Characterization of Molecular Interactions in the Bondline of Composites from Plasma-Treated Aluminum and Wood
Abstract
:1. Introduction
2. Results
2.1. Comparison of Untreated with Plasma-Treated Beech Wood
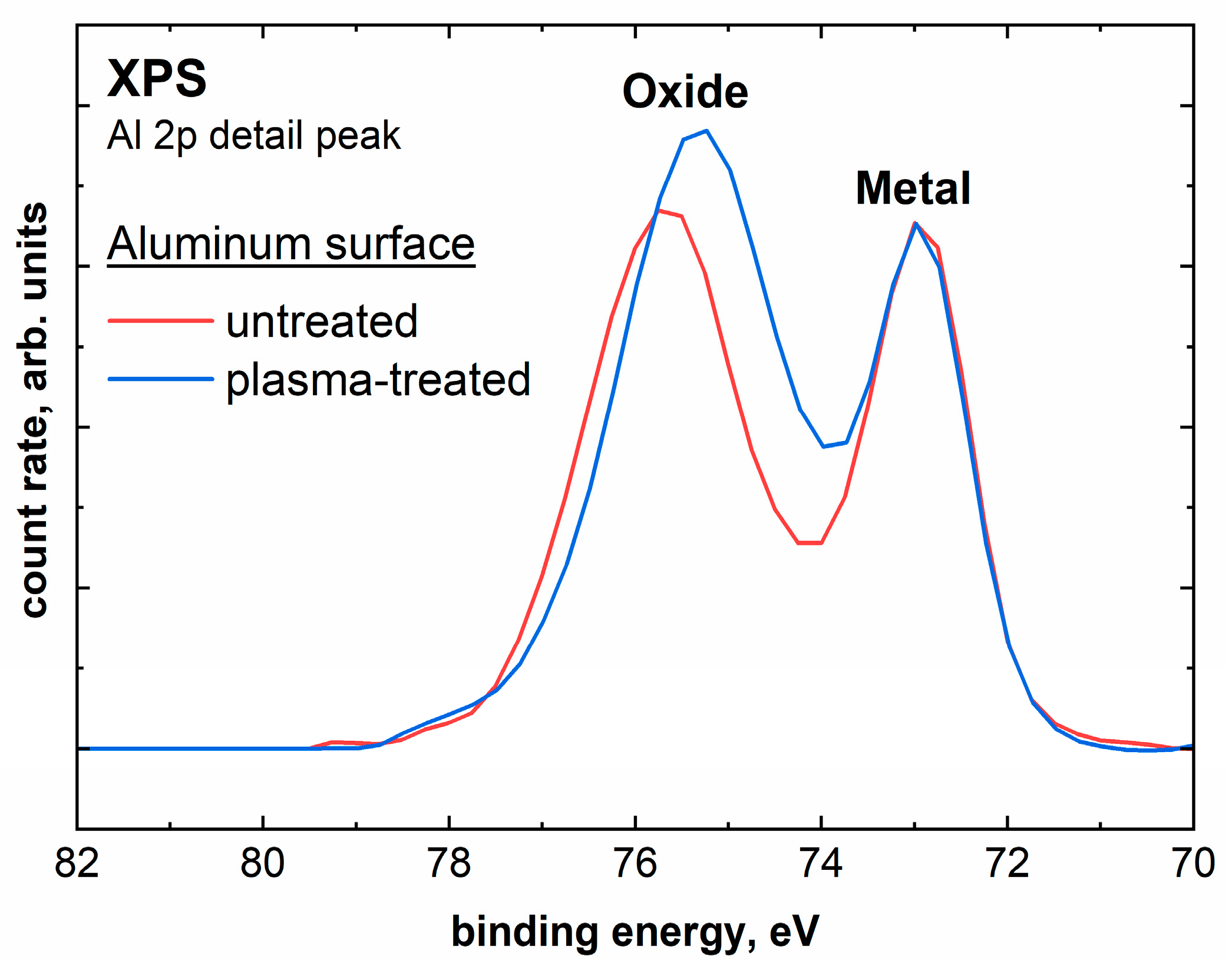
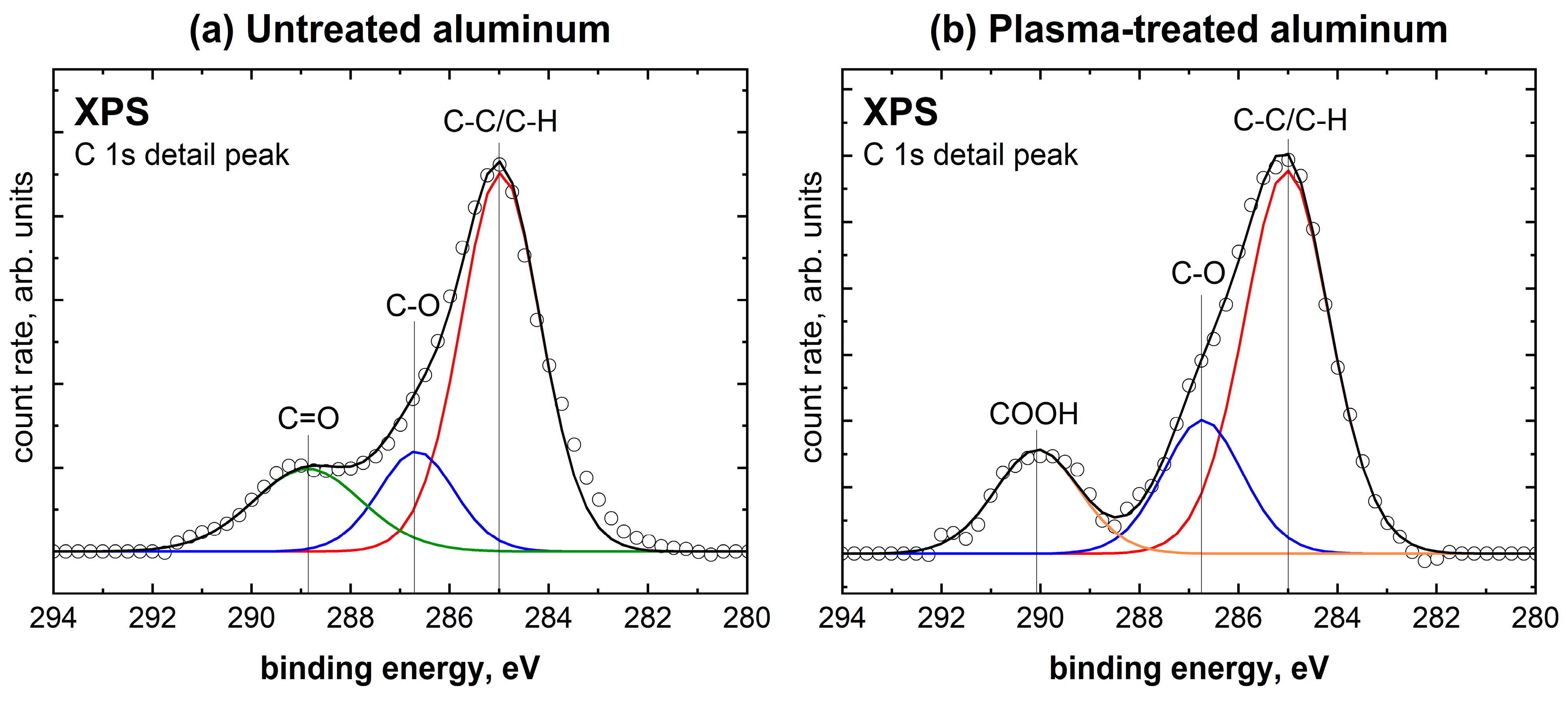
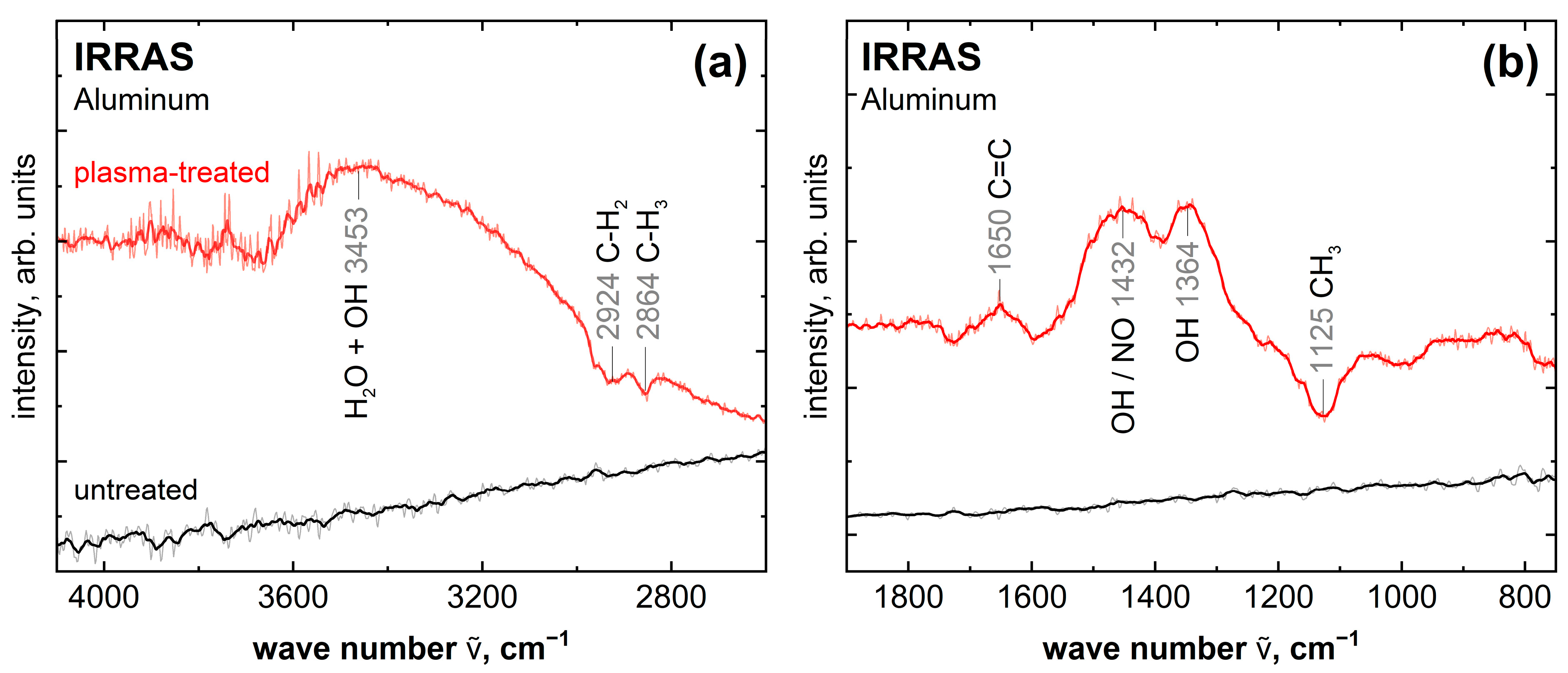
2.2. Comparison of Untreated with Plasma-Treated Aluminum Samples
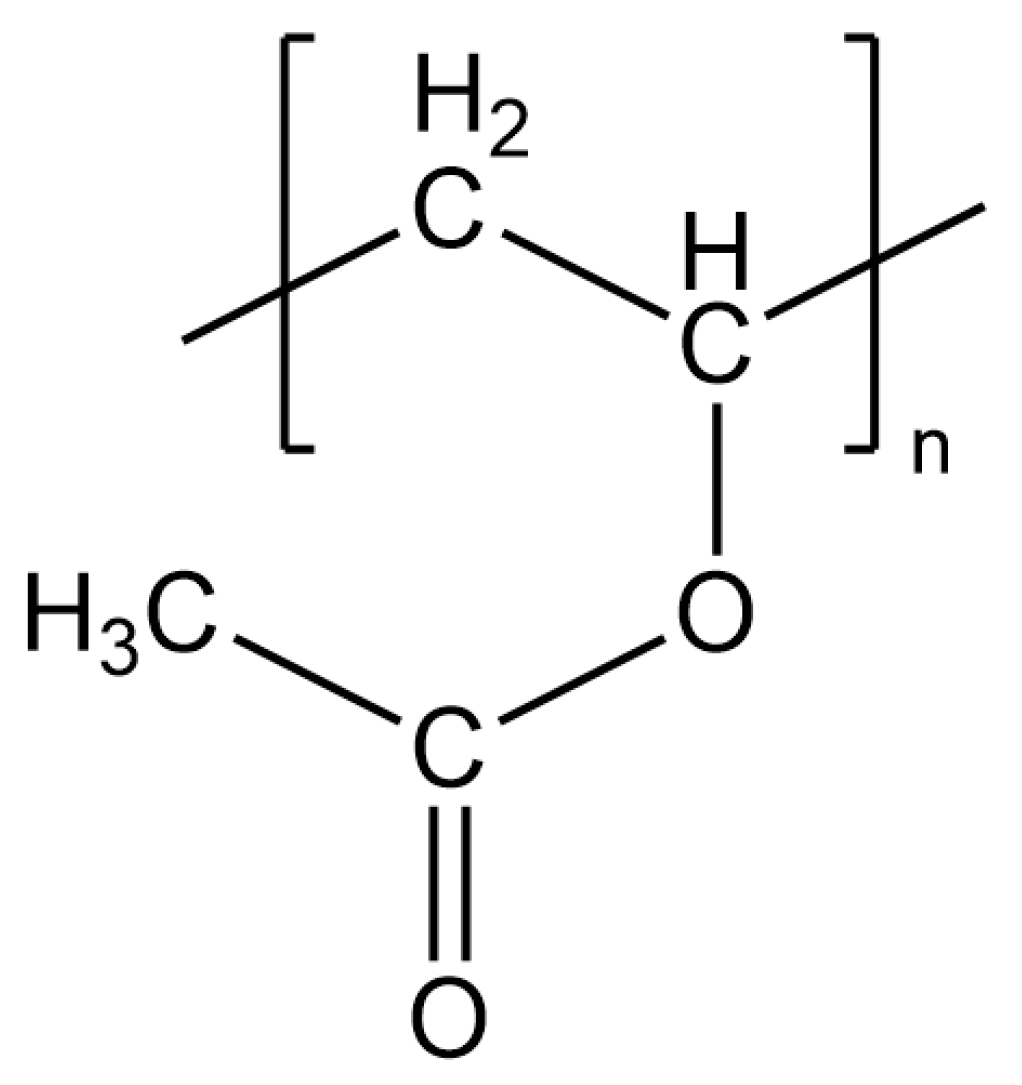
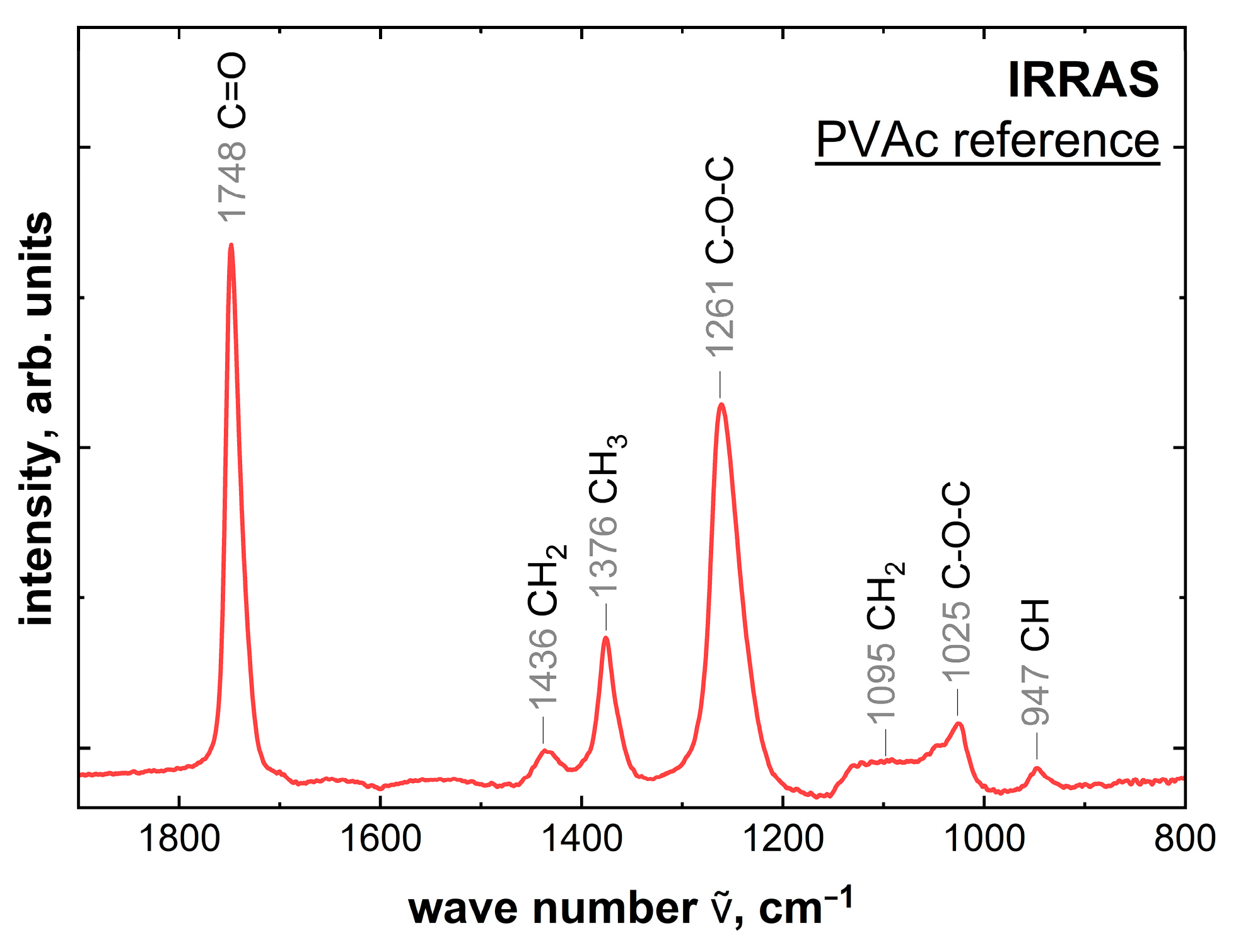
2.3. Measurements of the PVAc Reference
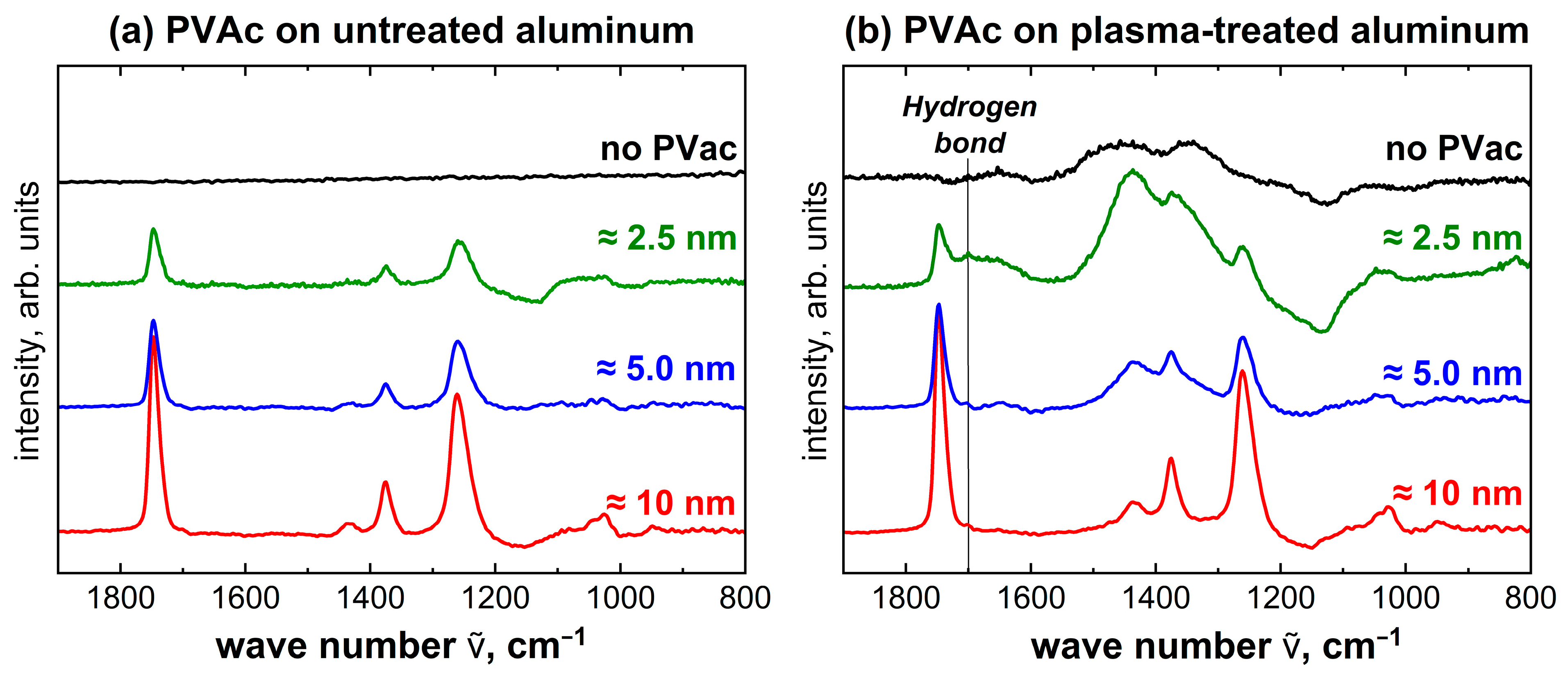
2.4. Characterization of the Interface between PVAc and Untreated or Plasma-Treated Aluminum
3. Discussion
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Characterization Methods
4.2.1. Atomic Force Microscopy (AFM)
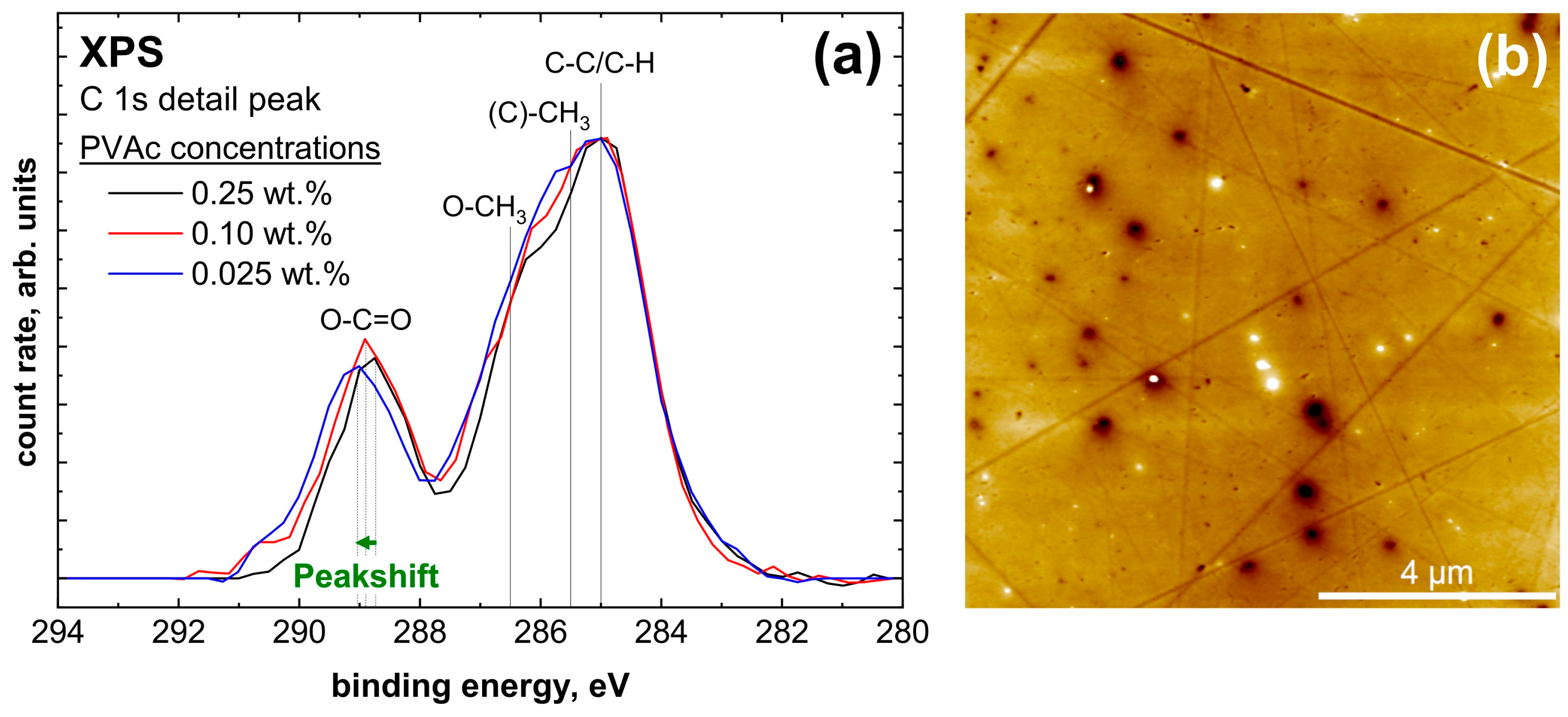
4.2.2. X-ray Photoelectron Spectroscopy (XPS)
4.2.3. Infrared Reflection Adsorption Spectroscopy (IRRAS)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czajkowski, Ł.; Olek, W.; Weres, J. Effects of Heat Treatment on Thermal Properties of European Beech Wood. Eur. J. Wood Prod. 2020, 78, 425–431. [Google Scholar] [CrossRef]
- Xie, Y.; Fu, Q.; Wang, Q.; Xiao, Z.; Militz, H. Effects of Chemical Modification on the Mechanical Properties of Wood. Eur. J. Wood Prod. 2013, 71, 401–416. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, S. Thermal Insulation Properties of Wood-Based Sandwich Panel for Use as Structural Insulated Walls and Floors. J. Wood Sci. 2006, 52, 75–83. