1. Introduction
In a study by Janus et al., it was observed, through in vitro permeability assessments using pig skin, that the ibuprofen racemate, when administered at a concentration of 0.01 g·cm
−3, exhibited a skin penetration rate of merely 6% [
1]. It is well-established that alterations in the compound’s structure can effectively modulate the solubility and permeability characteristics of ibuprofen.
The most straightforward modification involves the conversion of a carboxylic acid into its corresponding salt using a base, a process exemplified by the synthesis of ibuprofen sodium salt. Legg et al. conducted a study to investigate variations in absorption kinetics among different forms of ibuprofen, including ibuprofen sodium (IBUNa) and ibuprofen racemate (rac-IBU). Their research, involving 71 healthy adult participants, revealed that when administered on an empty stomach, IBUNa exhibited an approximately 30% higher maximum concentration in the body (C
max) compared to rac-IBU. Furthermore, the time to reach C
max (T
max) was notably earlier for IBUNa, with a difference of approximately 1.0–1.5 h [
2]. Additionally, there exist other known ibuprofen salts, such as potassium, calcium, magnesium, aluminum, zinc, and copper [
3,
4,
5,
6,
7].
The exploration of ion pair formation has been undertaken to enhance membrane permeability, thereby increasing the bioavailability of hydrophilic ionized molecules. According to this concept, when oppositely charged molecules interact, they form an ion pair, effectively neutralizing their overall electric charge. This transformation results in heightened lipophilicity of the ion pair compared to the individual ions, facilitating greater molecule penetration through various membranes, such as those found in the intestines, skin, or synthetic systems [
8,
9,
10].
Sarveiya et al. investigated the impact of alkylamine counterions, including ethylamine, diethylamine, triethylamine, and ethylenediamine, on the permeation of ibuprofen through a polydimethylsiloxane membrane, employing propylene glycol as a solvent. The control sample, sodium ibuprofenate, exhibited log P values of approximately 0.92 and a 3.09 μg·cm
−2 permeability. Their study revealed that the introduction of a counterion significantly influenced the partition coefficient and permeability of the drug through the membrane. Triethylammonium ibuprofenate demonstrated a permeation value of 48.14 μg·cm
−2 and a log P of 1.18, demonstrating the potential to enhance salt flow through lipophilic membranes using the ion pair approach. The degree of enhancement depends on factors such as lipophilicity, the extent of ion pairing, and the reduction in charge in the drug molecule [
8].
Another example of research aimed at increasing the solubility parameters of active pharmaceutical ingredients (API) in water comes from H. S. Alghurabi, who examined the influence of amino acid counterions, specifically lysine and arginine, on ibuprofen’s solubility. This work resulted in amino acid salts of ibuprofen with significantly increased solubility at room temperature. Notably, the introduction of this modification increased solubility by 1371 and 242 times, reaching 85.0 ± 8.9 mg·cm
−3 and 14.0 ± 3.7 mg·cm
−3 for arginine and lysine derivatives, respectively. The authors attribute this to the higher pKa value of arginine, owing to the guanidine moiety in its molecule, compared to lysine, which features an amino group at the end of its chain [
11].
In our previous study, ibuprofenate salts of L-valine esters were synthesized and evaluated for changes in solubility, partition coefficient, and permeability through pig skin. Our team successfully synthesized ethyl, isopropyl, propyl, butyl, pentyl, and hexyl esters of L-valine. The research revealed that the synthesis of such salts positively impacted the bioavailability of ibuprofen, with these compounds displaying improved solubility in water and phosphate buffers at pH 5.4 and 7.4. Notably, the propyl and isopropyl esters exhibited the highest permeability (677.41 and 604.51 μg IBU·cm
−2, respectively) compared to ibuprofen (302.84 μg IBU·cm
−2). The study’s authors also noted the release of L-valine alongside ibuprofen, highlighting its potential positive effects on bodily functions, including reduced fatigue and participation in pantothenic acid (vitamin B5) synthesis, which supports wound treatment [
1,
12].
One of our latest studies described ibuprofenate esters of isopropyl L-amino acids, resulting in 14 such compounds through a three-step reaction. The solvent-free method was employed for synthesis, effectively reducing the presence of volatile organic compounds and minimizing process costs. Furthermore, this reaction virtually eliminates by-products, resulting in a highly pure end product [
13]. The ibuprofen salts obtained significantly increased the solubility of the active compound in both water (25 °C) and PBS (pH = 7.4, 32 °C). Moreover, all derivatives showed 2.95 to 16.52 times greater permeability of the active substance compared to ibuprofen. The highest permeability was achieved with the ibuprofenate ester of isopropyl L-lysine, which also exhibited an increased transport rate and the highest accumulated mass of penetrated ibuprofen. It was also demonstrated that the amino acid derivatives of ibuprofen obtained, showed 3.3 to 6.7 times greater accumulation in the skin. These results suggest the potential application of the obtained compounds as alternatives to poorly soluble NSAIDs for skin applications [
13].
Ibuprofenates of alkyl amino acid esters, a relatively niche area of research, have garnered attention for their potential pharmaceutical applications, particularly in improving drug delivery and enhancing the therapeutic effects of ibuprofen. Ibuprofenates of alkyl amino acid esters have been explored as potential prodrugs to enhance the transdermal delivery of ibuprofen. These compounds are designed to improve the drug’s solubility, permeability through the skin, and overall bioavailability. Such formulations aim to provide more efficient and controlled drug release. One of the primary advantages of these derivatives is their ability to increase the solubility of ibuprofen. This can be particularly useful for developing formulations with higher drug concentrations, which may be beneficial for specific therapeutic applications.
