Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway
Abstract
:1. Introduction
2. Results
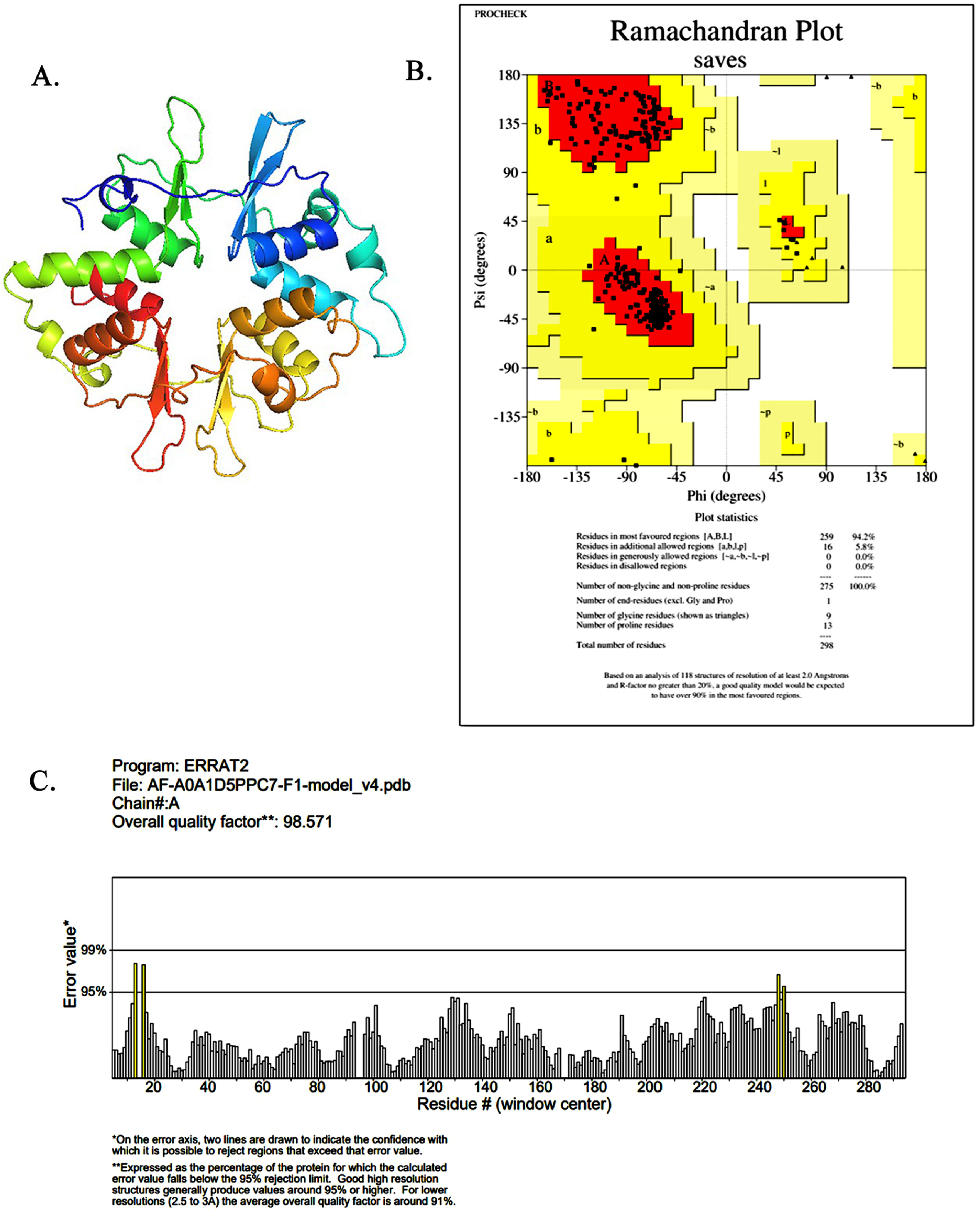
2.1. AMPK Model Building and Quality Assessment Visualization
2.2. Molecular Docking Analysis
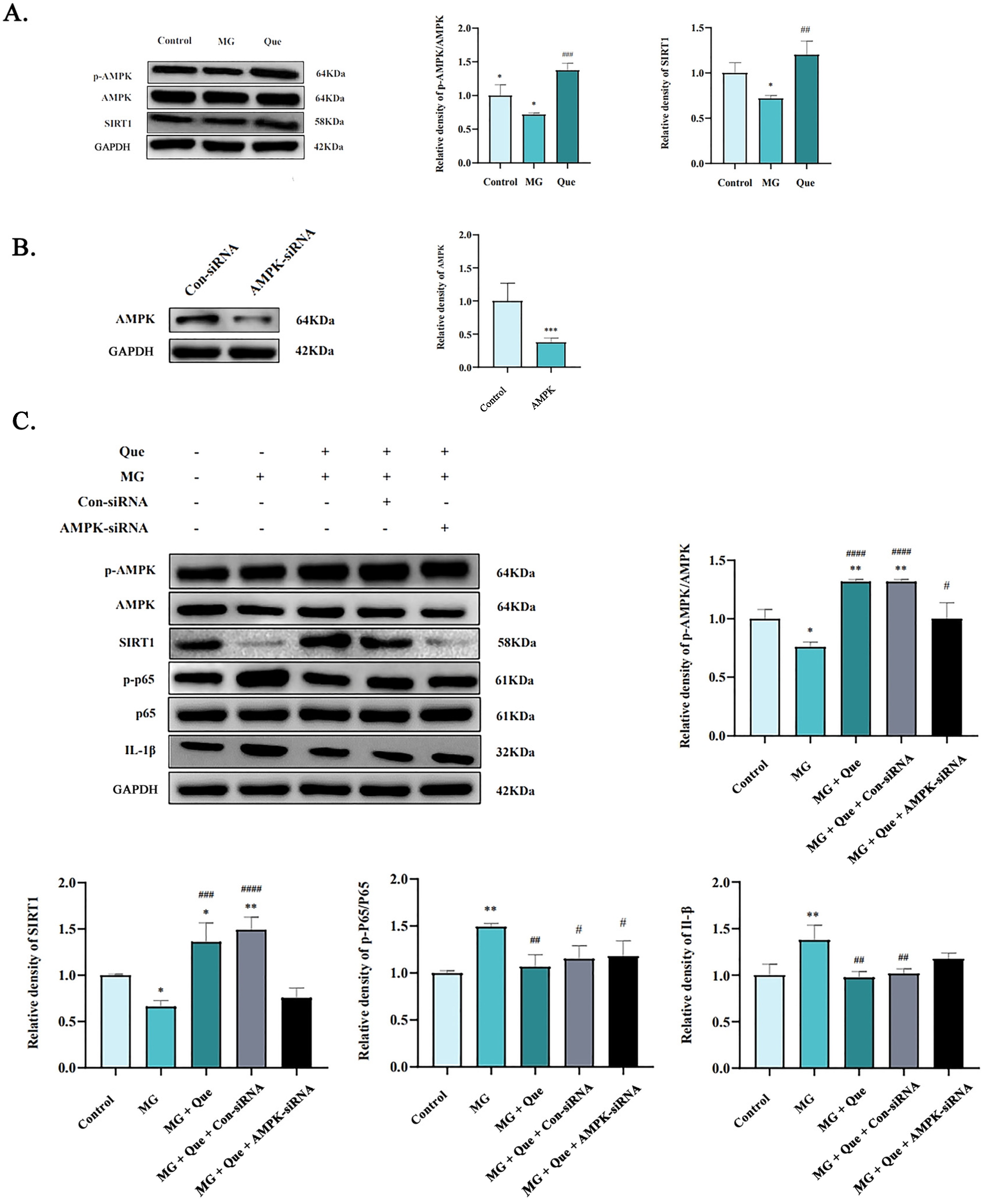
2.3. Quercetin Mitigates MG Infection-Induced Inhibition of AMPK Activity in CP-II Cells
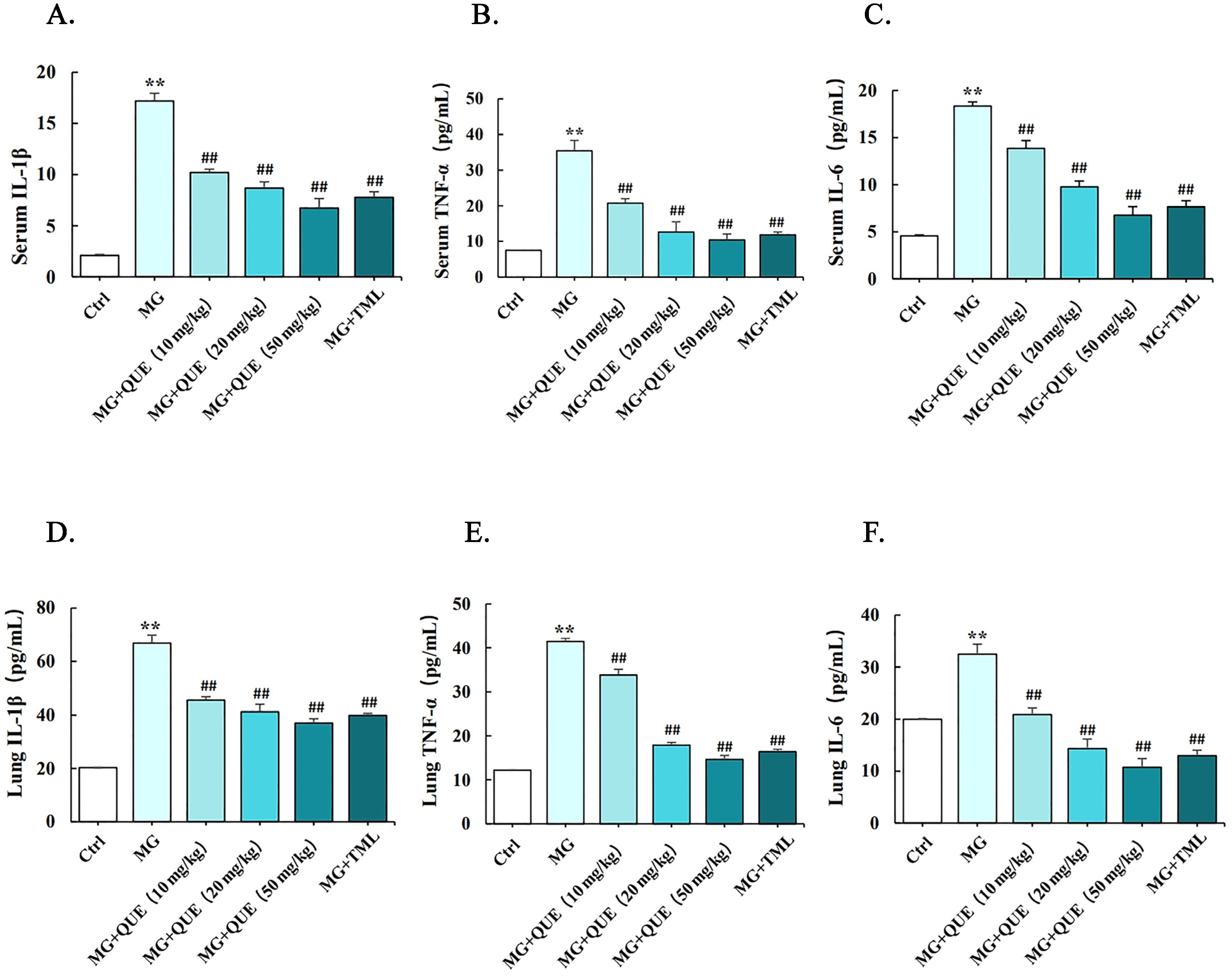
2.4. Quercetin Reduces MG-Induced Release of Proinflammatory Cytokine
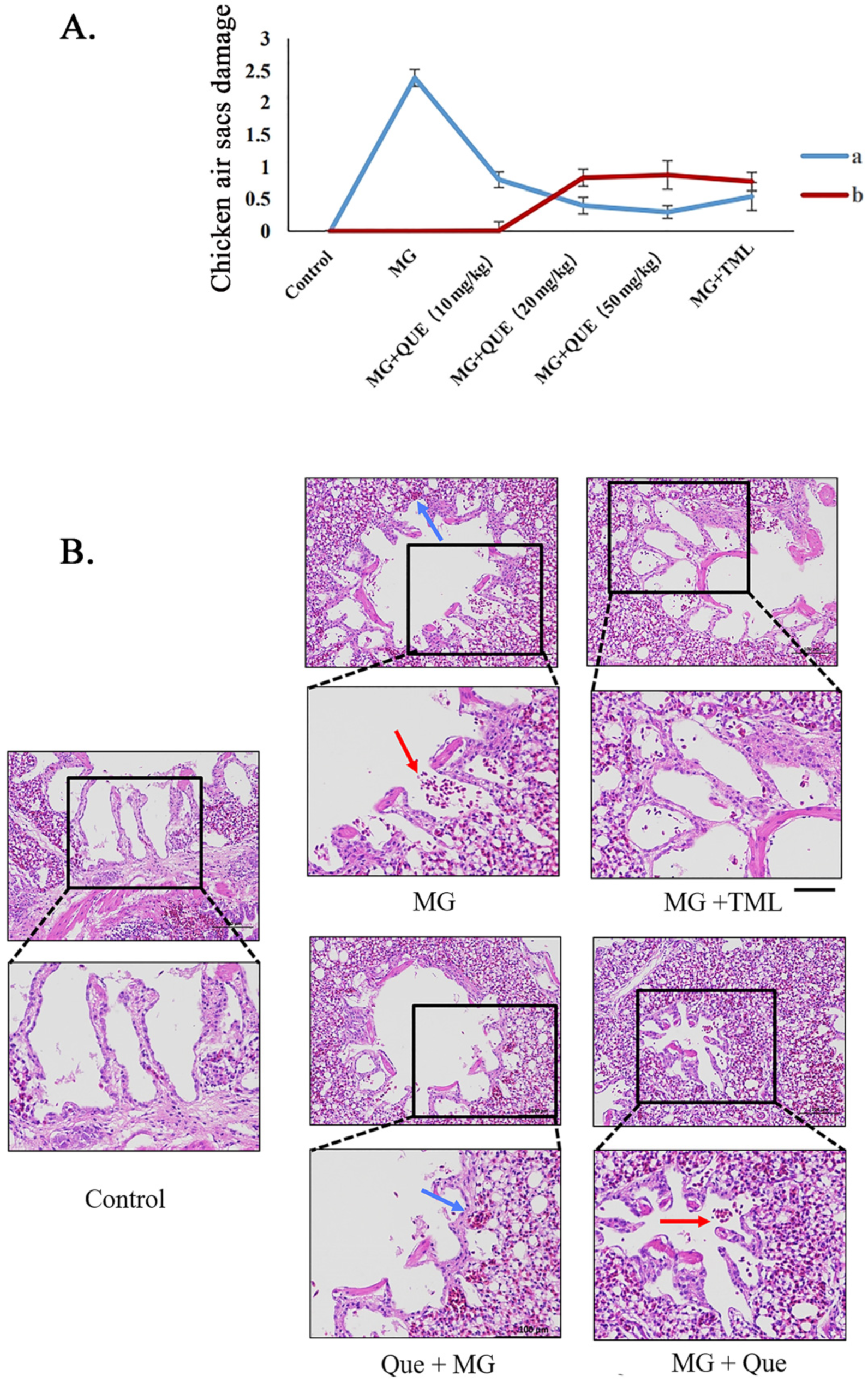
2.5. Quercetin Ameliorates Air Sac Diseases and Lung Damage following MG Infection
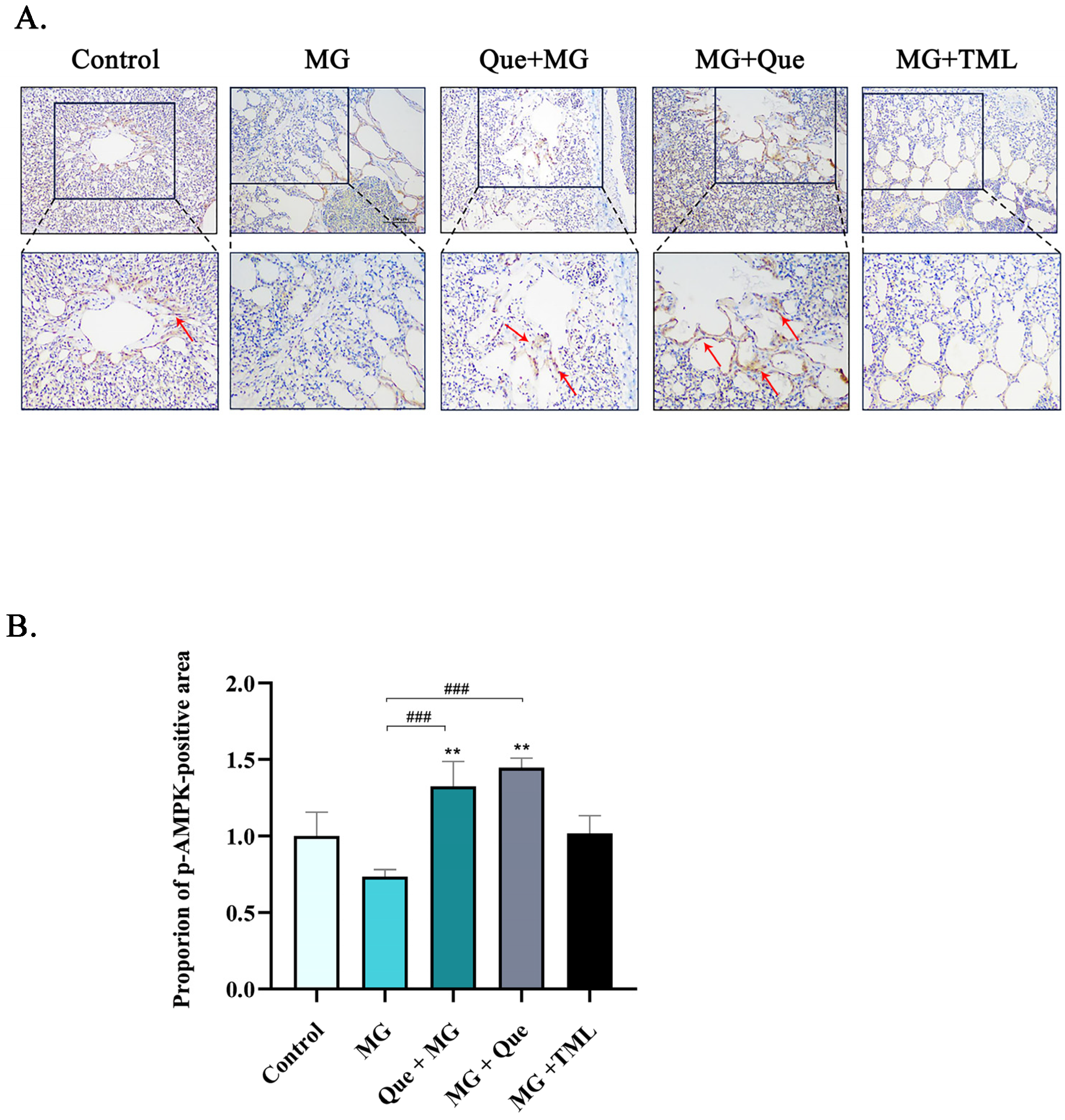
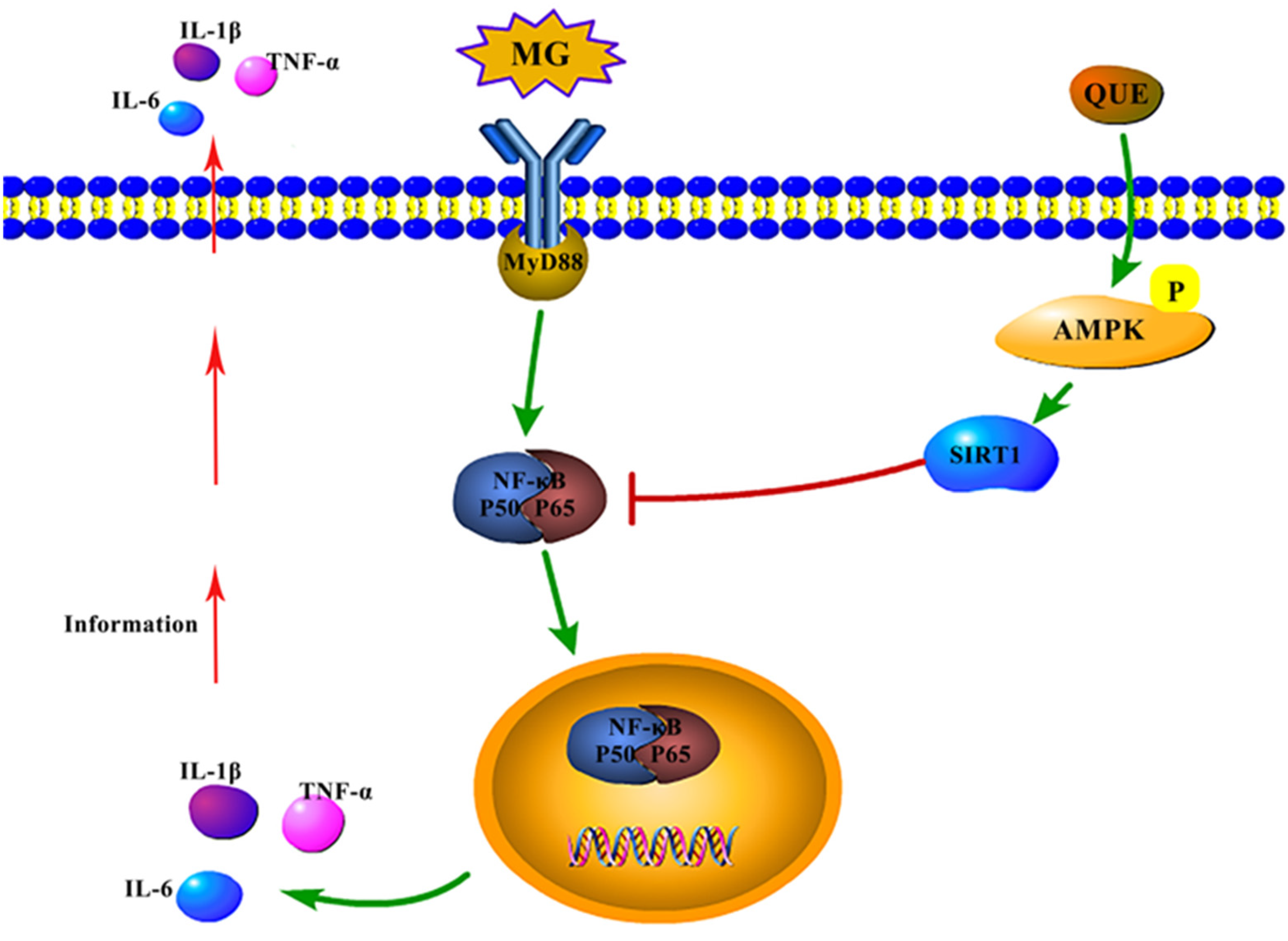
2.6. The Impact of Quercetin on the AMPK/SIRT1/NF-κB Pathway In Vivo and In Vitro under MG Infection
3. Discussion
4. Materials and Methods
4.1. Construction of the 3D Structure of AMPK-γ Proteins
4.2. Molecular Docking
4.3. Molecular Dynamic Simulations
4.4. Mycoplasma Strains and Culture Conditions
4.5. Cell Culture and Treatment
4.6. RNAi Transfection
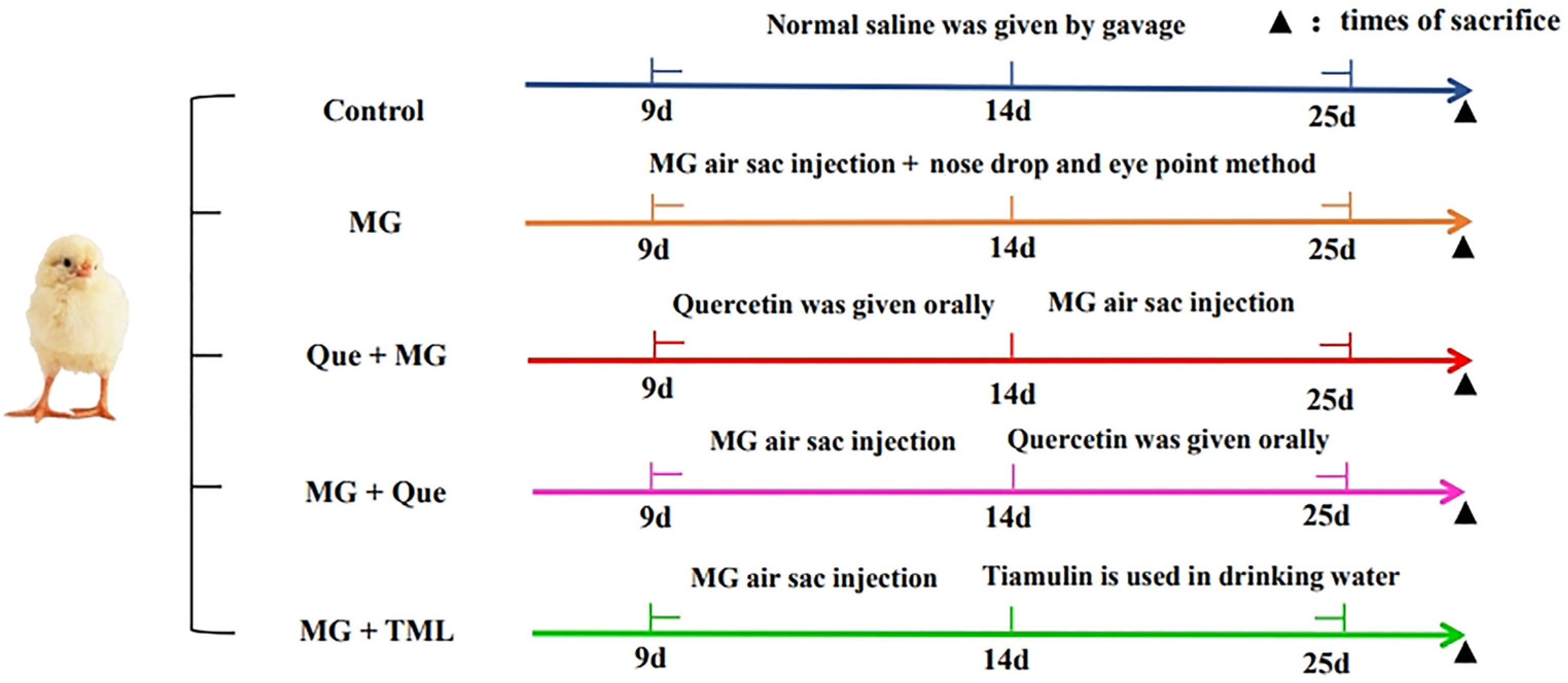
4.7. Experimental Chickens and Treatments
4.8. ELISA Assay
4.9. Western Blot
4.10. HE Staining
4.11. Immunohistochemistry
4.12. Ethical Statement
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthew, J.B. The Ring-Necked Pheasant (Phasianus colchicus) Industry within the United Kingdom and the Threat Posed by Mycoplasma gallisepticum: A Review. Vet. Sci. 2022, 9, 391. [Google Scholar]
