1,1,1,3,3,3-Hexafluoro-2-Propanol-Promoted Friedel–Crafts Reaction: Metal-Free Synthesis of C3-Difluoromethyl Carbinol-Containing Imidazo[1,2-a]pyridines at Room Temperature
Abstract
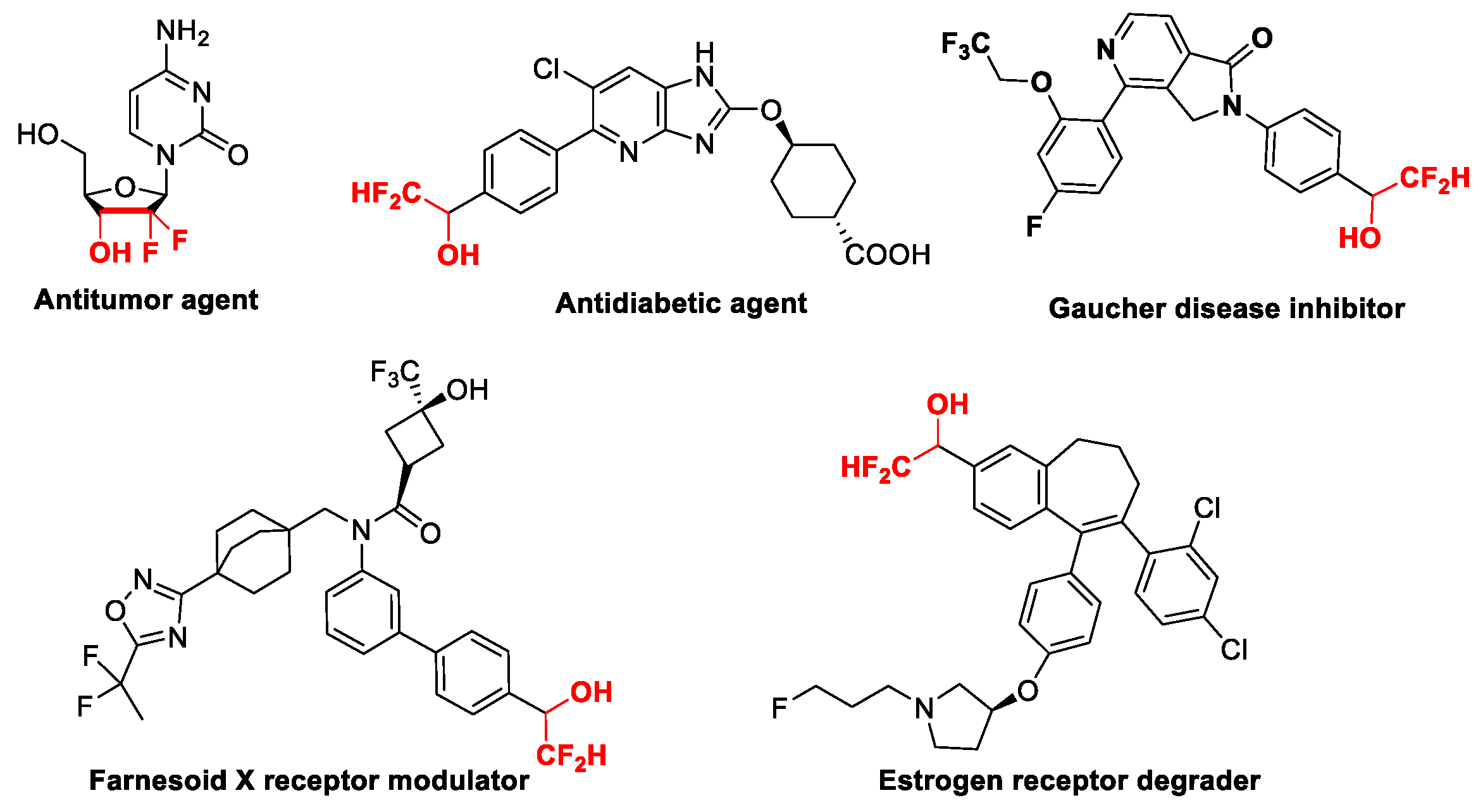
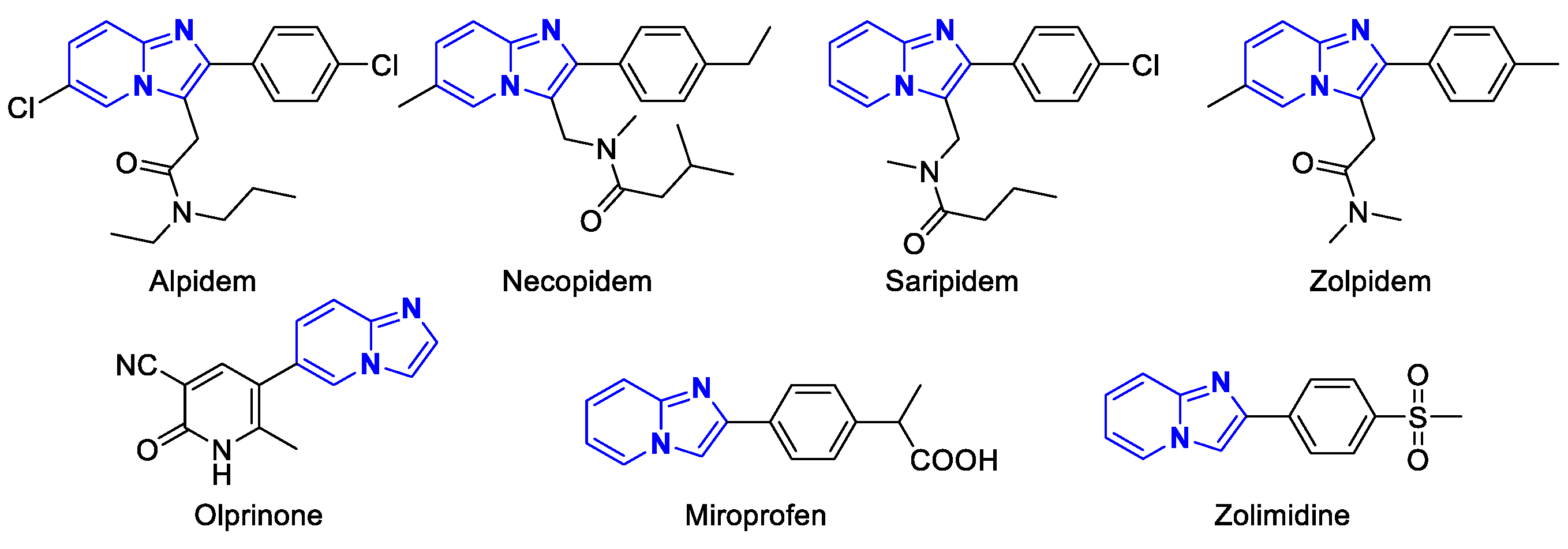
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
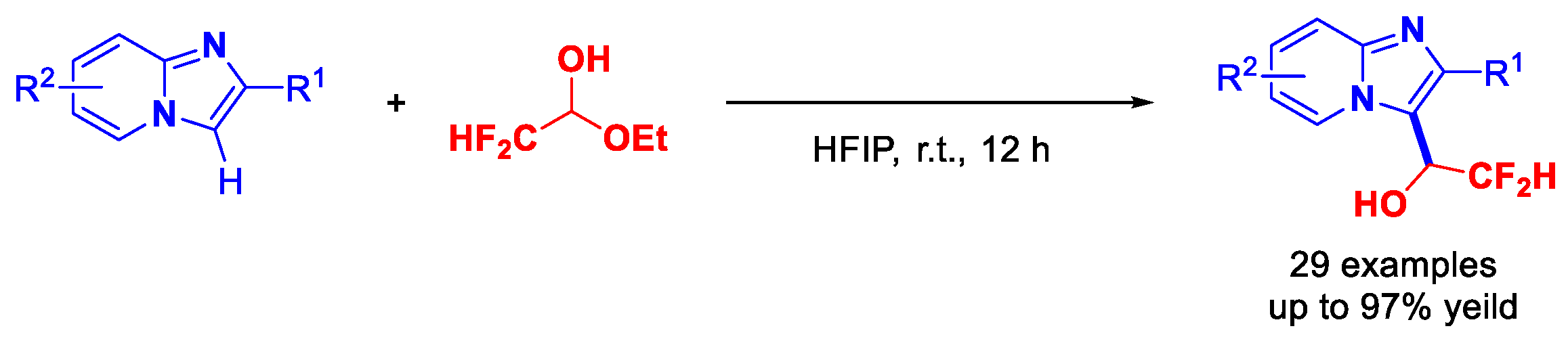
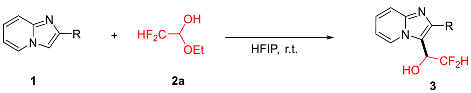
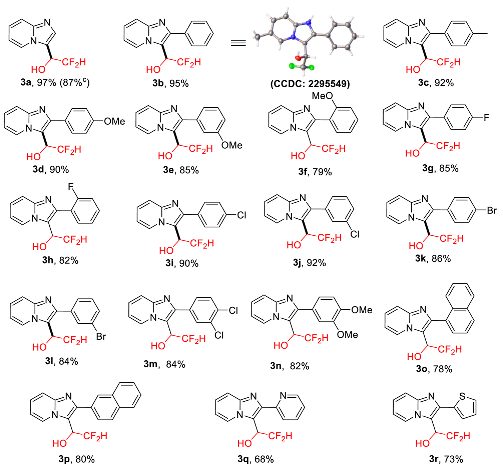
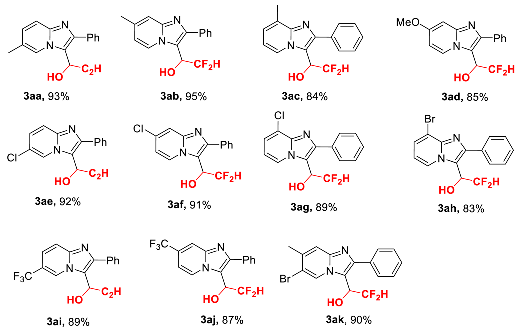
2.2. Scope of Reaction Substrates
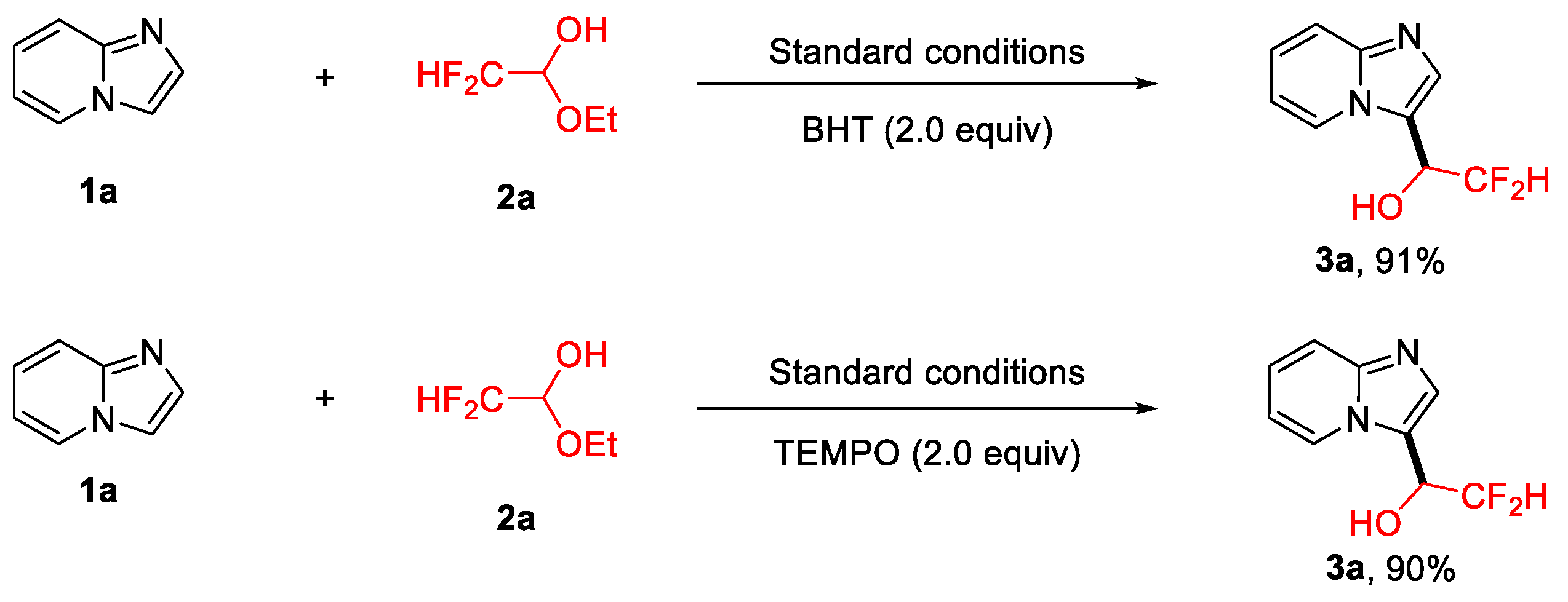
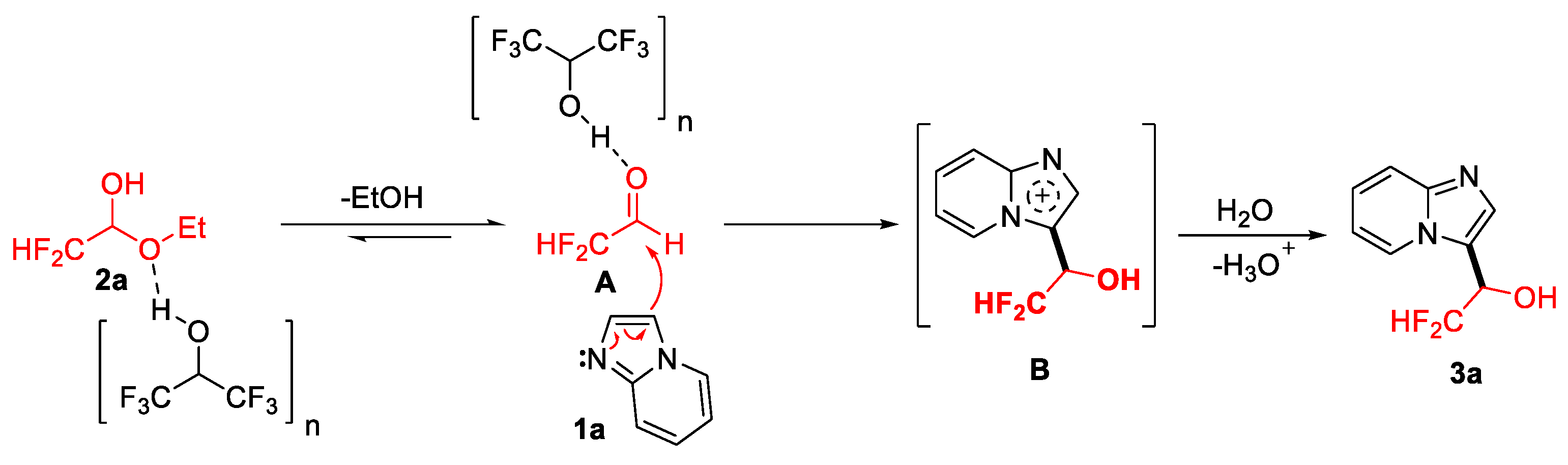
2.3. Mechanism Investigation
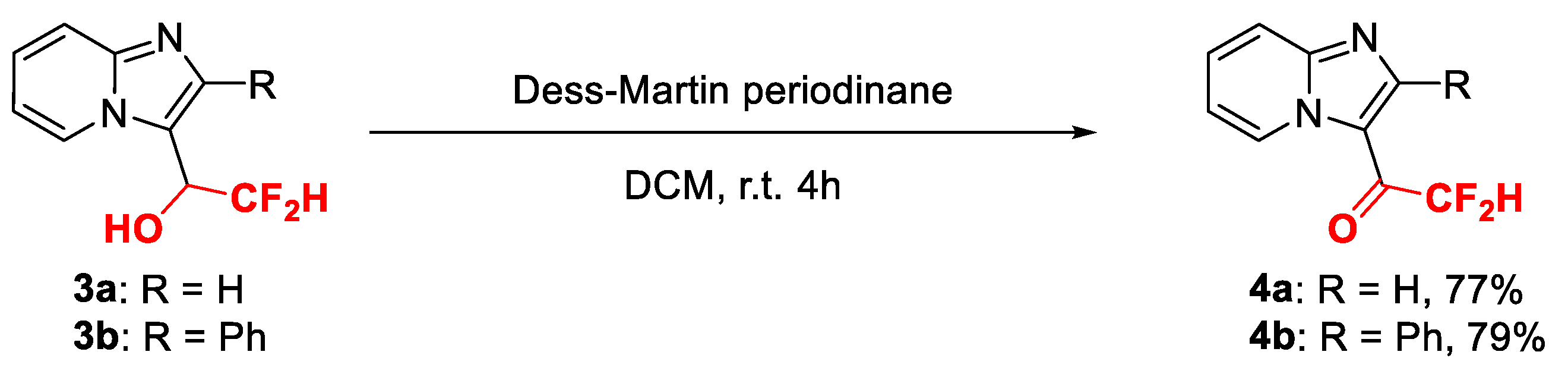
2.4. Synthetic Derivatization
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for Compounds 3a–3ak
3.3. Experimental Procedure for Compounds 4a and 4b
3.4. Characterization Data for All Products 3a–3ak and 4a–4b
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3a), white solid (38 mg, 97%); m.p. 160–162 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH: 5.16–5.23 (m, 1H), 6.10 (td, J = 55.6, 3.2 Hz, 1H), 6.85–6.90 (m, 1H), 7.23–7.28 (m, 1H), 7.49–7.56 (m, 2H), 8.41 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC: 65.7 (t, J = 26.0 Hz), 112.7, 115.1 (t, J = 243.0 Hz), 116.9, 119.9, 125.6, 125.7, 131.8, 146.2; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −126.14 (d, J = 285.8 Hz, 1F), 127.05 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C9H9F2N2O [M + H]+, 199.0677, found: 199.0669.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-phenyl-imidazo[1,2-a]pyridine (3b), white solid (52 mg, 95%); m.p. 161–163 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.31–5.38 (m, 1H), 6.14 (td, J = 55.6, 3.2 Hz, 1H), 7.18–7.23 (m, 1H), 7.30–7.35 (m, 3H), 7.44–7.50 (m, 3H), 8.63 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.2 (t, J = 25.0 Hz), 112.3, 115.4 (t, J = 245.0 Hz), 115.6, 116.5, 125.9, 127.3, 128.3, 128.4, 128.8, 133.1, 145.4, 145.5; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.70 (d, J = 282.0 Hz, 1F), −124.88 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H13F2N2O [M + H]+, 275.0990, found: 275.0979.