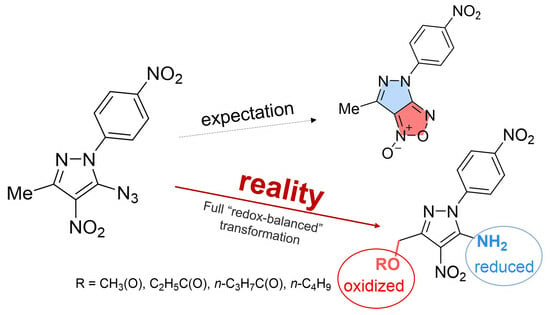
An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants
Abstract
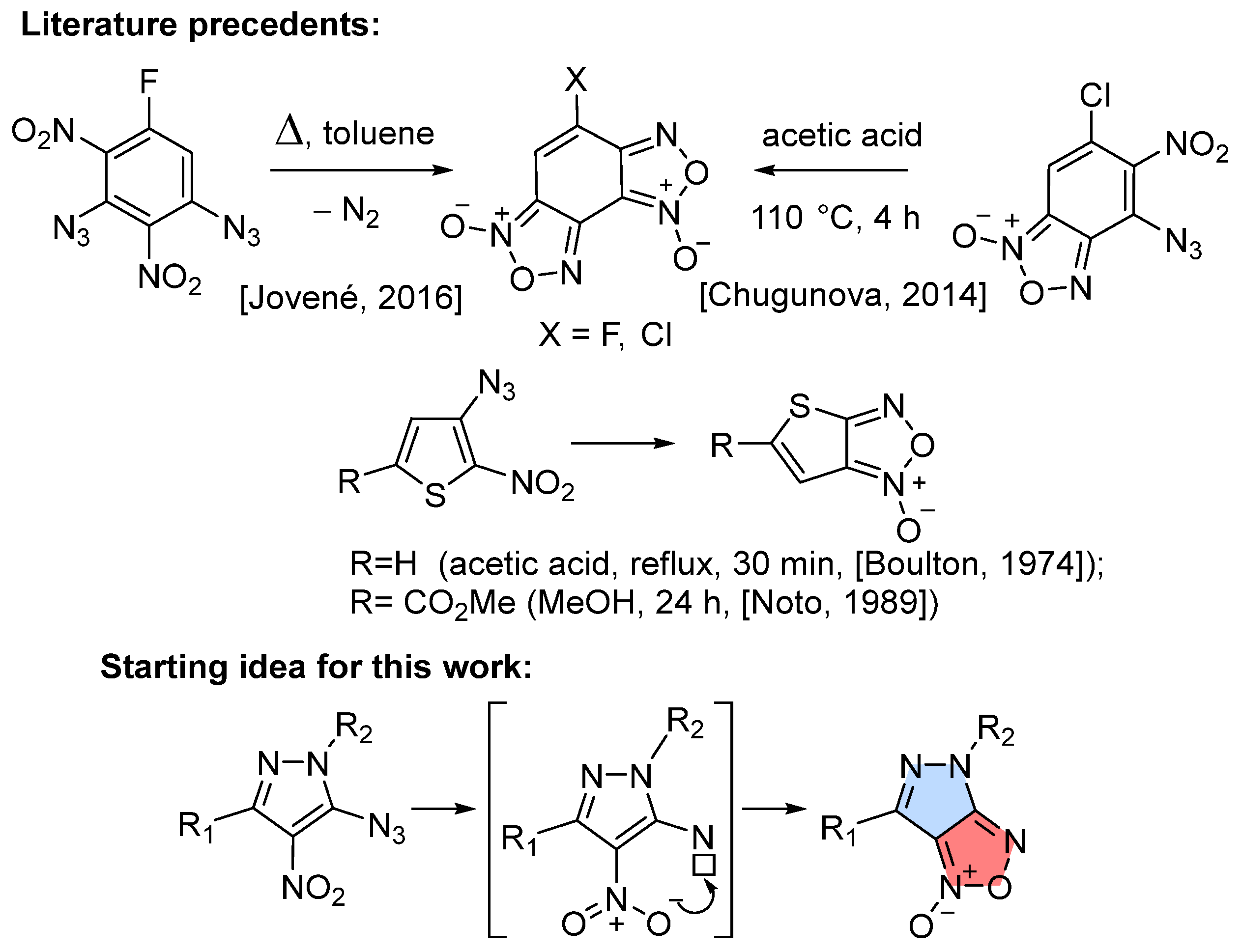
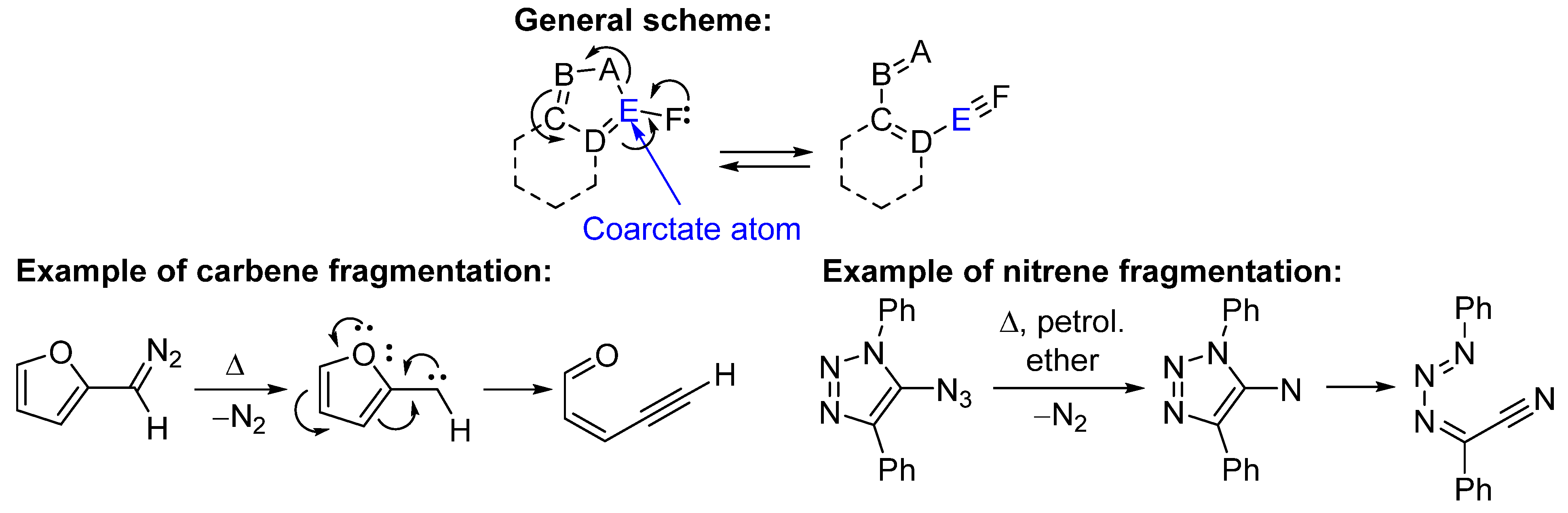
1. Introduction
2. Results and Discussion
3. Materials and Methods
Chemistry
- 5-Chloro-3-methyl-4-nitro-1-(4-nitrophenyl)-1H-pyrazole (3). Acetic anhydride (6 mL) was added to 5-chloro-3-methyl-1-phenyl-1H-pyrazole 2 (0.44 g, 2.3 mmol), the reaction mixture was cooled to 0 °C, and then fuming nitric acid (97–99%, 4 mL) was added dropwise. The reaction mixture was stirred at room temperature for 4 h and then poured over crushed ice. The obtained precipitate was filtered off, washed with cold water (100 mL), and dried under vacuum (0.06 mm Hg) at 40 °C to constant weight. The crude product was recrystallized from acetone to give the target compound. Yellow powder, yield 0.55 g (85%), m.p.: 148–150 °C. IR (ν, cm–1): 691, 787, 860, 1005, 1147, 1317, 1346 (NO2 symm), 1381, 1459, 1503, 1532 (NO2 asymm), 1553, 1596. 1H NMR (500 MHz, CDCl3): δ = 8.41 (d, J = 8.6 Hz, 2H), 7.83 (d, J = 8.6 Hz, 2H), 2.64 (s, 3H). 13C NMR (126 MHz, CDCl3): δ = 148.7, 147.9, 141.7, 131.2, 128.2, 126.0, 125.0, 14.7. Anal. calcd (%) for C10H7ClN4O4: C, 42.50; H, 2.50; Cl, 12.54; N, 19.82. Found: C, 42.54; H, 2.48; Cl, 12.53; N, 19.85.