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion Behavior and Mechanical Properties of Al-Zn-Mg Aluminum Alloy Weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Steiger, R.; Gehri, E.; Widmann, R. Pull-out Strength of Axially Loaded Steel Rods Bonded in Glulam Parallel to the Grain. Mater. Struct. 2007, 40, 69–78. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Meng, X.; Wang, F.; Wan, L.; Dong, Z.; Cao, J. Friction Rivet Joining towards High-Performance Wood-Metal Hybrid Structures. Compos. Struct. 2020, 247, 112472. [Google Scholar] [CrossRef]
- Le, H.-L.T.; Goniakowski, J.; Noguera, C.; Koltsov, A.; Mataigne, J.-M. Effects of Surface Hydroxylation on Adhesion at Zinc/Silica Interfaces. Phys. Chem. Chem. Phys. 2018, 20, 15581–15588. [Google Scholar] [CrossRef]
- Taheri, P.; Wielant, J.; Hauffman, T.; Flores, J.R.; Hannour, F.; De Wit, J.H.W.; Mol, J.M.C.; Terryn, H. A Comparison of the Interfacial Bonding Properties of Carboxylic Acid Functional Groups on Zinc and Iron Substrates. Electrochim. Acta 2011, 56, 1904–1911. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Xu, Z.; Zhang, Y.; Zhao, F.; Zhang, S. State of Research and Trends in Development of Wood Adhesives. For. Stud. China 2011, 13, 321–326. [Google Scholar] [CrossRef]
- Iždinský, J.; Reinprecht, L.; Sedliačik, J.; Kúdela, J.; Kučerová, V. Bonding of Selected Hardwoods with PVAc Adhesive. Appl. Sci. 2020, 11, 67. [Google Scholar] [CrossRef]
- Novosel, A.; Sedlar, T.; Miklečić, J.; Turkulin, H.; Lučić, L.; Mihulja, G.; Živković, V. Analysis of Bonding Mechanisms of Various Implants and Adhesives in Laminated Oak-Wood Elements. Polymers 2022, 14, 5373. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef] [PubMed]