- Jerstad, A.C.; Hamilton, C.M.; Smith, V.E. The Clinical Course of Infectious Sinusitis in Experimentally Infected Turkeys. Avian Dis. 1959, 3, 114–122. [Google Scholar] [CrossRef]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine 2006, 24, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Levisohn, S.; Kleven, S.H. Avian mycoplasmosis (Mycoplasma gallisepticum). Rev. Sci. Tech. (Int. Off. Epizoot.) 2000, 19, 425–442. [Google Scholar] [CrossRef]
- Kleven, S.H. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult. Sci. 1998, 77, 1146–1149. [Google Scholar] [CrossRef]
- Glisson, J.R.; Kleven, S.H. Mycoplasma gallisepticum Vaccination: Effects on Egg Transmission and Egg production. Avian Dis. 1984, 28, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Liao, Y.-F.; Du, Q. Research and application of quercetin-loaded nano drug delivery system. China J. Chin. Mater. Med. 2018, 43, 1978–1984. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Juan, S.-H.; Shen, S.-C.; Hsu, F.-L.; Chen, Y.-C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phos-phatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappaB-induced inflammatory mediators production in RAW 264.7cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian. Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Ok-Joo, S.; Seung, W.R. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, N.; Guo, X.; Niu, X.; Zhao, Z.; Wang, W.; Xie, X.; Lv, P. Dl-3-n-butylphthalide promotes remyelination and suppresses inflammation by regulating AMPK/SIRT1 and STAT3/NF-κB signaling in chronic cerebral hypoperfusion. Front. Aging Neurosci. 