Effect of Vacancy, As, and Sb Dopants on the Gold-Capturing Ability of Cu2S during Gold Collection in Matte Processes
Abstract
:1. Introduction
2. Results and Discussion
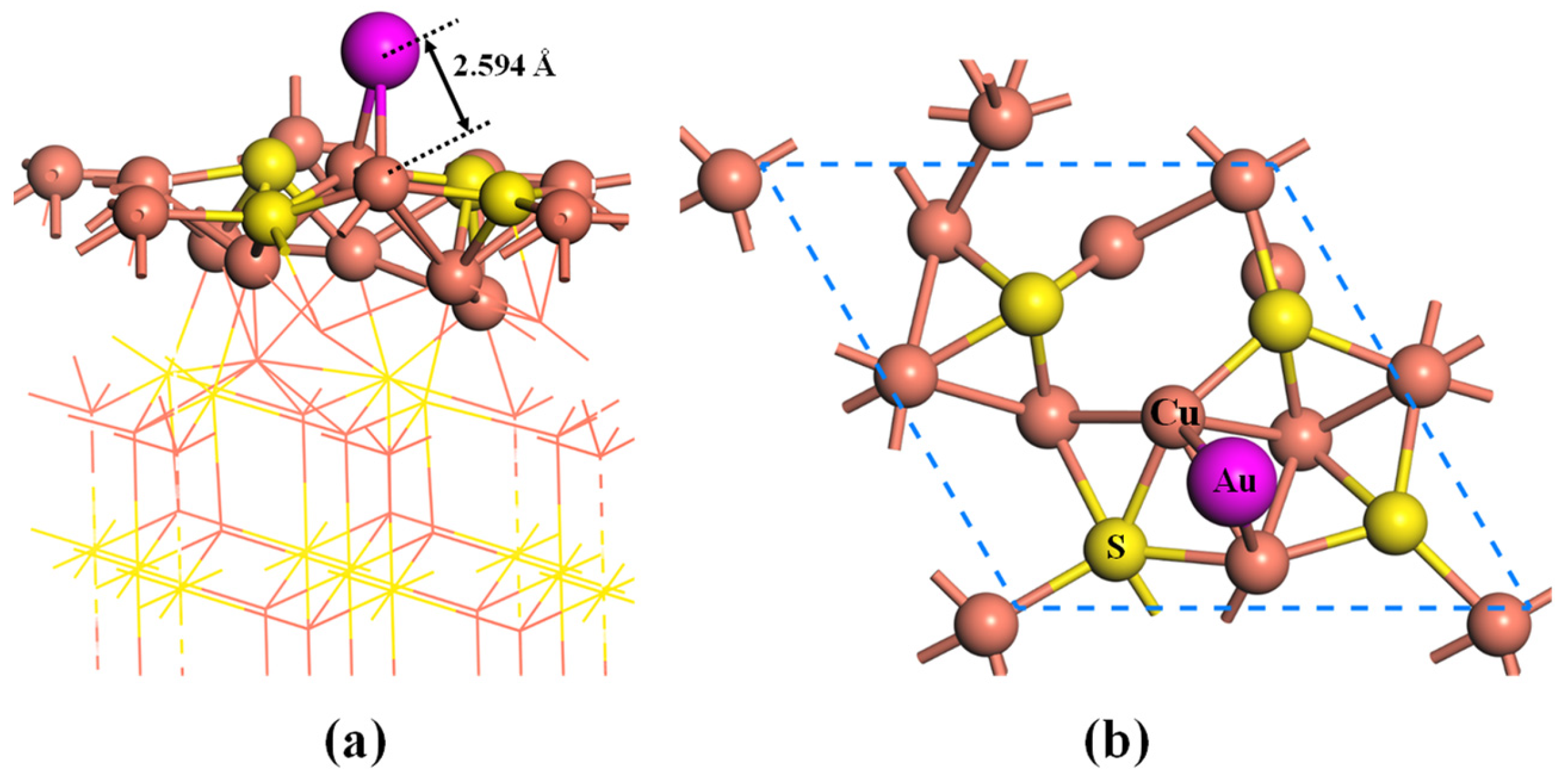
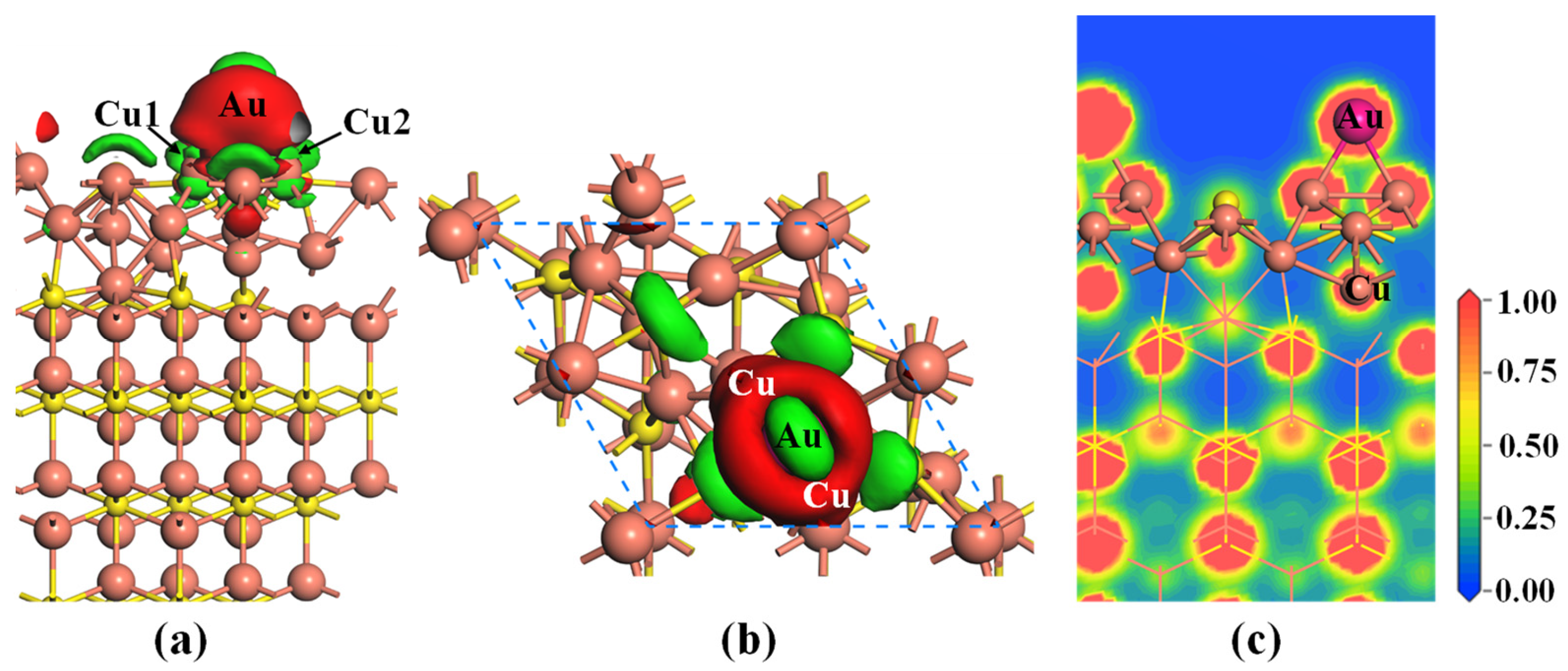
2.1. Adsorption of Au Atom on Pristine Cu2S(111)
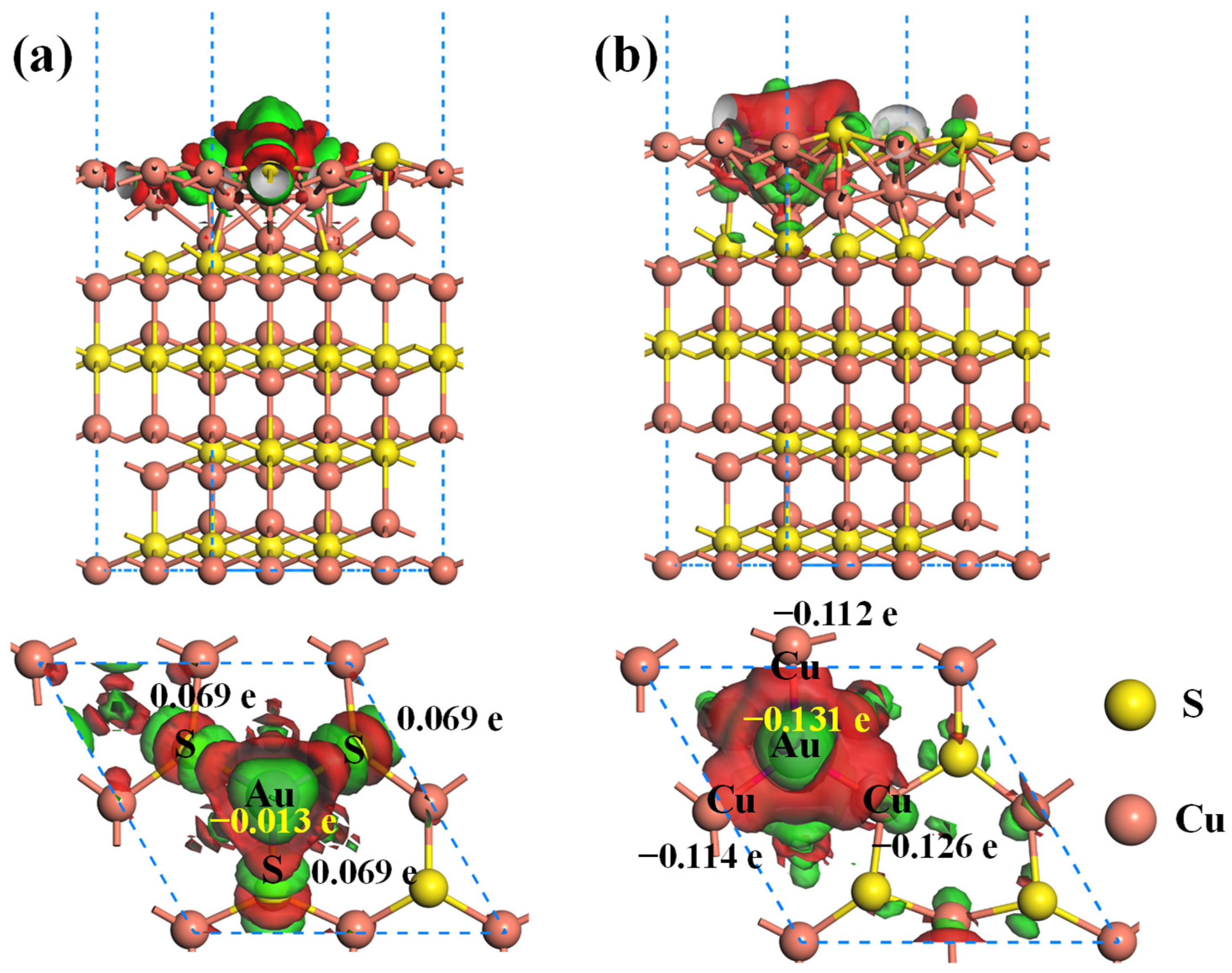
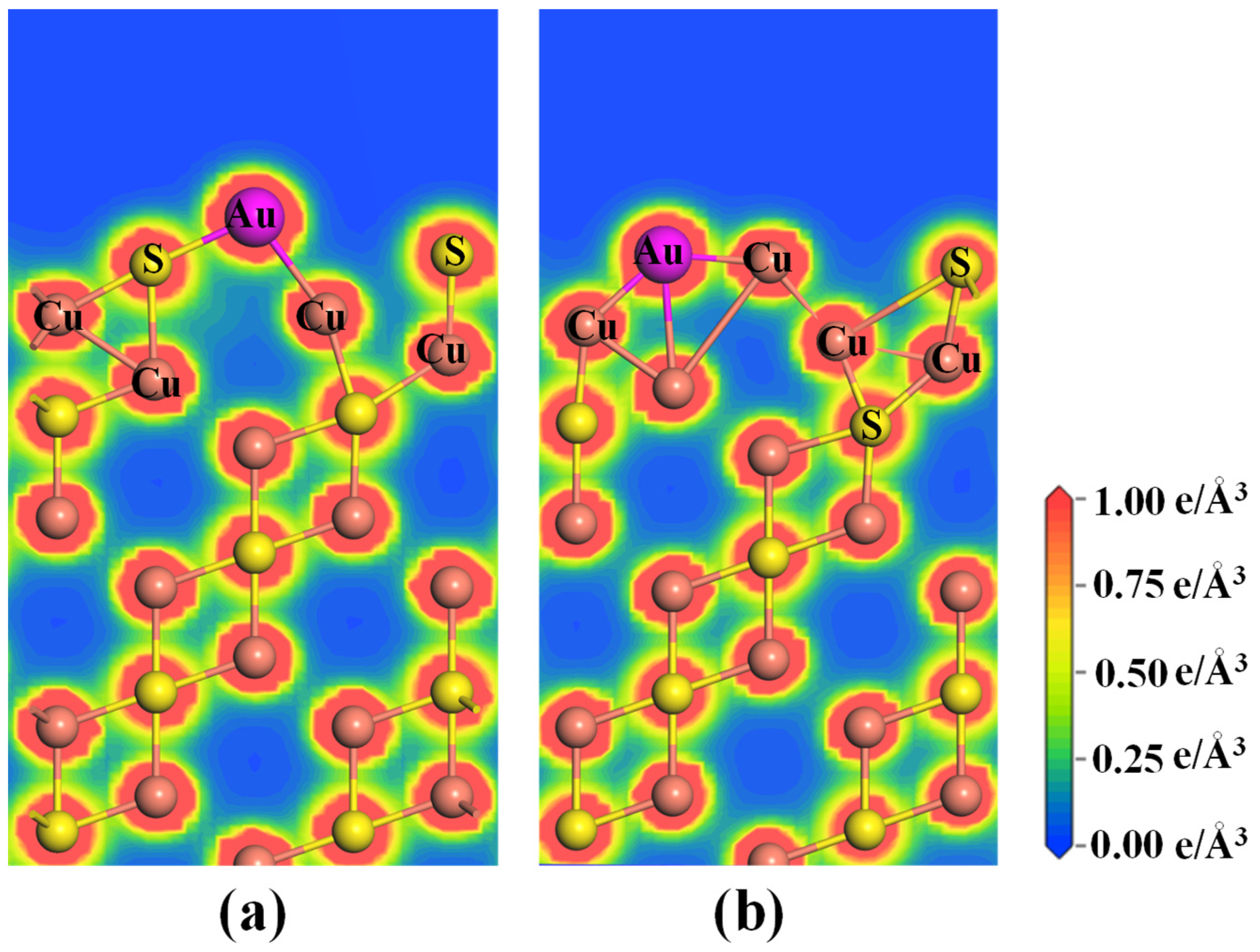
2.2. Effect of the Vacancy on the Au Adsorption of Cu2S(111)
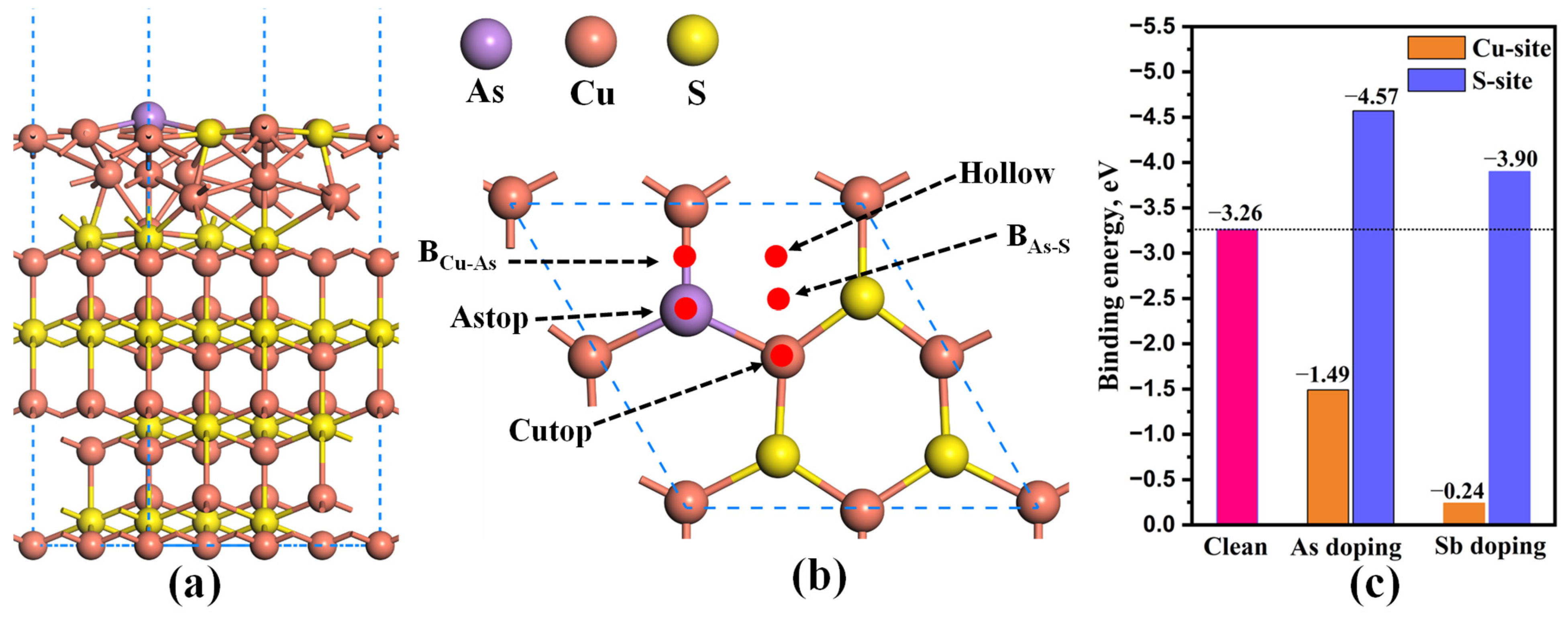
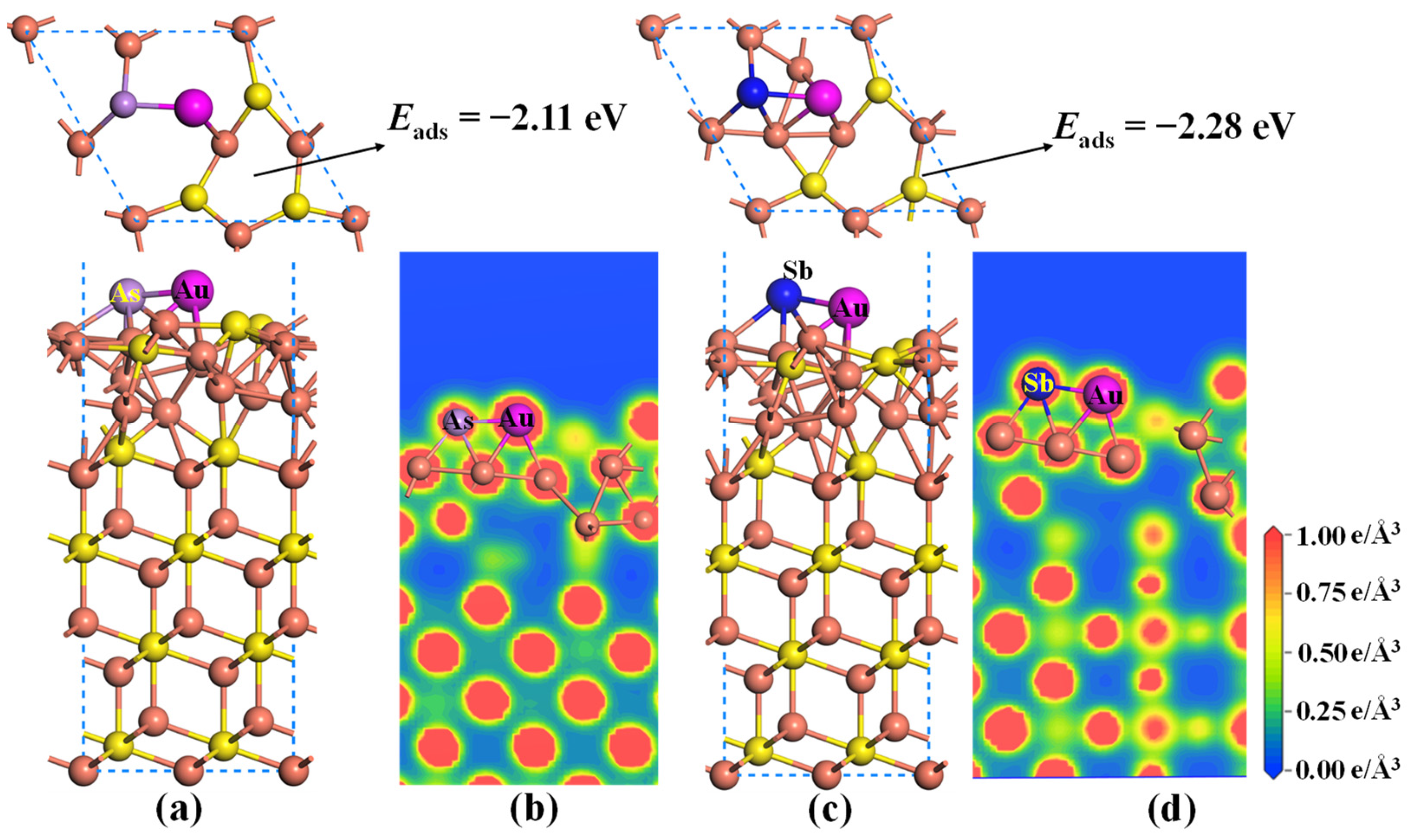
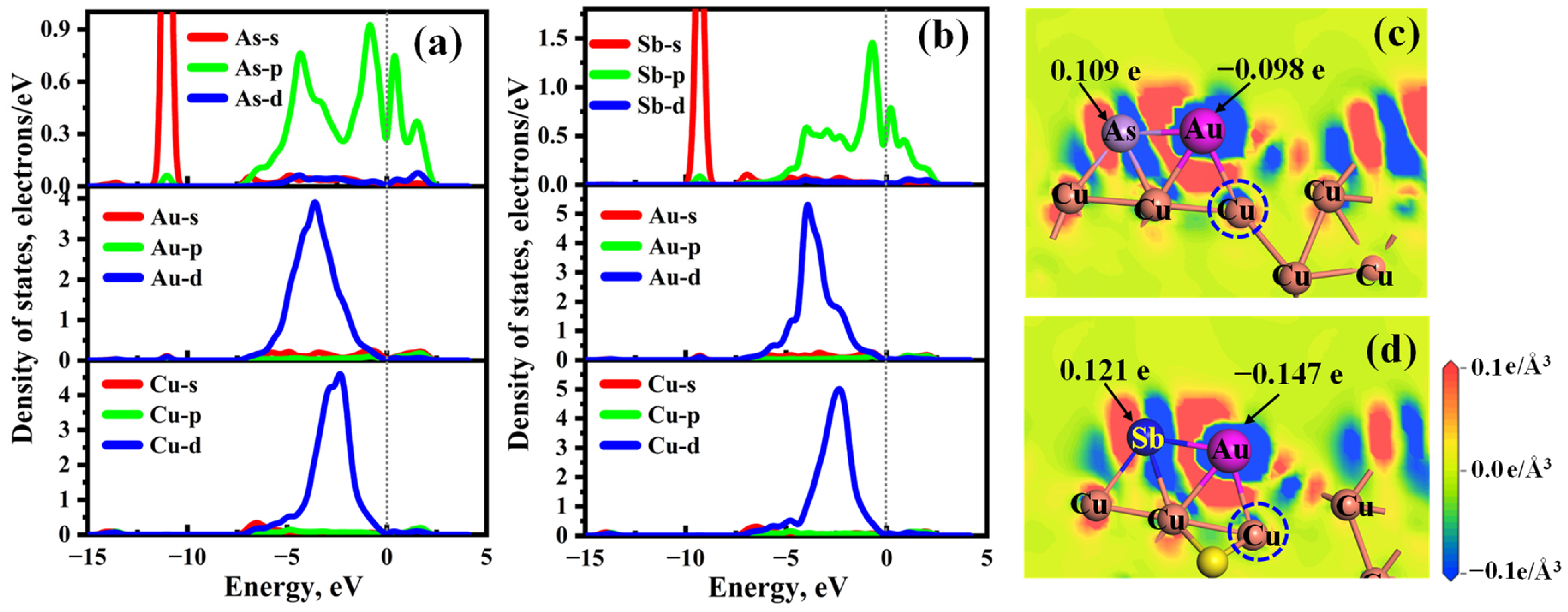
2.3. Effect of As and Sb Dopant on the Au Adsorption of Cu2S
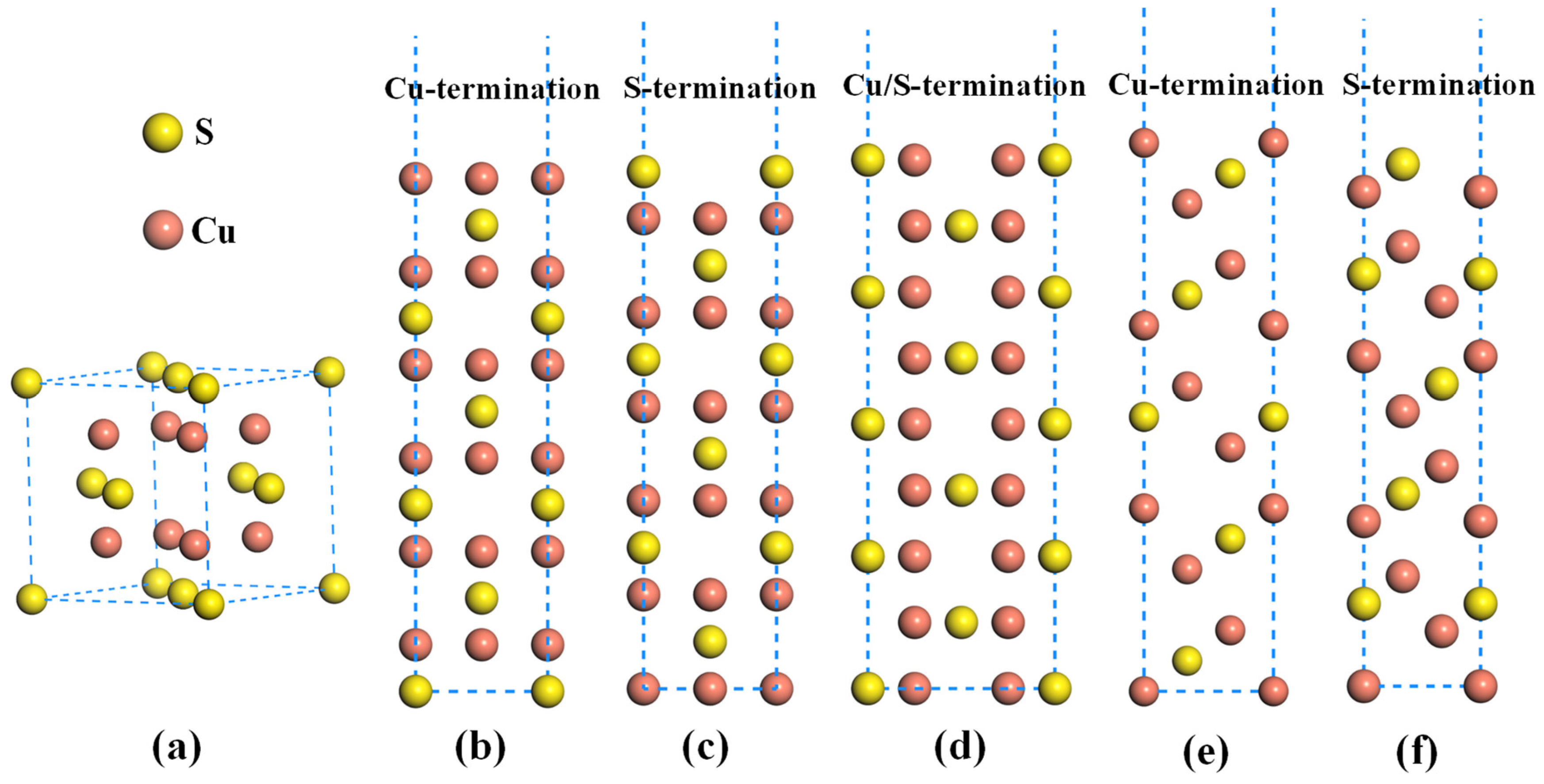
3. Calculation Method and Details
4. Conclusions
- (1)
- The Cu-terminated Cu2S(111) exhibits the lowest surface energy, resulting in its preferential exposure during the high-temperature smelting process.
- (2)
- Gold atom is preferentially adsorbed on the BCu-Cu site, with an adsorption energy of −1.99 eV. The strong chemisorption is mainly attributed to the hybridizations between Au-5d and Cu-3d orbitals.
- (3)
- The presence of Cu vacancy weakens the adsorption strength of the Au atom on the Cu2S(111), whereas the S vacancy notably enhances the adsorption strength. Thus, the S vacancy of Cu2S(111) can effectively improve the Au-capturing efficiency of Cu2S.