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(p-tolyl)-imidazo[1,2-a]pyridine (3c), white solid (53 mg, 92%); m.p. 187–189 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 2.35 (s, 3H), 5.31–5.38 (m, 1H), 6.13 (J = 55.6, 3.2 Hz, 1H), 6.75–6.80 (m, 1H), 7.13 (d, J = 7.6 Hz, 2H), 7.16–7.21 (m, 1H), 7.35 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 9.2 Hz, 1H), 8.61 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 21.2, 66.2 (t, J = 24.6 Hz), 112.2, 115.4 (t, J = 244.0 Hz), 116.4, 125.8, 127.3, 128.6, 128.8, 129.1, 129.3, 130.1, 138.1, 145.4; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.68 (d, J = 282.0 Hz, 1F), −124.96 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O [M + H]+, 289.1147, found: 289.1136.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(4-methoxyphenyl)-imidazo[1,2-a]pyridine (3d), white solid (55 mg, 90%); m.p. 178–180 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 3.83 (s, 3H), 5.29–5.36 (m, 1H), 6.14 (td, J = 55.6, 2.4 Hz, 1H), 6.71–6.81 (m, 1H), 6.86–6.90 (m, 2H), 7.81–7.21 (m, 1H), 7.40–7.46 (m, 3H), 8.62 (d, J = 4.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 55.2, 66.2 (t, J = 25.0 Hz), 112.2, 113.9, 115.1, 115.4 (t, J = 244.0 Hz), 116.3, 115.6, 125.8, 127.2, 130.1, 145.2, 145.4, 159.6; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.74 (d, J = 282.0 Hz, 1F), −124.87 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O2 [M + H]+, 305.1096, found: 305.1081.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(3-methoxyphenyl)-imidazo[1,2-a]pyridine (3e), white solid (52 mg, 85%); m.p. 176–178 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.34–5.42 (m, 1H), 6.13 (td, J = 55.6, 3.6 Hz, 1H), 6.75–6.79 (m, 1H), 6.84–6.87 (m, 1H), 7.00–7.05 (m, 2H), 7.14–7.23 (m, 2H), 7.41 (d, J = 7.2 Hz, 1H), 8.61 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 55.2, 66.3 (t, J = 26.0 Hz), 112.3, 112.9, 114.1, 114.2, 115.4 (t, J = 245.0 Hz), 115.5, 116.6, 1221.2, 125.9, 127.3, 129.5, 134.4, 145.3, 145.4, 159.4; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.62 (d, J = 282.0 Hz, 1F), −124.75 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O2 [M + H]+, 305.1096, found: 305.1081.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(2-methoxyphenyl)-imidazo[1,2-a]pyridine (3f), white solid (48 mg, 79%); m.p. 177–179 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 3.56 (s, 1H), 5.07–5.14 (m, 1H), 5.91 (td, J = 55.6, 1.6 Hz, 1H), 6.70–6.77 (m, 2H), 6.81 (d, J = 8.4 Hz, 1H), 7.09–7.16 (m, 2H), 7.21–7.25 (m, 1H), 7.37 (d, J = 8.8 Hz, 1H), 8.57 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 55.2, 66.5 (t, J = 24.0 Hz), 111.0, 112.1, 115.7 (t, J = 246.0 Hz),, 116.6, 116.9, 120.8, 122.1, 125.4, 127.2, 130.0, 132.1, 141.3, 145.6, 156.3; 19F NMR (376 MHz, CDCl3), δF, ppm: −123.06 (d, J = 278.2 Hz, 1F), −127.67 (d, J = 278.2 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O2 [M + H]+, 305.1096, found: 305.1081.