2020, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L.; Masi, S.; Ambrosini, S.; Mohammed, S.A.; Sciarretta, S.; Lüscher, T.F.; Paneni, F.; Costantino, S.; et al. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Zhou, T.; Shen, Q.; Qin, X. Exendin-4 promotes the vascular smooth muscle cell re-differentiation through AMPK/SIRT1/FOXO3a signaling pathways. Atherosclerosis 2018, 276, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lan, Z. Polydatin attenuates potassium oxonateinduced hyperuricemia and kidney inflammation by inhibiting NF-kappaB/NLRP3 inflammasome activation via the AMPK/ SIRT1 pathway. Food Funct. 2017, 8, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.; Godugu, C. Nimbolide ameliorates pancreatic inflammation and apoptosis by modulating NF-kappaB/ SIRT1 and apoptosis signaling in acute pancreatitis model. Int. Immunopharmacol. 2021, 90, 107246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Chen, R.R.; Li, J.H.; Chen, J.Y.; Li, W.; Niu, X.L.; Wang, F.F.; Wang, J.; Yang, J.X. Puerarin protects against myocardial ischemia/reperfusion damage by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 2020, 89, 107086. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zhang, Z.; Chi, Q.; Liu, Y.; Yang, L.; Xu, K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-κB/SIRT1/AMPK signaling pathways. Phytomedicine 2020, 78, 153305. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, S.; Wei, X.; Zhang, M.; Chen, Y.; Mao, X.; Chen, G.; Liu, C. Short-term moderate caloric restriction in a high-fat diet alleviates obesity via AMPK/SIRT1 signaling in white adipocytes and liver. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Shailendra, G.; Narendra, N.; Brian, S.; Benoit, V.; Avtar, K.S. Inderjit Singh. 5-Aminoimidazole-4-Carboxamide-1-beta-4-Ribofuranoside Inhibits Proinflammatory Response in Glial Cells: A Possible Role of AMP-Activated Protein Kinase. J. Neurosci. Jan. 2004, 24, 479–487. [Google Scholar]
- Bepari, A.K.; Reza, H.M. Identification of a novel inhibitor of SARS-CoV-2 3CL-PRO through virtual screening and molecular dynamics simulation. Peer J. 2021, 9, e11261. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Stefani, E.; Tondo, A.; Righetti, V.; Bottinelli, M.; Gavazzi, L.; Merenda, M.; Catania, S. Isolation and characterization of an atypical Mycoplasma gallisepticum strain showing a new mgc2 variant. Veter. Microbiol. 2023, 282, 109768. [Google Scholar] [CrossRef] [PubMed]
- Mahmmoud, E.N.; Hamad, M.A.; Khudhur, Z.N. Mycoplasma gallisepticum Detection of in broiler chickens by PCR. Open Vet. J. 2022, 12, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Marouf, S.; Khalf, M.A.; Alorabi, M.; El-Shehawi, A.M.; El-Tahan, A.M. Mycoplasma gallisepticum: A devastating organism for the poultry in-dustry in Egypt. Poult. Sci. 2022, 101, 101658. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Kathayat, D.; Ghanem, M.; Jung, K.; Closs, G.; Deblais, L.; Srivastava, V.; El-Gazzar, M.; Rajashekara, G. Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Veter. Microbiol. 2020, 247, 108799. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of querce-tin-magnesium complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, F.L.; Mao, Y.J.; Ying, L.L.; Zhou, B.; Li, Y. Quercetin decreases the triglyceride content through the PPAR signalling pathway in primary hepatocytes of broiler chickens. Biotechnol. Biotechnol. Equi. 2019, 33, 1000–1010. [Google Scholar] [CrossRef]
- Han, C.Y.; Tabassum, C.M.; Yao, J.Y.; Wang, M.; Yang, J.X.; Wang, S.N.; Zhou, B.; Li, Y. Effects of quercetin on performance and lipid metabolism in laying hens. J. Northeast Agric. Univ. (Engl. Ed.) 2017, 24, 29–36. [Google Scholar]
- Hsu, C.-C.; Peng, D.; Cai, Z.; Lin, H.-K. AMPK signaling and its targeting in cancer progression and treatment. Semin. Cancer Biol. 2022, 85, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1–AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, M.Q.; Ni, S.H.; Wang, M.; Liu, L.Y.; You, H.Y.; Wu, X.H.; Wang, Y.J.; Lu, L.; Wei, L.B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020, 867, 172797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung damage via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.S.; Gallo, E.S.; Borenstein, R. Multifaceted Role of AMPK