- (4)
- As/Sb atom preferentially substitutes for the S atom in the topmost layer of Cu2S(111). Doping As and Sb into the Cu2S(111) can enhance the Au adsorption and Au-capturing capabilities, and the Sb doping exhibits superior effectiveness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jorjani, E.; Sabzkoohi, H.A. Gold leaching from ores using biogenic lixiviants—A review. Curr. Res. Biotechnol. 2022, 4, 10–20. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Ma, C. Bioleaching of gold from waste printed circuit boards by Chromobacterium violaceum. J. Mater. Cycles Waste Manag. 2015, 17, 529–539. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Zhang, L. Pyrite enhanced chlorination roasting and its efficacy in gold and silver recovery from gold tailing. Sep. Purif. Technol. 2020, 250, 117168. [Google Scholar] [CrossRef]
- Chen, A.; Peng, Z.; Hwang, J.Y.; Ma, Y.; Liu, X.; Chen, X. Recovery of Silver and Gold from Copper Anode Slimes. JOM 2015, 67, 493–502. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 198–212. [Google Scholar] [CrossRef]
- Yu, R.L.; Wu, F.D.; Chen, A.; Shi, L.J.; Zeng, W.M.; Gu, G.H.; Qin, W.Q.; Qiu, G.Z. Effect of mixed moderately thermophilic adaptation on leachability and mechanism of high arsenic gold concentrate in an airlift bioreactor. J. Cent. South Univ. 2015, 22, 66–73. [Google Scholar] [CrossRef]
- Fernández, R.R.; Collins, A.; Marczak, E. Gold recovery from high-arsenic-containing ores at Newmont’s roasters. Min. Metall. Explor. 2010, 27, 60–64. [Google Scholar] [CrossRef]
- Chen, M.; Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Shi, J.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Precious Metal Distributions Between Copper Matte and Slag at High PSO2 in WEEE Reprocessing. Metall. Mater. Trans. B 2021, 52, 871–882. [Google Scholar] [CrossRef]
- Amdur, A.M.; Fedorov, S.A.; Yurak, V.V. Transfer of Gold, Platinum and Non-Ferrous Metals from Matte to Slag by Flotation. Metals 2021, 11, 1602. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, B.; Liu, Z.; Yang, H.; Zou, M.; Fu, Y. Effects of Fe/SiO2 Ratio and MgO Content on the Viscous Behaviors of the SiO2–FeO–MgO–12 Wt Pct Fe2O3–8 Wt Pct CaO–3 Wt Pct Al2O3 Slag System. Metall. Mater. Trans. B 2022, 53, 902–915. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wang, S.S.; Wang, Q.M.; Tian, Q.H.; Wang, Z.; Wang, Y.J.; Peng, G.M.; Zhao, B.J. Mechanism of gold collection in matte and distribution behavior of precious metals in oxygen-enriched smelting process. Trans. Nonferrous Met. Soc. China 2020, 30, 2951–2962. [Google Scholar]
- Wan, X.; Kleemola, L.; Klemettinen, L.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. On the Kinetic Behavior of Recycling Precious Metals (Au, Ag, Pt, and Pd) Through Copper Smelting Process. J. Sustain. Metall. 2021, 7, 920–931. [Google Scholar] [CrossRef]
- Chen, M.; Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Sukhomlinov, D.; Shi, J.; Taskinen, P.; Jokilaakso, A. Recovery of Precious Metals (Au, Ag, Pt, and Pd) from Urban Mining Through Copper Smelting. Metall. Mater. Trans. B 2020, 51, 1495–1508. [Google Scholar] [CrossRef]
- Shishin, D.; Hidayat, T.; Chen, J.; Hayes, P.C.; Jak, E. Experimental Investigation and Thermodynamic Modeling of the Distributions of Ag and Au between Slag, Matte, and Metal in the Cu–Fe–O–S–Si System. J. Sustain. Metall. 2019, 5, 240–249. [Google Scholar] [CrossRef]
