Phenylboronic Acid and Amino Bifunctional Modified Adsorbent for Quickly Separating Phenolic Acids from Crude Extract of Clerodendranthus spicatus and Evaluation of Their Antioxidant and Hypoglycemic Activities
Abstract
:1. Introduction
2. Results and Discussion
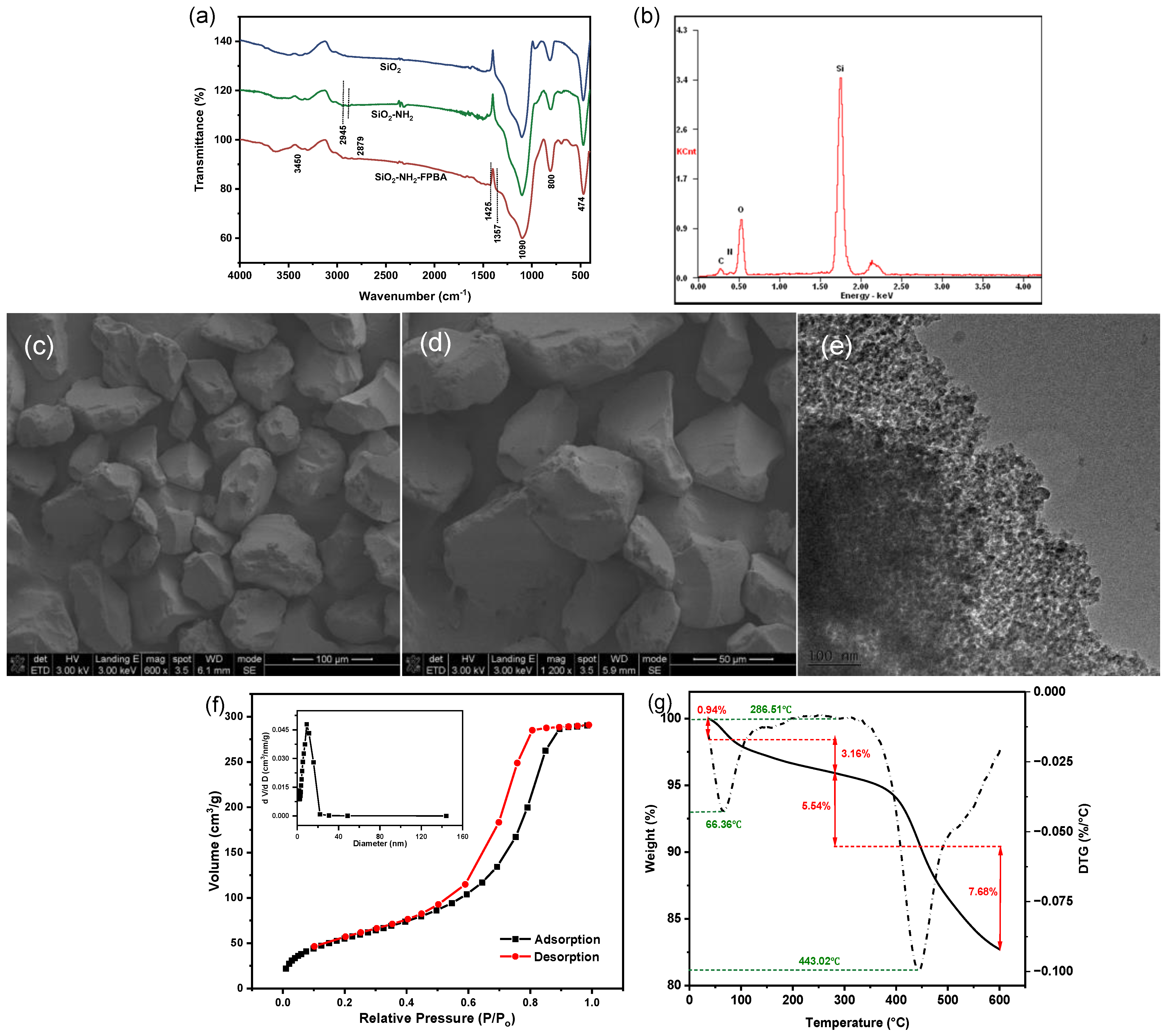
2.1. Characterization
2.2. Evaluation of the Adsorption Ability of SiO2-NH2-FPBA to GA, CA and PCA
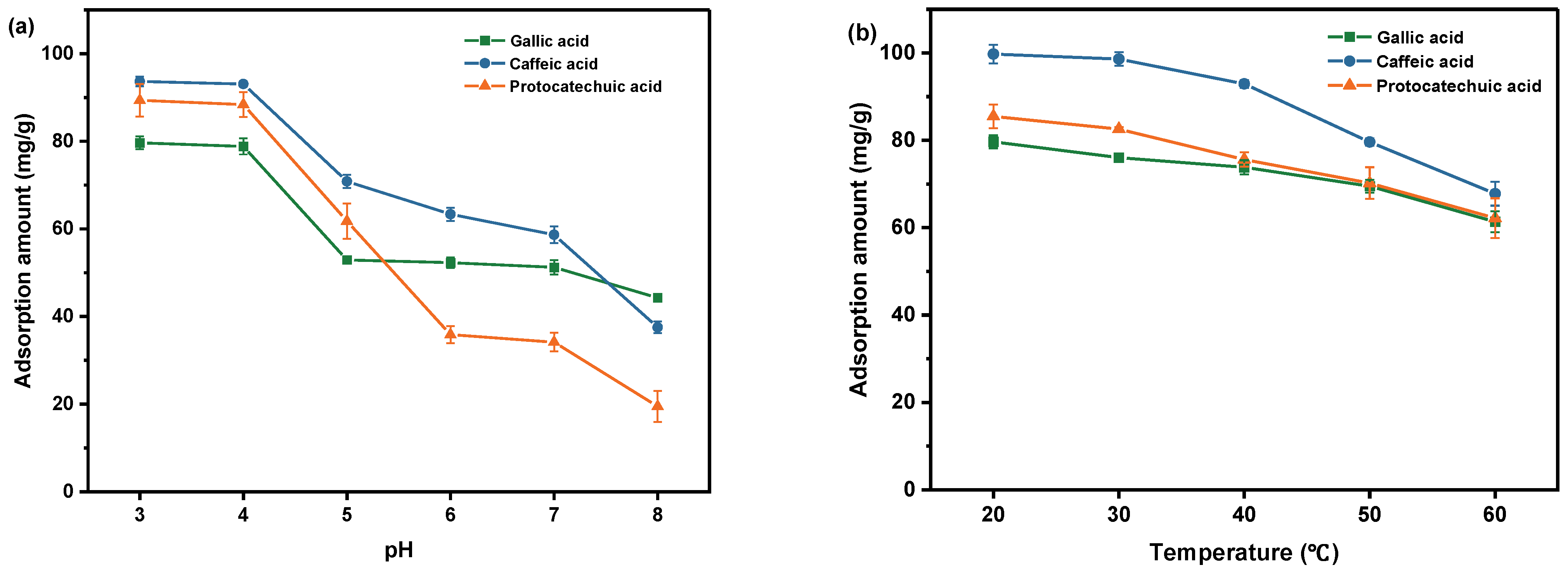
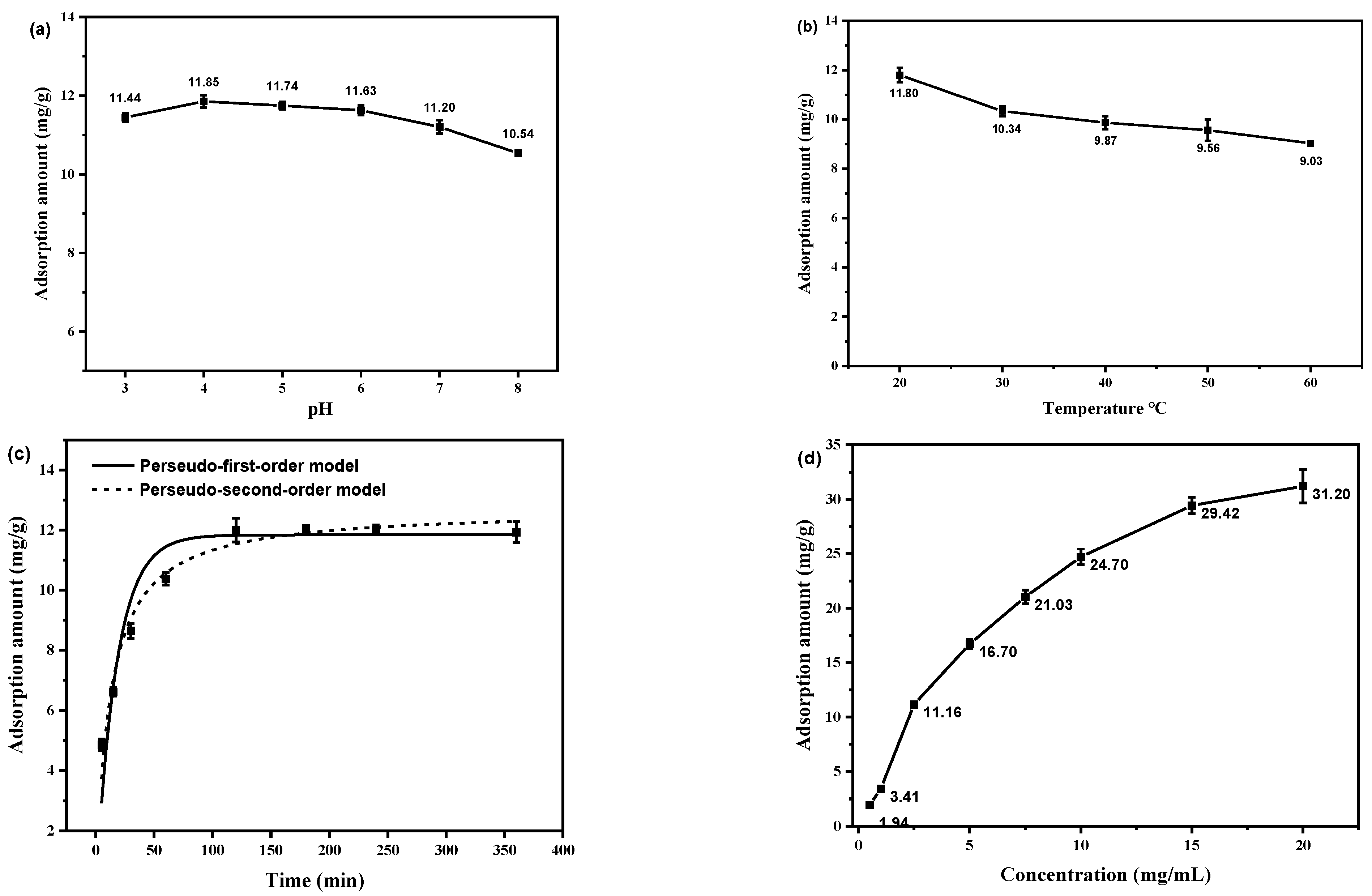
2.2.1. Effect of pH on Absorption
2.2.2. Effect of Temperature on Absorption
2.2.3. Effect of Time on Absorption
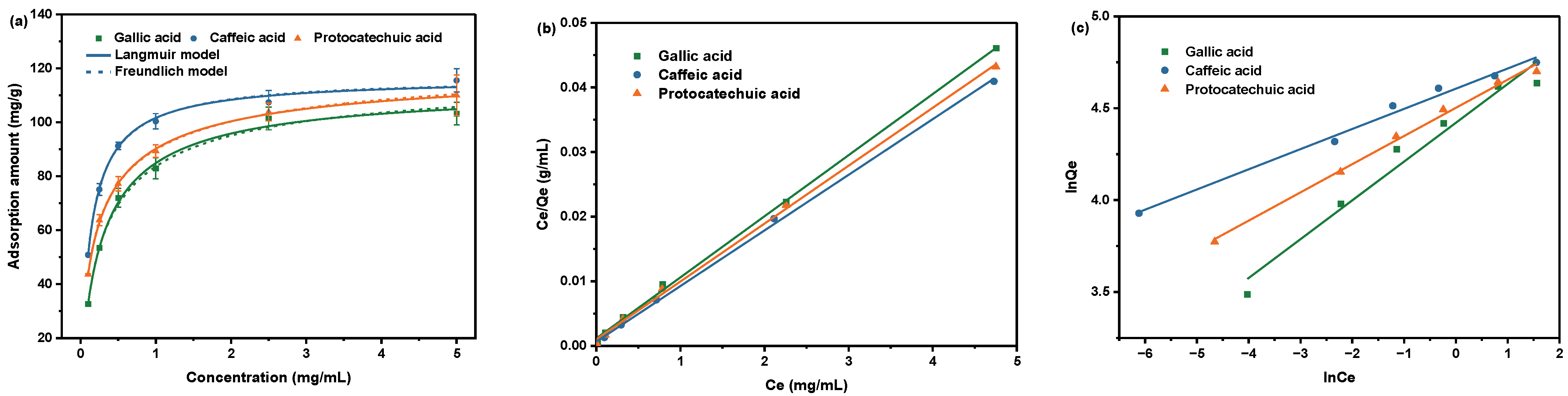
2.2.4. Effect of Concentration on Absorption
2.2.5. Exploration of Desorption Conditions
2.3. Comparison of SiO2-NH2-FPBA with the Reported Adsorbents
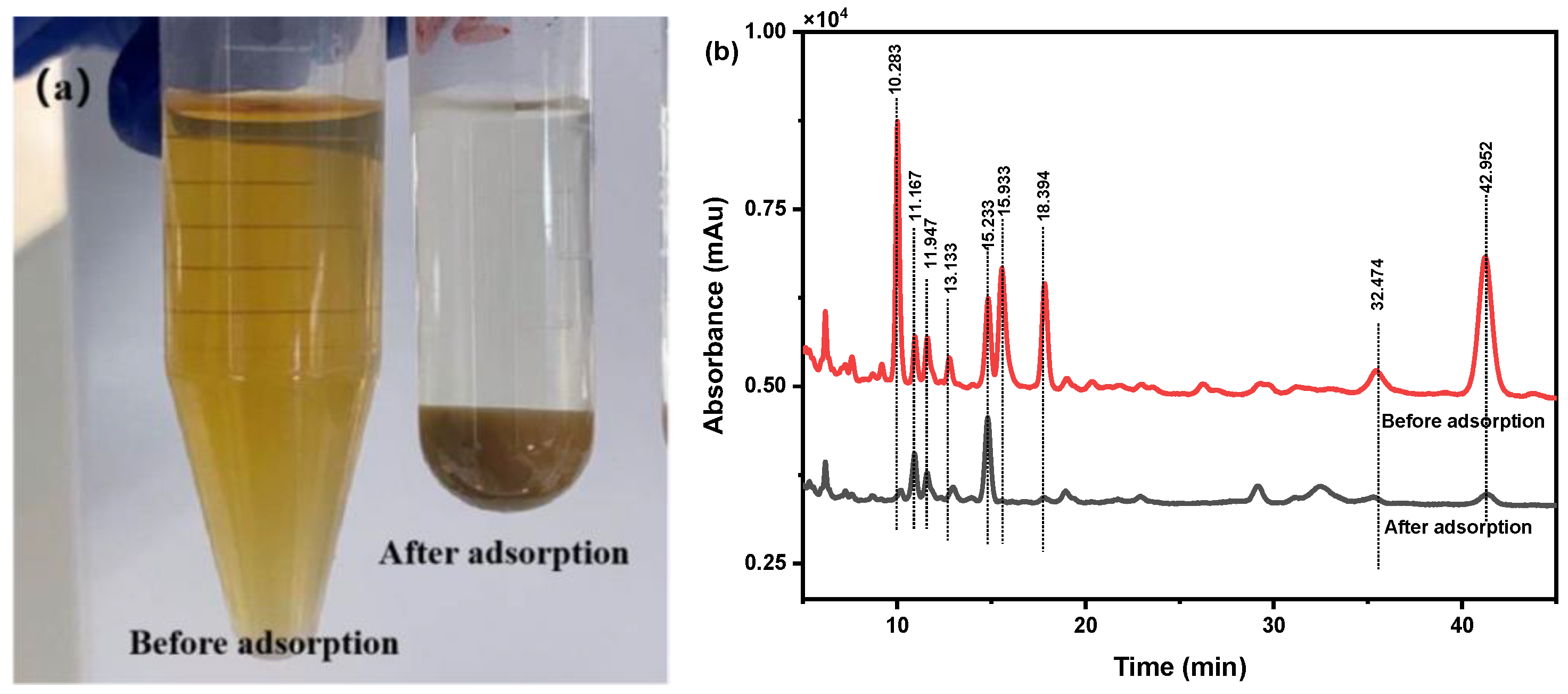
2.4. Evaluation of the Adsorption of SiO2-NH2-FPBA to Phenolic Acids in C. spicatus Crude Extract
2.4.1. Effect of pH on Absorption
2.4.2. Effect of Temperature on Absorption
2.4.3. Effect of Time on Absorption
2.4.4. Effect of Concentration on Absorption
2.4.5. Desorption Conditions
2.5. Identification of the Separated Phenolic Acids by Q Exactive HF LC-MS
2.6. Antioxidant and Hypoglycemic Activities of the Isolated Phenolic Acids from C. spicatus
3. Materials and Methods
3.1. Materials
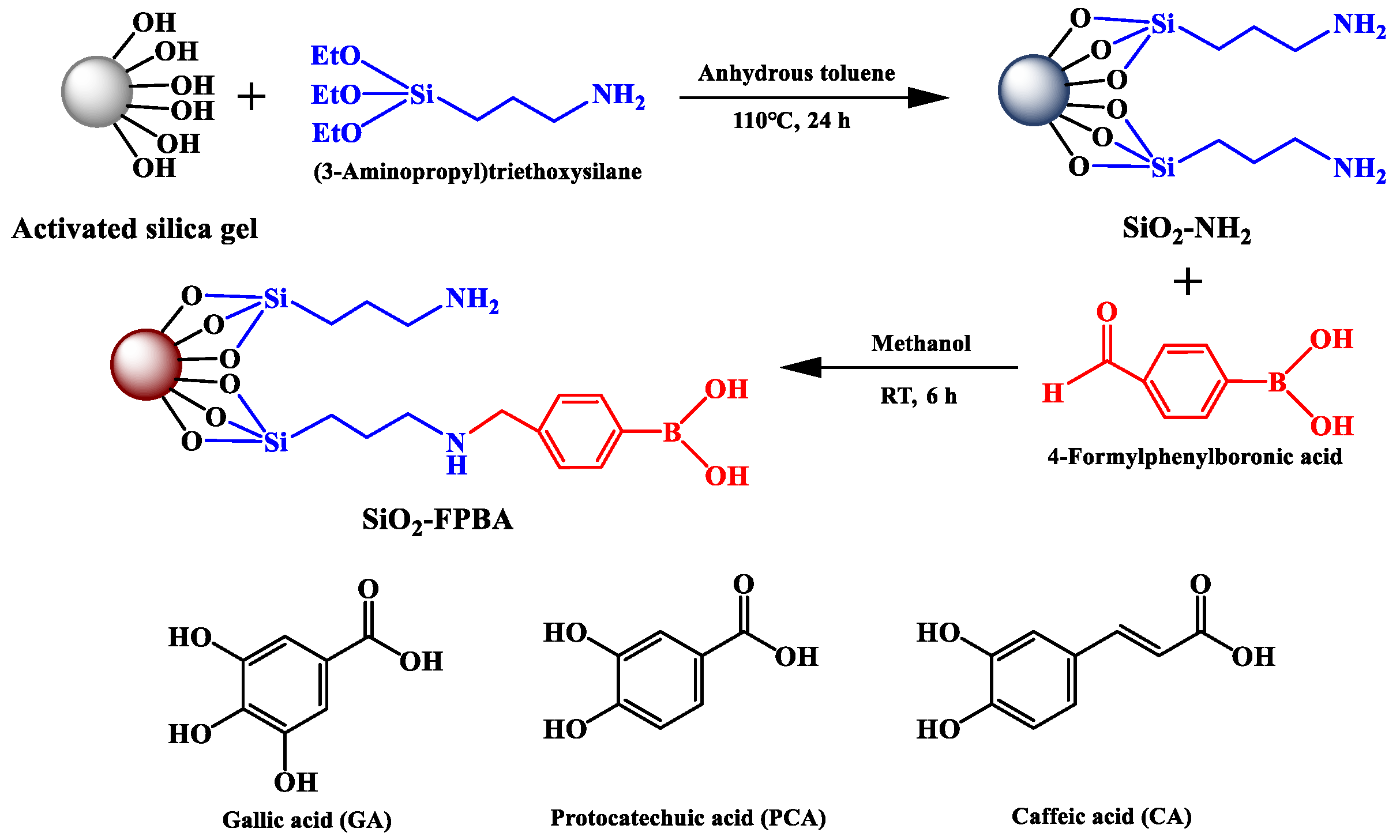
3.2. Preparation of Phenylboronic Acid and Amino Bifunctional Modified Absorbent
3.2.1. Preparation of Amino Modified Silica Gel (SiO2-NH2)
3.2.2. Preparation of Phenylboronic Acid and Amino Modified Adsorbent (SiO2-NH2-FPBA)
3.3. Adsorption Experiments on GA, CA and PCA
3.4. Adsorption of SiO2-NH2-FPBA to Phenolic Acids from C. spicatus Extract Solutions
3.5. Identification of the Separated Phenolic Acids by Q Exactive HF LC-MS
3.6. The Antioxidant and Hypoglycemic Activities of the Isolated Phenolic Acids In Vitro
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Brautigan, D.L.; Gielata, M.; Heo, J.; Kubicka, E.; Wilkins, L.R. Selective toxicity of caffeic acid in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 505, 612–617. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, H.J.; Hwang, J.S. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar]