- Nassr, M.; Krupa, I.; Ouederni, M.; Krishnamoorthy, S.K.; Popelka, A. An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers. Polymers 2023, 15, 916. [Google Scholar] [CrossRef] [PubMed]
- Žigon, J.; Kovač, J.; Zaplotnik, R.; Saražin, J.; Šernek, M.; Petrič, M.; Dahle, S. Enhancement of Strength of Adhesive Bond between Wood and Metal Using Atmospheric Plasma Treatment. Cellulose 2020, 27, 6411–6424. [Google Scholar] [CrossRef]
- Dahle, S.; Srinivasa, K.; Žigon, J.; Cheumani Yona, A.M.; Avramidis, G.; Viöl, W.; Petrič, M. Influence of Air Plasma Pretreatments on Mechanical Properties in Metal-Reinforced Laminated Wood. Front. Mater. 2022, 8, 796474. [Google Scholar] [CrossRef]
- Sawangrat, C.; Thipchai, P.; Kaewapai, K.; Jantanasakulwong, K.; Suhr, J.; Wattanachai, P.; Rachtanapun, P. Surface Modification and Mechanical Properties Improvement of Bamboo Fibers Using Dielectric Barrier Discharge Plasma Treatment. Polymers 2023, 15, 1711. [Google Scholar] [CrossRef]
- Radetic, M.; Jovancic, P.; Puac, N.; Petrovic, Z.L. Environmental Impact of Plasma Application to Textiles. J. Phys. Conf. Ser. 2007, 71, 012017. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, Z.; Wang, C.; Zang, C.; Zhang, Y.; Xu, L. Influence of DBD Plasma Pretreatment on the Deposition of Chitosan onto UHMWPE Fiber Surfaces for Improvement of Adhesion and Dyeing Properties. Appl. Surf. Sci. 2017, 396, 1571–1579. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Van Vlierberghe, S.; Dubruel, P.; Leys, C.; Gengembre, L.; Schacht, E.; Payen, E. Deposition of Polymethyl Methacrylate on Polypropylene Substrates Using an Atmospheric Pressure Dielectric Barrier Discharge. Prog. Org. Coat. 2009, 64, 230–237. [Google Scholar] [CrossRef]