in Viral Infections. Cells 2021, 10, 1118. [Google Scholar] [CrossRef]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Sang, A.; Wang, Y.; Wang, S.; Wang, Q.; Wang, X.; Li, X.; Song, X. Quercetin attenuates sepsis-induced acute lung damage via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell Signal 2022, 96, 110363. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, A.J.; Diks, S.H.; van der Poll, T.; Peppelenbosch, M.P.; Wieland, C.W. Kinase Activity Profiling of Pneumococcal Pneumonia. PLoS ONE 2011, 6, e18519. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.X.; Xiao, J.S.; Guang, X.Z.; Jing, M.; Babak, B.; Xu, Q. Local delivery of simvastatin maintains tooth anchorage during mechanical tooth moving via antiinflammation property and AMPK/MAPK/NF-kB inhibition. J. Cell Mol. Med. 2020, 25, 333–344. [Google Scholar]
- Wang, Y.; Yang, Z.; Yang, L.; Zou, Q.; Zhao, S.; Hu, N.; Chen, D.; Cui, R.; Ma, H. Liuweidihuang pill alleviates inflammation of the testis via AMPK/SIRT1/NF-kappaB pathway in aging rats. Evid. Based Complement. Altern. Med. 2020, 2020, 2792738. [Google Scholar]
- Yong, H.W.; Quan, L.; Ping, L.; Bei, L. GSK621 activates AMPK signaling to inhibit LPS-induced TNFα production. Bio. Chem. Biophys. Res. Commun. 2016, 480, 289–295. [Google Scholar]