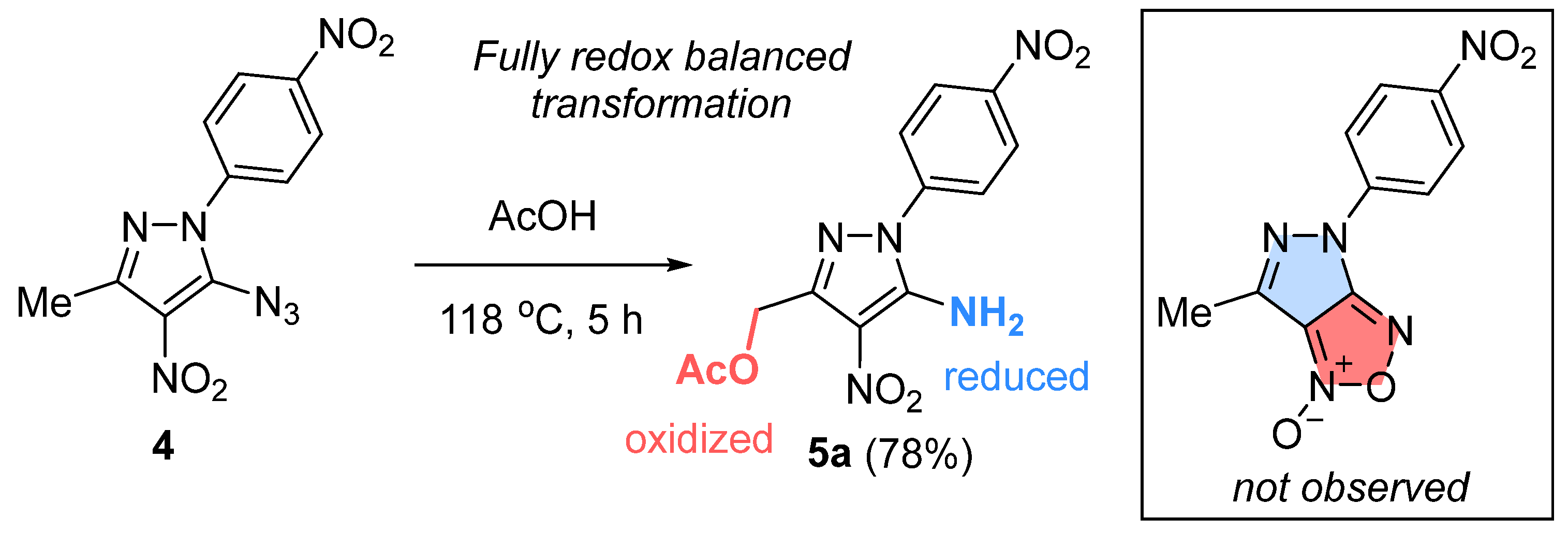
- 5-Azido-3-methyl-4-nitro-1-(4-nitrophenyl)-1H-pyrazole (4). To a solution of 5-chloro-3-methyl-4-nitro-1-(4-nitrophenyl)-1H-pyrazole 3 (0.50 g, 1.8 mmol) in acetone (5 mL) at room temperature was added a solution of sodium azide (0.15 g, 2.3 mmol) in 1 mL of water. The reaction mixture was stirred for 1 h (the reaction was monitored by thin-layer chromatography; eluent: toluene–ethylacetate (2:1, v/v)). After completion of the reaction, the solvent was removed under reduced pressure, washed with cold water, and dried in vacuum (0.06 mm Hg) at 40 °C to constant weight. Light brown powder, yield 0.45 g (86%), Rf 0.31, m.p.: 104–106 °C. IR (ν, cm–1): 690, 751, 822, 857, 1347 (NO2 symm), 1382, 1418, 1439, 1557 (NO2 asymm), 1561, 2152 (N3). 1H NMR (500 MHz, Acetone-d6): δ = 8.40–8.43 (m, 2H), 8.07–8.11 (m, 2H), 2.55 (s, 3H). 13C NMR (101 MHz, Acetone-d6): δ = 147.9, 147.6, 142.5, 138.5, 126.2, 125.2, 125.1, 14.4. Anal. calcd (%) for C10H7N7O4: C, 41.53; H, 2.44; N, 33.90. Found: C, 41.58; H, 2.47; N, 33.87.
