Metabolic Transformation of Gentiopicrin, a Liver Protective Active Ingredient, Based on Intestinal Bacteria
Abstract
:1. Instruction
2. Results
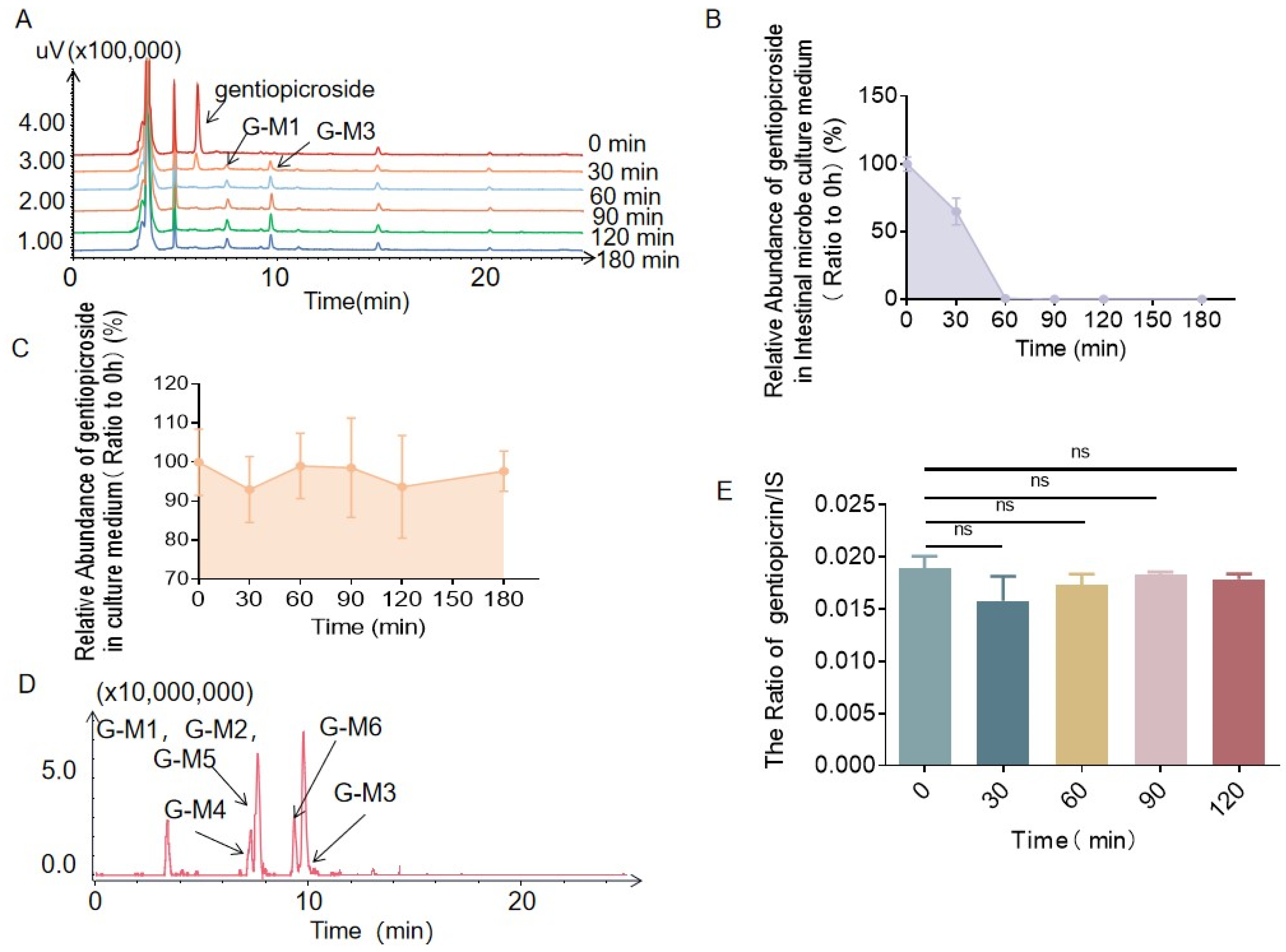
2.1. Biotransformation of Gentiopicrin by Intestinal Bacteria and Liver Microsomes
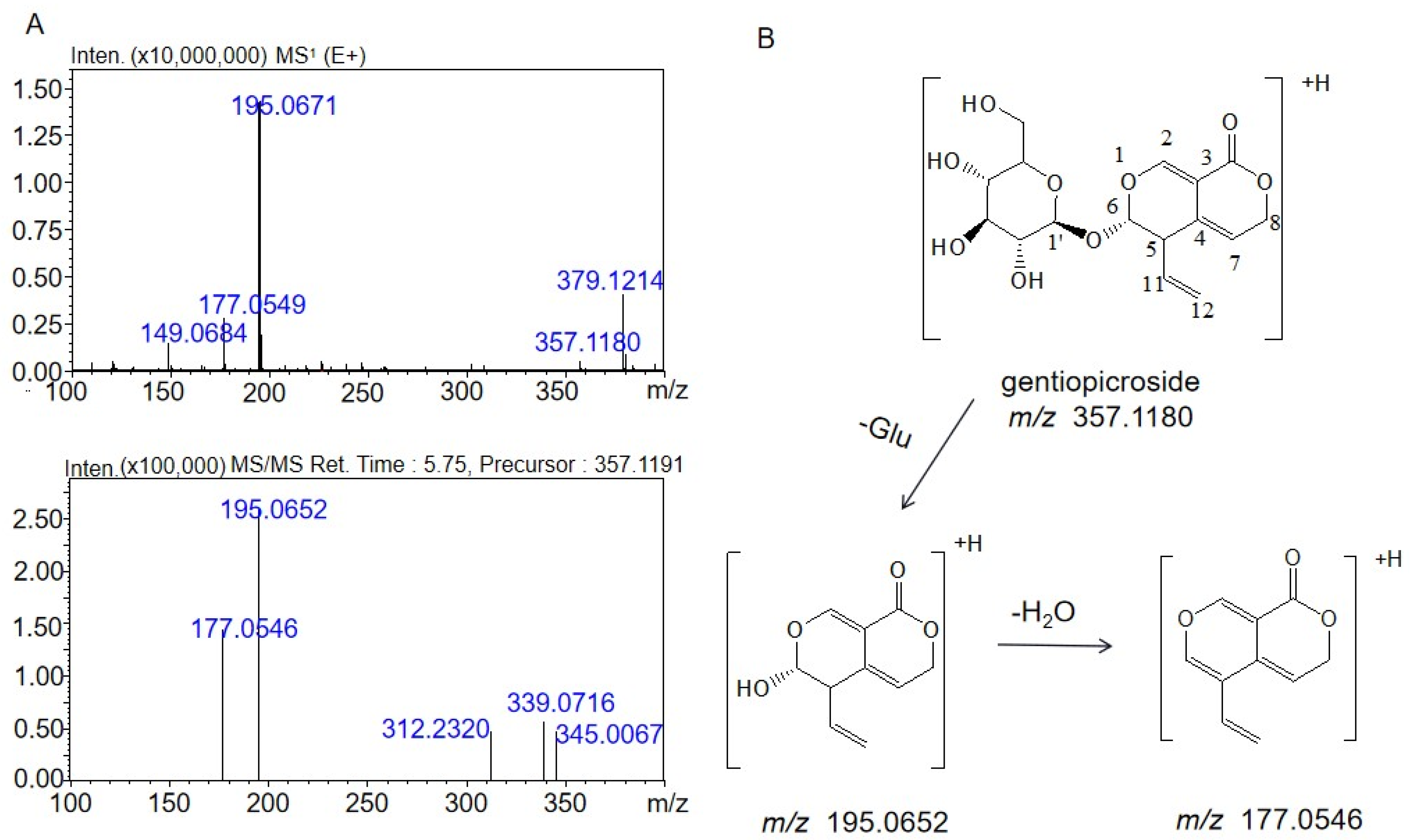
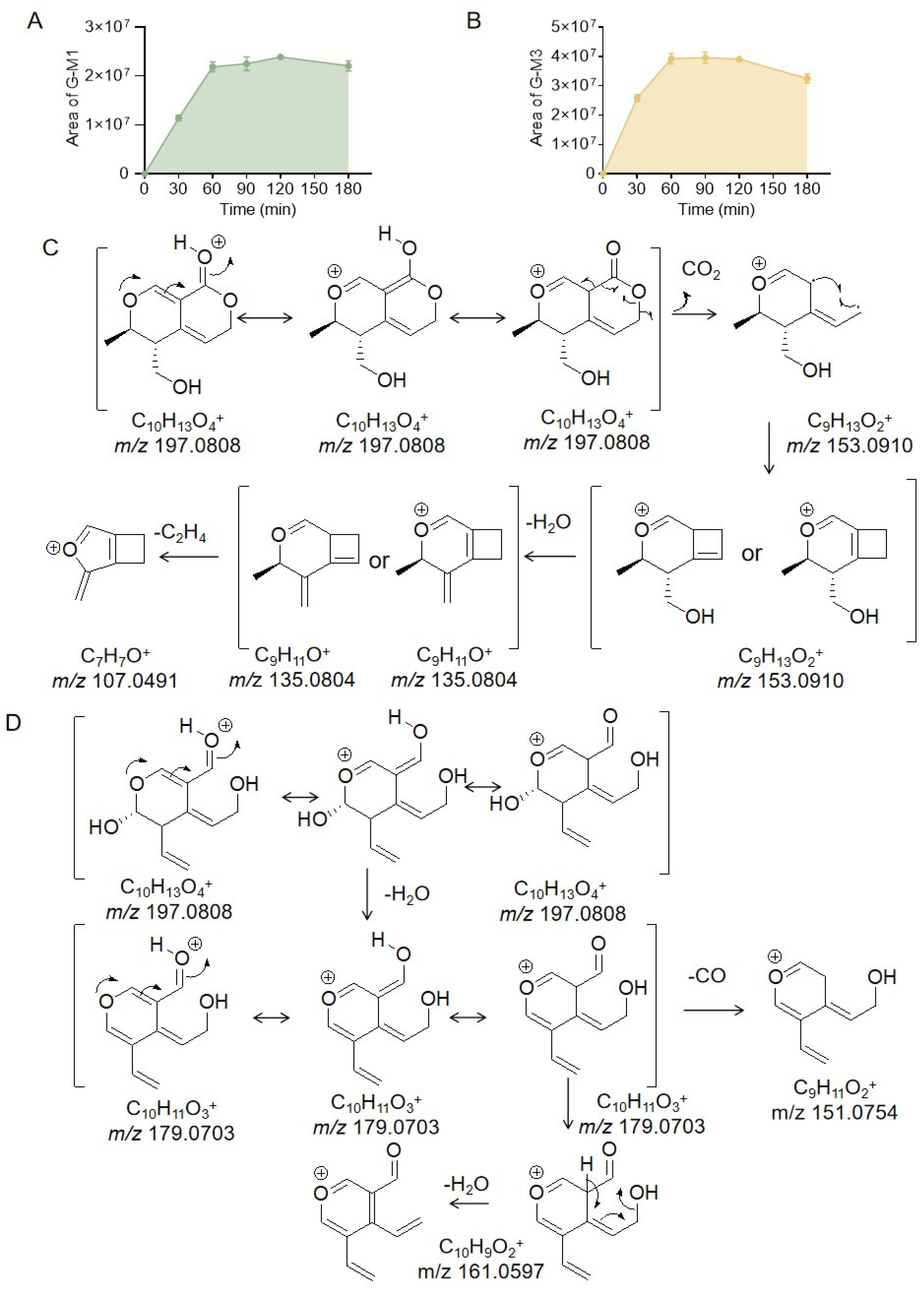
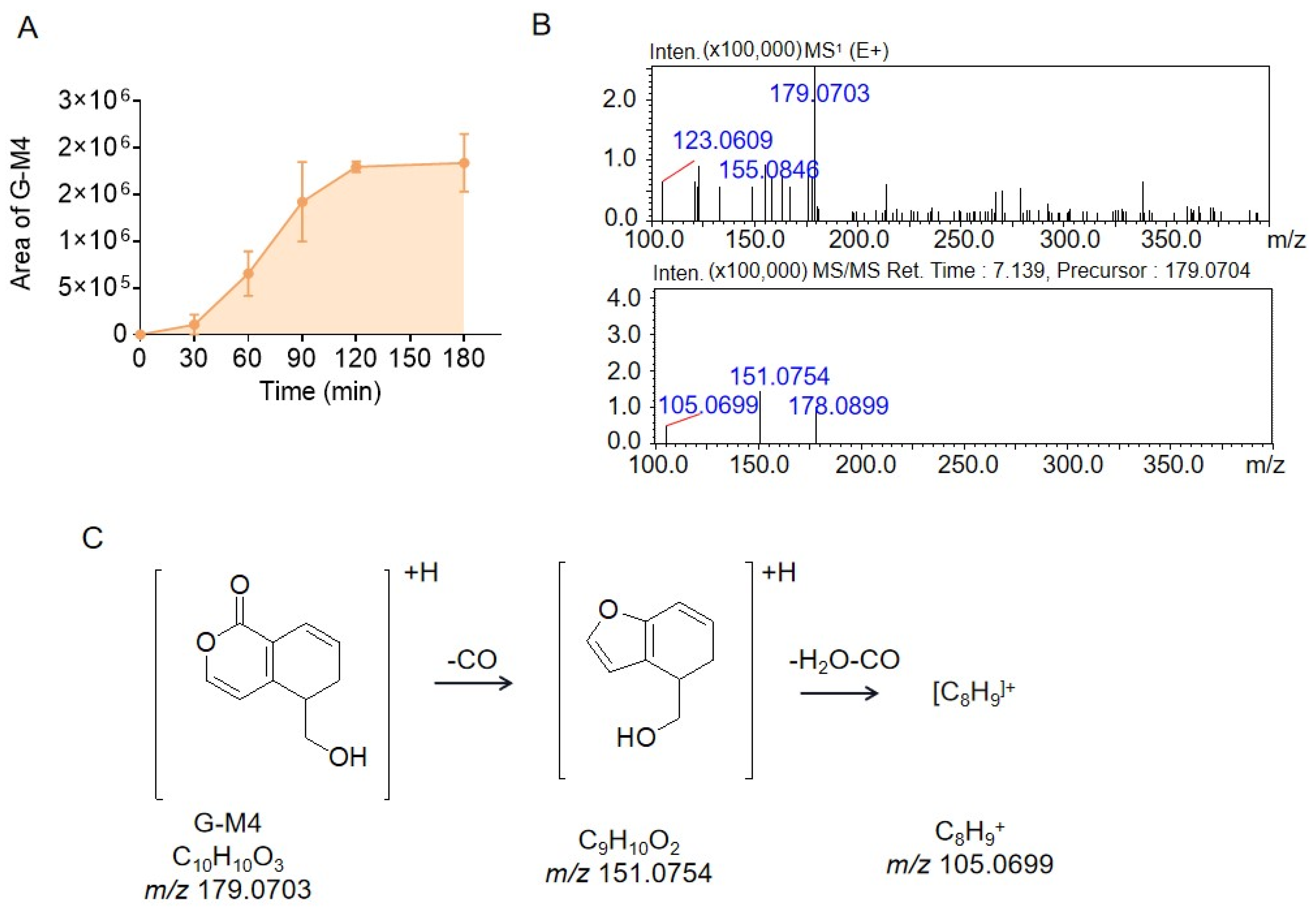
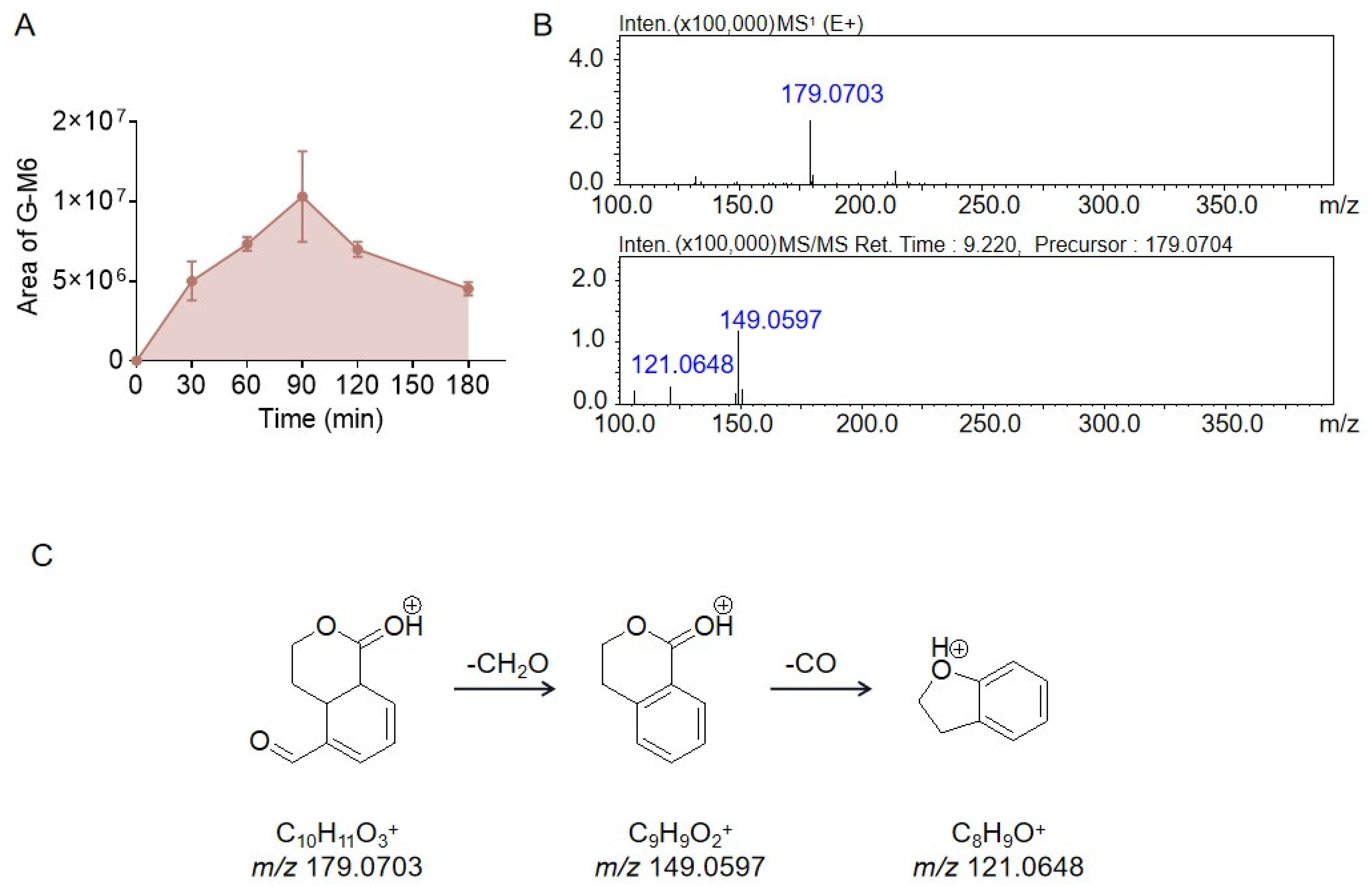
2.2. Identification of the Metabolites Generated by the Intestinal Bacteria
2.3. Proposed Metabolite Pathway of Gentiopicrin
3. Discussion
4. Materials and Methods
4.1. Instruments and Reagents
4.2. Animals
4.3. Identification of Gentiopicrin Metabolites by LC/MSn-IT-TOF
4.4. Determination of Gentiopicrin by LC-MS/MS
4.5. In Vitro Incubation of Gentiopicrin with Gut Microbiota
4.6. In Vitro Incubation of Gentiopicrin with Liver Microsomes
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China, 11th ed.; China Medical Science Press: Beijing, China, 2020; Volume I, pp. 808–809. [Google Scholar]