This publication is a continuation of research on amino acid alkyl ester ibuprofenates to increase the active substance’s bioavailability. In this research, we will obtain and characterize the obtained derivatives of alkyl esters of glutamic acid.
2. Results and Discussion
In our previous research, we explored the impact of varying amino acid alkyl ester chain lengths on the properties of synthesized L-valine alkyl ester ibuprofenates and examined the influence of different amino acids in amino acid isopropyl ester ibuprofenates. A diverse set of amino acids, including glycine, L-alanine, L-valine, L-isoleucine, L-leucine, L-serine, L-threonine, L-cysteine, L-methionine, L-aspartic acid, L-lysine, L-phenylalanine, and L-proline, were employed in these investigations. Our findings revealed that various structural changes in the molecule impact skin permeability and solubility in water and buffer solutions, biodegradability, and lipophilicity [
1,
13,
14].
Our preceding studies demonstrated that not all amino acid alkyl ester hydrochlorides (precursors for ibuprofen derivatives) can be obtained using trimethylsilyl chloride as the chlorinating agent [
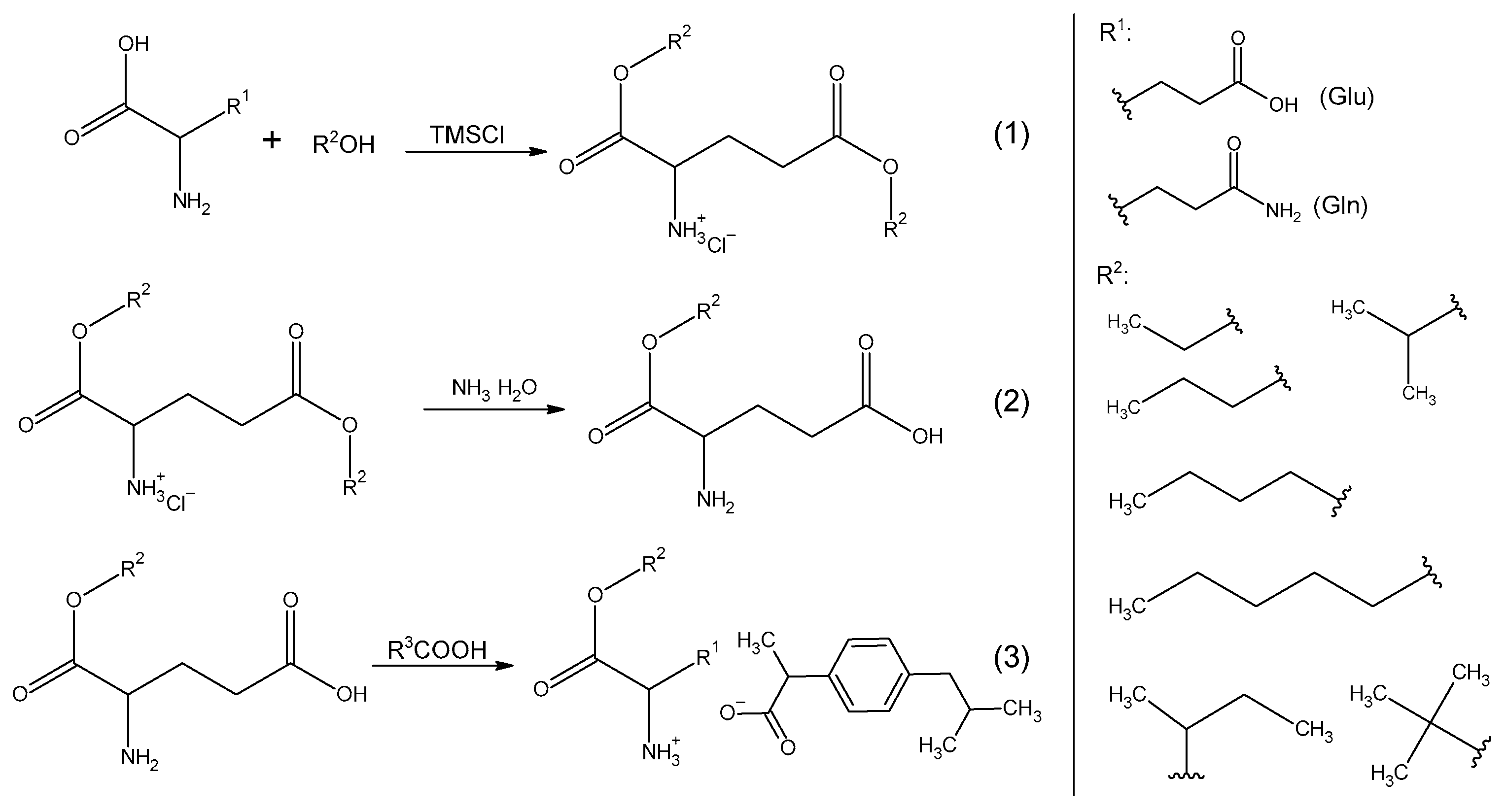
13]. In this publication, we employed chlorotri(methyl)silane as the chlorinating agent and focused on two specific amino acids: glutamine and glutamic acid. Notably, we observed that when an acid amide, L-glutamine, was used as the amino acid substrate, it led to the formation of acidic L-amino acid alkyl ester hydrochlorides. This observation provides evidence that the amide moiety undergoes acidic hydrolysis in response to the chlorinating agent’s influence. The same phenomenon was observed using asparagine and aspartic acid. Furthermore, the hydroxyl group, replacing the amino group, underwent esterification by alcohol, resulting in the esterification of both carboxyl groups of the amino acid. A similar reaction pattern was observed when utilizing L-glutamic acid for synthesis, where both carboxyl groups of the amino acid underwent esterification.
