL | 6-OHDA-induced SH-SY5Y human cells | Mitigation of ROS generation, mitochondrial dysfunctions, and DNA damage followed by reduction in Caspase-3 activity [45]. |
| Crossyne flava | 2.5, 5, and 10 µg/mL | MPP+-induced SH-SY5Y cells. | Inhibited ROS and ATP depletion followed by induction of apoptosis [46]. |
| Ganoderma lucidum extract | 800 μg/mL and 400 mg/kg | Neuro-2a cells and mouse model | Regulating autophagy, mitochondrial function, and apoptosis [47]. |
| Geranium robertianum aqueous extract | 0–200 µg/mL | MPP+-induced SH-SY5Y | Antioxidant and apoptosis inhibitory properties [48]. |
| Hidrox® with Hydroxytyrosol | 10 mg/kg, i.p. | Rotenone induced mice | Improves neuroinflammation, oxidative stress, and apoptosis [49]. |
| Liuwei Dihuang Pills (enriched with quercetin, stigmasterol, kaempferol, and β-sitosterol) | Not available | Network pharmacology (in silico) | Regulates AKT1, VEGFA, and IL6, G protein-coupled amine receptor activity, ROS, membrane raft, MAPK signaling pathway, and cellular senescence [50]. |
| Myrica esculenta leaves methanol extract | 50, 100, and 200 mg/kg, orally for one week | Haloperidol-induced rats | Escalation of cellular antioxidants [51]. |
| Polyscias fruticosa leaves extract | 1, 2, 4, 8, and 16 mg/mL | Drosophila melanogaster model (dUCH knockdown) | Ameliorate dopaminergic neuron degeneration [52]. |
| Sphaerocoryne affinis fruit water extract | 3, 6, 12, and 18 mg/mL. | DPPH and fly model | Ameliorate the locomotor disabilities and degeneration of dopaminergic neurons [53]. |
| Compounds | Botanical Sources | Dose | Study Model | Mechanism of Action |
|---|---|---|---|---|
| Polyphenols | ||||
| Quercetin | Onions, apples, tea, brassica vegetables, and nuts | 30 mg/kg for 30 days | Acrolein (3 mg/kg for 30 days) induced rats | Protects cerebellum tissues from neurotoxicity and oxidative stress [54]. |
| Apigenin | Grape fruit, parsley, celery, and oranges | 50 mg/kg apigenin, 5 days | MPTP (25 mg/kg for 5 days) induced mouse | Reverses the expressions and concentrations of TNF-α, IL-1β, IL-6, IL-10, and TGF-β [55]. |
| Chlorogenic acid | Coffee (Coffea arabica) | 1, 5, 10, 20, 40, and 100 µg/mL (in vitro); 50 mg/kg, orally for 13 weeks (in vivo). | In silico, in vitro (GLUTag cell line), and in vivo (rotenone-induced PD mice). | Acts as GLP-1 secretagogue [56]. |
| Coffee, honeysuckle, and Eucommia | 75, 150, and 300 μM | MPTP zebrafish (6-OHDA-treated SHSY5Y cells). | Boosting the autophagy in neuronal cells [57]. | |
| Hydroxytyrosol | Extra virgin olive oil | 250 µg/mL | C. elegans models | Improvements in locomotive behavior and the attenuation of autofluorescence [58]. |
| Virgin olive oil | 1, 10, 25, and 50 μM | Murine microglial BV2 cell line | Microglial activation, expression of NADPH oxidase, MAPKs, and production of ROS [59]. | |
| Extra virgin olive oil | 0.1–200 µM (in vitro) and 50 mg/kg (in vivo). | In silico, in vitro (platelet MAO-B activity) and MPTP-induced mouse model | MAO-B inhibition (IC50: 7.78 μM), improved DA and motor impairments [60]. | |
| Methanol extract of Buddleja cordata and extra virgin olive oil | 1.5 mg/kg | MPP+ induced rats | Inhibitory effect on the MAO isoforms (MAO A and MAO B) [61]. | |
| Micellar nanocarriers | 10–200 μM | In vitro (hCMEC/D3-SH-SY5Y) cells (rotenone) | Improves oxidative stress by 12% and 9%, respectively, compared to the corresponding free drug [62]. | |
| Schisandrin B | Schisandra chinensis | 100 μM | 6-OHDA-induced SH-SY5Y cells and mice | Inhibitis the negative modulation of miR-34a on Nrf2 pathway [63]. |
| Ginkgolic acid | Ginkgo biloba leaves | 10, 40, and 80 μM | KCl-induced SH-SY5Y cells | Promotes autophagy-dependent clearance of α-syn aggregates [64]. |
| Pinocembrin-7-methylether | Pigeon pea, thai ginger, honey, and propolis | Up to 200 μM | 6-OHDA-induced SH-SY5Y cells and zebrafsh | Activation of Nrf2/ARE/HO-1 signaling cascades [65]. |
| Chrysin | Passion flowers (Oroxylum indicum, Passiflora incarnata and Passiflora caerulea), Scutellaria baicalensis, mushrooms, bee propolis, and honey | Up to 500 mg/kg | In various in vitro and in vivo models | Increasing the expression of Nrf2, activates MEF2D, suppresses the MPP-induced upregulation of c-caspase and Bax, as well as the downregulation of anti-apoptotic protein Bcl 2. Additionally, enhances the production of neurotrophic factors and increase dopamine levels in the striatum via MAO-B [66]. |
| Vanillin | Natural vanilla | 100, 200, 300, 400, and 500 nM (in vitro) and 5, 10, or 20 mg/kg (in vivo) | LPS-induced murine microglial BV-2 cells and rats | Reduces over expression of iNOS, COX-2, IL-1β, and IL-6 through regulating ERK1/2, p38 and NF-κB signaling [67]. |