- Zhang, Y.; Yang, X.M.; Wang, S.; Song, S.; Yang, X.D. Gentiopicroside prevents alcoholic liver damage by improving mitochondrial dysfunction in the rat model. Phytother Res. 2021, 35, 2230–2251. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Shang, Y.; Jin, Q.; Wu, Y.L.; Liu, J.; Qiao, C.Y.; Zhan, Z.Y.; Ye, H.; Nan, J.X.; Lian, L.H. Gentiopicroside Ameliorates the Progression from Hepatic Steatosis to Fibrosis Induced by Chronic Alcohol Intake. Biomol. Ther. 2020, 28, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Ning, Y.Y.; Zhao, Y.Y.; Sun, W.J.; Thorimbert, S.; Dechoux, L.; Sollogoub, M.; Zhang, Y.M. Research Progress of Natural Product Gentiopicroside—A Secoiridoid Compound. Mini-Rev. Med. Chem. 2017, 17, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.M.; Sun, X.H.; Lin, Z.Y.; Yang, Y.; Zhang, M.; Xu, Z.C.; Liu, P.Q.; Liu, Z.Q.; Huang, H.Q. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, Z.T.; Bligh, S.W.A.; White, K.N.; White, C.J.B. Pharmacokinetics and tissue distribution of gentiopicroside following oral and intravenous administration in mice. Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 199–203. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Fu, J.; Ma, S.R.; Peng, R.; Yu, J.B.; Cong, L.; Pan, L.B.; Zhang, Z.G.; Tian, H.; Che, C.T.