When used to synthesize tert-butyl alcohol, obtaining the expected alkyl ester hydrochloride was impossible. Probably due to steric reasons and the geometry of the alcohol molecule, it was not possible to obtain an ester. As is known, tertiary alcohols are less reactive than primary or secondary alcohols, so the esterification process may be more difficult when using them. In order to obtain tert-butyl ester hydrochlorides, more aggressive reaction conditions or other synthesis methods would have to be used.
Following a three-step reaction sequence, L-glutamic acid alkyl ester ibuprofenates were successfully synthesized, with yields ranging from 73% to 91%. In the final step of this process, the corresponding L-glutamic acid alkyl ester was combined with ibuprofen in the presence of chloroform.
The identity of the obtained compounds, i.e., amino acid alkyl ester hydrochlorides and amino acid alkyl ester ibuprofenates, was confirmed on the basis of analyses of
1H,
13C NMR, and FTIR spectra. Individual spectra and the interpretation of the spectra, chemical shifts of the signals, their multiplicity, integration, coupling constants (for proton spectra), and chemical shifts of the signals (for carbon spectra) are presented in the
Supplementary Materials (Figures S1–S34). Integration in the
1H NMR spectra was performed with respect to the proton located on the α carbon derived from the amino acid (proton designation 4), which occurs at a shift of approximately 4.5 ppm.
The formation of amino acid alkyl ester hydrochlorides was confirmed by detecting a signal in the 1H NMR spectrum, indicating ammonium group protonation and the desired ionic compound formation. It was noticed that the effect of revealing the signal coming from the ammonium group increased with the increase in the length of the alkyl chain. Additionally, the signal was more intense in compounds with branched chains (isopropyl, sec-butyl) than in their straight-chain counterparts.
Based on the analysis of 1H NMR spectra, it was confirmed that in the case of the syntheses of ibuprofenate alkyl esters of L-amino acids, one of the ester bonds was hydrolyzed, which led to obtaining a salt with one carboxyl group of the amino acid esterified. Additionally, in the 1H NMR spectra, signals from protons in the ammonium group were not visible.
The obtained compounds exhibited characteristic absorption bands in the range of 1230 cm−1, 1460 cm−1, and 1740 cm−1, corresponding to C-O, C-N, and C=O stretching vibrations, characteristic of α-amino acids. Additionally, the bands at 2800–3000 cm−1 indicated overlapping C-H and N-H stretching vibrations. The appearance of a symmetrical vibration band at around 1380 cm−1 confirmed the ionic structure of ibuprofen salts. Although an asymmetric vibration band around 1600 cm−1 was suggested, it was partially obscured by C=O stretching vibrations. These vibrations, along with the characteristic functional groups, confirmed the identity and ionic structure of the obtained hydrochlorides and ibuprofenates of alkyl esters of L-amino acids.
In the UV–Vis spectroscopy analysis, the obtained L-amino acid alkyl ester hydrochlorides showed one characteristic absorption band, showing the maximum absorption at a wavelength in the range of 210.4–220.6 nm. However, in the case of L-glutamic acid alkyl ester ibuprofenates, two absorption bands are visible, with their highest values at 223.2–230.0 nm and 259.2–264.0 nm, respectively. It is worth mentioning that ibuprofen shows maximum absorbance at a wavelength of 260 nm.
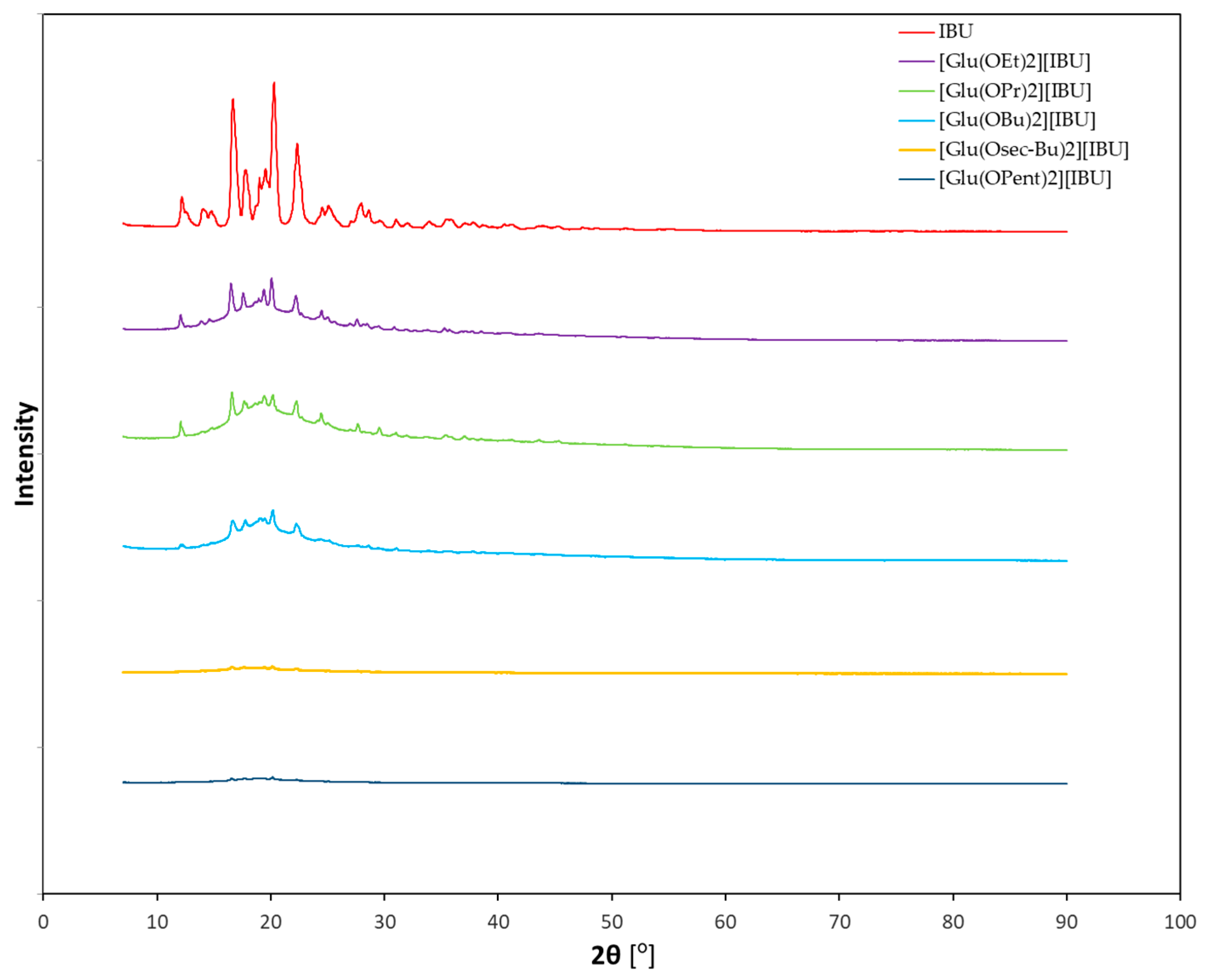
Figure 1 shows the diffractograms of ibuprofenates of L-glutamic acid alkyl esters, while the individual diffractograms of hydrochlorides and ibuprofenates are presented in the
Supplementary Materials (Figures S35–S44). It was noticed that the diffractograms of the obtained ibuprofen salts differ significantly from the diffractogram of the standard—ibuprofen. As the alkyl chain of the ester increases, the intensity of the reflections decreases, indicating that the crystalline character disappears and the derivatives with a longer chain are amorphous.