- 5-Amino-4-nitro-1-(4-nitrophenyl)-1H-pyrazol-3-yl)methyl acetate (5a). Gray pearlescent solid (0.08 g) was obtained in 78% yield. Rf 0.17, m.p.: 198–199 °C. IR (ν, cm–1): 820, 863, 1032, 1253, 1346 (NO2 symm), 1464, 1520, 1599 (NO2 asymm), 1637 (CO), 1721 (C=O), 3293, 3408 (NH2). 1H NMR (400 MHz, Acetone-d6): δ = 8.46 (d, J = 9.0 Hz, 2H), 7.99 (d, J = 9.0 Hz, 2H), 7.35 (br.s, 2H), 5.33 (s, 2H), 2.08 (s, 3H). 13C NMR (101 MHz, Acetone-d6): δ = 169.6, 147.1, 144.7, 142.3, 125.1, 124.9, 116.9, 58.7, 19.6. Anal. calcd (%) for C12H11N5O6: C, 44.87; H, 3.45; N, 21.80. Found: C, 44.83; H, 3.52; N, 21.85. ESI, m/z for C12H11N5O6: 319.99 [M − H]−.
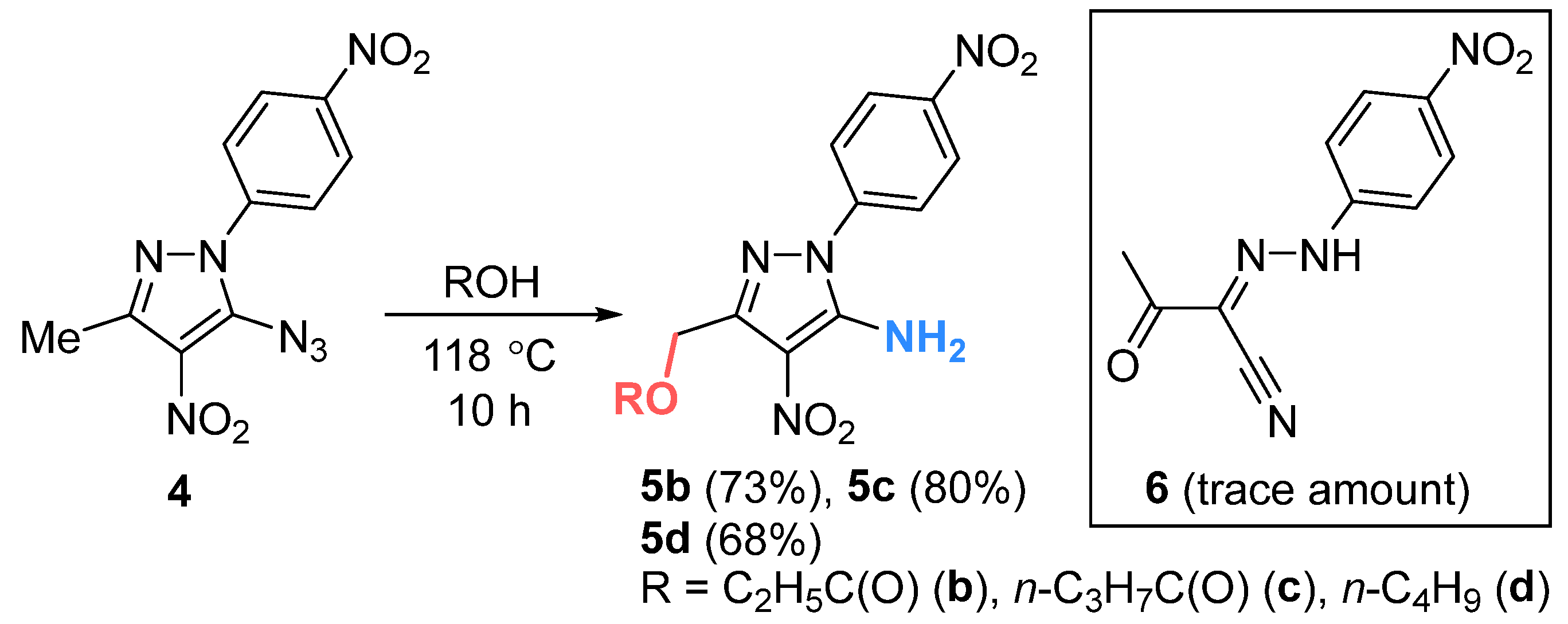
- (5-Amino-4-nitro-1-(4-nitrophenyl)-1H-pyrazol-3-yl)methyl propionate (5b). Orange oil, yield 0.085 g (73%). Rf 0.15. IR (ν, cm–1): 694, 753, 819, 860, 1099, 1182, 1291, 1347 (NO2 symm), 1460, 1526, 1598 (NO2 asymm), 1635 (CO), 1708, 1738 (C=O), 3328, 3430 (NH2). 1H NMR (400 MHz, Acetone-d6): δ = 8.38–8.42 (m, 2H), 7.91–7.97 (m, 2H), 7.36 (br.s, 2H), 5.31 (s, 2H), 2.38 (q, J = 7.6 Hz, 2H), 1.11 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, Acetone-d6): δ = 173.9, 147.9, 145.7, 143.1, 125.9(4), 125.9(1), 125.6, 117.7, 59.5, 27.7, 9.4. Anal. calcd (%) for C13H13N5O6: C, 46.57; H, 3.91; N, 20.89. Found: C, 46.72; H, 4.02; N, 20.92. ESI, m/z for C13H13N5O6: 334.04 [M − H]−.
