Efficient Demulsification Performance of Emulsified Condensate Oil by Hyperbranched Low-Temperature Demulsifiers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Emulsified Condensate Characterization
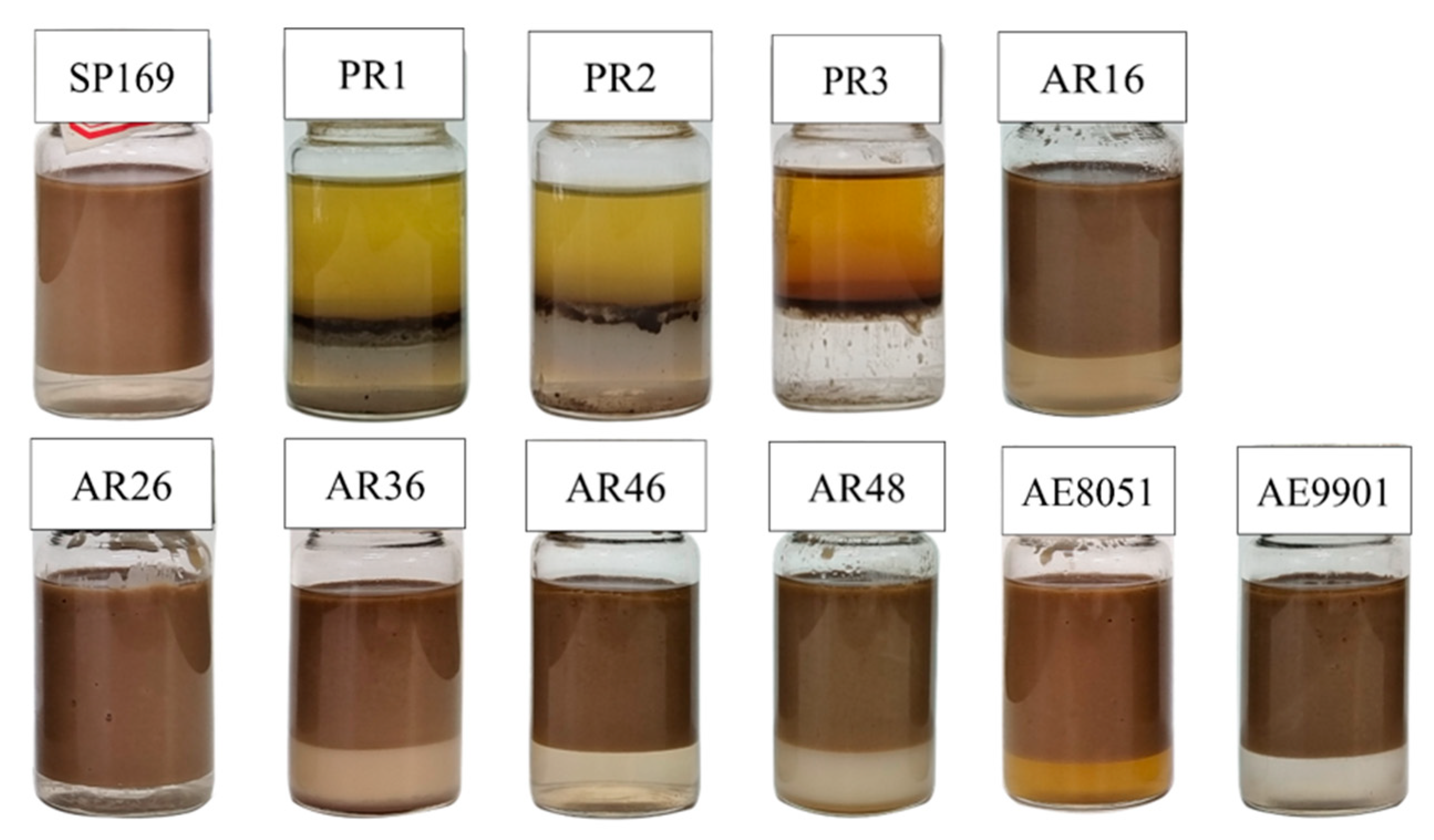
2.2. Effect of Different Surfactant Demulsifiers on the Demulsification of Emulsified Condensate Oil
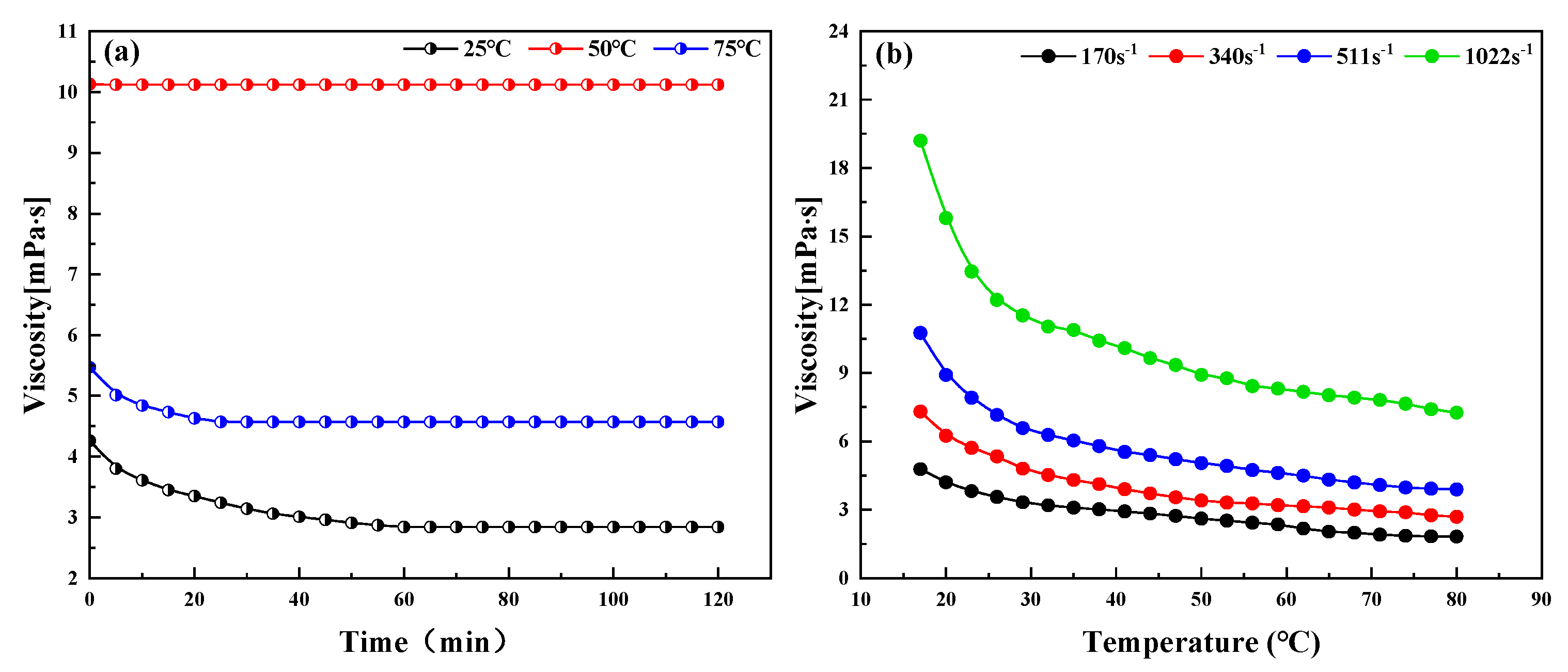
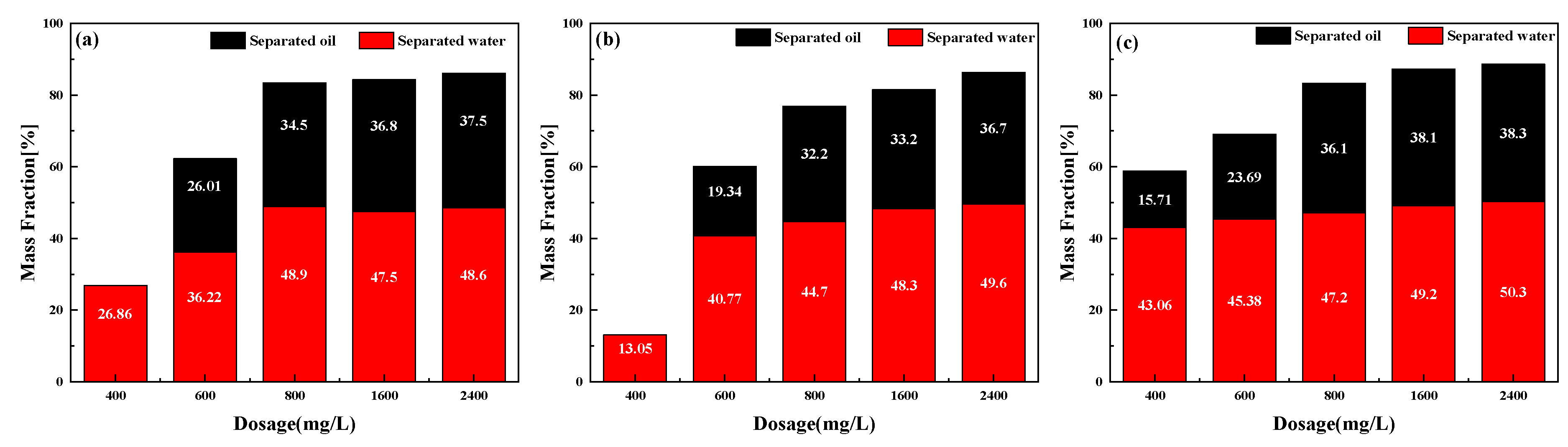
2.3. Effect of Different Temperature and Demulsifier Concentration on the Demulsification of Emulsified Condensate Oil
2.4. Demulsifier Performance and Dehydration Mechanisms of the Emulsified Condensate Oil
2.5. The Experiment of On-Site Low-Temperature Demulsification
3. Materials and Methods
3.1. Materials
3.2. Relative Solubility Number Determination
3.3. Bottle Tests
3.4. Measurement of Viscosity–Temperature Curve and Abnormal Point of Dehydrated Crude Oil
3.5. Measurement of Clay Particle Size
3.6. Observation of Emulsion Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.S. Key production technologies of low-permeability gas reservoirs and their development trend. Nat. Gas Ind. 2006, 26, 38–41. [Google Scholar] [CrossRef]
- Hu, W.R.; Wei, Y.; Bao, J.W. Development of the theory and technology for low permeability reservoirs in China. Pet. Explor. Dev. 2018, 45, 685–697. [Google Scholar] [CrossRef]
- Liu, Y.K.; Wang, F.J.; Wang, Y.M. The mechanism of hydraulic fracturing assisted oil displacement to enhance oil recovery in low and medium permeability reservoirs. Pet. Explor. Dev. 2022, 49, 864–873. [Google Scholar] [CrossRef]
- Jian, O.Y.; Yan, M. Quantitative effect of droplet size and emulsion viscosity on the storage stability of asphalt emulsion. Constr. Build. Mater. 2022, 342, 127994. [Google Scholar]
- Sun, N.N.; Jiang, H.Y.; Su, R.Y. Experimental Study on Synergistic Demulsification of Microwave-Magnetic Nanoparticles. ACS Omega 2022, 7, 35523–35531. [Google Scholar] [CrossRef] [PubMed]
- Elena, V.; Marina, O.; Larisa, B. Microwave Irradiation in Technologies of Wastewater and Wastewater Sludge Treatment: A Review. Water 2021, 13, 1784. [Google Scholar]