The results of the optical, specific, and molar rotation of the obtained L-glutamic acid alkyl ester hydrochlorides and ibuprofenates are summarized in
Table 1. All obtained and L-glutamic acid alkyl ester hydrochlorides and ibuprofenates rotate the plane of polarized light to the right (+), with the hydrochlorides showing higher optical rotation values than their ibuprofenate counterparts. The highest value was demonstrated by [Glu(OiPr)
2][HCl]. Amino acid alkyl ester hydrochlorides show a much higher rotation angle than the amino acids from which they are obtained. A racemic mixture of ibuprofen was used for the syntheses, which is why this substrate could not rotate polarized light. The obtained ibuprofenates have a specific rotation of light within +2° (except for the isopropyl derivative +9.8°).
All compounds showed thermal stability up to over 160 °C. It was noticed that as the alkyl chain of the ester increases, the thermal stability of the compounds increases, which confirms the literature reports [
1]. Additionally, compounds with branched alkyl chains, such as isopropyl and
sec-butyl chains, further increased thermal stability compared to their n-propyl and n-butyl isomers. In the case of ibuprofenates, during heating, the fastest decomposition was [Glu(OEt)][IBU], which was characterized by the onset of decomposition at a temperature of 194.2 °C, while [Glu(OiPr)][IBU] decomposed only at 222.9 °C, which was the highest value among all ibuprofen salts tested. As in the case of hydrochlorides, the first derivative of mass loss over time was determined, thanks to which the temperatures of the fastest decomposition of the sample were determined. The highest temperature is 247.7 °C—[Glu(OBu)][IBU], and the lowest is 228.3 °C—[Glu(OEt)][IBU].
DSC analyses determined the temperatures of phase transformations occurring due to temperature changes in the sample. Individual thermograms are listed in the
Supplementary Materials (Figures S58 and S59), and the values of phase transformation temperatures and their enthalpy energies are listed in
Table 2. Hydrochlorides of alkyl esters of L-glutamic acid melted at temperatures lower than the above-mentioned derivatives of L-aspartic acid. Melting points were >100 °C. All hydrochlorides had glass transition temperatures from approximately −45 to −17 °C. No crystallization temperatures were determined for ibuprofenates of L-glutamic acid alkyl esters, while melting points were determined for ethyl, propyl, and butyl derivatives (50.43, 59.02, 71.05 °C, respectively). Glass transition temperatures of approximately −50 °C were determined for all ibuprofenates. Moreover, it has been noted that some L-glutamic acid alkyl ester ibuprofenates have significantly lower melting points than the corresponding L-glutamic acid alkyl ester hydrochlorides. For example, [Glu(OEt)
2][HCl] melts at a temperature of about 101 °C, and [Glu(OEt)][IBU] melts at half that temperature—about 50 °C. Interestingly, in the case of [Glu(OBu)
2][HCl], it is the opposite—the hydrochloride has a melting point of about 64 °C, and ibuprofenate [Glu(OBu)][IBU]—71 °C.
All L-glutamic acid alkyl ester ibuprofenates showed a positive log P value, although lower than ibuprofen, which confirms that the introduction of the L-amino acid alkyl ester cation reduces the log P value. The obtained salts can be classified as medium-lipophilic compounds. Ibuprofen has the highest lipophilic character (log P = 3.37), and [Glu(OEt)][IBU] has the lowest (log P = 2.37). The partition coefficient and water solubility parameters of the obtained L-glutamic acid alkyl ester ibuprofenates are listed in
Table 3. The solubility of the compound in g·dm
−3 and the solubility in terms of the active substance (ibuprofen) in g IBU·dm
−3 are presented.
