Anodic Oxidation of Tungsten under Illumination-Multi-Method Characterization and Modeling at the Molecular Level
Abstract
:1. Introduction
2. Results
2.1. General Electrochemical Behavior of W in Sulfate-Fluoride Solutions
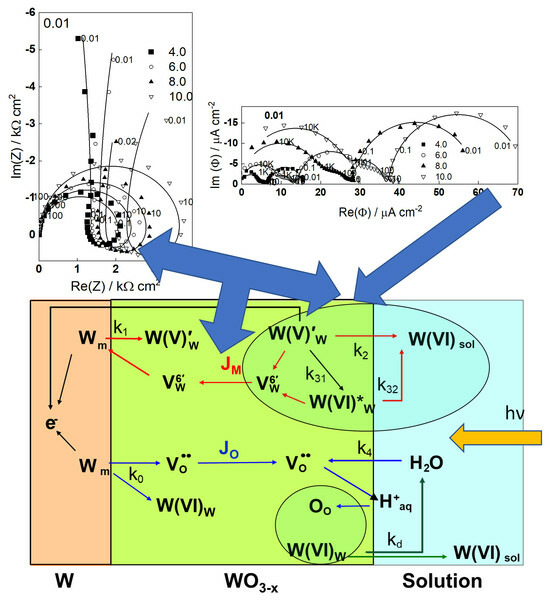
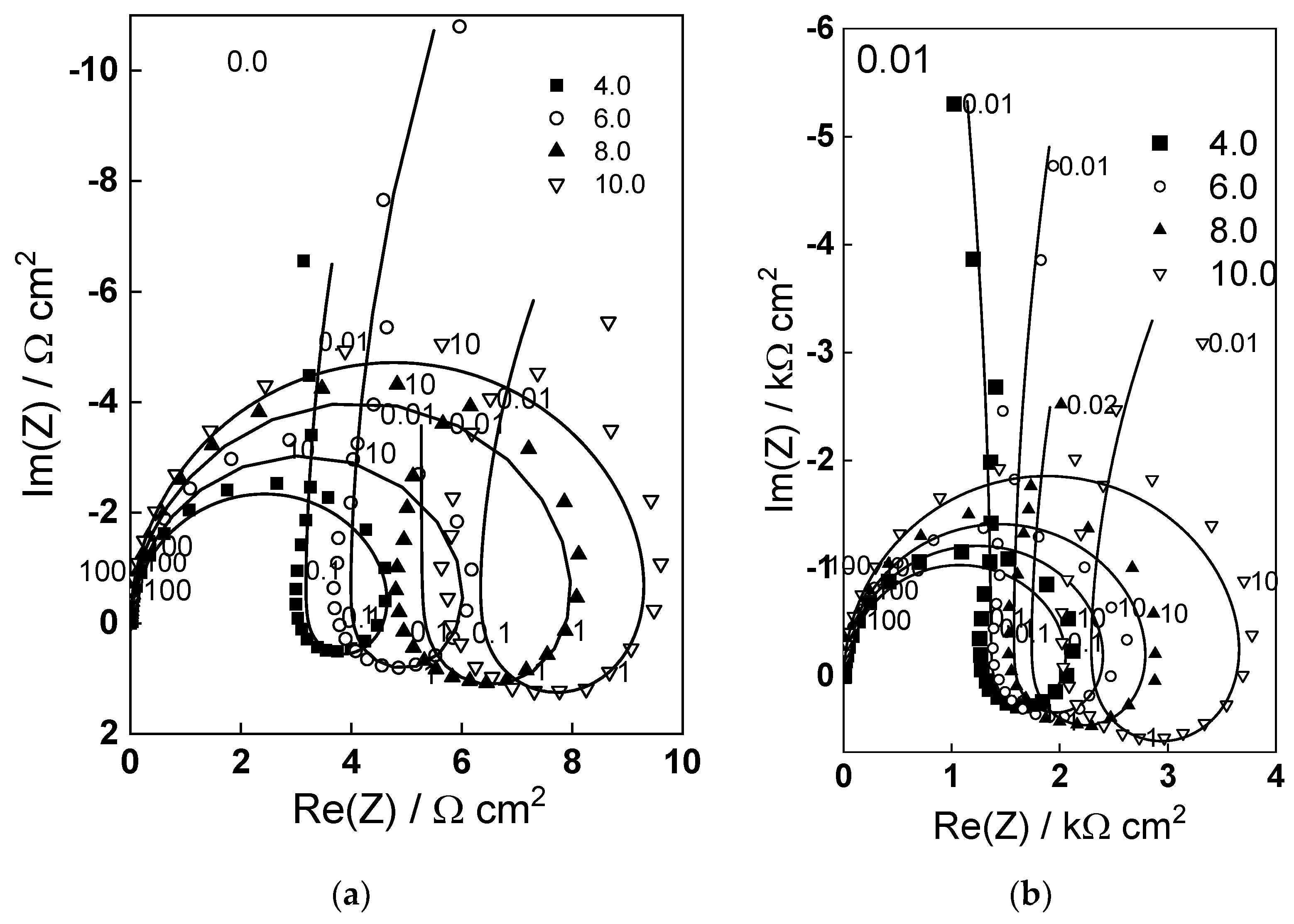
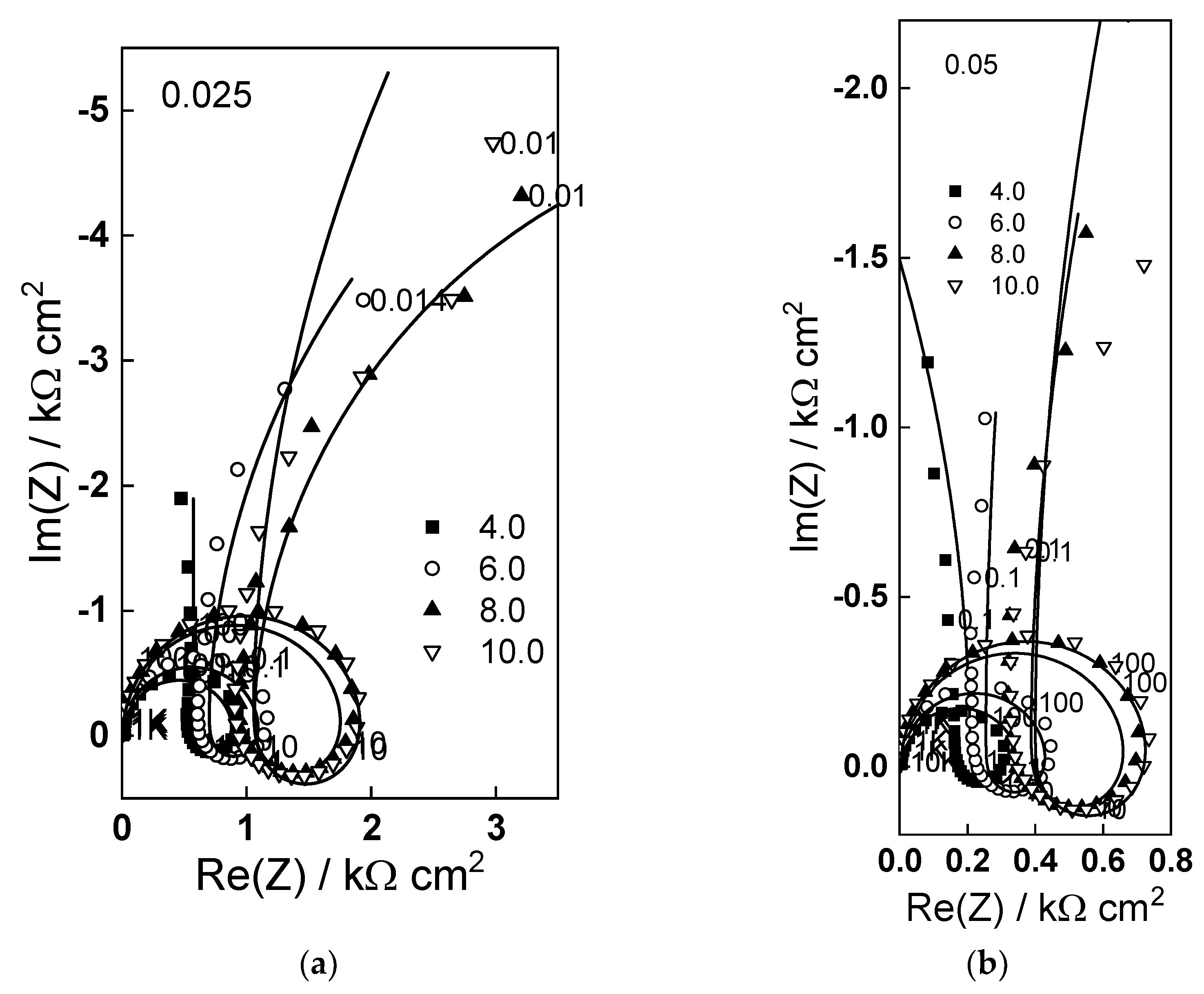
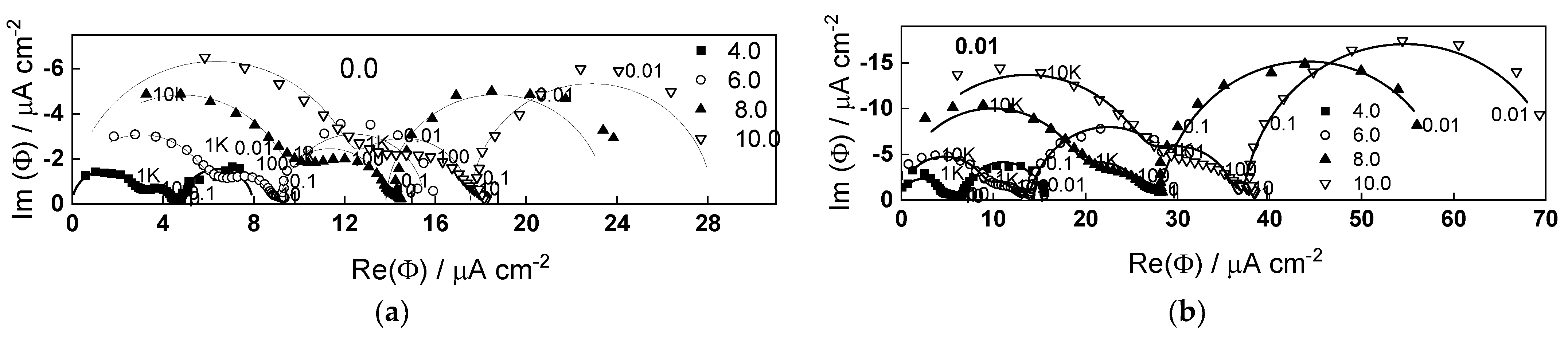
2.2. Electrochemical Impedance Spectroscopy
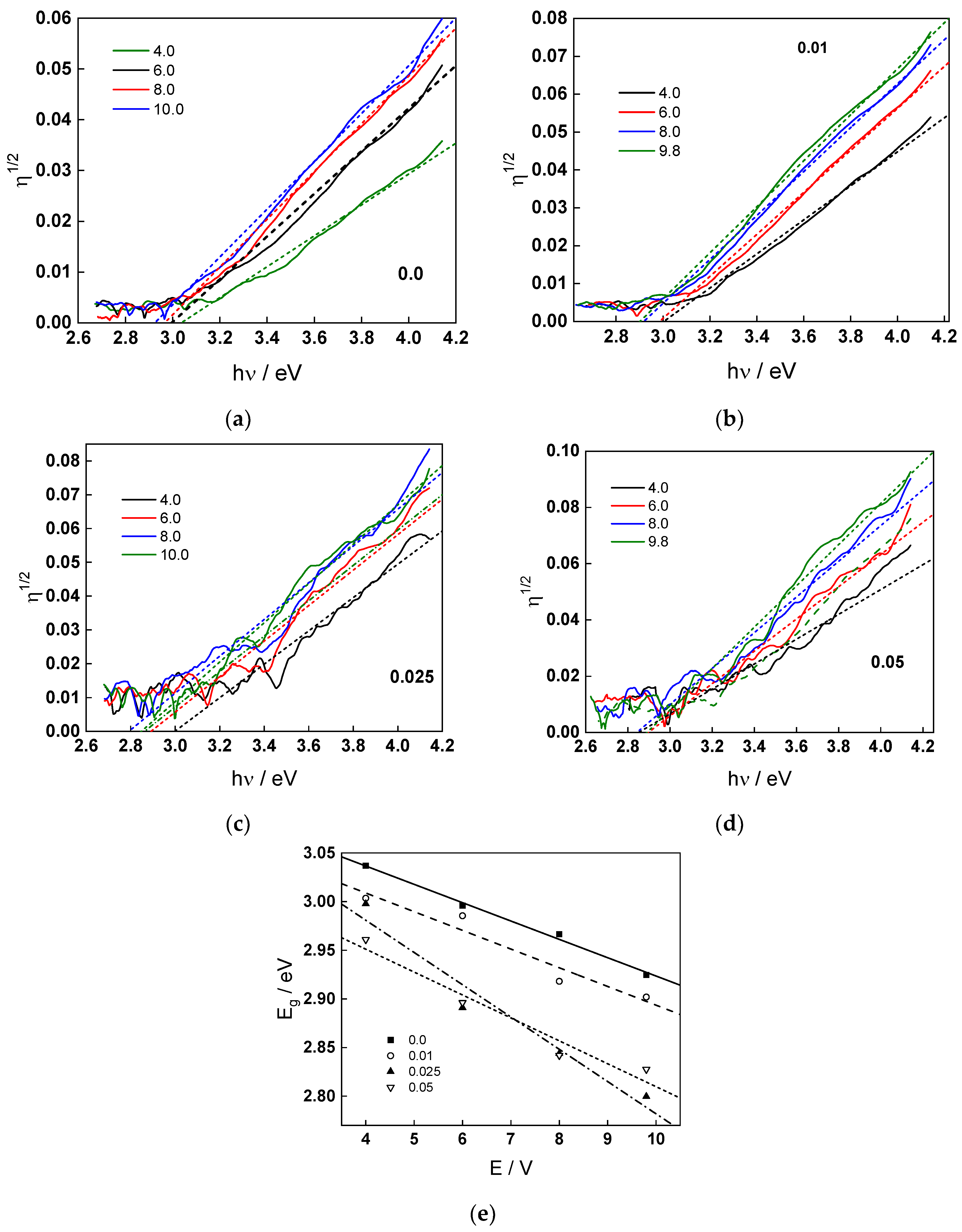
2.3. Energy Spectra of the Photocurrent
2.4. IMPS Response during Film Growth and Dissolution
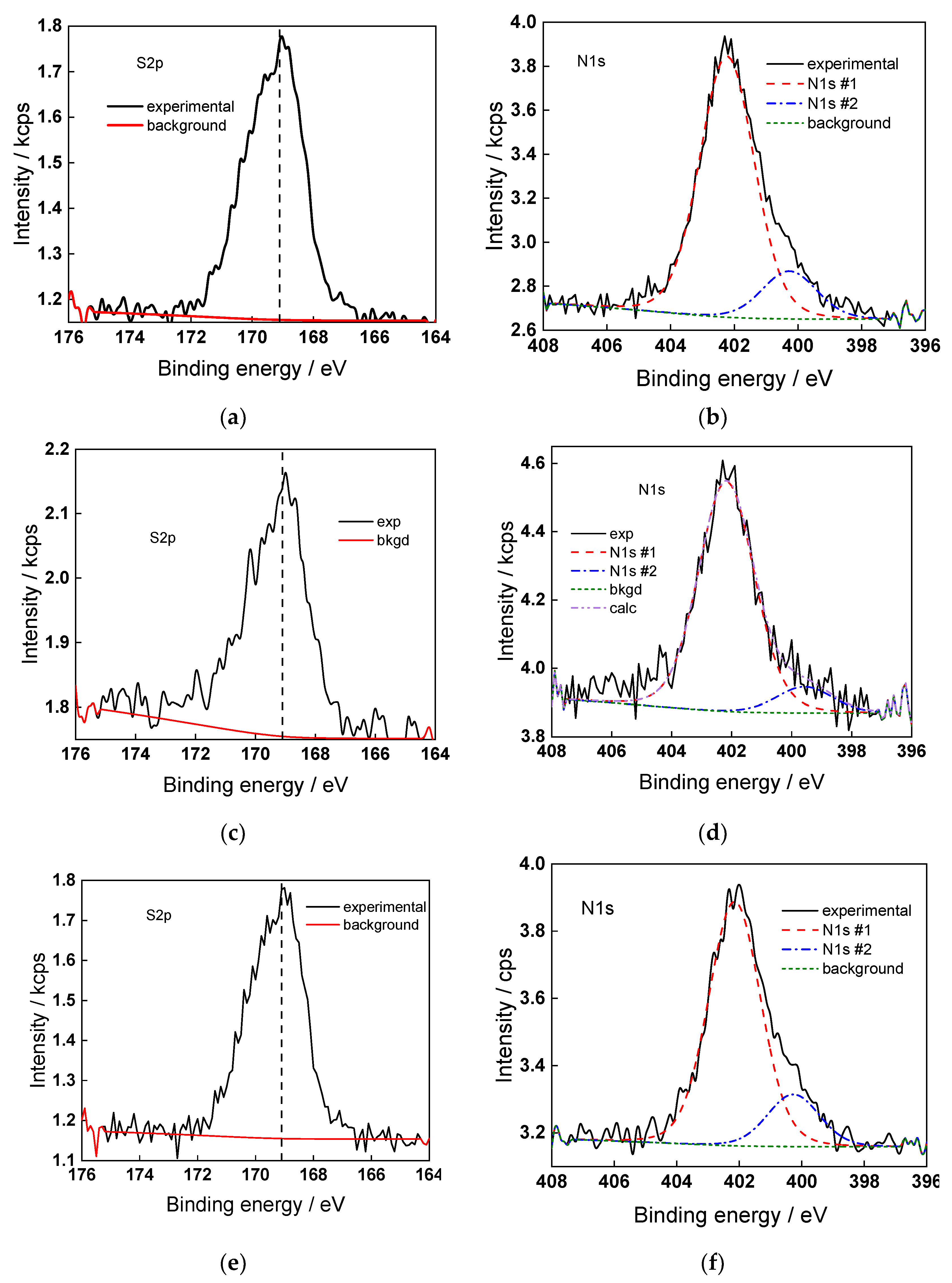
2.5. Surface Composition of the Oxides
3. Discussion
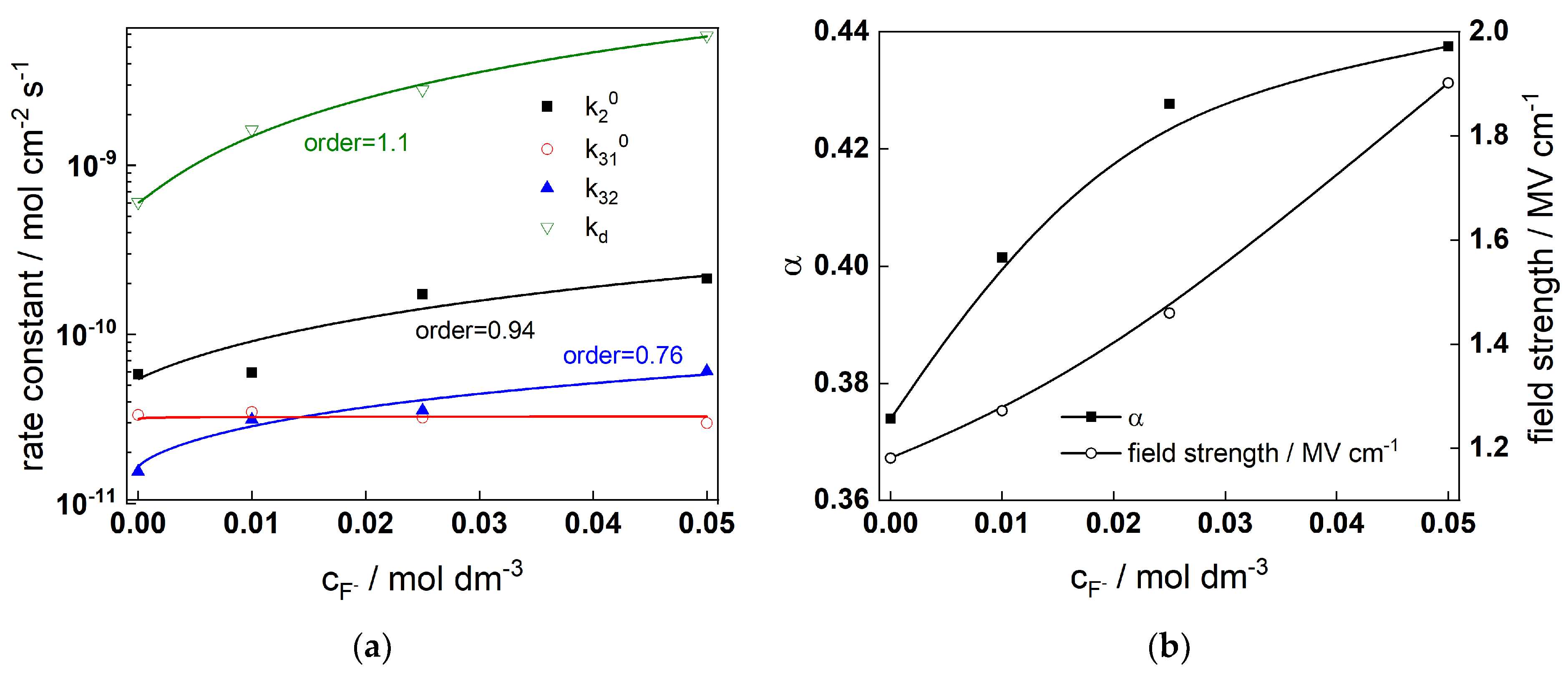
3.1. A Model for Film Growth and Tungsten Dissolution
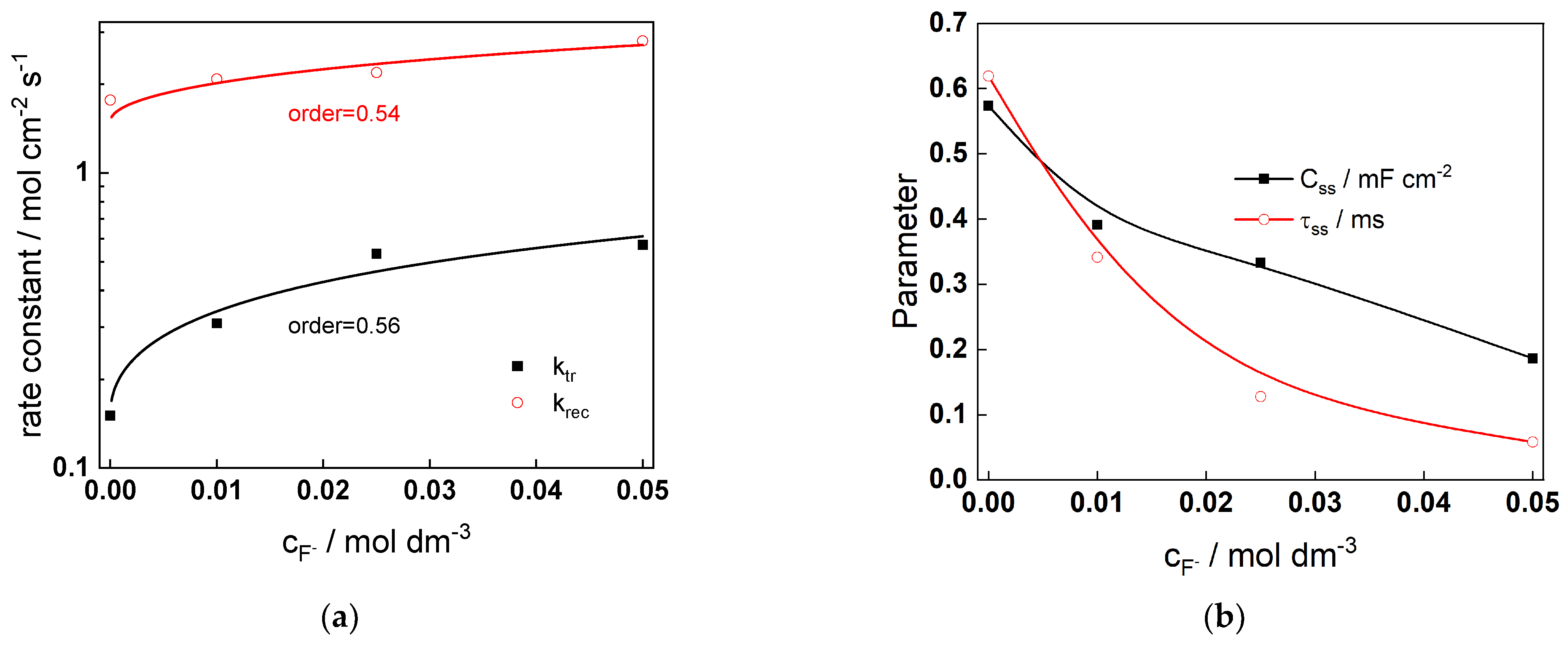
3.2. Interpretation of the IMPS Response
4. Materials and Methods
5. Conclusions
- Electrochemical data (current vs. potential curves and impedance spectra in a wide range of potentials and fluoride concentrations) give the possibility to estimate the rate constants at the oxide/electrolyte interface and the transport parameters through the oxide layer. Reaction orders vs. fluoride indicate the active participation of the anion in the oxidation and dissolution processes.
- The validity of the obtained estimates is in accordance with surface composition data obtained by XPS. Conversely to what is observed with titanium, the concentration of fluoride at the surface is rather low, i.e., it can be regarded as a catalytic species.