- Nahid, H.; Hu, G.J.; Li, J.B. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules 2020, 25, 4915. [Google Scholar]
- Liu, J.; Huang, X.F.; Lu, L.J. Turbiscan Lab® Expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. J. Hazard. Mater. 2011, 190, 214–221. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Fang, S.W. Dissipative particle dynamics investigation of demulsification process and mechanism of comb-like block polyether. Polym. Adv. Technol. 2018, 29, 3171–3180. [Google Scholar] [CrossRef]
- Meor, M.H.S.B.; Abdul, L.A.; Afiqah, T.A.K. A review of demulsification technique and mechanism for emulsion liquid membrane applications. J. Dispers. Sci. Technol. 2022, 43, 910–927. [Google Scholar]
- Bai, Y.P.; Zhao, L.; Shao, L. Hybrid emulsifiers enhancing polymerization stabilities and properties of pressure sensitive adhesives. J. Appl. Polym. Sci. 2010, 115, 1125–1130. [Google Scholar] [CrossRef]
- Wu, Y.F.; Qu, X. Influence of Different Types of Emulsifiers on Properties of Emulsified Asphalt Binder and Its Evaporation Residue by Molecular Dynamics Simulation. Adv. Mater. Sci. Eng. 2021, 2021, 3313460. [Google Scholar] [CrossRef]
- Ayça, K.; Ayfer, S.E. Film and Surface Properties of Water-Based Latexes Prepared by Semicontinuous Emulsion Polymerization. Macromol. Symp. 2011, 302, 266–272. [Google Scholar]
- Jean, L.S.; Ana, M.F. Emulsion Stabilization, Breaking, and Inversion Depends upon Formulation: Advantage or Inconvenience in Flow Assurance. Energy Fuels 2012, 26, 4027–4033. [Google Scholar]
- Yogesh, D.; Rohit, K.S.; Ranvijay, S.; Naiya, T.K. Application of a novel surface-active green demulsifier for demulsification of field crude oil emulsion. Sep. Sci. Technol. 2023, 58, 1654–1678. [Google Scholar]
- Mwakasala, B.T.; Kang, W.L.; Yin, X. Demulsifier performance at low temperature in a low permeability reservoir. Pet. Sci. Technol. 2016, 34, 1905–1912. [Google Scholar] [CrossRef]