- Wang, S.; Jin, X.; Chen, H.; Han, M.; Bao, J.; Niu, D.; Wang, Y.; Li, R.; Wu, Z.; Li, J. Quercetin alleviates Mycoplasma gallisepticum-induced inflammatory damage and oxidative stress through inhibition of TLR2/MyD88/NF-κB pathway in vivo and in vitro. Microb. Pathog. 2023, 176, 106006. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 2000, 54, 960–963. [Google Scholar] [CrossRef]
- Carullo, G.; Spizzirri, U.G.; Malivindi, R.; Rago, V.; Motta, M.F.; Lofaro, D.; Restuccia, D.; Aiello, F. Development of Quercetin-DHA Ester-Based Pectin Conjugates as New Functional Supplement: Effects on Cell Viability and Migration. Nutraceuticals 2022, 2, 278–288. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Wang, H.Z.; Lu, Z.Y.; Li, Y.; Liu, T.; Zhao, L.L.; Gao, T.Q.; Lu, X.L.; Gao, B. Virtual Screening of Novel 24-Dehydroxysterol Reductase (DHCR24) Inhibitors and the Biological Evaluation of Irbesartan in Cholesterol-Lowering Effect. Molecules 2023, 28, 2643. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Xie, D.Y.; Chen, Y.; Tian, E.J.; Muhammad, I.; Chen, X.P.; Miao, Y.S.; Hu, W.J.; Wu, Z.; Ni, H.; et al. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum -infected RAW264. 7 cells. Mol. Immunol. 2017, 87, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Zhang, W.; Liu, Y.; Wang, J.; Wu, Z.; Shan, S.W.A.; Li, R.; Miao, Y.; Chen, C.; Li, J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in chicken bursa of fabricius through modulation of autophagy and inhibited inflammation and apoptosis. J. Sci. Food Agric. 