- Electronic carrier dynamics during film growth and dissolution is assessed using IMPS measurements in a wide range of potentials. A qualitative relationship between phenomenological parameters obtained from a generalized interpretation of the IMPS response and the sequence of charge transfer reactions at the interface is tentatively proposed.
- Further extension of this type of measurements is needed in order to quantify the relation between photo-induced charge transfer/recombination of electronic carriers, on one hand, and generation, transport, and consumption of ionic point defects, on the other.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N.; Chan, E.S. Nanostructured Tungsten Trioxide Thin Films Synthesized for Photo-electrocatalytic Water Oxidation: A review. ChemSusChem 2014, 7, 2974–2997. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Chen, C.; Xu, W.; Li, L. Tungsten Trioxide Nanostructures for Photoelectrochemical Water Splitting: Material Engineering and Charge Carrier Dynamic Manipulation. Adv. Funct. Mater. 2019, 29, 1809036. [Google Scholar] [CrossRef]
- Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. [Google Scholar] [CrossRef]
- Mukherjee, N.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of nanoporous tungsten oxide by galvanostatic anodization. J. Mater. Res. 2003, 18, 2296–2299. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Sieber, I.; Taveira, L.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-organized porous WO3 formed in NaF electrolytes. Electrochem. Commun. 2005, 7, 295–298. [Google Scholar] [CrossRef]