The lipophilicity of the obtained compounds can be arranged in ascending order: [Glu(OEt)][IBU] < [Glu(OPr][IBU] < [Glu(OPent)][IBU] < [Glu(OiPr)][IBU] < [Glu (OBu)][IBU] < [Glu(Osec-Bu)][IBU] < [IBU]. It can be seen that with the increase in the alkyl chain length, with the exception of [Glu(OPent)][IBU]), lipophilicity increases. The salt of L-glutamic acid alkyl ester with the longest alkyl chain showed a lipophilicity value lower than the n-butyl derivative. This is quite an unusual trend. Branching of the alkyl chain increases the lipophilicity of the compound—the same tendency in the case of derivatives with a chain length of C3 and C4.
The solubility of the compounds per active mass of ibuprofen was higher than the standard (ibuprofen). Ibuprofenate, which showed the highest solubility in water at 25 °C ([Glu(OiPr)][IBU]—0.203 ± 0.006 g IBU·dm−3), dissolved approximately three times better than ibuprofen. However, the compound that achieved the lowest solubility value ([Glu(Osec-Bu)][IBU]—0.093 ± 0.012 g IBU·dm−3) was characterized by a solubility 1.24 times higher than the standard. The solubility of compounds can be arranged in ascending order as follows: IBU < [Glu(Osec-Bu)][IBU] < [Glu(OEt)][IBU] < [Glu(OPent)][IBU] < Glu(OBu)][IBU] < [Glu(OPr)][IBU] < [Glu(OiPr)][IBU]. There is no typical relationship between the length of the alkyl chain, according to which the longer the chain, the lower the solubility of the compound in water.
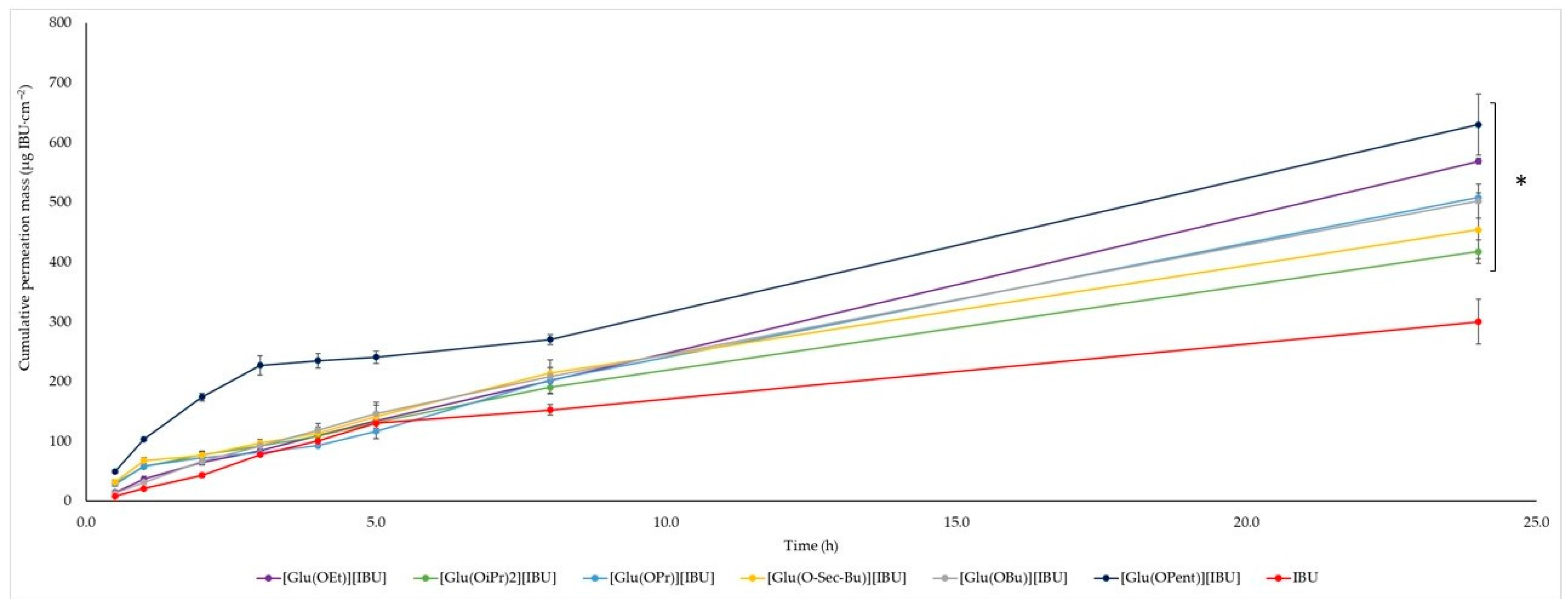