- Zhao, H.; Jia, J.; Bai, M.; Miao, M. Updated pharmacological effects of total phenolic acid on the meningeal microcirculation in mice. J. Healthc. Eng. 2022, 2022, 8441050–8441055. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; Zahran, F.; Troconis, D.; Villalva, M.; Reglero, G.; Fornari, T. Selective precipitation of phenolic compounds from Achillea millefolium L. extracts by supercritical anti-solvent technique. J. Supercrit. Fluids 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Luo, J.; Zeuner, B.; Morthensen, S.T.; Meyer, A.S.; Pinelo, M. Separation of phenolic acids from monosaccharides by low-pressure nanofiltration integrated with laccase pre-treatments. J. Membr. Sci. 2015, 482, 83–91. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Counce, R.M.; Counce, R.W.; Watson, J.S.; Labbé, N.; Tao, J. Recovery of Phenolic Compounds from Switchgrass Extract. ACS Sustain. Chem. Eng. 2018, 6, 374–379. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H.; Wang, J.; Liu, Z.; Bi, J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B 2009, 877, 733–737. [Google Scholar] [CrossRef]
- Byun, H.-S.; Chun, D. Adsorption and separation properties of gallic acid imprinted polymers prepared using supercritical fluid technology. J. Supercrit. Fluids 2017, 120, 249–257. [Google Scholar] [CrossRef]
- Simanaviciute, D.; Klimaviciute, R.; Rutkaite, R. Equilibrium adsorption of caffeic, chlorogenic and rosmarinic acids on cationic cross-linked starch with quaternary ammonium groups. Int. J. Biol. Macromol. 2017, 95, 788–795. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Airado-Rodríguez, D. Revalorization of agro-industrial effluents based on gallic acid recovery through a novel anionic resin. Process Saf. Environ. Prot. 2018, 115, 17–26. [Google Scholar] [CrossRef]