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.W.; Zhao, Z.X.; He, C.Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.S.; Li, X.Y.; Wen, B.Y.; et al. Transforming berberine into its intestine-absorbable form by gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- El-Sedawy, A.I.; Hattori, M.; Kobashi, K.; Namba, T. Metabolism of gentiopicroside (gentiopicrin) by human intestinal bacteria. Chem. Pharm. Bull. 1989, 37, 2435–2457. [Google Scholar] [CrossRef]
- Zeng, W.L.; Han, H.; Tao, Y.Y.; Yang, L.; Wang, Z.T.; Chen, K.X. Identification of bio-active metabolites of gentiopicroside byUPLC/Q-TOF MS and NMR. Biomed. Chromatogr. 2013, 27, 1129–1136. [Google Scholar] [CrossRef]
- Wang, Z.G.; Tang, S.H.; Jin, Y.; Zhang, Y.; Hattori, M.; Zhang, H.L.; Zhang, N. Two main metabolites of gentiopicroside detected in rat plasma by LC–TOF-MS following 2,4-dinitrophenylhydrazine derivatization. J. Pharm. Biomed. Anal. 2015, 107, 1–6. [Google Scholar] [CrossRef]
- Zeng, W.L.; Li, W.K.; Han, H.; Tao, Y.Y.; Yang, L.; Wang, Z.T.; Chena, K.X. Microbial Biotransformation of Gentiopicroside by the Endophytic Fungus Penicillium crustosum 2T01Y01. Appl. Environ. Microbiol. 