- de Tacconi, N.R.; Chenthamarakshan, C.R.; Yogeeswaran, G.; Watcharenwong, A.; Zoysa, S.; Basit, N.; Rajeshwar, K. Nanoporous TiO2 and WO3 Films by Anodization of Titanium and Tungsten Substrates: Influence of Process Variables on Morphology and Photoelectrochemical Response. J. Phys. Chem. B 2006, 110, 25347–25355. [Google Scholar] [CrossRef]
- Watcharenwong, A.; Chanmanee, W.; Tacconi, N.; Chenthamarakshan, C.R.; Kajitvichyanukul, P.; Rajeshwar, K. Anodic growth of nanoporous WO3 films: Morphology, photoelectrochemical response and photocatalytic activity for methylene blue and hexavalent chrome conversion. J. Electroanal. Chem. 2008, 612, 112–120. [Google Scholar] [CrossRef]
- Reyes-Gil, K.; Wiggenhorn, C.; Brunschwig, B.; Lewis, N. Comparison between the Quantum Yields of Compact and Porous WO3 Photoanodes. J. Phys. Chem. C 2013, 117, 14947–14957. [Google Scholar] [CrossRef]
- Syrek, K.; Zych, M.; Zaraska, L.; Sulka, G. Influence of annealing conditions on anodic tungsten oxide layers and their photoelectrochemical activity. Electrochim. Acta 2017, 231, 61–68. [Google Scholar] [CrossRef]
- Ahmadi, E.; Ng, C.; Razak, K.; Lockman, Z. Preparation of anodic nanoporous WO3 film using oxalic acid as electrolyte. J. Alloys Compd. 2017, 704, 518–527. [Google Scholar] [CrossRef]