The permeability profiles for ibuprofen and its salts are shown in
Figure 2 and
Table 4—the cumulative mass and permeability parameters of ibuprofen and L-glutamic acid alkyl ester ibuprofenates after 24 h of testing. The compound [Glu(OPent)][IBU], showed the highest skin permeability value after 24 h of testing (630.443 ± 51.226 μg IBU∙cm
−2), and the lowest was the parent ibuprofen (300.183 ± 37.443 μg IBU∙cm
−2). Permeability values after 24 h of permeation testing for the obtained compounds can be ordered from the highest to the lowest, respectively: [Glu(OPent)][IBU] > [Glu(OEt)][IBU] > [Glu(OPr)][IBU] > [Glu (O
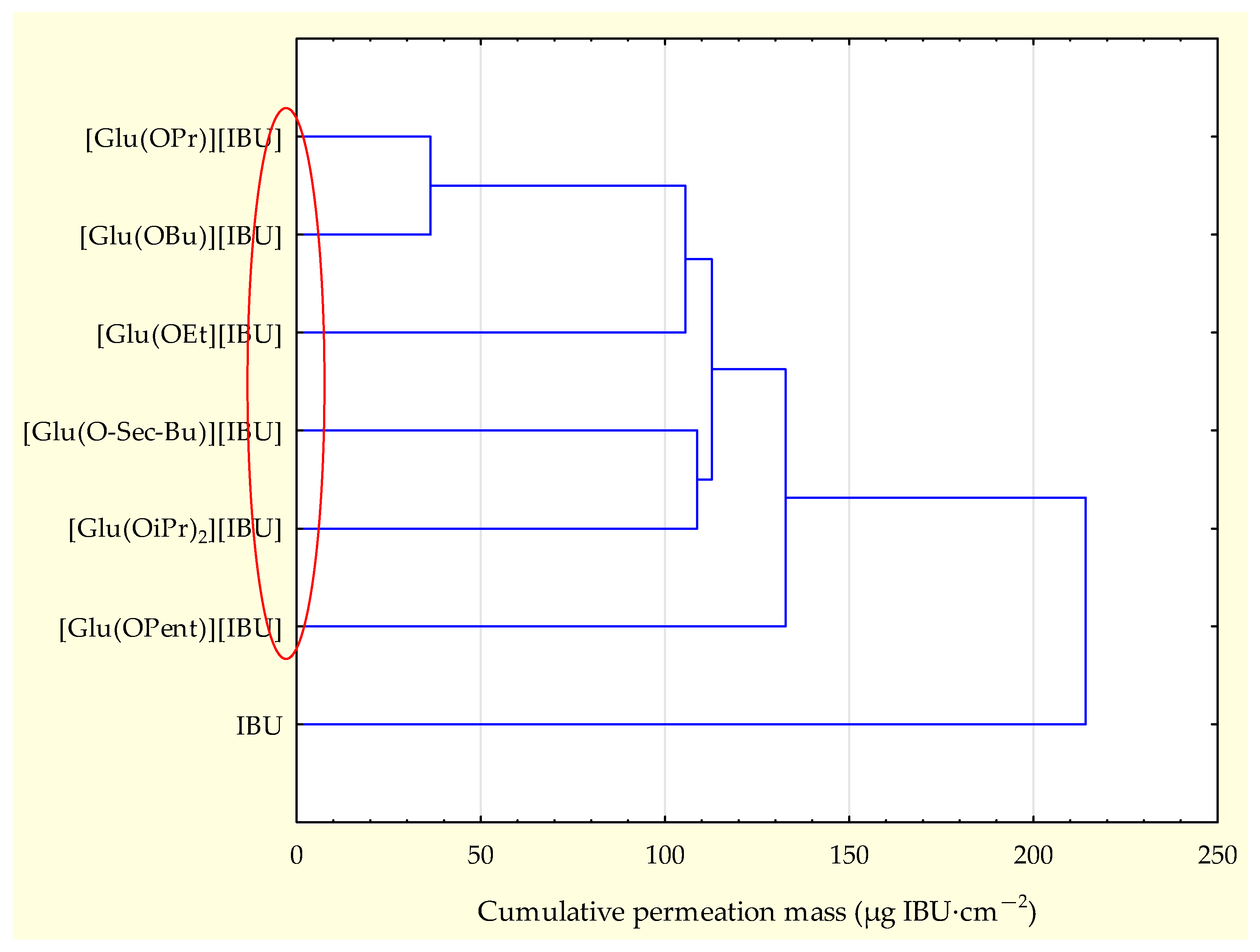
sec-Bu)][IBU] > [Glu(OBu)][IBU] > [Glu(OiPr)][IBU] > IBU. The accumulated mass of all analyzed derivatives after 24 h of penetration was significantly higher compared to pure IBU. This is confirmed by the cluster analysis test, where all derivatives form a separate cluster (
Figure 3). The compound [Glu(OPent)][IBU], permeated to the highest extent throughout the period of the study, as shown in the box plot (
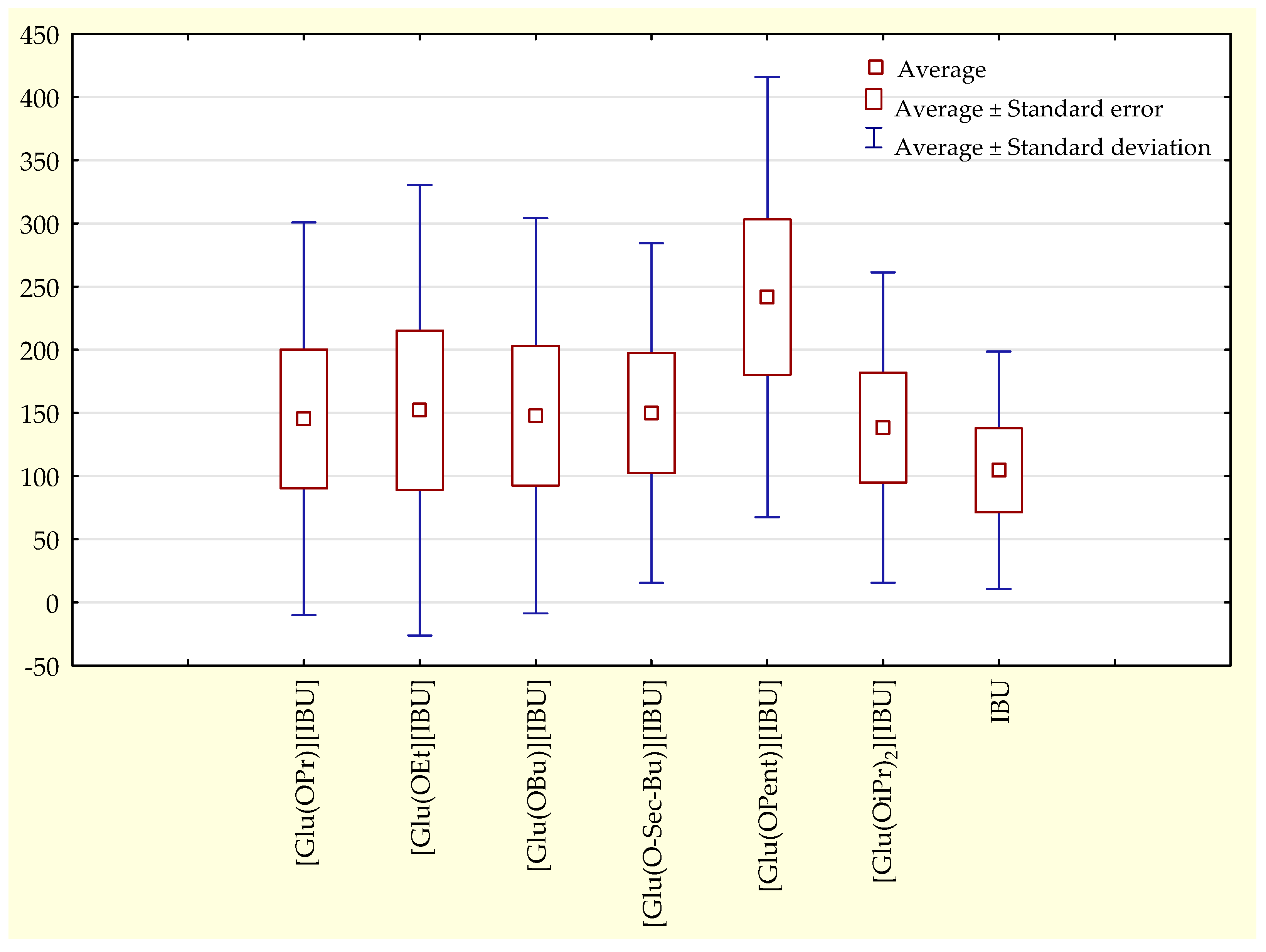
Figure 4).
The steady-state permeation values of [Glu(OPr)][IBU], [Glu(OiPr)][IBU], and [Glu(Osec-Bu)][IBU] were similar to those of ibuprofen, [Glu(OPent)][IBU] showed the highest Jss and Kp values (57.098 and 5.267, respectively), the lowest values were shown by the isopropyl derivative (26.451 and 2.686). The delay time, diffusion coefficient, and skin