- (5-Amino-4-nitro-1-(4-nitrophenyl)-1H-pyrazol-3-yl)methyl butyrate (5c). Orange oil, yield 0.096 g (80%). Rf 0.27. IR (ν, cm–1): 753, 769, 821, 860, 1012, 1110, 1178, 1290, 1347 (NO2 symm), 1459, 1526, 1598 (NO2 asymm), 1634 (CO), 1705, 1734 (C=O), 3324, 3434 (NH2). 1H NMR (600 MHz, Acetone-d6): δ = 8.42–8.48 (m, 2H), 7.95–8.01 (m, 2H), 7.36 (br.s, 2H), 5.33 (s, 2H), 2.35 (t, J = 7.4 Hz, 2H), 1.65 (q, J = 7.4 Hz, 2H), 0.95 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, Acetone-d6): δ = 173.1, 148.0, 147.9, 145.7, 143.2, 125.9(5), 125.8(9), 125.7, 59.4, 36.3, 19.1, 13.8. Anal. calcd (%) for C14H15N5O6: C, 48.14; H, 4.33; N, 20.05. Found: C, 48.20; H, 4.37; N, 20.01. ESI, m/z for C14H15N5O6: 348.05 [M − H]−.
- 3-(Butoxymethyl)-4-nitro-1-(4-nitrophenyl)-1H-pyrazol-5-amine (5d). Orange oil, yield 0.075 g (68%). Rf 0.30. IR (ν, cm–1): 694, 753, 770, 820, 860, 1013, 1111, 1172, 1290, 1346 (NO2 symm), 1457, 1525, 1598 (NO2 asymm), 1634 (CO), 1702 (C=O), 3330, 3430 (NH2). 1H NMR (400 MHz, Acetone-d6): δ = 8.41–8.45 (m, 2H), 7.94–7.99 (m, 2H), 7.29 (br.s, 2H), 4.68 (s, 2H), 3.59 (t, J = 6.5 Hz, 2H), 1.62–1.53 (m, 2H), 1.48–1.32 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, Acetone-d6): δ = 147.9, 147.7(4), 147.6(8), 143.4, 125.9, 125.6, 117.9, 71.3, 65.9, 32.5, 19.9, 14.1. Anal. calcd (%) for C14H17N5O5: C, 50.15; H, 5.11; N, 20.89. Found: C, 50.23; H, 5.17; N, 20.82. ESI,) m/z for C14H17N5O5: 334.08 [M − H]−.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.J.; Shimazumi, R.; Driscoll, J.L.; Dherange, B.D.; Park, D.-I.; Levin, M.D. Aromatic nitrogen scanning by ipso-selective nitrene internalization. Science 2023, 381, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.H. Reduction of Organic Azides to Primary Amines with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1951, 73, 5865–5866. [Google Scholar] [CrossRef]
- Rolla, F. Sodium borohydride reactions under phase-transfer conditions: Reduction of azides to amines. J. Org. Chem. 1982, 47, 4327–4329. [Google Scholar] [CrossRef]
- Ranu, B.C.; Sarkar, A.; Chakraborty, R. Reduction of Azides with Zinc Borohydride. J. Org. Chem. 1994, 59, 4114–4116. [Google Scholar] [CrossRef]
- Becher, J.; Pluta, K.; Krake, N.; Brøndum, K.; Christensen, N.J.; Vinader, M.V. Syntheses of o-Aminohetarenecarbaldehydes via Azides. Synthesis 1989, 1989, 530–533. [Google Scholar] [CrossRef]
- Kale, A.; Medishetti, N.; Kanugala, S.; Kumar C, G.; Atmakur, K. Na2S-promoted reduction of azides in water: Synthesis of pyrazolopyridines in one pot and evaluation of antimicrobial activity. Org. Biomol. Chem. 2019, 17, 3186–3194. [Google Scholar] [CrossRef]
- Zelenay, B.; Besora, M.; Monasterio, Z.; Ventura-Espinosa, D.; White, A.J.P.; Maseras, F.; Díez-González, S. Copper-mediated reduction of azides under seemingly oxidising conditions: Catalytic and computational studies. Catal. Sci. Technol. 2018, 8, 5763–5773. [Google Scholar] [CrossRef]
- Staudinger, H.; Meyer, J. Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef]
- van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; van Delft, F.L. Organophosphorus-Catalysed Staudinger Reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Lenting, P.E.; Mecinović, J. Sustainable organophosphorus-catalysed Staudinger reduction. Green Chem. 2018, 20, 4418–4422. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Wolf, J.J.; Mecinović, J. Catalytic Staudinger Reduction at Room Temperature. J. Org. Chem. 2019, 84, 6536–6545. [Google Scholar] [CrossRef] [PubMed]
- Bakulev, V.A.; Shafran, Y.M.; Beliaev, N.A.; Beryozkina, T.V.; Volkova, N.N.; Joy, M.N.; Fan, Z. Heterocyclic azides: Advances in their chemistry. Russ. Chem. Rev. 2022, 91, RCR5042. [Google Scholar] [CrossRef]
- Tsyrenova, B.; Khrustalev, V.; Nenajdenko, V. 2 H -Bis-1,2,3-triazolo-isoquinoline: Design, Synthesis, and Photophysical Study. J. Org. Chem. 2020, 85, 7024–7035. [Google Scholar] [CrossRef]
- Tsyrenova, B.; Nenajdenko, V. Synthesis and Spectral Study of a New Family of 2,5-Diaryltriazoles Having Restricted Rotation of the 5-Aryl Substituent. Molecules 2020, 25, 480. [Google Scholar] [CrossRef]