- Du, N.; Cao, S.; Yu, Y. Research on the adsorption property of supported ionic liquids for ferulic acid, caffeic acid and salicylic acid. J. Chromatogr. B 2011, 879, 1697–1703. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, Q.; Ma, G.; Zhang, X.; Yuan, J.; Wu, H.; Liu, H.; Yang, J.; Xu, X. Four new phenolic acids from Clerodendranthus spicatus. Phytochem. Lett. 2014, 8, 16–21. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Guo, R.; Li, J. Phenolic acid derivatives with neuroprotective effect from the aqueous extract of Clerodendranthus spicatus. J. Asian Nat. Prod. Res. 2017, 19, 974–980. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589–126604. [Google Scholar] [CrossRef]
- Cagnon, B.; Chedeville, O.; Cherrier, J.F.; Caqueret, V.; Porte, C. Evolution of adsorption kinetics and isotherms of gallic acid on an activated carbon oxidized by ozone: Comparison to the raw material. J. Taiwan Inst. Chem. Eng. 2011, 42, 996–1003. [Google Scholar] [CrossRef]
- Li, N.; Ng, T.B.; Wong, J.H.; Qiao, J.X.; Zhang, Y.N.; Zhou, R.; Chen, R.R.; Liu, F. Separation and purification of the antioxidant compounds, caffeic acid phenethyl ester and caffeic acid from mushrooms by molecularly imprinted polymer. Food Chem. 2013, 139, 1161–1167. [Google Scholar] [CrossRef]
- Fan, D.; Li, H.; Shi, S.; Chen, X. Hollow molecular imprinted polymers towards rapid, effective and selective extraction of caffeic acid from fruits. J. Chromatogr. A 2016, 1470, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chai, Z.; Zhu, Y.; Li, C.; Liang, X. Preparation and characterization of magnetic chitosan-modified diatomite for the removal of gallic acid and caffeic acid from sugar solution. Carbohydr. Polym. 2019, 219, 316–327. [Google Scholar] [CrossRef]
- Chai, Z.; Li, C.; Zhu, Y.; Song, X.; Chen, M.; Yang, Y.; Chen, D.; Liang, X.; Wu, J. Arginine-modified magnetic chitosan: Preparation, characterization and adsorption of gallic acid in sugar solution. Int. J. Biol. Macromol. 2020, 165, 506–516. [Google Scholar] [CrossRef]
- Nie, L.; Lu, J.; Zhang, W.; He, A.; Yao, S. Ionic liquid-modified silica gel as adsorbents for adsorption and separation of water-soluble phenolic acids from Salvia militiorrhiza Bunge. Sep. Purif. Technol. 2015, 155, 2–12. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Amine-modified SBA-15 and MCF mesoporous molecular sieves as promising sorbents for natural antioxidant. Modeling of caffeic acid adsorption. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 411–421. [Google Scholar] [CrossRef]