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(4-fluorophenyl)-imidazo[1,2-a]pyridine 2,2- (3g), white solid (49 mg, 85%); m.p. 208–210 °C; 1H-NMR (400 MHz, CD3OD), δH, ppm: 5.28–5.35 (m, 1H), 6.33 (td, J = 55.6, 3.6 Hz, 1H), 6.92–6.96 (m, 1H), 7.18–7.24 (m, 2H), 7.35–7.39 (m, 1H), 7.55 (d, J = 9.2 Hz, 1H), 7.63–7.67 (m, 2H), 8.75 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CD3OD), δC, ppm: 66.1 (t, J = 25.0 Hz), 112.3, 115.1 (t, J = 22.0 Hz), 115.7 (t, J = 243.0 Hz), 115.8, 116.1, 126.4, 127.5, 129.6 (d, J = 3.0 Hz), 130.8 (d, J = 9.0 Hz), 145.5, 144.2, 163.0 (d, J = 246.0 Hz); 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −115.40, −125.45 (d, J = 285.8 Hz, 1F), −126.28 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12F3N2O [M + H]+, 293.0896, found: 293.0885.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(2-fluorophenyl)-imidazo[1,2-a]pyridine (3h), white solid (48 mg, 82%); m.p. 173–175 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 5.04–5.11 (m, 1H), 5.95 (td, J = 55.6, 2.0 Hz, 1H), 6.68–6.72 (m, 1H), 6.92–7.00 (m, 2H), 7.09–7.13 (m, 1H), 7.17–7.23 (m, 1H), 7.26–7.31 (m, 1H), 8.37 (d, J = 9.2 Hz, 1H), 8.57 (d, J = 6.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 66.4 (t, J = 24.0 Hz), 112.4, 115.4 (t, J = 246.0 Hz), 115.6, 115.8, 116.6, 120.9 (d, J = 15.0 Hz), 124.3 (d, J = 4.0 Hz), 126.0, 127.5, 130.4 (d, J = 8.0 Hz), 132.0, 138.9, 145.9, 159.4 (d, J = 245.0 Hz); 19F NMR (376 MHz, CDCl3), δF, ppm: −123.88 (d, J = 285.8 Hz, 1F), −124.66 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12F3N2O [M + H]+, 293.0896, found: 293.0885.
- (±)-2-(4-Chlorophenyl)-3-(2,2-difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3i), white solid (55 mg, 90%); m.p. 198–200 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.26–5.32 (m, 1H), 6.16 (td, J = 55.6, 3.2 Hz, 1H), 6.80–6.85 (m, 1H), 7.22–7.31 (m, 3H), 7.40 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 9.2 Hz, 1H), 8.60 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.3 (t, J = 26.0 Hz), 112.6, 115.2 (t, J = 245.0 Hz), 115.6, 116.6, 126.2, 127.3, 128.7, 129.9, 131.5, 134.4, 144.2, 145.5; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.74 (d, J = 282.0 Hz, 1F), −124.57 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12ClF2N2O [M + H]+, 309.0601, found: 309.0616.