- Shcherbakov, D.; Baev, D.; Kalinin, M.; Dalinger, A.; Chirkova, V.; Belenkaya, S.; Khvostov, A.; Krut’ko, D.; Medved’ko, A.; Volosnikova, E.; et al. Design and Evaluation of Bispidine-Based SARS-CoV-2 Main Protease Inhibitors. ACS Med. Chem. Lett. 2022, 13, 140–147. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, X.; Qian, X.; Zeng, T.; Sang, N.; Liu, H.; Zhou, Y.; Tao, L.; Zhou, X.; Su, N.; et al. Bifunctional Naphtho[2,3-d][1,2,3]triazole-4,9-dione Compounds Exhibit Antitumor Effects In Vitro and In Vivo by Inhibiting Dihydroorotate Dehydrogenase and Inducing Reactive Oxygen Species Production. J. Med. Chem. 2020, 63, 7633–7652. [Google Scholar] [CrossRef]
- Kitamura, M.; Sakata, R.; Tashiro, N.; Ikegami, A.; Okauchi, T. Synthesis of Diazonaphthoquinones from Naphthols by Diazo-Transfer Reaction. Bull. Chem. Soc. Jpn. 2015, 88, 824–833. [Google Scholar] [CrossRef]
- Chugunova, E.; Gazizov, A.; Islamov, D.; Burilov, A.; Tulesinova, A.; Kharlamov, S.; Syakaev, V.; Babaev, V.; Akylbekov, N.; Appazov, N.; et al. The Reactivity of Azidonitrobenzofuroxans towards 1,3-Dicarbonyl Compounds: Unexpected Formation of Amino Derivative via the Regitz Diazo Transfer and Tautomerism Study. Int. J. Mol. Sci. 2021, 22, 9646. [Google Scholar] [CrossRef]
- Tsyrenova, B.D.; Khrustalev, V.N.; Nenajdenko, V.G. Synthesis of blue light emitting heterocycles via cyclization of 2-pyridine derived 4-azido-r1,2,3-triazoles. Org. Biomol. Chem. 2021, 19, 8140–8152. [Google Scholar] [CrossRef]
- Ivanov, S.M.; Mironovich, L.M.; Rodinovskaya, L.A.; Shestopalov, A.M. The first stable examples of compounds containing both diazonium and acyl azide, and synthesis of a new pyrazino[2′,3′:3,4]pyrazolo[5,1-c][1,2,4]triazin-4(6H)-one heterocyclic system. Tetrahedron Lett. 2017, 58, 1851–1853. [Google Scholar] [CrossRef]
- Vil’, V.A.; Barsegyan, Y.A.; Kuhn, L.; Terent’ev, A.O.; Alabugin, I.V. Creating, Preserving, and Directing Carboxylate Radicals in Ni-Catalyzed C(sp3)–H Acyloxylation of Ethers, Ketones, and Alkanes with Diacyl Peroxides. Organometallics 2023, 42, 2598–2612. [Google Scholar] [CrossRef]
- Kuhn, L.; Vil’, V.A.; Barsegyan, Y.A.; Terent’ev, A.O.; Alabugin, I.V. Carboxylate as a Non-innocent L-Ligand: Computational and Experimental Search for Metal-Bound Carboxylate Radicals. Org. Lett. 2022, 24, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Li, X.; Tang, M.; Gao, B.; Huang, G. An Efficient Method for the α-Acetoxylation of Ketones. Synthesis 2007, 2007, 1165–1168. [Google Scholar] [CrossRef]
- Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. Iodobenzene-Catalyzed α-Acetoxylation of Ketones. In Situ Generation of Hypervalent (Diacyloxyiodo)benzenes Using m-Chloroperbenzoic Acid. J. Am. Chem. Soc. 2005, 127, 12244–12245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, F.; Yao, L.; Shi, T.; Tang, B.; Kuninobu, Y.; Wang, Z. C–N and C–O Bond Formation in Copper-Catalyzed/Mediated sp3 C–H Activation: Mechanistic Studies from Experimental and Computational Aspects. J. Org. Chem. 2020, 85, 9713–9726. [Google Scholar] [CrossRef]
- Wang, Z.; Kuninobu, Y.; Kanai, M. Copper-Mediated Direct C(sp3)–H and C(sp2)–H Acetoxylation. Org. Lett. 2014, 16, 4790–4793. [Google Scholar] [CrossRef]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. A Highly Selective Catalytic Method for the Oxidative Functionalization of C−H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef]
- Desai, L.V.; Hull, K.L.; Sanford, M.S. Palladium-Catalyzed Oxygenation of Unactivated sp3 C−H Bonds. J. Am. Chem. Soc. 2004, 126, 9542–9543. [Google Scholar] [CrossRef]
- Wang, D.-H.; Hao, X.-S.; Wu, D.-F.; Yu, J.-Q. Palladium-Catalyzed Oxidation of Boc-Protected N-Methylamines with IOAc as the Oxidant: A Boc-Directed sp3 C−H Bond Activation. Org. Lett. 2006, 8, 3387–3390. [Google Scholar] [CrossRef]
- Desai, L.V.; Malik, H.A.; Sanford, M.S. Oxone as an Inexpensive, Safe, and Environmentally Benign Oxidant for C−H Bond Oxygenation. Org. Lett. 2006, 8, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khaskin, E.; Anderson, N.P.; Zavalij, P.Y.; Vedernikov, A.N. Catalytic aerobic oxidation of substituted 8-methylquinolines in PdII-2,6-pyridinedicarboxylic acid systems. Chem. Commun. 2008, 31, 3625–3627. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Lee, M.; Dunn, A.L.; Sanford, M.S. Palladium-Catalyzed C–H Bond Acetoxylation via Electrochemical Oxidation. Org. Lett. 2018, 20, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Kumar, S.; Kumar, N.; Roy, P. Synthesis anti-inflammatory and anticancer activity evaluation of some pyrazole and oxadiazole derivatives. Med. Chem. Res. 2012, 21, 3043–3052. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Liu, J.-C.; Zhang, W.; Li, Y.-R.; Li, C.; Zheng, C.-J.; Piao, H.-R. Synthesis and Antimicrobial Evaluation of (Z)-5-((3-phenyl-1H-pyrazol-4-yl)methylene)-2-thioxothiazolidin-4-one Derivatives. Med. Chem. 2016, 12, 751–759. [Google Scholar] [CrossRef]