- Seah, M.P. The Quantitative Analysis of Surfaces by XPS: A Review. Surf. Interface Anal. 1980, 2, 222–239. [Google Scholar] [CrossRef]
- Hoffmann, F. Infrared Reflection-Absorption Spectroscopy of Adsorbed Molecules. Surf. Sci. Rep. 1983, 3, 107. [Google Scholar] [CrossRef]
- Moritz, P.; Bürger, F.; Wegewitz, L.; Maus-Friedrichs, W. Preparation Techniques of Thin Cyanoacrylate Adhesive Films for Interface Analysis. J. Adhes. 2022, 98, 963–978. [Google Scholar] [CrossRef]
- Moritz, P.; Bürger, F.; Höfft, O.; Wegewitz, L.; Maus-Friedrichs, W. Bonding Mechanism of Cyanoacrylates on SiO 2 and Au: Spectroscopic Studies of the Interface. J. Phys. Chem. C 2021, 125, 23409–23417. [Google Scholar] [CrossRef]
- Nguila Inari, G.; Pétrissans, M.; Dumarcay, S.; Lambert, J.; Ehrhardt, J.J.; Šernek, M.; Gérardin, P. Limitation of XPS for Analysis of Wood Species Containing High Amounts of Lipophilic Extractives. Wood Sci. Technol. 2011, 45, 369–382. [Google Scholar] [CrossRef]
- Avramidis, G.; Klarhöfer, L.; Maus-Friedrichs, W.; Militz, H.; Viöl, W. Influence of Air Plasma Treatment at Atmospheric Pressure on Wood Extractives. Polym. Degrad. Stab. 2012, 97, 469–471. [Google Scholar] [CrossRef]
- Johansson, L.; Campbell, J.M.; Hänninen, T.; Ganne-Chèdeville, C.; Vuorinen, T.; Hughes, M.; Laine, J. XPS and the Medium-dependent Surface Adaptation of Cellulose in Wood. Surf. Interface Anal. 2012, 44, 899–903. [Google Scholar] [CrossRef]