partition coefficient were calculated only for IBU and [Glu(OBu)][IBU]. In other cases, cross-talk profiles (no delay time) made calculations impossible. In this case, a steady state was not obtained. Therefore, the steady-state slope could not be determined on the graph, and the intercepts in the trend line equations of these compounds showed positive values. The fact that the delay time parameter was not calculated indicates that most of the L-glutamic acid alkyl ester ibuprofenates obtained penetrate the skin immediately, without any delay.
The permeation rates were also determined, which are visible in
Figure 5. The graph shows the amount of ibuprofen mass per hour calculated between measurement points. It can be seen that the permeation rate increases for all compounds from the point 0.0–0.5 to 0.5–1 and then decreases. Within the first two hours, the highest value is reached by [Glu(OPent)][IBU], twice as high as ibuprofen, and the lowest among ibuprofenates is [Glu(OiPr)][IBU] (417.994 μg IBU∙cm
−2), which penetrated almost 40% better than ibuprofen (300.183 μg IBU∙cm
−2). The permeation rate of compounds from the point 0.0–1 to 1.0–2.0 drops dramatically, with [Glu(OPent)][IBU] maintaining the highest value among the rest. After 24 h, the highest value of the permeation rate was demonstrated by [Glu(OEt)][IBU] (difference of approximately 0.5 μg∙h
−1 compared to [Glu(OPent)][IBU]. The rest of the compounds achieved similar values, and the lowest value shows [IBU].