- Ostapenko, T.; Weyland, M.; Eremin, A. Filaments formed in the hexagonal columnar liquid crystal phase of star-shaped oligobenzoates(Article). Liq. Cryst. 2013, 40, 345–353. [Google Scholar] [CrossRef]
- Xia, C.M.; Luo, Y.W. Modification of bitumen emulsion via heterocoagulation with SIS triblock copolymer latex. J. Appl. Polym. Sci. 2017, 134, 45510. [Google Scholar] [CrossRef]
- Srijaroonrat, P.; Julien, E.; Aurelle, Y. Unstable Secondary Oil/Water Emulsion Treatment Using Ultrafiltration: Fouling Control by Back Flushing. Membr. Sci. 1999, 159, 11–20. [Google Scholar] [CrossRef]
- Bessler, D.U.; Chilingarian, G.V.; John, O. Surface Operation in Petroleum Production; Chilingarian, G.V., Ed.; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Bock, J.; Jacques, D.F.; Valint, P.L., Jr.; Pacansky, T.J.; Yang, H.W.-H. Hydrophobically Functionalized Cationic Polymers. US 0464957, 30 May 1989. [Google Scholar]
- Zhai, M.J.; Wu, M.; Wang, C.Y. A novel silica-supported polyether polysiloxane quaternary ammonium demulsifier for highly efficient fine-sized oil droplet removal of oil-in-water emulsions. RSC Adv. 2020, 10, 18918–18926. [Google Scholar] [CrossRef]
- Wang, J.; Hu, F.L.; Li, C.Q. Synthesis of dendritic polyether surfactants for demulsification. Sep. Purif. Technol. 2010, 73, 349–354. [Google Scholar] [CrossRef]
- Li, Z.W.; Yin, S.; Tan, G.R. Synthesis and properties of novel branched polyether as demulsifiers for polymer flooding. Colloid Polym. Sci. 2016, 294, 1943–1958. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, X.; Zhang, H.M. Interfacial and demulsifying behavior of novel fluorinated hyperbranched polymers. J. Surfactants Deterg. 2014, 17, 977–984. [Google Scholar] [CrossRef]
- Kang, W.L.; Yin, X.; Yang, H.B.; Zhao, Y.; Huang, Z.; Hou, X.; Sarsenbekuly, B.; Zhu, Z.; Wang, P.; Zhang, X. Demulsification performance, behavior and mechanism of different demulsifiers on the light crude oil emulsions. Colloids Surf. A Phys. Eng. Asp. 2018, 545, 197–204. [Google Scholar] [CrossRef]