- (±)-2-(3-Chlorophenyl)-3-(2,2-difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3j), white solid (57 mg, 92%); m.p. 174–176 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.28–5.26 (m, 1H), 6.15 (td, J = 55.6, 3.6 Hz, 1H), 6.78–6.83 (m, 1H), 7.20–7.23 (m, 1H), 7.25–7.32 (m, 2H), 7.35–7.38 (m, 1H), 7.44–7.51 (m, 2H), 8.63 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.1 (t, J = 25.0 Hz), 112.6, 115.3 (t, J = 245.0 Hz), 115.9, 116.5, 126.2, 126.9, 127.4, 128.3, 128.7, 129.7, 134.3, 134.9, 143.8, 145.5; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.86 (d, J = 285.8 Hz, 1F), −124.63 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12ClF2N2O [M + H]+, 309.0601, found: 309.0616.
- (±)-2-(4-Bromophenyl)-3-(2,2-difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3k), white solid (60 mg, 86%); m.p. 190–192 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.24–5.31 (m, 1H), 6.16 (td, J = 55.6, 3.6 Hz, 1H), 6.80–6.85 (m, 1H), 7.22–7.26 (m, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.43–7.47 (m, 3H), 8.59 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.2 (t, J = 25.0 Hz), 112.5, 115.2 (t, J = 245.0 Hz), 115.7, 116.6, 122.7, 126.2, 127.3, 130.2, 131.6, 131.9, 144.2, 145.5; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.74 (d, J = 282.0 Hz, 1F), −124.56 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15BrF2N2O [M + H]+, 353.0096, found: 353.0107.
- (±)-2-(3-Bromophenyl)-3-(2,2-difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3l), white solid (59 mg, 84%); m.p. 186–188 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.27–5.35 (m, 1H), 6.01–6.16 (m, 1H), 6.78–6.82 (m, 1H), 7.17–7.22 (m, 2H), 7.38–7.45 (m, 3H), 7.67 (s, 1H), 8.62 (d, J = 6.4 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.22 (t, J = 25.0 Hz), 112.6, 115.3 (t, J = 245.0 Hz), 115.9, 116.6, 122.5, 126.3, 127.4, 130.0, 131.3, 131.7, 135.2, 143.7, 145.6; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.88 (d, J = 285.8 Hz, 1F), −124.66 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12BrF2N2O [M + H]+, 353.0096, found: 353.0107.