- Qian, J.; Xu, X.; Su, J.; Zeng, W.; Han, B.; Hao, X.; Kai, G. A strategy for effective recovery of salvianolic acid a from Salvia miltiorrhiza (Danshen) through multiple interactions. Compos. B Eng. 2022, 231, 109563–109574. [Google Scholar] [CrossRef]
- Su, J.J.; Qian, J.; Zeng, W.H.; Wang, Y.; Kai, G.Y. Effective adsorption of salvianolic acids with phenylboronic acid functionalized polyethyleneimine-intercalated montmorillonite. Sep. Purif. Technol. 2023, 311, 123304–123315. [Google Scholar] [CrossRef]
- Guan, S.C.; Fan, G.Y. Diterpenoids from aerial parts of Clerodendranthus spicatus and their cytotoxic activity. Helvetica. Chimica. Acta. 2015, 97, 1708–1713. [Google Scholar] [CrossRef]
- Shafaei, A.; Halim, N.; Zakaria, N. Analysis of free amino acids in different extracts of orthosiphon stamineus leaves by high-performance liquid chromatography combined with solid-phase extraction. Pharmacogn. Mag. 2017, 13, 385–391. [Google Scholar]
- Luo, Y.; Cheng, L.Z.; Luo, Q.; Yan, Y.M.; Wang, S.M.; Sun, Q.; Cheng, Y.X. New ursane-type triterpenoids from Clerodendranthus spicatus. Fitoterapia 2017, 119, 69–74. [Google Scholar] [CrossRef]
- Chen, X.; Ma, G.; Huang, Z.; Tongyu, W.; Xu, X.; Zhong, X. Identification of water-soluble phenolic acids from Clerodendranthus spicatus. Chin. Trad. Herb. Drugs 2017, 48, 2614–2618. [Google Scholar]
- David, A.E.; Wang, N.S.; Yang, V.C.; Yang, A.J. Chemically surface modified gel (CSMG): An excellent enzyme-immobilization matrix for industrial processes. J. Biotechnol. 2006, 125, 395–407. [Google Scholar] [CrossRef]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel 2021, 291, 120126–120133. [Google Scholar] [CrossRef]