- Král, P.; Ráhel’, J.; Stupavská, M.; Šrajer, J.; Klímek, P.; Mishra, P.K.; Wimmer, R. XPS Depth Profile of Plasma-Activated Surface of Beech Wood (Fagus Sylvatica) and Its Impact on Polyvinyl Acetate Tensile Shear Bond Strength. Wood Sci. Technol. 2015, 49, 319–330. [Google Scholar] [CrossRef]
- Di, M.W.; Liu, Y. Stability and ageing of plasma treated Wood/Polyethylene Composites surfaces. Adv. Mater. Res. 2010, 150–151, 829–833. [Google Scholar]
- Sherwood, P.M.A. Introduction to Studies of Aluminum and Its Compounds by XPS. Surf. Sci. Spectra 1998, 5, 1–3. [Google Scholar] [CrossRef]
- Brunelli, K.; Pezzato, L.; Napolitani, E.; Gross, S.; Magrini, M.; Dabalà, M. Effects of Atmospheric Pressure Plasma JET Treatment on Aluminium Alloys. Surf. Eng. 2014, 30, 636–642. [Google Scholar] [CrossRef]
- Strohmeier, B.R. Improving the Wettability of Aluminum Foil with Oxygen Plasma Treatments. J. Adhes. Sci. Technol. 1992, 6, 703–718. [Google Scholar] [CrossRef]
- Kim, M.C.; Yang, S.H.; Boo, J.-H.; Han, J.G. Surface Treatment of Metals Using an Atmospheric Pressure Plasma Jet and Their Surface Characteristics. Surf. Coat. Technol. 2003, 174–175, 839–844. [Google Scholar] [CrossRef]
- Belkind, A.; Gershman, S. Plasma cleaning of surfaces. Vac. Coat. Technol. 2008, 9, 46–57. [Google Scholar]
- Pinson, S.J.M.; Collins, J.; Thompson, G.E.; Alexander, M.R. Atmospheric Pressure Plasma Cleaning of Aluminium. Trans. IMF 2001, 79, 155–159. [Google Scholar] [CrossRef]