- Chandrakantha, B.; Isloor, A.M.; Shetty, P.; Isloor, S.; Malladi, S.; Fun, H.K. Synthesis, characterization and antimicrobial activity of novel ethyl 1-(N-substituted)-5-phenyl-1H-pyrazole-4-carboxylate derivatives. Med. Chem. Res. 2012, 21, 2702–2708. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Shamsuzzaman Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; El Rhayam, Y.; Karrouchi, K.; Cherrah, Y.; Tarib, A.; Ansar, M.; Faouzi, M.E.A. Identification of the new progress on Pyrazole Derivatives Molecules as Antimicrobial and Antifungal Agents. West Afr. J. Med. 2022, 39, 1217–1244. [Google Scholar]
- Pasin, J.S.M.; Ferreira, A.P.O.; Saraiva, A.L.L.; Ratzlaff, V.; Andrighetto, R.; Machado, P.; Marchesan, S.; Zanette, R.A.; Bonacorso, H.G.; Zanatta, N.; et al. Antipyretic and antioxidant activities of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles in rats. Braz. J. Med. Biol. Res. 2010, 43, 1193–1202. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Saad, H.A.; Osman, N.A.; Moustafa, A.H. Synthesis and Analgesic Activity of Some New Pyrazoles and Triazoles Bearing a 6,8-Dibromo-2-methylquinazoline Moiety. Molecules 2011, 16, 10187–10201. [Google Scholar] [CrossRef]
- dos Santos Fernandes, G.F.; de Souza, P.C.; Marino, L.B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R.; Chung, M.C.; Pavan, F.R.; dos Santos, J.L. Synthesis and biological activity of furoxan derivatives against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2016, 123, 523–531. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Z.-W.; Ling, Y.; He, L.-Q.; Huang, P.; Gu, H.-X.; Hu, R.-F. Design, synthesis and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med. Chem. Res. 2018, 27, 1198–1205. [Google Scholar] [CrossRef]
- Abdelall, E.K.A. Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg. Chem. 2020, 94, 103441. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Ritter, H.; Licht, H.H. Synthesis and reactions of dinitrated amino and diaminopyridines. J. Heterocycl. Chem. 1995, 32, 585–590. [Google Scholar] [CrossRef]
- Fogel’zang, A.E.; Egorshev, V.Y.; Sinditskii, V.P.; Dutov, M.D. Organic azide structure and combustion trends. Combust. Explos. Shock Waves 1990, 26, 558–564. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Yao, Q. Synthesis and quantitative structure–activity relationship (QSAR) analysis of some novel oxadiazolo[3,4-d]pyrimidine nucleosides derivatives as antiviral agents. Bioorg. Med. Chem. Lett. 2015, 25, 241–244. [Google Scholar] [CrossRef]
- Jovené, C.; Jacquet, M.; Chugunova, E.A.; Kharlamov, S.V.; Goumont, R. Synthesis and 1-oxide/3-oxide interconversion of 4-substituted benzodifuroxans: A thorough NMR and theoretical study of the structure of 4-fluoro- and 4-chloro-benzodifuroxan. Tetrahedron 2016, 72, 2057–2063. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Voloshina, A.D.; Mukhamatdinova, R.E.; Serkov, I.V.; Proshin, A.N.; Gibadullina, E.M.; Burilov, A.R.; Kulik, N.V.; Zobov, V.V.; Krivolapov, D.B.; et al. The Study of the Biological Activity of Amino-Substituted Benzofuroxans. Lett. Drug Des. Discov. 2014, 11, 502–512. [Google Scholar] [CrossRef]
- Boulton, A.J.; Middleton, D. Furazans and furazan oxides. V. Tropono[4,5-c]-, thieno[2,3-c]-, and biphenyleno[2,3-c]furazan oxides. J. Org. Chem. 1974, 39, 2956–2962. [Google Scholar] [CrossRef]
- Noto, R.; Rainieri, R.; Arnone, C. Effect of the nature of the starting aromatic ring on the cyclization of o-nitroaryl azides: Kinetic and thermodynamic studies of the conversion of two azido(methoxycarbonyl)nitrothiophenes into methoxycarbonylthienofurazan oxides. J. Chem. Soc. Perkin Trans. 2 1989, 2, 127–130. [Google Scholar] [CrossRef]