| Ferulic acid | Rhizoma Ligustici wallichii, Angelica sinensis, and Asafoetida giantfennel | 50 μM | 6-OHDA-induced C. elegans models | Autophagy induction [68]. |
| Heptamethoxyflavone | Orange and grapefruit | 3–10 µM (in vitro) and 1.2–100 mg/kg (in vivo) | In various in vitro and in vivo models | Regulates IL-1β expression and suppresses MK-801-induced locomotive hyperactivity [69]. |
| Kaempferitrin | Cinnamomum osmophloeum | 25 µM (in vitro) and 2–5 mg/kg (in vivo) | In various in vitro and in vivo models | Prevents H2O2-induced oxidative stress [69]. |
| Vitexin | Hawthorn, pearl millet, mung bean, pigeon pea, mosses and tartary buckwheat sprouts | 10–50 µM (in vitro) and 1–100 mg/kg (in vivo) | In various in vitro and in vivo models | Regulates PI3/AKT, mTOR pathway; enhanced effect of TPv1 and NR2B pathway, suppresses CDPK II, prevents lipid peroxidation by TBHP; and inhibits effect of CYP2C11 and CYP3A1 [69]. |
| Amentoflavone | Cnestis ferruginea, Hypericum perforatum, Viburnum and Ginkgo species. | 0.1–60 µM (in vitro) and 0.1–100 µg/mL (in vivo) | In various in vitro and in vivo models | Inhibits COX and phospholipase A2, activate p38-AKT signaling pathway and inhibits production of prostaglandins E2 [69]. |
| Mangiferin | Swertia minor and Mangifera pajang | Up to 100 mg/kg | In various in vitro and in vivo models | Counteracts the neurotoxic effect of MPTP, rotenone, and 6-OHDA, etc. [70]. |
| Myricetin | Myrica nagi | Up to 100 mg/kg | In various in vitro and in vivo models | Protective effect against amyloid-beta, MPTP, rotenone, and 6-OHDA, etc. [71]. |
| Terpenes | ||||
| Asiatic acid | Centella asiatica | 10–100 nM | LPS-induced BV2 microglia cells and MPP+-induced SH-SY5Y cells, | Protects dopaminergic neurons from neuroinflammation by suppressing NLRP3 inflammasome activation in microglia cells as well as protecting dopaminergic neurons directly [72]. |
| Paeoniflorin | Herbaceous peony | 30 mg/kg | Network pharmacology and MPTP-induced mice | Inhibits apoptosis in hippocampal neurons of the CA1 and CA3, and upregulates PSD-95 as well as SYN protein levels. Similar protective effects were observed upon JNK/p53 pathway inhibition using SP600125 [73]. |
| Madecassoside | Centella asiatica | 15, 30, 60 mg/kg | MPTP-induced rats | Reversing the depletion of DA, antioxidant activity, increasing ratio of Bcl-2/Bax, increasing protein expression of BDNF [74]. |
| Loganin | Cornus officinalis fruits | 50 mg/kg | MPTP-induced mice | Reduce inflammation, autophagy, and apoptosis [75]. |
| Perillyl alcohol | Mentha haplocalyx | 200 µM | LPS and MPTP induced in vitro and in vivo study | Attenuates NLRP3 inflammasome activation and rescues dopaminergic neurons [76]. |
| Alkaloids | ||||
| 2-(Quinoline-8-carboxamido) benzoic acid | Aspergillus sp. | Various dose | MPP+-induced Caenorhabditis elegans | Modulates the formation of neurotoxic α-synuclein ameliorated induced dopaminergic neurodegeneration [77]. |
| Melodicochine A | Stems and leaves of Melodinus cochinchinensis | 0.72 to 17.89 μM | 6-hydroxydopamine-induced SH-SY5Y cells | Neuroprotection [78]. |
| Berberine | Coptis chinensis and Berberis vulgaris | 100–200 mg/kg | Mice and bacteria | Improves brain dopa/dopamine levels [79]. |
| Caffeine (1,3,7-trimethylxanthin) | Seeds and leaves of coffee (Coffea arabica), tea (Camellia sinensis) and cocoa (Theobroma cacao L.) | 20 mg/kg (varied dose) | In various in vitro and in vivo models | Exhibits antioxidant properties and inhibits lipid peroxidation [80]. |
| Lycopodium | Lycopodiaceae plants | 50 mg/kg | Rotenone-induced rat | Reduction in pro-inflammatory response and α-synuclein expression. Also, synergistically enhances antioxidant defense via multimodal action [81]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, N.; Anand, S.; Shah, K.; Chauhan, N.S.; Sethiya, N.K.; Singhal, M. Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease. Molecules 2023, 28, 7588. https://doi.org/10.3390/molecules28227588
Kumari N, Anand S, Shah K, Chauhan NS, Sethiya NK, Singhal M. Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease. Molecules. 2023; 28(22):7588. https://doi.org/10.3390/molecules28227588
Chicago/Turabian StyleKumari, Nitu, Santosh Anand, Kamal Shah, Nagendra Singh Chauhan, Neeraj K. Sethiya, and Manmohan Singhal. 2023. "Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease" Molecules 28, no. 22: 7588. https://doi.org/10.3390/molecules28227588
APA StyleKumari, N., Anand, S., Shah, K., Chauhan, N. S., Sethiya, N. K., & Singhal, M. (2023). Emerging Role of Plant-Based Bioactive Compounds as Therapeutics in Parkinson’s Disease. Molecules, 28(22), 7588. https://doi.org/10.3390/molecules28227588