- Mui, T.S.M.; Silva, L.L.G.; Prysiazhnyi, V.; Kostov, K.G. Surface Modification of Aluminium Alloys by Atmospheric Pressure Plasma Treatments for Enhancement of Their Adhesion Properties. Surf. Coat. Technol. 2017, 312, 32–36. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Zajickova, L.; Slavicek, P. Aluminium Surface Activation Using Atmospheric-pressure Plasma. Proc. Contrib. Pap. 2009, 2, 182–187. [Google Scholar]
- Kim, H.-S.; Kang, W.S.; Kim, G.-H.; Hong, S.H. Metal Surface Oxidation by Using Dielectric Barrier Discharge. Thin Solid Film. 2010, 518, 6394–6398. [Google Scholar] [CrossRef]
- Bónová, L.; Zahoranová, A.; Kováčik, D.; Zahoran, M.; Mičušík, M.; Černák, M. Atmospheric Pressure Plasma Treatment of Flat Aluminum Surface. Appl. Surf. Sci. 2015, 331, 79–86. [Google Scholar] [CrossRef]
- Endo, K.; Inoue, C.; Kobayashi, N.; Aida, M. Spectra Analysis of the XPS Core and Valence Energy Levels of Polymers by an Ab Initio Mo Method Using Simple Model Molecules. J. Phys. Chem. Solids 1994, 55, 471–478. [Google Scholar] [CrossRef]
- Terui, Y.; Hirokawa, K. Fourier Transform Infrared Emission Spectra of Poly(Vinyl Acetate) Enhanced by the Island Structure of Gold. Vib. Spectrosc. 1994, 6, 309–314. [Google Scholar] [CrossRef]
- Guo, L.; Sato, H.; Hashimoto, T.; Ozaki, Y. FTIR Study on Hydrogen-Bonding Interactions in Biodegradable Polymer Blends of Poly(3-Hydroxybutyrate) and Poly(4-Vinylphenol). Macromolecules 2010, 43, 3897–3902. [Google Scholar] [CrossRef]
- Possart, W.; Schlett, V. XPS of the Interphase Between PMMA and Metals. A Study of Degradation and Interaction. J. Adhes. 1995, 48, 25–46. [Google Scholar] [CrossRef]