- Avarmaa, K.; Johto, H.; Taskinen, P. Distribution of Precious Metals (Ag, Au, Pd, Pt, and Rh) Between Copper Matte and Iron Silicate Slag. Metall. Mater. Trans. B 2016, 47, 244–255. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium Distribution of Precious Metals Between Slag and Copper Matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Roghani, G.; Takeda, Y.; Itagaki, K. Phase equilibrium and minor element distribution between FeOx-SiO2-MgO-based slag and Cu2S-FeS matte at 1573 K under high partial pressures of SO2. Metall. Mater. Trans. B 2000, 31, 705–712. [Google Scholar] [CrossRef]
- Morris, D.F.C.; Khan, M.A. Application of solvent extraction to the refining of precious metals—III: Purification of gold. Talanta 1968, 15, 1301–1305. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, M.; Ci, S.N.; Liu, Q.; Zhao, X.P.; Qian, P.; Qu, X.H. First-principles simulations of the interface adhesion and wettability: Cu(111)/TiC(111) versus Cu(111)/WC(0001). Phys. B Condens. Matter 2022, 646, 414336–414342. [Google Scholar] [CrossRef]
- Karimadom, B.R.; Meyerstein, D.; Mizrahi, A.; Kornweitz, H. On the mechanism of dehalogenation of methyl halides (Br and Cl) on Ag(111) and Au(111) surfaces: A DFT study. Appl. Surf. Sci. 2023, 615, 156059–156067. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, G.; Pang, Q.; He, S.; Deng, F.; Xu, Y.; Yao, H. Adsorption and catalytic oxidation of elemental mercury over regenerable magnetic FeCe mixed oxides modified by non-thermal plasma treatment. Chem. Eng. J. 2019, 358, 1454–1463. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Mu, X.; Xu, M.; Yu, J.; Yang, G.; Luo, X.; Zhao, H.; Wu, T. Development of Pdn/g-C3N4 adsorbent for Hg0 removal—DFT study of influences of the support and Pd cluster size. Fuel 2019, 254, 115537. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Wang, B.; Cheng, H.; Cheng, X.; Huang, Z. DFT study on Al-doped defective graphene towards adsorption of elemental mercury. Appl. Surf. Sci. 2018, 427, 547–553. [Google Scholar] [CrossRef]
- Xiong, H.H.; Gan, L.; Tong, Z.F.; Zhang, H.H.; Zhou, Y. Investigation of iron adsorption on composite transition metal carbides in steel by first-principles calculation. J. Phys. Chem. Solids 2018, 116, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Z.; Cheng, Y.; Zhou, M.; Chen, H.; Zhao, K.; Shi, X. Chemical bonding engineering for high-symmetry Cu2S-based materials with high thermoelectric performance. Mater. Today Phys. 2023, 32, 101028–101037. [Google Scholar] [CrossRef]