- Syrek, K.; Zaraska, L.; Zych, M.; Sulka, G. The effect of anodization conditions on the morphology of porous tungsten oxide layers formed in aqueous solution. J. Electroanal. Chem. 2018, 829, 106–115. [Google Scholar] [CrossRef]
- Zych, M.; Syrek, K.; Zaraska, L.; Sulka, G. Improving Photoelectrochemical Properties of Anodic WO3 Layers by Optimizing Electrosynthesis Conditions. Molecules 2020, 25, 2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Paulose, M.; Neupane, R.; Schaffer, L.; Rana, D.; Su, J.; Guo, L.; Varghese, O. Nanoporous WO3 films synthesized by tuning anodization conditions for photoelectrochemical water oxidation. Sol. Energy Mater. Solar Cells 2020, 209, 110472. [Google Scholar] [CrossRef]
- Karastoyanov, V.; Bojinov, M. Anodic oxidation of tungsten in sulfuric acid solution—Influence of hydrofluoric acid addition. Mater. Chem. Phys. 2008, 112, 2702–2710. [Google Scholar] [CrossRef]
- Karastoyanov, V.; Bojinov, M. Mechanism of anodic oxidation of tungsten in neutral sulphate-fluoride solutions. J. Solid State Electrochem. 2009, 13, 309–320. [Google Scholar] [CrossRef]
- Stancheva, M.; Bojinov, M. Influence of Fluoride Content on the Barrier Layer Formation and Titanium Dissolution in Ethylene Glycol—Water Electrolytes. Electrochim. Acta 2012, 78, 65–74. [Google Scholar] [CrossRef]
- Bojinov, M.; Stancheva, M. Coupling between dissolution and passivation revisited—Kinetic parameters of anodic oxidation of titanium alloys in a fluoride-containing electrolyte. J. Electroanal. Chem. 2015, 737, 150–161. [Google Scholar] [CrossRef]