2014, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Song, C.Q. Studies on the Metabolism of Gentiopcroside by Rat Intestinal Flora. Chin. J. Chin. Mater. Med. 2000, 11, 673–676. [Google Scholar]
- Kuang, H.X.; Wu, G.S.; Liu, H.; Wu, Q.; Wang, Q.H.; Wang, Z.B. Progress in biotransformation and pharmacological activities of gentiopicroside and gentianine. Tianjin J. Tradit. Chin. Med. 2016, 7, 385–389. [Google Scholar]
- Xiong, K.; Gao, T.T.; Zhang, T.; Wang, Z.T.; Han, H. Simultaneous determination of gentiopicroside and its two active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1065–1066, 1–7. [Google Scholar] [CrossRef]
- Han, H.; Xiong, A.Z.; He, C.Y.; Liu, Q.; Yang, L.; Wang, Z.T. Combination of UHPLC/Q-TOF-MS, NMR spectroscopy, and ECD calculation for screening and identification of reactive metabolites of gentiopicroside in humans. Anal. Bioanal. Chem. 2014, 406, 1781–1793. [Google Scholar] [CrossRef]
- Hassan, N.; Ahamad, J.; Amin, S.; Mujeeb, M.; Mir, S.R. Rapid preparative isolation of erythrocentaurin from Enicostemma littorale by medium-pressure liquid chromatography, its estimation by high-pressure thin-layer chromatography, and its α-amylase inhibitory activity. J. Sep. Sci. 2015, 38, 592–598. [Google Scholar] [CrossRef]
- Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Synthesis of erythrocentaurin derivatives as a new class of hepatitis B virus inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1568–1571. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Gupta, A.; Saha, S.; Khanna, S. Therapies to modulate gut microbiota: Past, present and future. World J. Gastroenterol. 2020, 26, 777–788. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Liu, C.; Han, Z.Z.; Han, H.; Yang, L.; Wang, Z.T. Microbial Biotransformation of Iridoid Glycosides from Gentiana Rigescens by Penicillium Brasilianum. Chem. Biodivers. 2020, 17, e2000676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, J.M.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the Molecular Mechanisms of the Interplay Between Herbal Medicines and Gut Microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- ElKaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]


| Compounds | Reaction | Molecular Formula | Fragment Characteristics | ||
|---|---|---|---|---|---|
| MS1/[M + H]+ | MS/MS | MS3 | |||
| Gentiopicrin | - | C16H20O9 | 357.1180 | 195.0650 | 177.0545 |
| G-M1/G-M2 | Hydrolysis + Reduction | C10H12O4 | 197.0808 | 179.0703 153.0910 135.0804 | 107.0491 |
| G-M3 | Hydrolysis | C10H12O4 | 197.0808 | 179.0703 | 161.0597 151.0754 |
| G-M4 | Hydrolysis + Reduction | C10H10O3 | 179.0703 | 151.0754 | 105.0699 |
| G-M5 | Hydrolysis + Dehydration | C10H10O3 | 179.0703 | 149.0597 | 117.0335 |
| G-M6 | Hydrolysis + Reduction | C10H10O3 | 179.0703 | 149.0597 | 121.0648 |
| Position | 1H-NMR(G-M1) | 1H-NMR (G-M2) | 13C-NMR (G-M1) | 13C-NMR (G-M2) |
|---|---|---|---|---|
| 2 | 7.50 (d, J = 1.2) | 7.58 (d, J = 1.2) | 152.9 | 154.7 |
| 3 | 102.9 | 103.6 | ||
| 4 | 126.4 | 128.9 | ||
| 5 | 2.45 (ddd, J = 7.8; 6.0; 3.0) | 2.70 (m) | 45.9 | 44.1 |
| 6 | 4.64 (dq, J = 6.6; 3.0) | 4.44 (dq, J = 6.6; 3.6) | 74.9 | 76.9 |
| 7 | 5.52 (ddd, J = 3.6; 3.0; 1.2) | 5.53 (ddd, J = 3.6; 3.0; 1.2) | 115.8 | 114.3 |
| 8 | 5.07 (dd, J = 17.2; 3.0); 4.97 (dd, J = 17.2; 3.6) | 5.01 (dd, J = 11.2; 3.0); 4.97 (dd, J = 11.2; 3.6) | 70.6 | 70.6 |
| 10 | 166.8 | 166.9 | ||
| 11 | 3.60 (dd, J = 14.4; 7.8); 3.52 (dd, J = 14.4; 6.0) | 3.74 (dd, J = 14.4; 6.0); 3.62 (dd, J = 14.4; 7.8) | 62.5 | 59.7 |