- Jang, E.H.; Pack, S.P.; Kim, I.; Chung, S. A systematic study of hexavalent chromium adsorption and removal from aqueous environments using chemically functionalized amorphous and mesoporous silica nanoparticles. Sci. Rep. 2020, 10, 5558–5579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.; Zhang, K.; Liu, T.; Hu, Y.; Chen, X.; Wang, C.; Huang, X.; Kong, L.; Liu, J. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 2018, 9, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Balbuena, P.B.; Gubbins, K.G. Theoretical interpretation of adsorption behavior of simple fluids in slit pores. Langmuir 1993, 10, 1801–1814. [Google Scholar]
- Zheng, X.; Qin, Y.; Meng, X.; Jin, Z.; Fan, L.; Wang, J. Synthesis of polyethylene glycol functional bonded silica gel for selective recognition and separation of alpha-cyclodextrin. J. Chromatogr. A 2021, 1639, 461917–461924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, M.C.; Chang, Z.Y.; Li, H.B. Study on the graft modification mechanism of macroporous silica gel surface based on silane coupling agent vinyl triethoxysilane. RSC Adv. 2021, 11, 25158–25169. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.A.A.; Rahman, R.A.; Yusof, A.H.M.; Sulaiman, N.J.; Sukmawati, D.; Syukri, M.S.M. Adsorption kinetics of immobilized laccase on magnetically-separable hierarchically-ordered mesocellular mesoporous silica. Bioresour. Technol. Rep. 2023, 22, 101445–101449. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, K.; Dixit, U. Adsorption, kinetics and thermodynamics of phenol removal by ultrasound-assisted sulfuric acid-treated pea (Pisum sativum) shells. Sustain. Chem. Pharm. 2021, 22, 100491–100499. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, D.D.; Zhang, J.W.; Gao, D.; Yang, F.Q.; Chen, H.; Xia, Z.N. Amino-terminated supramolecular cucurbit [6] uril pseudorotaxane complexes immobilized on magnetite@silica nanoparticles: A highly efficient sorbent for salvianolic acids. Talanta 2019, 195, 354–365. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Qin, B.; Zhang, B.; Ma, J.G.; Hu, Y.Q.; Han, L.; He, M.F.; Liu, C.Y. Specific enrichment of caffeic acid from Taraxacum mon-golicum Hand.-Mazz. by pH and magnetic dual-responsive molecularly imprinted polymers. Anal. Chim. Acta 2020, 1096, 193–202. [Google Scholar] [CrossRef]