- Bojinov, M.; Betova, I.; Karastoyanov, V. Modelling barrier film growth and dissolution on titanium based on EIS, XPS and photocurrent data. Electrochim. Acta 2020, 344, 136137. [Google Scholar] [CrossRef]
- Bojinov, M.; Betova, I.; Karastoyanov, V.; Stancheva, M. Parameterization and Extension of a Model of Oxide Growth by a Multi-Method Approach. J. Electrochem. Soc. 2021, 168, 031502. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.; Li, W.; Li, J.; Li, Y.; Shi, O.; Chen, O. Highly Efficient Photoelectrochemical Hydrogen Generation Using ZnxBi2S3+x Sensitized Platelike WO3 Photoelectrodes. ACS Appl. Mater. Interfaces 2015, 7, 10763–10770. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; He, H.; Li, Y.; Chen, O. Electrochemical Doping Induced In Situ Homo-species for Enhanced Photoelectrochemical Performance on WO3 Nanoparticles Film Photoelectrodes. Electrochim. Acta 2016, 210, 251–260. [Google Scholar] [CrossRef]
- Rodríguez-Perez, M.; Rodríguez-Gutierrez, I.; Vega-Poot, A.; García-Rodríguez, R.; Rodríguez-Gattorno, G.; Oskam, G. Charge transfer and recombination kinetics at WO3 for photoelectrochemical water oxidation. Electrochim. Acta 2017, 258, 900–908. [Google Scholar] [CrossRef]
- Bai, S.; Yang, X.; Liu, C.; Xiang, X.; Luo, R.; He, J.; Chen, A. An integrating photoanode of WO3/Fe2O3 heterojunction decorated with NiFe-LDH to improve PEC water splitting efficiency. ACS Sustain. Chem. Eng. 2018, 6, 12906–12913. [Google Scholar] [CrossRef]