| 12 | 1.30 (d, J = 6.6) | 1.36 (d, J = 6.6) | 18.8 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Yu, H.; Guo, Q.; Wang, Y.; Xu, H.; Lu, J.; Hu, J.; Wang, Y. Metabolic Transformation of Gentiopicrin, a Liver Protective Active Ingredient, Based on Intestinal Bacteria. Molecules 2023, 28, 7575. https://doi.org/10.3390/molecules28227575
Fu J, Yu H, Guo Q, Wang Y, Xu H, Lu J, Hu J, Wang Y. Metabolic Transformation of Gentiopicrin, a Liver Protective Active Ingredient, Based on Intestinal Bacteria. Molecules. 2023; 28(22):7575. https://doi.org/10.3390/molecules28227575
Chicago/Turabian StyleFu, Jie, Hang Yu, Qinglan Guo, Yanan Wang, Hui Xu, Jinyue Lu, Jiachun Hu, and Yan Wang. 2023. "Metabolic Transformation of Gentiopicrin, a Liver Protective Active Ingredient, Based on Intestinal Bacteria" Molecules 28, no. 22: 7575. https://doi.org/10.3390/molecules28227575
APA StyleFu, J., Yu, H., Guo, Q., Wang, Y., Xu, H., Lu, J., Hu, J., & Wang, Y. (2023). Metabolic Transformation of Gentiopicrin, a Liver Protective Active Ingredient, Based on Intestinal Bacteria. Molecules, 28(22), 7575. https://doi.org/10.3390/molecules28227575