- Faisal, W.; Almomani, F. A critical review of the development and demulsification processes applied for oil recovery from oil in water emulsions. Chemosphere 2021, 291 Pt 3, 133099. [Google Scholar] [CrossRef] [PubMed]
- Tsabet, È.; Fradette, L. Effect of the properties of oil, particles, and water on the production of Pickering emulsions. Chem. Eng. Res. Des. Trans. Inst. Chem. Eng. 2015, 97, 9–17. [Google Scholar] [CrossRef]
- Zong, J.; Yuan, F.; Zhan, M.S. Numerical simulation of a mechanical flocculation process with multi-chambers in series. Water Sci. Technol. 2023, 87, 1945–1960. [Google Scholar] [CrossRef]
- Dickinson, E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2019, 96, 209–223. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Wu, J.Y.; Xu, Y.M.; Tadeusz, D. Development of a method for measurement of relative solubility of nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 229–237. [Google Scholar] [CrossRef]
- Bhupati, R.; Bhattacharyya, D.G. Water Soluble Polymer as Water-in-Oil Demulsifier. US 683619, 14 April 1914. [Google Scholar]
- Yuan, T.; Meng, J.Q.; Hao, T.Y. A scalable method toward superhydrophilic and underwater superoleophobic PVDF membranes for effective oil/water emulsion separation. ACS Appl. Mater. Interfaces 2015, 7, 14896–14904. [Google Scholar] [CrossRef]
- Yan, N.; Masliyah, J.H. Characterization and demulsification of solids-stabilized oil-in-water emulsions Part 2. Demulsification by the addition of fresh oil. Coloids Surf. A Physicochem. Eng. Asp. 1995, 96, 243–252. [Google Scholar] [CrossRef]
- Menon, V.B.; Wasan, D.T. A Review of the Factors affecting the stability of solids-stabilized Emulsion. Sep. Sci. Technol. 1988, 23, 21–31. [Google Scholar] [CrossRef]
- Song, X.Y.; Shi, P.; Duan, M. Investigation of demulsification efficiency in water-in-crude oil emulsions using dissipative particle dynamics. RSC Adv. 2015, 5, 62971–62981. [Google Scholar] [CrossRef]
- Jana, H.; Peter, K.; Ivan, C. Natural rubber nanocomposites with organo-modified bentonite. Clays Clay Miner. 2009, 57, 444–451. [Google Scholar]








| Parameter | Mass Ratio/% | |