- Pletincx, S.; Marcoen, K.; Trotochaud, L.; Fockaert, L.-L.; Mol, J.M.C.; Head, A.R.; Karslioğlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. Unravelling the Chemical Influence of Water on the PMMA/Aluminum Oxide Hybrid Interface In Situ. Sci. Rep. 2017, 7, 13341. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.F.; Leadley, S.R.; Castle, J.E.; Blomfield, C.J. Adsorption of PMMA on Oxidized Al and Si Substrates: An Investigation by High-Resolution X-Ray Photoelectron Spectroscopy. Langmuir 2000, 16, 2292–2300. [Google Scholar] [CrossRef]
- Moritz, P.; Höfft, O.; Wegewitz, L.; Maus-Friedrichs, W. Interaction of Cyanoacrylate with Metal Oxide Surfaces (Cu, Al). ChemPhysChem 2023, 24, e202300076. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Progress in Quantitative Surface Analysis by X-Ray Photoelectron Spectroscopy: Current Status and Perspectives. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 331–346. [Google Scholar] [CrossRef]
- Powel, C.J.; Jablonski, A. NIST Electron Inelastic-Mean-Free-Path Database, Version 1.2; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
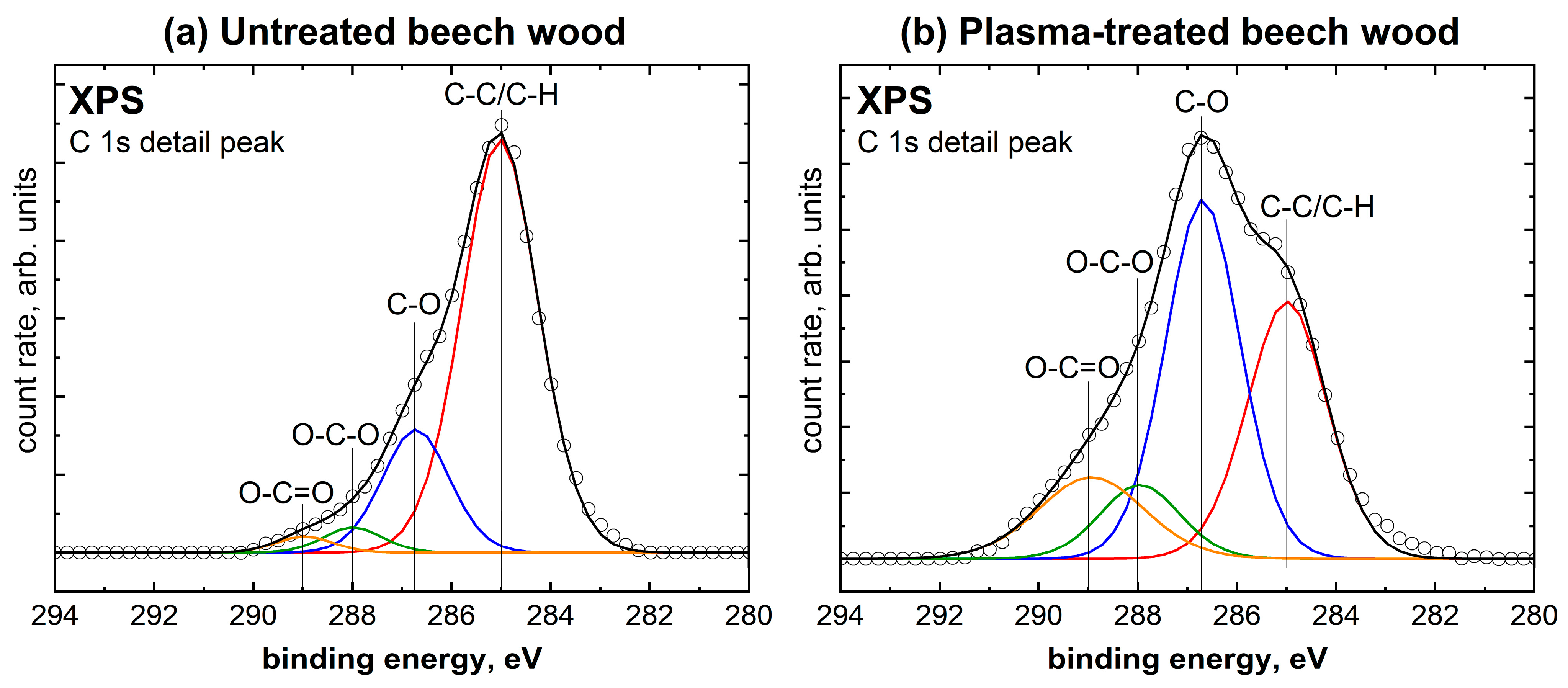


| Species | Untreated Beech Wood | Plasma-Treated Beech Wood |
|---|---|---|
| C-C/C-H | 74.7% | 33.2% |
| C-O | 19.6% | 43.3% |
| O-C-O | 3.5% | 9.5% |
| O-C=O | 2.2% | 14.0% |
| Species | Untreated Aluminum | Plasma-Treated Aluminum |
|---|---|---|
| C-C/C-H | 64.4% | 62.8% |
| C-O | 16.2% | 20.3% |
| C=O | 19.4% | - |
| COOH | - | 16.9% |
| Species | Position | FWHM | Proportion |
|---|---|---|---|
| Aliphatic C-C/C-H | 285.00 eV | 1.65 eV | 25.1% |
| (C)-CH3 | 285.46 eV | 1.75 eV | 24.9% |