- Liu, B.; Dong, B.; Yuan, X.; Kuang, Q.; Zhao, Q.; Yang, M.; Liu, J.; Zhao, B. Enrichment and separation of chlorogenic acid from the extract of Eupatorium adenophorum Spreng by macroporous resin. J. Chromatogr. B 2016, 1008, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N.; Gao, X.D.; Xue, Z.H.; Wang, Y.J.; Lu, Y.P.; Zhang, M.; Panichayupakaranant, P.; Chen, H.X. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int. J. Biol. Macromol. 2020, 163, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.Y.; Liu, J.Y.; Li, R.L.; Zhou, J.N.; Li, M.Y.; Lu, J.Y.; Zhao, G.Y.; Li, X.; Sui, W.J.; et al. Steam explosion improves extractability, antioxidant activity and α-glucosidase inhibitory activity of the constituents of Java tea (Clerodendranthus spicatus). Innov. Food Sci. Emerg. 2023, 86, 103350. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, Y.; Jia, Y.N.; Xue, Z.H.; Chen, Z.Q.; Zhang, M.; Panichayupakaranant, P.; Yang, S.Y.; Chen, H.X. Chrysophyllum cainito. L alleviates diabetic and complications by playing antioxidant, antiglycation, hypoglycemic roles and the chemical profile analysis. J. Ethnopharmacol. 2021, 281, 114569. [Google Scholar] [CrossRef]
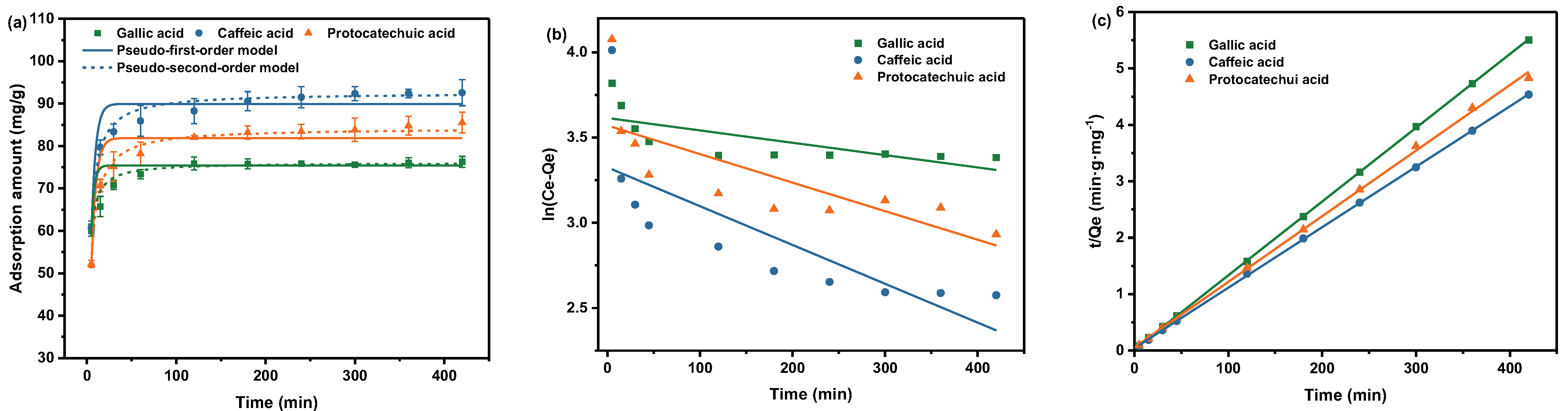


| Phenolic Acid Type | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | Qm (mg/g) | R2 | k1 (g·mg−1min−1) | Qm (mg/g) | R2 | |
| Gallic acid | 0.0007246 | 37.13 | 0.5330 | 0.006863 | 76.45 | 0.9999 |
| Caffeic acid | 0.002280 | 27.81 | 0.6038 | 0.002738 | 93.28 | 0.9999 |
| Protocatechuic acid | 0.001680 | 35.55 | 0.5832 | 0.002461 | 85.94 | 0.9992 |
| Phenolic Acid Type | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|
| Q0 | KL | RL2 | KF | n | RF2 | |
| Gallic acid | 105.7 | 8.298 | 0.9989 | 83.19 | 4.736 | 0.9573 |
| Caffeic acid | 116.2 | 13.48 | 0.9989 | 100.2 | 9.109 | 0.9889 |
| Protocatechuic acid | 111.6 | 8.960 | 0.9987 | 90.25 | 6.517 | 0.9943 |
| Adsorbent | Compounds * | Adsorption Capacity (mg/g) | Time (min) | Reference |
|---|---|---|---|---|
| SILs | FA, CA, SA | 53.2–72.2 | - | [12] |
| Fe3O4@SiO2 | CA | 21.10 | 35 | [18] |
| MCMD | GA, CA | 31.95, 27.64 | 720 | [19] |
| AMCS | GA | 48.38 | 720 | [20] |