|---|---|---|
| Mass density/Kg‧m−3 at 20 °C | 891 | |
| Viscosity/mPa‧s at 20 °C | 4.6 | |
| Mass fraction of water | 0.507 | |
| Mass fraction of alkane | C5–C21 | 0.308 |
| Naphthenic hydrocarbon | 0.076 | |
| Mass fraction of salt | Sodium chloride | 0.014 |
| Calcium chloride | 0.019 | |
| Mass fraction of argillaceous | 0.056 | |
| Surfactants | 0.02 |
| NO. | Demulsifier Series | Name | RSN | R | x,y,z |
|---|---|---|---|---|---|
| 1 | SP | SP169 | 10.3 | 18 | (200–800, 400–1000, 600–1200) |
| 2 | PR | PR-1 | 13.3 | - | (300–500, 200–500, 300–500) |
| 3 | PR-2 | 12.6 | - | (500–800, 500–700, 500–700) | |
| 4 | PR-3 | 12.8 | - | (500–800, 700–1000, 700–1000) | |
| 5 | AR | AR16 | 12.4 | 6 | (300–1000, 200–400, 0) |
| 6 | AR26 | 12.8 | 6 | (300–1000, 300–500, 0) | |
| 7 | AR36 | 13.6 | 6 | (300–1000, 400–600, 0) | |
| 8 | AR46 | 15 | 6 | (300–1000, 500–700, 0) | |
| 9 | AR48 | 15.2 | 6 | (300–1000, 600–800, 0) | |
| 10 | AE | AE9901 | 13 | 12 | (300–600, 200–500, 0) |
| 11 | AE8051 | 17 | 12 | (500–1000, 500–800, 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Li, Q.; Ma, Q.; Xu, B.; Zou, T. Efficient Demulsification Performance of Emulsified Condensate Oil by Hyperbranched Low-Temperature Demulsifiers. Molecules 2023, 28, 7524. https://doi.org/10.3390/molecules28227524
Jiang S, Li Q, Ma Q, Xu B, Zou T. Efficient Demulsification Performance of Emulsified Condensate Oil by Hyperbranched Low-Temperature Demulsifiers. Molecules. 2023; 28(22):7524. https://doi.org/10.3390/molecules28227524
Chicago/Turabian StyleJiang, Shaohui, Qingsong Li, Qiang Ma, Botao Xu, and Tao Zou. 2023. "Efficient Demulsification Performance of Emulsified Condensate Oil by Hyperbranched Low-Temperature Demulsifiers" Molecules 28, no. 22: 7524. https://doi.org/10.3390/molecules28227524
APA StyleJiang, S., Li, Q., Ma, Q., Xu, B., & Zou, T. (2023). Efficient Demulsification Performance of Emulsified Condensate Oil by Hyperbranched Low-Temperature Demulsifiers. Molecules, 28(22), 7524. https://doi.org/10.3390/molecules28227524