- Hu, J.; Jian, X.; Yang, T.; Peng, X. Investigation on the interface characteristic between WC(001) and diamond(111) by first-principles calculation. Diam. Relat. Mater. 2022, 123, 108864–108872. [Google Scholar] [CrossRef]
- Xiong, H.; Gan, L.; Li, M. Improving the oxidation resistance of WSi2 by boron doping: A DFT study. Mater. Today Commun. 2020, 25, 101312–101319. [Google Scholar] [CrossRef]
- Tong, T.; Linghu, Y.; Wu, G.; Wang, C.; Wu, C. Nitric oxide electrochemical reduction reaction on transition metal-doped MoSi2N4 monolayers. Phys. Chem. Chem. Phys. 2022, 24, 18943–18951. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Lou, F.; Guo, K.; Yu, Z. Non-precious metal activated MoSi2N4 monolayers for high-performance OER and ORR electrocatalysts: A first-principles study. Appl. Surf. Sci. 2022, 579, 152234–152245. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, C.; Negro, M.; Calegari, F.; Sansone, G.; Nisoli, M.; De Silvestri, S.; Stagira, S. Generalized molecular orbital tomography. Nat. Phys. 2011, 7, 822–826. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Miao, S.; Ding, J.; Yu, Y.; Zhang, J. A complete catalytic reaction scheme for Hg0 oxidation by HCl over RuO2/TiO2 catalyst. J. Hazard. Mater. 2019, 373, 660–670. [Google Scholar] [CrossRef] [PubMed]