| Fe3O4@NH2 (CB [6]) NH2 MNPs | SAB, FA, RA, SAA, LA, CA | 21.96–36.36 | 25 | [39] |
| pH-MMIPs | CA | 11.50 | - | [40] |
| NKA-II resin | CGA | 66.86 | 300 | [41] |
| SiO2-NH2 | CA, PCA, GA | 81.19, 38.15, 58.45 | 120 | This work |
| SiO2-NH2-FPBA | CA, PCA, GA | 93.64, 89.35, 79.66 | 120 | This work |
| No. | Rt (min) | Compound Identified | Molecular Formula | Molecular Weight | [M-H]− m/z | Err [ppm] | MS/MS |
|---|---|---|---|---|---|---|---|
| 1 | 6.73 | Dihydroferulic acid | C10H12O4 | 196.0736 | 195.0658 | 3.007 | 195.05064, 59.01374 |
| 2 | 6.85 | Chlorogenic acid | C16H18O9 | 354.0945 | 353.1023 | −0.095 | 191.03650 |
| 3 | 6.93 | Salicylic acid | C7H6O3 | 138.0313 | 137.0241 | 1.119 | 119.02329, 93.03446 |
| 4 | 7.99 | 2,5-Dihydroxybenzaldehyde | C7H6O3 | 138.0317 | 137.0241 | 4.017 | 137.02419, 109.02918 |
| 5 | 8.10 | Gallic acid | C7H6O5 | 170.0212 | 169.1230 | 1.325 | 125.02416 |
| 6 | 8.14 | Rosmarinic acid | C18H16O8 | 360.0845 | 359.2044 | 1.475 | 197.04518, 179.03462, 161.02415, 135.04495, 123.04496, 72.99299 |
| 7 | 8.17 | Succinic acid | C4H6O4 | 118.0266 | 117.3994 | 4.574 | 117.01916, 114.40305, 99.00867, 73.02940 |
| 8 | 8.24 | 4-Hydroxybenzaldehyde | C7H6O2 | 122.0368 | 121.0292 | 4.663 | 121.02927, 108.02168, 93.03434 |
| 9 | 8.88 | 3,5-O-dimethyl gallic acid | C9H10O5 | 198.0528 | 197.8075 | 2.651 | 197.80759 |
| 10 | 10.42 | Caffeic acid ethyl ester | C11H12O4 | 208.0736 | 207.1385 | 2.834 | 207.02722, 179.03477, 135.04494 |
| 11 | 10.62 | Protocatechuic acid | C7H6O4 | 154.0267 | 153.0190 | 4.154 | 153.01906, 109.02934 |
| 12 | 10.64 | Dihydrocaffeic acid | C9H10O4 | 182.0579 | 181.0502 | 2.964 | 181.05038, 137.06052, 109.02934, 59.01374 |
| 13 | 11.26 | Caffeic acid | C9H8O4 | 180.0423 | 179.1928 | 3.276 | 135.04494 |
| 14 | 11.29 | Vanillic acid | C8H8O4 | 168.0423 | 167.0459 | 3.510 | 167.033474, 152.01125, 138.92929, 108.02152 |
| 15 | 12.07 | Protocatechuic aldehyde | C7H6O3 | 138.0317 | 137.5061 | 4.017 | 137.02415 |
| 16 | 18.76 | Yunnaneic acid D | C27H24O12 | 540.1267 | 539.1385 | 0.875 | 491.10147, 297.07635, 179.03484, 161.02408, 135.04500 |