2021, 101, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Wang, H.Z.; Zhang, X.J.; Huang, X.T.; Jiang, S.; Li, Y.; Liu, T.; Lu, X.L.; Gao, B. High fat diet induces brain injury and neuronal apoptosis via down-regulating 3-β hydroxycholesterol 24 reductase (DHCR24). Cell Tissue Res. 2023, 393, 471–487. [Google Scholar] [CrossRef]



| Complexes (Kcal/mol) | ΔGtotal | ΔGvdw | ΔGele | ΔGgas | ΔGpolar | ΔGnonpolar | ΔGsolv |
|---|---|---|---|---|---|---|---|
| Que-AMPK | −53.216 | −136.123 | −63.101 | 199.224 | 166.718 | −20.710 | 146.008 |
| ZMP-AMPK | −4.545 | −128.428 | −185.410 | −313.838 | 331.144 | −21.852 | 309.292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wang, H.; Ishfaq, M.; Han, Y.; Zhang, X.; Li, X.; Wang, B.; Lu, X.; Gao, B. Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway. Molecules 2023, 28, 7388. https://doi.org/10.3390/molecules28217388
Lu Z, Wang H, Ishfaq M, Han Y, Zhang X, Li X, Wang B, Lu X, Gao B. Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway. Molecules. 2023; 28(21):7388. https://doi.org/10.3390/molecules28217388
Chicago/Turabian StyleLu, Ziyin, Haozhen Wang, Muhammad Ishfaq, Yufang Han, Xiujin Zhang, Xiang Li, Baoqi Wang, Xiuli Lu, and Bing Gao. 2023. "Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway" Molecules 28, no. 21: 7388. https://doi.org/10.3390/molecules28217388
APA StyleLu, Z., Wang, H., Ishfaq, M., Han, Y., Zhang, X., Li, X., Wang, B., Lu, X., & Gao, B. (2023). Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway. Molecules, 28(21), 7388. https://doi.org/10.3390/molecules28217388