- Fidalgo, M.; Ricardo Pires, J.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke—Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: A new hope for deadly amyotrophic lateral sclerosis. Drugs Today 2018, 54, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.K.; Chakka, S.K.; Scovell, I.; Neschadim, A.; Bello, A.M.; Salum, N.; Katsman, Y.; Bareau, M.C.; Branch, D.R.; Kotra, L.P. Structure–activity relationships of pyrazole derivatives as potential therapeutics for immune thrombocytopenias. Bioorg. Med. Chem. 2014, 22, 2739–2752. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Timasheva, R.E.; Gibadullina, E.M.; Burilov, A.R.; Goumont, R. First Synthesis of Benzotrifuroxan at Low Temperature: Unexpected Behavior of 5,7-Dichloro-4,6-dinitrobenzo-furoxan with Sodium Azide. Propellants Explos. Pyrotech. 2012, 37, 390–392. [Google Scholar] [CrossRef]
- dos Santos, M.S.; Oliveira, M.L.V.; Bernardino, A.M.R.; de Léo, R.M.; Amaral, V.F.; de Carvalho, F.T.; Leon, L.L.; Canto-Cavalheiro, M.M. Synthesis and antileishmanial evaluation of 1-aryl-4-(4,5-dihydro-1H-imidazol-2-yl)-1H-pyrazole derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 7451–7454. [Google Scholar] [CrossRef]
- Hassaneen, H.M.E.; Hassaneen, H.M.; Elnagdi, M.H. Enamines in Heterocyclic Synthesis: A Route to 4-Substituted Pyrazoles and Condensed Pyrazoles. Z. Naturforschung B 2004, 59, 1132–1136. [Google Scholar] [CrossRef]
- Kvaskoff, D.; Lüerssen, H.; Bednarek, P.; Wentrup, C. Phenylnitrene, phenylcarbene, and pyridylcarbenes. Rearrangements to cyanocyclopentadiene and fulvenallene. J. Am. Chem. Soc. 2014, 136, 15203–15214. [Google Scholar] [CrossRef]
- Sankaranarayanan, J.; Rajam, S.; Hadad, C.M.; Gudmundsdottir, A.D. The ability of triplet nitrenes to abstract hydrogen atoms. J. Phys. Org. Chem. 2010, 23, 370–375. [Google Scholar] [CrossRef]
- Voskresenska, V.; Wilson, R.M.; Panov, M.; Tarnovsky, A.N.; Krause, J.A.; Vyas, S.; Winter, A.H.; Hadad, C.M. Photoaffinity Labeling via Nitrenium Ion Chemistry: Protonation of the Nitrene Derived from 4-Amino-3-nitrophenyl Azide to Afford Reactive Nitrenium Ion Pairs. J. Am. Chem. Soc. 2009, 131, 11535–11547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falvey, D.E.; Gudmundsdottir, A.D. Nitrenes and Nitrenium Ions; Wiley Series of Reactive Intermediates in Chemistry and Biology; Wiley: Hoboken, NJ, USA, 2013; ISBN 9780470390597. [Google Scholar]
- Carra, C.; Bally, T.; Albini, A. Role of conformation and electronic structure in the chemistry of ground and excited state o-pyrazolylphenylnitrenes. J. Am. Chem. Soc. 2005, 127, 5552–5562. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bettinetti, G.; Minoli, G. Photodecomposition of Some Para-Substituted 2-Pyrazolylphenyl Azides. Substituents Affect the Phenylnitrene S−T Gap More Than the Barrier to Ring Expansion. J. Am. Chem. Soc. 1999, 121, 3104–3113. [Google Scholar] [CrossRef]
- Tomioka, H.; Ichikawa, N.; Komatsu, K. Photochemistry of 2-(methoxycarbonyl)phenyl azide studied by matrix-isolation spectroscopy. A new slippery energy surface for phenylnitrene. J. Am. Chem. Soc. 1993, 115, 8621–8626. [Google Scholar] [CrossRef]
- Winkler, M. Singlet−Triplet Energy Splitting and Excited States of Phenylnitrene. J. Phys. Chem. A 2008, 112, 8649–8653. [Google Scholar] [CrossRef]
- McCulla, R.D.; Burdzinski, G.; Platz, M.S. Ultrafast Study of the Photochemistry of 2-Azidonitrobenzene. Org. Lett. 2006, 8, 1637–1640. [Google Scholar] [CrossRef]
- Becher, J.; Brøndum, K.; Krake, N.; Pluta, K.; Simonsen, O.; Molina, P.; Begtrup, M. An unexpected ring opening–ring closure reaction of 5-azido-4-formylpyrazole. J. Chem. Soc. Chem. Commun. 1988, 8, 541–542. [Google Scholar] [CrossRef]
- Dehaen, W.; Becher, J. Synthesis of 5-amino-4-cyanopyrazoles via ring opening-ring closure of 5-azido-4-iminomethylpyrazoles isolation of the intermediate. Tetrahedron Lett. 1991, 32, 3565–3568. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Bresch, S.; Dos Passos Gomes, G. Orbital hybridization: A key electronic factor in control of structure and reactivity. J. Phys. Org. Chem. 2015, 28, 147–162. [Google Scholar] [CrossRef]
- Juaristi, E.; dos Passos Gomes, G.; Terent’ev, A.O.; Notario, R.; Alabugin, I.V. Stereoelectronic Interactions as a Probe for the Existence of the Intramolecular α-Effect. J. Am. Chem. Soc. 2017, 139, 10799–10813. [Google Scholar] [CrossRef]