| O-CH3 | 286.66 eV | 1.67 eV | 25.4% |
| O-C=O | 289.10 eV | 1.45 eV | 24.6% |
| Wave Number | Species |
|---|---|
| 1748 cm−1 | C=O (symmetric stretching mode) |
| 1436 cm−1 | C-H2 (deformation mode) |
| 1376 cm−1 | C-H3 (deformation mode) |
| 1261 cm−1 | C-O-C (antisymmetric stretching mode) |
| 1095 cm−1 | C-H2 (wagging mode) |
| 1025 cm−1 | C-O-C (symmetric stretching mode) |
| 947 cm−1 | C-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, S.J.; Moritz, P.; Höfft, O.; Wegewitz, L.; Maus-Friedrichs, W.; Dahle, S. Characterization of Molecular Interactions in the Bondline of Composites from Plasma-Treated Aluminum and Wood. Molecules 2023, 28, 7574. https://doi.org/10.3390/molecules28227574
Zimmermann SJ, Moritz P, Höfft O, Wegewitz L, Maus-Friedrichs W, Dahle S. Characterization of Molecular Interactions in the Bondline of Composites from Plasma-Treated Aluminum and Wood. Molecules. 2023; 28(22):7574. https://doi.org/10.3390/molecules28227574
Chicago/Turabian StyleZimmermann, Sascha Jan, Philipp Moritz, Oliver Höfft, Lienhard Wegewitz, Wolfgang Maus-Friedrichs, and Sebastian Dahle. 2023. "Characterization of Molecular Interactions in the Bondline of Composites from Plasma-Treated Aluminum and Wood" Molecules 28, no. 22: 7574. https://doi.org/10.3390/molecules28227574
APA StyleZimmermann, S. J., Moritz, P., Höfft, O., Wegewitz, L., Maus-Friedrichs, W., & Dahle, S. (2023). Characterization of Molecular Interactions in the Bondline of Composites from Plasma-Treated Aluminum and Wood. Molecules, 28(22), 7574. https://doi.org/10.3390/molecules28227574