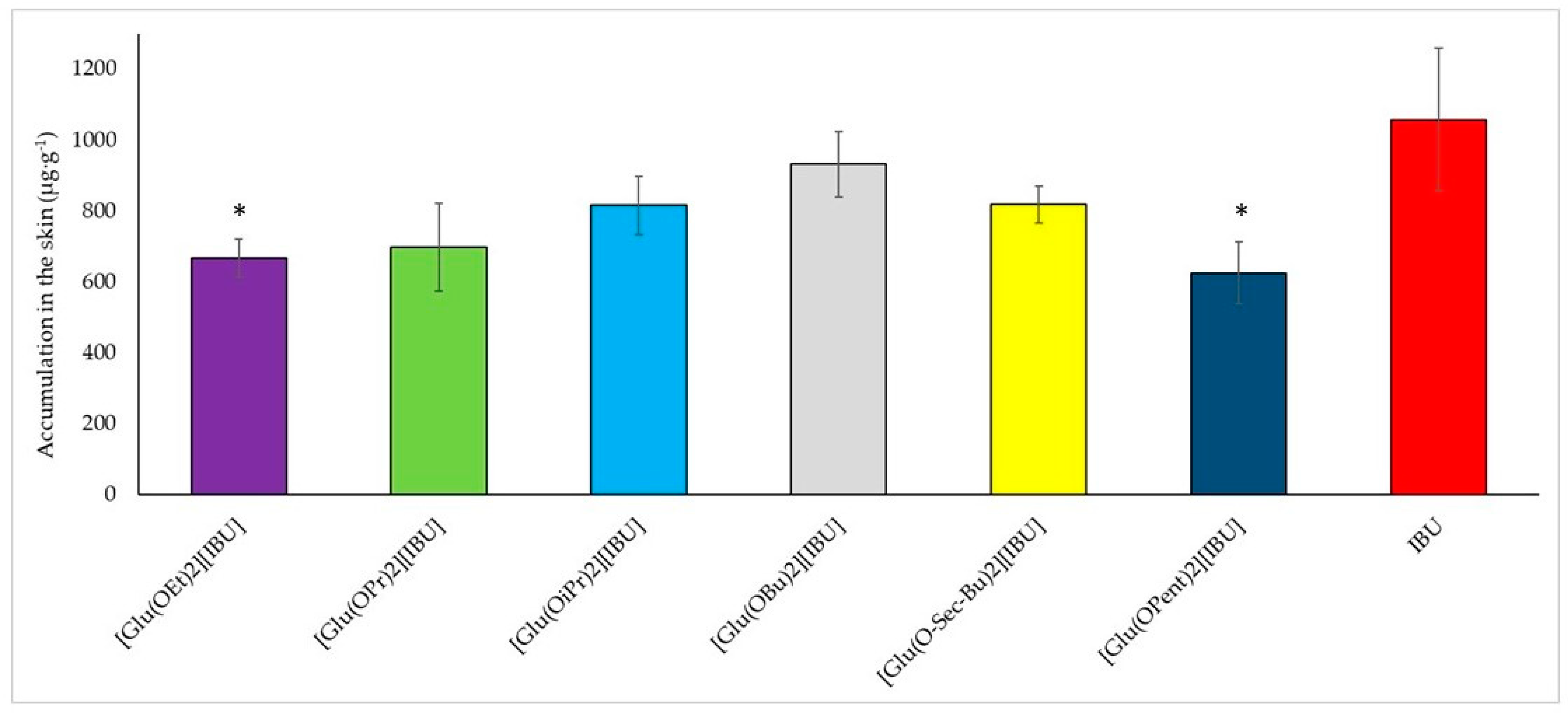
In addition to penetrating, the compounds can accumulate in the skin.
Figure 6 shows a graph of the accumulation of the compound in pig skin depending on the type of compound used for testing. The lowest accumulation was recorded for the compound [Glu(OPent)][IBU] (625.895 ± 87.275 µg IBU·g
−1 pig skin), and the highest for ibuprofen (1057.468 ± 200.589 µg IBU·g
−1 pig skin). The highest value for the obtained derivative was recorded for the compound [Glu(OBu)][IBU] (931.823 ± 93.449 µg IBU·g
−1 pig skin), which is equal to approximately 90% of the accumulated mass for ibuprofen. A visible relationship indicates that as the length of the alkyl chain increases, the accumulation in the skin increases and reaches a maximum at the butyl chain. It was also noticed that the accumulation in the skin decreases as the permeability increases. Compounds with the highest rate of penetration through pig skin, such as [Glu(OPent)][IBU] and [Glu(OEt)][IBU], achieved the lowest accumulation values in the skin. IBU, which had the lowest penetration rate among the tested compounds, was also characterized by the highest accumulation in the skin.
4. Conclusions
This study systematically investigated the impact of introducing L-glutamic acid alkyl ester moieties into the ibuprofen structure, shedding light on their physicochemical properties and skin permeability behavior. The obtained L-glutamic acid alkyl ester ibuprofenates exhibited a decrease in lipophilicity, as evidenced by the lower octanol/water partition coefficients ranging from 2.374 to 3.191 compared to ibuprofen. This reduction in lipophilicity was attributed to the incorporation of the amino acid alkyl ester cation, indicating that these compounds can be classified as medium-lipophilic substances.
Furthermore, the ibuprofen salts displayed significantly enhanced solubility in water compared to the parent ibuprofen. Among them, [Glu(OiPr)][IBU] exhibited the highest solubility, approximately three times higher than that of ibuprofen. This enhanced solubility is important for pharmaceutical applications, as it can potentially lead to improved bioavailability and efficacy.
Additionally, the skin permeability studies revealed that the L-glutamic acid alkyl ester ibuprofenates exhibited higher permeation rates through pig skin compared to ibuprofen. The compound [Glu(OPent)][IBU], demonstrated the highest skin penetration value after 24 h, indicating its potential as a promising transdermal delivery system. Interestingly, it was observed that compounds with higher permeability rates also showed lower accumulation in the skin. This finding suggests that increased permeability might lead to reduced skin retention, which could be advantageous for minimizing localized side effects.
Furthermore, the study highlighted a relationship between the length of the alkyl chain and skin accumulation. As the length of the alkyl chain increased, the accumulation in the skin also increased until it reached a maximum at the butyl chain, after which it decreased. This observation provides valuable insights for the design of transdermal drug delivery systems, allowing for the optimization of skin permeability and reduced skin retention.
In summary, the incorporation of L-glutamic acid alkyl ester moieties into the ibuprofen structure not only enhanced the water solubility of the compound but also significantly improved its skin permeability properties. These findings have important implications for the development of novel transdermal drug delivery systems, offering potential advancements in the field of pharmaceutical sciences. Further research could focus on exploring the therapeutic applications of these ibuprofen salts and their efficacy in vivo, paving the way for innovative and efficient drug delivery strategies.