- (±)-2-(3,4-Dichlorophenyl)-3-(2,2-difluoro-1-hydroxy)ethyl-imidazo[1,2-a]pyridine (3m), white solid (57 mg, 84%); m.p. 205–207 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.26–5.34 (m, 1H), 6.18 (td, J = 55.6, 3.6 Hz, 1H), 6.83–6.87 (m, 1H), 7.25–7.30 (m, 1H), 7.38–7.53 (m, 3H), 7.68 (s, 1H), 8.63 (d, J = 6.4 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD=7:1), δC, ppm: 66.2 (t, J = 26.0 Hz), 112.7, 115.2 (t, J = 245.0 Hz), 116.0, 116.6, 126.5, 127.4, 128.0, 130.5, 132.5, 132.6, 133.2, 142.9, 145.6; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.85 (d, J = 285.8 Hz, 1F), −124.66 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H11Cl2F2N2O [M + H]+, 343.0211, found: 343.0213.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(3,4-dimethoxyphenyl)-imidazo[1,2-a]pyridine (3n), white solid (55 mg, 82%); m.p. 178–180 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 3.86 (s, 3H), 3.90 (s, 3H), 5.35–5.42 (m, 1H), 6.19 (td, J = 55.6, 3.6 Hz, 1H), 6.78–6.86 (m, 2H), 7.04 (d, J = 8.0 Hz, 1H), 7.16 (s, 1H), 7.19–7.23 (m, 1H), 7.46 (d, J = 8.8 Hz, 1H), 8.61 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 55.7, 55.7, 66.3 (t, J = 25.0 Hz), 110.9, 112.1, 112.3, 115.1, 115.4 (t, J = 245.0 Hz), 116.3, 121.2, 125.8, 125.9, 127.2, 145.3, 145.4, 148.7, 149.0; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.54 (d, J = 282.0 Hz, 1F), −124.53 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C17H17F2N2O3 [M + H]+, 335.1202, found: 335.1195.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(naphthalen-1-yl)-imidazo[1,2-a]pyridine (3o), white solid (51 mg, 78%); m.p. 212–214 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 4.68–4.76 (m, 1H), 5.45 (td, J = 55.6, 2.8 Hz, 1H), 6.67–6.71 (m, 1H), 6.94–6.99 (m, 2H), 7.06–7.10 (m, 1H), 7.15–7.19 (m, 1H), 7.25–7.30 (m, 2H), 7.41 (d, J = 8.4 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 8.46 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 66.0 (t, J = 25.0 Hz), 112.3, 115.2 (t, J = 245.0 Hz), 116.4, 117.2, 124.9, 125.6, 125.9, 125.9, 126.2, 127.3, 128.0, 128.3, 129.0, 130.2, 132.2, 133.4, 143.8, 145.3; ESI-HRMS, m/z: Calcd for C19H15F2N2O [M + H]+, 325.1147, found: 325.1154.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(naphthalen-2-yl)-imidazo[1,2-a]pyridine (3p), white solid (52 mg, 80%); m.p. 210–212 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.38–5.42 (m, 1H), 6.14 (td, J = 55.6, 3.2 Hz, 1H), 6.57–6.61 (m, 1H), 6.96–7.01 (m, 1H), 7.33 (d, J = 8.8 Hz, 1H), 7.47–7.51 (m, 3H), 7.65 (d, J = 8.4 Hz, 1H), 7.73–7.77 (m, 2H), 7.85 (s, 1H), 8.53 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD=7:1), δC, ppm: 66.3 (t, J = 25.0 Hz), 112.2, 115.4 (t, J = 245.0 Hz), 115.8, 116.3, 125.8, 126.3, 126.3, 126.4, 127.2, 127.6, 127.8, 128.1, 128.3, 130.4, 132.9, 133.0, 145.2, 145.5; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.52 (d, J = 285.8 Hz, 1F), −124.60 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C19H15F2N2O [M + H]+, 325.1147, found: 325.1154.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(pyridin-2-yl)-imidazo[1,2-a]pyridine (3q), white solid (37 mg, 68%); m.p. 165–167 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 5.25–5.32 (m, 1H), 5.87 (td, J = 55.6, 5.6 Hz, 1H), 6.78–6.82 (m, 1H), 7.14–7.23 (m, 2H), 7.56 (d, J = 9.2 Hz, 1H), 7.78–7.82 (m, 1H), 8.03 (d, J = 6.8 Hz, 1H), 8.38 (d, J = 8.0 Hz, 1H), 8.45 (d, J = 4.0 Hz, 1H), 9.82 (br, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 65.8 (t, J = 26.0 Hz), 113.3, 115.9 (t, J = 244.0 Hz), 118.0, 120.1, 123.0, 123.0, 124.0, 125.6, 138.4, 142.6, 145.1, 147.2, 152.6; 19F NMR (376 MHz, CDCl3), δF, ppm: −123.20 (d, J = 282.0 Hz, 1F), −127.07 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C14H12F2N3O [M + H]+, 276.0943, found: 276.0962.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-(thiophen-2-yl)-imidazo[1,2-a]pyridine (3r), white solid (41 mg, 73%); m.p. 163–165 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.46–5.54 (m, 1H), 6.10 (td, J = 55.6, 3.2 Hz, 1H), 6.69–6.73 (m, 1H), 6.96–6.99 (m, 1H), 7.11–7.16 (m, 1H), 7.19 (d, J = 3.6 Hz, 1H), 7.27 (d, J = 5.2 Hz, 1H), 7.40 (d, J = 9.2 Hz, 1H), 8.57 (d, J = 7.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.4 (t, J = 25.0 Hz), 112.4, 114.9, 115.4 (t, J = 246.0 Hz), 116.4, 126.2, 126.3, 126.7, 127.4, 127.6, 135.5, 139.1, 145.7; 19F NMR (376 MHz, CDCl3), δF, ppm: −123.44 (d, J = 282.0 Hz, 1F), −127.37 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C13H11F2N2OS [M + H]+, 281.0555, found: 281.0566.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-6-methyl-2-phenyl-imidazo[1,2-a]pyridine (3aa), white solid (54 mg, 93%); m.p. 212–214 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 2.30 (s, 3H), 5.28–5.36 (m, 1H), 6.13 (td, J = 55.6, 4.0 Hz, 1H), 7.00 (d, J = 9.2 Hz, 1H), 7.26–7.31 (m, 4H), 7.38–7.41 (m, 2H), 8.35 (s, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 18.4, 66.3 (t, J = 25.0 Hz), 115.1, 115.2 (t, J = 245.0 Hz), 115.8, 122.0, 124.8, 128.0, 128.4, 128.7, 129.0, 133.1, 144.5, 145.2; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.27 (d, J = 282.0 Hz, 1F), −124.93 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O [M + H]+, 289.1147, found: 289.1136.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-7-methyl-2-phenyl-imidazo[1,2-a]pyridine (3ab), white solid (55 mg, 95%); m.p. 186–188 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 2.32 (s, 3H), 5.26–5.34 (m, 1H), 6.18 (td, J = 55.6, 3.6 Hz, 1H), 7.55 (d, J = 8.8 Hz, 1H), 7.17–7.29 (m, 6H), 8.43 (d, J = 9.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 21.3, 66.4 (t, J = 25.0 Hz), 114.8, 115.1, 115.3 (t, J = 245.0 Hz), 126.5, 128.1, 128.4, 128.6, 132.7, 137.3, 144.8, 145.7; 19F NMR (376 MHz, CDCl3), δF, ppm: −123.73 (d, J = 282.0 Hz, 1F), −124.81 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O [M + H]+, 289.1147, found: 289.1136.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-8-methyl-2-phenyl-imidazo[1,2-a]pyridine (3ac), white solid (49 mg, 84%); m.p. 184–186 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 2.53 (s, 3H), 5.21–5.28 (m, 1H), 6.08 (td, J = 55.6, 1.6 Hz, 1H), 6.70–6.74 (m, 1H), 7.03 (d, J = 6.0 Hz, 1H), 7.28–7.32 (m, 3H), 7.45–7.48 (m, 2H), 8.48 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 16.9, 66.2 (t, J = 25.0 Hz), 112.4, 115.2 (t, J = 245.0 Hz), 115.9, 124.8, 124.9, 126.7, 128.1, 128.3, 129.1, 133.3, 145.2, 145.9; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.51 (d, J = 282.0 Hz, 1F), −124.57 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O [M + H]+, 289.1147, found: 289.1136.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-7-methoxy-2-phenyl-imidazo[1,2-a]pyridine (3ad), white solid (52 mg, 85%); m.p. 201–203 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 3.86 (s, 3H), 5.27–5.32 (m, 1H), 5.99–6.28 (m, 1H), 6.78–6.81 (m, 1H), 7.31–7.42 (m, 3H), 7.49–7.59 (m, 2H), 8.46 (d, J = 4.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 55.5, 66.2 (t, J = 25.0 Hz), 93.7, 107.3, 114.3, 115.4 (t, J = 245.0 Hz), 127.8, 128.1, 128.4, 128.7, 133.3, 145.0, 147.3, 158.8; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.98 (d, J = 282.0 Hz, 1F), −125.18 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H15F2N2O2 [M + H]+, 305.1096, found: 305.1081.