- Nigst, T.A.; Antipova, A.; Mayr, H. Nucleophilic Reactivities of Hydrazines and Amines: The Futile Search for the α-Effect in Hydrazine Reactivities. J. Org. Chem. 2012, 77, 8142–8155. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent′ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef] [PubMed]
- Herges, R. Coarctate and Pseudocoarctate Reactions: Stereochemical Rules. J. Org. Chem. 2015, 80, 11869–11876. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliff, L.D.; McClintock, S.P.; Haley, M.M. Reactions in the conjugated “ene-ene-yne” manifold: Five-membered ring fragmentation and ring formation via coarctate/pseudocoarctate mechanisms. Chem. Soc. Rev. 2008, 37, 343–364. [Google Scholar] [CrossRef]
- Hu, C.; Mena, J.; Alabugin, I.V. Design principles of the use of alkynes in radical cascades. Nat. Rev. Chem. 2023, 7, 405–423. [Google Scholar] [CrossRef]
- Plas, H.C. The Sn (ANRORC) Mechanism: A new Mechanism for Nucleophilic Substitution. Acc. Chem. Res. 1978, 2, 462–468. [Google Scholar] [CrossRef]
- Nikitina, A.F.; Sheridan, R.S. Geometry and aromaticity in highly strained heterocyclic allenes: Characterization of a 2,3-didehydro-2H-thiopyran. Org. Lett. 2005, 7, 4467–4470. [Google Scholar] [CrossRef]
- Secrieru, A.; O’Neill, P.M.; Cristiano, M.L.S. Revisiting the Structure and Chemistry of 3(5)-Substituted Pyrazoles. Molecules 2019, 25, 42. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Liebman, J.F. The annular tautomerism of imidazoles and pyrazoles: The possible existence of nonaromatic forms. Struct. Chem. 2006, 17, 439–444. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Gold, B.; Mikhailovskaya, T.F.; Alabugin, I.V. Strain control in nucleophilic cyclizations: Reversal of exo-selectivity in cyclizations of hydrazides of acetylenyl carboxylic acids by annealing to a pyrazole scaffold. J. Phys. Org. Chem. 2012, 25, 998–1005. [Google Scholar] [CrossRef]
- APEX2, Version 2.1. SAINTPlus, Data Reduction and Correction Program (Version 7.31A), Bruker Advansed X-ray Solutions. BrukerAXS Inc.: Madison, WI, USA, 2006.
- Sheldrick, G.M. SADABS, Program for Empirical X-ray Absorption Correction; Bruker-Nonius: Delft, The Netherlands, 1990–2004.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Young, T.A.; Silcock, J.J.; Sterling, A.J.; Duarte, F. autodE: Automated Calculation of Reaction Energy Profiles—Application to Organic and Organometallic Reactions. Angew. Chem. Int. Ed. 2021, 60, 4266–4274. [Google Scholar] [CrossRef]
- Riniker, S.; Landrum, G.A. Better Informed Distance Geometry: Using What We Know To Improve Conformation Generation. J. Chem. Inf. Model. 2015, 55, 2562–2574. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview20; Université de Sherbrooke: Sherbrooke, QC, Canda, 2020; Available online: http://www.cylview.org (accessed on 8 October 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chugunova, E.; Gazizov, A.S.; Islamov, D.; Matveeva, V.; Burilov, A.; Akylbekov, N.; Dobrynin, A.; Zhapparbergenov, R.; Appazov, N.; Chabuka, B.K.; et al. An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants. Molecules 2023, 28, 7335. https://doi.org/10.3390/molecules28217335
Chugunova E, Gazizov AS, Islamov D, Matveeva V, Burilov A, Akylbekov N, Dobrynin A, Zhapparbergenov R, Appazov N, Chabuka BK, et al. An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants. Molecules. 2023; 28(21):7335. https://doi.org/10.3390/molecules28217335
Chicago/Turabian StyleChugunova, Elena, Almir S. Gazizov, Daut Islamov, Victoria Matveeva, Alexander Burilov, Nurgali Akylbekov, Alexey Dobrynin, Rakhmetulla Zhapparbergenov, Nurbol Appazov, Beauty K. Chabuka, and et al. 2023. "An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants" Molecules 28, no. 21: 7335. https://doi.org/10.3390/molecules28217335
APA StyleChugunova, E., Gazizov, A. S., Islamov, D., Matveeva, V., Burilov, A., Akylbekov, N., Dobrynin, A., Zhapparbergenov, R., Appazov, N., Chabuka, B. K., Christopher, K., Tonkoglazova, D. I., & Alabugin, I. V. (2023). An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants. Molecules, 28(21), 7335. https://doi.org/10.3390/molecules28217335