| 17 | 20.31 | Rosmarinic acid-Glc | C24H26O13 | 522.1373 | 521.1290 | 0.973 | 359.09784, 341.08820, 323.07614, 197.04514, 179.03461 |
| 18 | 21.38 | Salvianolic aid A | C26H22O10 | 494.1140 | 493.2072 | 0.763 | 295.06058, 267.06546, 203.03455, 185.02414, 135.04495, 109.02928 |
| 19 | 23.22 | Dicaffeoyltartaric acid | C22H18O12 | 474.0798 | 473.0689 | 1.102 | 179.03491, 161.02411, 135.04504 |
| 20 | 23.99 | Lithospermic acid | C27H22O12 | 538.1111 | 537.1029 | 0.971 | 493.11276, 161.02406, 135.04492 |
| 21 | 24.35 | Clerodendranoic acid | C29H26O12 | 566.1424 | 565.1553 | 0.923 | 357.09732 |
| 22 | 64.45 | Asiatic acid | C30H48O5 | 488.3493 | 487.3419 | −0.668 | 487.34143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Song, Q.; Zhang, X.; Zheng, P.; Zhao, R.; Li, Y.; Chen, H. Phenylboronic Acid and Amino Bifunctional Modified Adsorbent for Quickly Separating Phenolic Acids from Crude Extract of Clerodendranthus spicatus and Evaluation of Their Antioxidant and Hypoglycemic Activities. Molecules 2023, 28, 7539. https://doi.org/10.3390/molecules28227539
Cheng M, Song Q, Zhang X, Zheng P, Zhao R, Li Y, Chen H. Phenylboronic Acid and Amino Bifunctional Modified Adsorbent for Quickly Separating Phenolic Acids from Crude Extract of Clerodendranthus spicatus and Evaluation of Their Antioxidant and Hypoglycemic Activities. Molecules. 2023; 28(22):7539. https://doi.org/10.3390/molecules28227539
Chicago/Turabian StyleCheng, Mengqi, Qianyi Song, Xiaoyu Zhang, Pingyi Zheng, Ran Zhao, Youxin Li, and Haixia Chen. 2023. "Phenylboronic Acid and Amino Bifunctional Modified Adsorbent for Quickly Separating Phenolic Acids from Crude Extract of Clerodendranthus spicatus and Evaluation of Their Antioxidant and Hypoglycemic Activities" Molecules 28, no. 22: 7539. https://doi.org/10.3390/molecules28227539
APA StyleCheng, M., Song, Q., Zhang, X., Zheng, P., Zhao, R., Li, Y., & Chen, H. (2023). Phenylboronic Acid and Amino Bifunctional Modified Adsorbent for Quickly Separating Phenolic Acids from Crude Extract of Clerodendranthus spicatus and Evaluation of Their Antioxidant and Hypoglycemic Activities. Molecules, 28(22), 7539. https://doi.org/10.3390/molecules28227539