- Rodríguez-Gutierrez, I.; Djatoubai, E.; Rodríguez-Perez, M.; Su, J.; Rodríguez-Gattorno, G.; Vayssieres, L.; Oskam, G. Photoelectrochemical water oxidation at FTO|WO3@CuWO4 and FTO|WO3@CuWO4jBiVO4 heterojunction systems: An IMPS analysis. Electrochim. Acta 2019, 308, 317–327. [Google Scholar] [CrossRef]
- Amano, F.; Koga, S. Influence of light intensity on the steady-state kinetics in tungsten trioxide particulate photoanode studied by intensity-modulated photocurrent spectroscopy. J. Electroanal. Chem. 2020, 860, 113891. [Google Scholar] [CrossRef]
- Rodríguez-Gutierrez, I.; Djatoubai, E.; Su, J.; Vega-Poot, A.; Rodríguez-Gattorno, G.; Souza, F.; Oskam, G. An intensity-modulated photocurrent spectroscopy study of the charge carrier dynamics of WO3/BiVO4 heterojunction systems. Sol. Energy Mater. Sol. Cells 2020, 208, 110378. [Google Scholar] [CrossRef]
- Lin, H.; Long, X.; An, Y.; Yang, S. In situ growth of Fe2WO6 on WO3 nanosheets to fabricate heterojunction arrays for boosting solar water splitting. J. Chem. Phys. 2020, 152, 214704. [Google Scholar] [CrossRef] [PubMed]
- Goosens, A.; Macdonald, D.D. A Photoelectrochemical Impedance Spectroscopic Study of Passive Metals. Electrochim. Acta 1993, 38, 1965–1968. [Google Scholar] [CrossRef]
- Goosens, A.; Macdonald, D.D. A photoelectrochemical impedance spectroscopic study of passive tungsten. J. Electroanal. Chem 1993, 352, 65–81. [Google Scholar] [CrossRef]