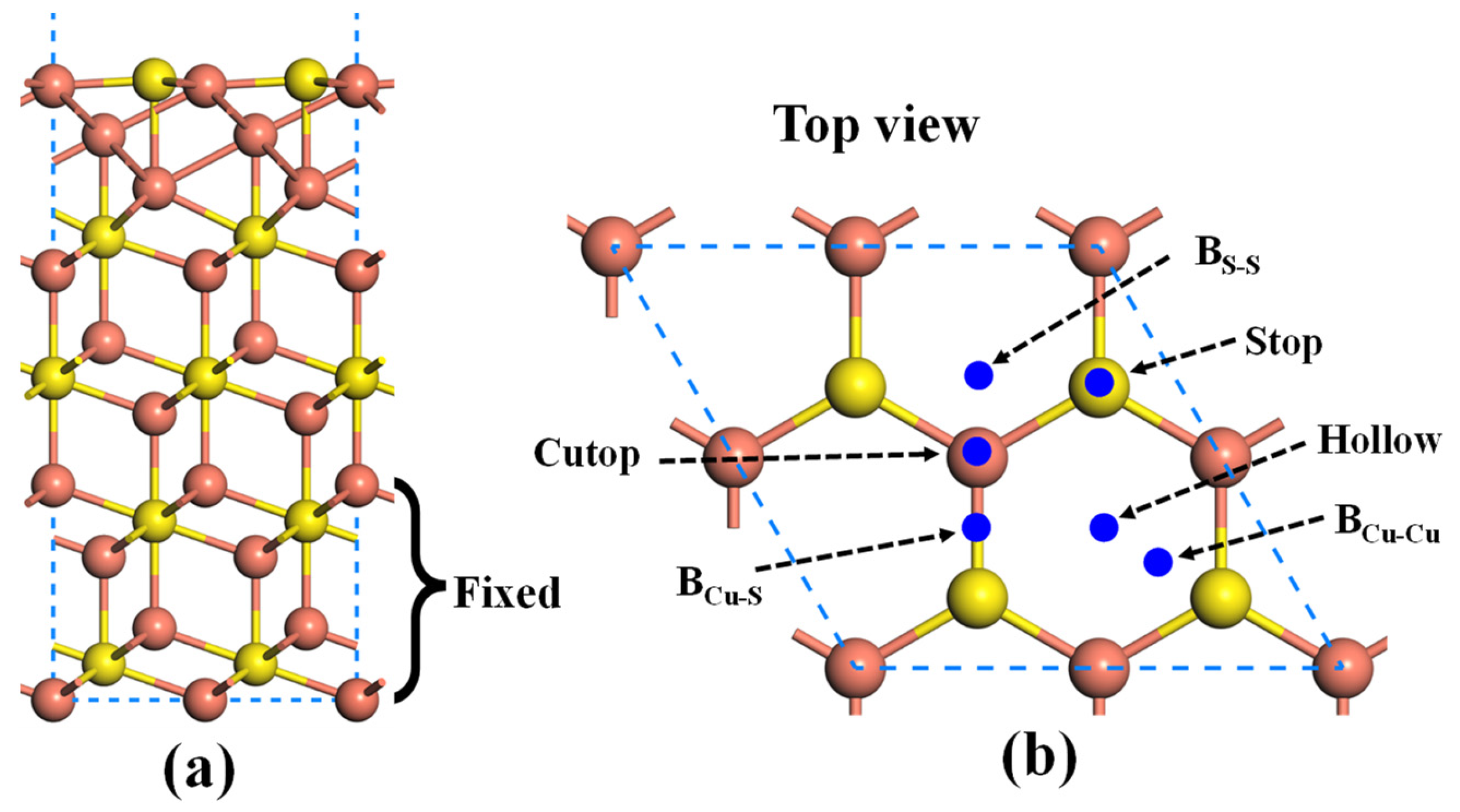



| Adsorption Sites | Stop | BCu-Cu | BCu-S | BCu-S | Cutop | Hollow |
|---|---|---|---|---|---|---|
| Eads | −1.54 | −1.99 | −1.84 | −1.81 | −1.67 | −1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Xiong, H.; Gan, L. Effect of Vacancy, As, and Sb Dopants on the Gold-Capturing Ability of Cu2S during Gold Collection in Matte Processes. Molecules 2023, 28, 7390. https://doi.org/10.3390/molecules28217390
Huang H, Xiong H, Gan L. Effect of Vacancy, As, and Sb Dopants on the Gold-Capturing Ability of Cu2S during Gold Collection in Matte Processes. Molecules. 2023; 28(21):7390. https://doi.org/10.3390/molecules28217390
Chicago/Turabian StyleHuang, Hui, Huihui Xiong, and Lei Gan. 2023. "Effect of Vacancy, As, and Sb Dopants on the Gold-Capturing Ability of Cu2S during Gold Collection in Matte Processes" Molecules 28, no. 21: 7390. https://doi.org/10.3390/molecules28217390
APA StyleHuang, H., Xiong, H., & Gan, L. (2023). Effect of Vacancy, As, and Sb Dopants on the Gold-Capturing Ability of Cu2S during Gold Collection in Matte Processes. Molecules, 28(21), 7390. https://doi.org/10.3390/molecules28217390