- (±)-6-Chloro-3-(2,2-difluoro-1-hydroxy)ethyl-2-phenyl-imidazo[1,2-a]pyridine (3ae), white solid (57 mg, 92%); m.p. 215–217 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.30–5.38 (m, 1H), 6.16 (td, J = 55.6, 3.2 Hz, 1H), 7.18–7.22 (m, 1H), 7.36–7.42 (m, 3H), 7.46 (d, J = 9.2 Hz, 1H), 7.51–7.54 (m, 2H), 8.72 (s, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.3 (t, J = 25.0 Hz), 115.3 (t, J = 245.0 Hz), 116.2, 116.9, 120.5, 125.3, 127.2, 128.5, 128.6, 128.8, 132.8, 144.0, 146.3; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.99 (d, J = 282.0 Hz, 1F), −124.97 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12ClF2N2O [M + H]+, 309.0601, found: 309.0616.
- (±)-7-Chloro-3-(2,2-difluoro-1-hydroxy)ethyl-7-chloro-2-phenyl-imidazo[1,2-a]pyridine (3af), white solid (56mg, 91%); m.p. 219–221 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.34–5.41 (m, 1H), 6.16 (t, J = 55.6 Hz, 1H), 6.83 (d, J = 7.2 Hz, 1H), 7.33–7.47 (m, 3H); 7.59 (d, J = 4.4 Hz, 2H), 8.62 (d, J = 7.2 Hz, 1H); 13C-NMR (100 MHz, DMSO-d6), δC, ppm: 65.8 (t, J = 24.0 Hz), 113.8, 116.0, 116.3 (t, J = 243.0 Hz), 116.8, 128.8, 128.9, 129.0, 129.2, 131.3, 133.8, 145.2, 146.0; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −124.13 (d, J = 285.8 Hz, 1F), −125.03 (d, d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12ClF2N2O [M + H]+, 309.0601, found: 309.0616.
- (±)-8-Chloro-3-(2,2-difluoro-1-hydroxy)ethyl-8-chloro-2-phenyl-imidazo[1,2-a]pyridine (3ag), white solid (55 mg, 89%); m.p. 233–235 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.32–5.39 (m, 1H), 6.08 (td, J = 55.6, 3.2 Hz, 1H), 6.78–6.82 (m, 1H), 7.34–7.45 (m, 4H), 7.62 (d, J = 6.4 Hz, 2H), 8.64 (d, J = 7.2 Hz, 1H); 13C-NMR (100 MHz, DMSO-d6), δC, ppm: 65.9 (t, J = 25.0 Hz), 112.5, 116.2 (t, J = 243.0 Hz), 118.4, 121.8, 125.2, 127.2, 128.9, 129.2, 129.2, 133.8, 142.5, 145.6; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −124.06 (d, J = 282.0 Hz, 1F), −125.11 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12ClF2N2O [M + H]+, 309.0601, found: 309.0616.
- (±)-8-Bromo-3-(2,2-difluoro-1-hydroxy)ethyl-2-phenyl-imidazo[1,2-a]pyridine (3ah), white solid (58 mg, 83%); m.p. 259–261 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.32–5.38 (m, 1H), 6.01–6.30 (m, 1H), 6.73–6.77 (m, 1H), 7.41–7.46 (m, 3H), 7.55 (d, J = 6.8 Hz, 1H), 7.64 (d, J = 6.8 Hz, 2H), 8.69 (d, J = 6.0 Hz, 1H); 13C-NMR (100 MHz, DMSO-d6), δC, ppm: 66.0 (t, J = 25.0 Hz), 110.6, 113.0, 116.2 (t, J = 243.0 Hz), 118.4, 127.6, 128.6, 128.8, 129.1, 129.2, 133.8, 143.1, 145.6; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −123.98 (d, J = 282.0 Hz, 1F), −125.16 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C15H12BrF2N2O [M + H]+, 353.0096, found: 353.0107.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-phenyl-6-(trifluoromethyl)-imidazo[1,2-a]pyridine (3ai), white solid (61 mg, 89%); m.p. 193–195 °C; 1H-NMR (400 MHz, CDCl3:CD3OD = 7:1), δH, ppm: 5.35–5.43 (m, 1H), 6.17 (td, J = 55.6, 2.8 Hz, 1H), 7.33–7.39 (m, 4H), 7.48–7.51 (m, 2H), 7.59 (d, J = 9.8 Hz, 1H), 9.02 (s, 1H); 13C-NMR (100 MHz, CDCl3:CD3OD = 7:1), δC, ppm: 66.1 (t, J = 25.0 Hz), 115.5 (t, J = 245.0 Hz), 116.5 (q, J = 34.0 Hz), 116.9, 117.3, 121.6–121.8 (m), 123.5 (q, J = 270.0 Hz), 126.8 (q, J = 5.0 Hz), 128.6, 128.7, 128.7, 132.4, 145.4, 147.1; 19F NMR (376 MHz, CDCl3:CD3OD = 7:1), δF, ppm: −62.05, −123.79 (d, J = 282.0 Hz, 1F), −125.09 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H12F5N2O [M + H]+, 343.0864, found: 343.0857.
- (±)-3-(2,2-Difluoro-1-hydroxy)ethyl-2-phenyl-7-(trifluoromethyl)-imidazo[1,2-a]pyridine (3aj), white solid (60 mg, 87%); m.p. 206–208 °C; 1H-NMR (400 MHz, CD3OD), δH, ppm: 5.37–5.45 (m, 1H), 6.36 (td, J = 55.6, 3.6 Hz, 1H), 7.18 (dd, J = 6.4, 3.2 Hz, 1H), 7.44–7.54 (m, 3H), 7.66–7.69 (m, 2H); 7.93 (s, 1H), 8.96 (d, J = 7.2 Hz, 1H); 13C-NMR (CD3OD), δC, ppm: 66.1 (t, J = 25.0 Hz), 107.6–107.7 (m), 113.8 (q, J = 4.0 Hz), 115.8 (t, J = 245.0 Hz), 118.0 (t, J = 3.0 Hz), 123.4 (q, J = 272.0 Hz), 127.5 (q, J = 34.0 Hz), 128.4, 128.6, 128.8, 129.0, 132.7, 143.7, 147.3; 19F NMR (376 MHz, CD3OD), δF, ppm: −65.30, −125.65 (d, J = 285.8 Hz, 1F), −126.62 (d, J = 285.8 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H12F5N2O [M + H]+, 343.0864, found: 343.0857.
- (±)-6-Bromo-3-(2,2-difluoro-1-hydroxy)ethyl-7-methyl-2-phenyl-6-(trifluoro-methyl)-imidazo[1,2-a]pyridine (3ak), white solid (66 mg, 90%); m.p. 237–239 °C; 1H-NMR (400 MHz, DMSO-d6), δH, ppm: 5.32–5.39 (m, 1H), 6.59 (t, J = 53.2 Hz, 1H), 6.86–6.88 (m, 1H), 7.40–7.51 (m, 3H), 7.54–7.70 (m, 3H), 8.85 (s, 1H); 13C-NMR (100 MHz, DMSO-d6), δC, ppm: 22.4, 65.8 (t, J = 24.0 Hz), 110.5, 116.0, 116.3 (t, J = 243.0 Hz), 116.6, 127.4, 127.7, 129.0, 129.1, 134.0, 135.7, 144.7, 145.6; 19F NMR (376 MHz, DMSO-d6), δF, ppm: −123.85 (d, J = 282.0 Hz, 1F), −124.80 (d, J = 282.0 Hz, 1F); ESI-HRMS, m/z: Calcd for C16H14BrF2N2O [M + H]+, 367.0252, found: 367.0244.