- González-Borrero, P.P.; Sato, F.; Medina, A.N.; Baesso, M.L.; Bento, A.C.; Baldissera, G.; Persson, C.; Niklasson, G.A.; Granqvist, C.G.; Ferreira da Silva, A. Optical band-gap determination of nanostructured WO3 film. Appl. Phys. Lett. 2010, 96, 061909. [Google Scholar] [CrossRef]
- Tsarenko, A.; Gorshenkov, M.; Yatsenko, A.; Zhigunov, D.; Butova, V.; Kaichev, V.; Ulyankina, A. Electrochemical Synthesis-Dependent Photoelectrochemical Properties of Tungsten Oxide Powders. Chem. Eng. 2022, 6, 31. [Google Scholar] [CrossRef]
- Johansson, M.; Mattsson, A.; Lindquist, S.-E.; Niklasson, G.; Österlund, L. The Importance of Oxygen Vacancies in Nanocrystalline WO3−x Thin Films Prepared by DC Magnetron Sputtering for Achieving High Photoelectrochemical Efficiency. J. Phys. Chem. C 2017, 121, 7412–7420. [Google Scholar] [CrossRef]
- Hirst, J.; Müllera, S.; Peeters, D.; Sadlo, A.; Mai, L.; Reyes, O.; Friedrich, D.; Mitoraj, D.; Devi, A.; Beranek, R.; et al. Comparative Study of Photocarrier Dynamics in CVD-deposited CuWO4, CuO, and WO3 Thin Films for Photoelectrocatalysis. Zeitschrift für Physikalische Chemie 2020, 234, 699–717. [Google Scholar] [CrossRef]
- Butler, M. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Searson, P.; Latanision, R.; Stimming, U.J. Analysis of the photoelectrochemical response of the passive film on iron in neutral solutions. J. Electrochem. Soc. 1988, 135, 1358–1363. [Google Scholar] [CrossRef]
- Asami, K.; Chen, S.-C.; Habazaki, H.; Hashimoto, K. The surface characterization of titanium and titanium-nickel alloys in sulfuric acid. Corros. Sci. 1993, 35, 43–49. [Google Scholar] [CrossRef]