- 3-(2,2-Difluoroacetyl)-imidazo[1,2-a]pyridine (4a), white solid (15 mg, 77%); m.p. 102–104 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 6.26 (t, J = 53.6 Hz, 1H), 7.21–7.25 (m, 1H), 7.63–7.68 (m, 1H), 7.87 (d, J = 9.2 Hz, 1H), 8.63 (s, 1H), 9.62 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 111.1 (t, J = 252.0 Hz), 116.2, 118.1, 119.9, 129.0, 131.0, 146.0 (t, J = 6.0 Hz), 149.7, 177.0 (t, J = 26.0 Hz); 19F NMR (376 MHz, CDCl3), δ, ppm: −121.40; ESI-HRMS, m/z: Calcd for C9H7F2N2O [M + H]+, 197.0521, found: 197.0539.
- 3-(2,2-Difluoroacetyl)-2-phenyl-imidazo[1,2-a]pyridine (4b), white solid (22 mg, 79%); m.p. 121–123 °C; 1H-NMR (400 MHz, CDCl3), δH, ppm: 5.91 (t, J = 53.2 Hz, 1H), 7.23–7.26 (m, 1H), 7.52–7.58 (m, 3H), 7.62–7.65 (m, 2H), 7.66–7.72 (m, 1H), 7.85 (d, J = 8.8 Hz, 1H), 9.78 (d, J = 6.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3), δC, ppm: 106.0 (t, J = 244.0 Hz), 116.2, 117.7, 119.0, 128.8, 129.4, 129.7, 130.2, 131.6, 133.7, 148.5, 157.5, 177.2 (t, J = 25.0 Hz); 19F NMR (376 MHz, CDCl3), δF, ppm: −124.48; ESI-HRMS, m/z: Calcd for C15H11F2N2O [M + H]+, 273.0834, found: 273.0830.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; et al. CF2H, a functional group-dependent hydrogen-bond donor: Is it a more or less lipophilic bioisostere of OH, SH and CH3? J. Med. Chem. 2019, 62, 5628–5637. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, D.; Yang, Y.; You, H.; Sun, M.; Wang, Y.; Shen, X.; Liu, Z.Q. Free-radical-promoted Dehydrogenative coupling of polyfluorinated alcohol with quinone, chromone, and coumarin. Org. Lett. 2020, 22, 4844–4847. [Google Scholar] [CrossRef] [PubMed]
- Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. Difluoromethyl bioisostere: Examining the “lipophilic hydrogen bond donor” concept. J. Med. Chem. 2017, 60, 797–804. [Google Scholar] [CrossRef]
- Selmi-Higashi, E.; Zhang, J.L.; Cambeiro, X.C.; Arseniyadis, S. Synthesis of α-difluoromethyl aryl ketones through a photoredox difluoromethylation of enol silanes. Org. Lett. 2021, 23, 4239–4243. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, B.; Pei, C.C.; Li, J.Y.; Zou, D.P.; Wu, Y.J.; Wu, Y.S. Visible-light-induced radical difluoromethylation/cyclization of unactivated alkenes: Access to CF2H-substituted quinazolinones. Org. Lett. 2021, 23, 7787–7791. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, X.X.; Chen, J.Y.; Yang, C.; Pan, Y.L.; Cheng, J.P.; Meng, Q.B.; Li, X. Direct C-H difluoromethylation of heterocycles via organic photoredox catalysis. Nat. Commun. 2020, 11, 638. [Google Scholar] [CrossRef]
- Hertel, L.W.; Boder, G.B.; Kroin, J.S.; Rinzel, S.M.; Poore, G.A.; Todd, G.C.; Grindey, G.B. Evaluation of the antitumor activity of gemcitabine (2′,2′-Difluoro-2′-deox-ycytidine). Cancer Res. 1990, 50, 4417–4422. [Google Scholar]
- Rudd, M.T.; Bennett, D.J.; Wai, J.; Meng, Z. Homobispiperidinyl Derivatives as Liver X Receptor Beta Agonists, Compositions, and Their Use. Patent WO2017095758A1, 8 June 2017. [Google Scholar]
- Tanaka, Y.; Kikuchi, F.; Yamamoto, T.; Nakamura, M.; Takami, K.; Murakami, M.; Daini, M.; Wada, Y.; Kakegawa, K.; Kasahara, T.; et al. Heterocyclic Compound for Gaucher Disease. Patent WO2021020363, 4 February 2021. [Google Scholar]
- Wacker, D.A.; Nara, S.J.; Cheruku, S.; Sarkunam, K.; Jaipuri, F.A.; Thangavel, S.; Narayan, R.; Bandreddy, S.R.; Jogi, S.; Kathi, P.K. Substituted Bicyclic Compounds as Farnesoid X Receptor Modulators and Their Preparation. Patent WO2020168143, 20 August 2020. [Google Scholar]
- Boisnard, S.; El-Ahmad, Y.; Fett, E.; Halley, F.; Nicolai, E.; Tabart, M.; Terrier, C.; Vivet, B. Preparation of Novel Substituted 6,7-Dihydro-5H-benzo[7]annulene Compounds as Inhibitors and Degraders of Estrogen Receptors. Patent WO2021063967, 8 April 2021. [Google Scholar]
- Zhang, Y.X.; Yan, W.T.; Wang, Y.K.; Weng, Z.Q. Copper-catalyzed synthesis of indol-3-yl α-(difluoromethyl)-α-(trifluoromethyl)carbinols: Construction of difluoromethylated sp3 carbon centers. Org. Lett. 2017, 19, 5478–5481. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.Y.; Pei, C.C.; Li, J.Y.; Zou, D.P.; Wu, Y.J.; Wu, Y.S. Ruthenium-catalyzed ortho-C–H hydroxyfluoroalkylation of arenes with fluorinated alcohols. J. Org. Chem. 2022, 87, 14364–14373. [Google Scholar] [CrossRef]
- Devi, N.; Singh, D.; K. Rawal, R.; Bariwal, J.; Singh, V. Medicinal attributes of imidazo[1,2-a]pyridine derivatives: An update. Curr. Top. Med. Chem. 2016, 16, 2963–2994. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kayakiri, H.; Satoh, S.; Inoue, T.; Sawada, Y.; Inamura, N.; Asano, M.; Aramori, I.; Hatori, C.; Sawai, H.; et al. A Novel Class of Orally Active Non-Peptide Bradykinin B2 Receptor Antagonists. 4. Discovery of Novel Frameworks Mimicking the Active Conformation. J. Med. Chem. 1998, 41, 4587–4598. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Design, synthesis and structure-affinity relationships of aryloxyanilide derivatives as novel peripheral benzodiazepine receptor ligands. Bioorg. Med. Chem. 2004, 12, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.M.; Salunke, D.B.; Yoo, E.; Mutz, C.A.; Balakrishna, R.; David, S.A. Antibacterial activities of Groebke–Blackburn–Bienaymé-derived imidazo[1,2-a]pyridin-3-amines. Biorg. Med. Chem. 2012, 20, 5850–5863. [Google Scholar] [CrossRef]
- Feng, S.; Hong, D.; Wang, B.; Zheng, X.; Miao, K.; Wang, L.; Yun, H.; Gao, L.; Zhao, S.; Shen, H.C. Discovery of Imidazopyridine Derivatives as Highly Potent Respiratory Syncytial Virus Fusion Inhibitors. ACS Med. Chem. Lett. 2015, 6, 359–362. [Google Scholar] [CrossRef]
- Ismail, M.A.; Arafa, R.K.; Wenzler, T.; Brun, R.; Tanious, F.A.; Wilson, W.D.; Boykin, D.W. Synthesis and antiprotozoal activity of novel bis-benzamidino imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines. Biorg. Med. Chem. 2008, 16, 683–691. [Google Scholar] [CrossRef]