- Peter, L.; Ponomarev, E.; Fermin, D. Intensity-modulated photocurrent spectroscopy: Reconciliation of phenomenological analysis with multistep electron transfer mechanisms. J. Electroanal. Chem. 1997, 427, 79–96. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| α2 | 0.33 ± 0.011 |
| α31 | 0.33 ± 0.010 |
| a/nm | 0.32 ± 0.012 |
| βO/nmol cm−2 | 0.11 ± 0.003 |
| β/nmol cm−2 | 0.3 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojinov, M.; Penkova, Y.; Betova, I.; Karastoyanov, V. Anodic Oxidation of Tungsten under Illumination-Multi-Method Characterization and Modeling at the Molecular Level. Molecules 2023, 28, 7387. https://doi.org/10.3390/molecules28217387
Bojinov M, Penkova Y, Betova I, Karastoyanov V. Anodic Oxidation of Tungsten under Illumination-Multi-Method Characterization and Modeling at the Molecular Level. Molecules. 2023; 28(21):7387. https://doi.org/10.3390/molecules28217387
Chicago/Turabian StyleBojinov, Martin, Yoanna Penkova, Iva Betova, and Vasil Karastoyanov. 2023. "Anodic Oxidation of Tungsten under Illumination-Multi-Method Characterization and Modeling at the Molecular Level" Molecules 28, no. 21: 7387. https://doi.org/10.3390/molecules28217387
APA StyleBojinov, M., Penkova, Y., Betova, I., & Karastoyanov, V. (2023). Anodic Oxidation of Tungsten under Illumination-Multi-Method Characterization and Modeling at the Molecular Level. Molecules, 28(21), 7387. https://doi.org/10.3390/molecules28217387