- Kim, O.; Jeong, Y.; Lee, H.; Hong, S.-S.; Hong, S. Design and synthesis of imidazopyridine analogues as inhibitors of phosphoinositide 3-kinase signaling and angiogenesis. J. Med. Chem. 2011, 54, 2455–2466. [Google Scholar] [CrossRef]
- Ma, C.H.; Chen, M.; Feng, Z.W.; Zhang, Y.; Wang, J.; Jiang, Y.-Q.; Yu, B. Functionalization of imidazo[1,2-a]pyridines via radical reactions. New J. Chem. 2021, 45, 9302–9314. [Google Scholar] [CrossRef]
- Ji, X.-M.; Wei, L.; Chen, F.; Tang, R.-Y. Direct trifluoromethylation of imidazoheterocycles in a recyclable medium at room temperature. RSC Adv. 2015, 38, 29766–29773. [Google Scholar] [CrossRef]
- Nipate, D.S.; Jaspal, S.; Shinde, V.N.; Rangan, K.; Kumar, A. TEMPO-mediated cross-dehydrogenative coupling of indoles and imidazo[1,2-a]pyridines with fluorinated alcohols. Org. Lett. 2021, 23, 1373–1377. [Google Scholar] [CrossRef]
- Lefebvre, Q.; Hoffmann, N.; Rueping, M. Photoorganocatalysed and visible light photoredox catalysed trifluoromethylation of olefins and (hetero)aromatics in batch and continuous flow. Chem. Commun. 2016, 52, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- Tfilla, P.; Baeza, A.; Najera, C. Fluorinated Alcohols As Promoters for the Metal-Free Direct Substitution Reaction of Allylic Alcohols with Nitrogenated, Silylated, and Carbon Nucleophiles. J. Org. Chem. 2012, 77, 7344–7354. [Google Scholar]
- Pérez, J.M.; Maquilón, C.; Ramón, D.J.; Baeza, A. Hexafluoroisopropanol-Promoted Metal-Free Allylation of Silyl Enol Ethers with Allylic Alcohols. Asian J. Org. Chem. 2017, 6, 1440–1444. [Google Scholar] [CrossRef]
- Yang, J.G.; Gui, J.; Mu, M.M.; Liu, S.M.; Li, J.S.; Ren, J.; Wang, Z.M. Synthesis of difluoromethylated carbinols via a HFIP-promoted hydroxy-difluoromethylation of aniline, indole, and pyrrole derivatives with difluoroacetaldehyde ethyl hemiacetal. J. Org. Chem. 2023, 88, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, H.F.; Armaly, A.M.; Cacioppo, J.G.; Coombs, T.C.; Koehn, K.R.K.; Norwood, V.M., IV; Aubé, J. HFIP in organic synthesis. Chem. Rev. 2022, 122, 12544–12747. [Google Scholar] [CrossRef]
- Chen, Y.X.; Wang, Y.R.; Zhong, R.; Li, J.S. HFIP promoted C3 alkylation of lawsone and 4-hydroxycoumarin with alcohols by dehydrative cross-coupling. J. Org. Chem. 2020, 85, 10638–10647. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Hong, P.; Li, J.; Wang, Z.; Ren, J. Synthesis of 2,2-Difluoro-3-hydroxy-1,4-diketones via an HFIP-Catalyzed Mukaiyama Aldol Reaction of Glyoxal Monohydrates with Difluoroenoxysilanes. J. Org. Chem. 2022, 87, 1144–1153. [Google Scholar] [CrossRef]
- CCDC: 2295549 (for 3b) Contain the Supplementary Crystallographic Data for This Paper. These Data Are Provided Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 11 October 2023).
- Li, J.; Xi, W.; Liu, S.; Ruan, C.; Zheng, X.; Yang, J.; Wang, L.; Wang, Z. HFIP-catalyzed diflfluoroalkylation of propargylic alcohols to access tetrasubstituted diflfluoroalkyl allenes. Org. Lett. 2021, 23, 7264–7269. [Google Scholar] [CrossRef]
- Li, G.-X.; Qu, J. Friedel-Crafts alkylation of arenes with epoxides promoted by fluorinated alcohols or water. Chem. Commun. 2010, 46, 2653–2655. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Gui, J.; Xiong, D.; Li, J.; Wang, Z.; Ren, J. HFIP-Promoted selective hydroxyalkylation of aniline derivatives with arylglyoxal hydrates. J. Org. Chem. 2022, 87, 6352–6361. [Google Scholar] [CrossRef]
- Obermayer, D.; Znidar, D.; Glotz, G.; Stadler, A.; Dallinger, D.; Kappe, C.O. Design and performance validation of a conductively heated sealed-vessel reactor for organic Synthesis. J. Org. Chem. 2016, 81, 11788–11801. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Ganguly, B.; Kar, B.; Dwivedi, S.; Das, S. Green procedure for highly efficient, rapid synthesis of imidazo[1,2-a]pyridine and its late stage functionalization. Synth. Commun. 2018, 48, 1076. [Google Scholar] [CrossRef]
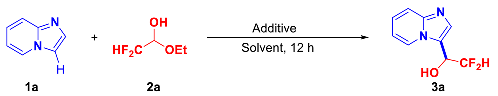 | |||
|---|---|---|---|
| Entry | Additive (Equiv.) | Solvent | Yield (%) [b] |
| 1 | TFA (10%) | DCM | trace |
| 2 | TsOH (10%) | DCM | trace |
| 3 | BF3·OEt2 | DCM | trace |
| 4 | Y(OTf)3 (10%) | DCM | trace |
| 5 | Sc(OTf)3 (10%) | DCM | trace |
| 6 | HFIP (10%) | DCM | trace |
| 7 | HFIP (1.0) | DCM | 50 |
| 8 | HFIP (2.0) | DCM | 73 |
| 9 | HFIP | 97 | |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Liu, Z.; Guo, X.; Wu, L.; Chen, Z.; Yang, K. 1,1,1,3,3,3-Hexafluoro-2-Propanol-Promoted Friedel–Crafts Reaction: Metal-Free Synthesis of C3-Difluoromethyl Carbinol-Containing Imidazo[1,2-a]pyridines at Room Temperature. Molecules 2023, 28, 7522. https://doi.org/10.3390/molecules28227522
Gao J, Liu Z, Guo X, Wu L, Chen Z, Yang K. 1,1,1,3,3,3-Hexafluoro-2-Propanol-Promoted Friedel–Crafts Reaction: Metal-Free Synthesis of C3-Difluoromethyl Carbinol-Containing Imidazo[1,2-a]pyridines at Room Temperature. Molecules. 2023; 28(22):7522. https://doi.org/10.3390/molecules28227522
Chicago/Turabian StyleGao, Juanjuan, Zhaowen Liu, Xiaohua Guo, Longhui Wu, Zhixi Chen, and Kai Yang. 2023. "1,1,1,3,3,3-Hexafluoro-2-Propanol-Promoted Friedel–Crafts Reaction: Metal-Free Synthesis of C3-Difluoromethyl Carbinol-Containing Imidazo[1,2-a]pyridines at Room Temperature" Molecules 28, no. 22: 7522. https://doi.org/10.3390/molecules28227522
APA StyleGao, J., Liu, Z., Guo, X., Wu, L., Chen, Z., & Yang, K. (2023). 1,1,1,3,3,3-Hexafluoro-2-Propanol-Promoted Friedel–Crafts Reaction: Metal-Free Synthesis of C3-Difluoromethyl Carbinol-Containing Imidazo[1,2-a]pyridines at Room Temperature. Molecules, 28(22), 7522. https://doi.org/10.3390/molecules28227522






