Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition
Abstract
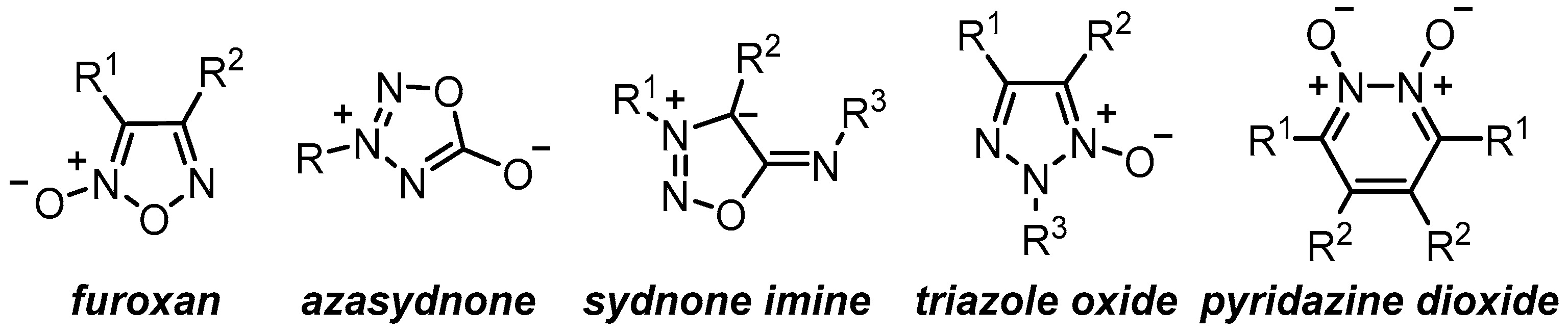
:1. Introduction
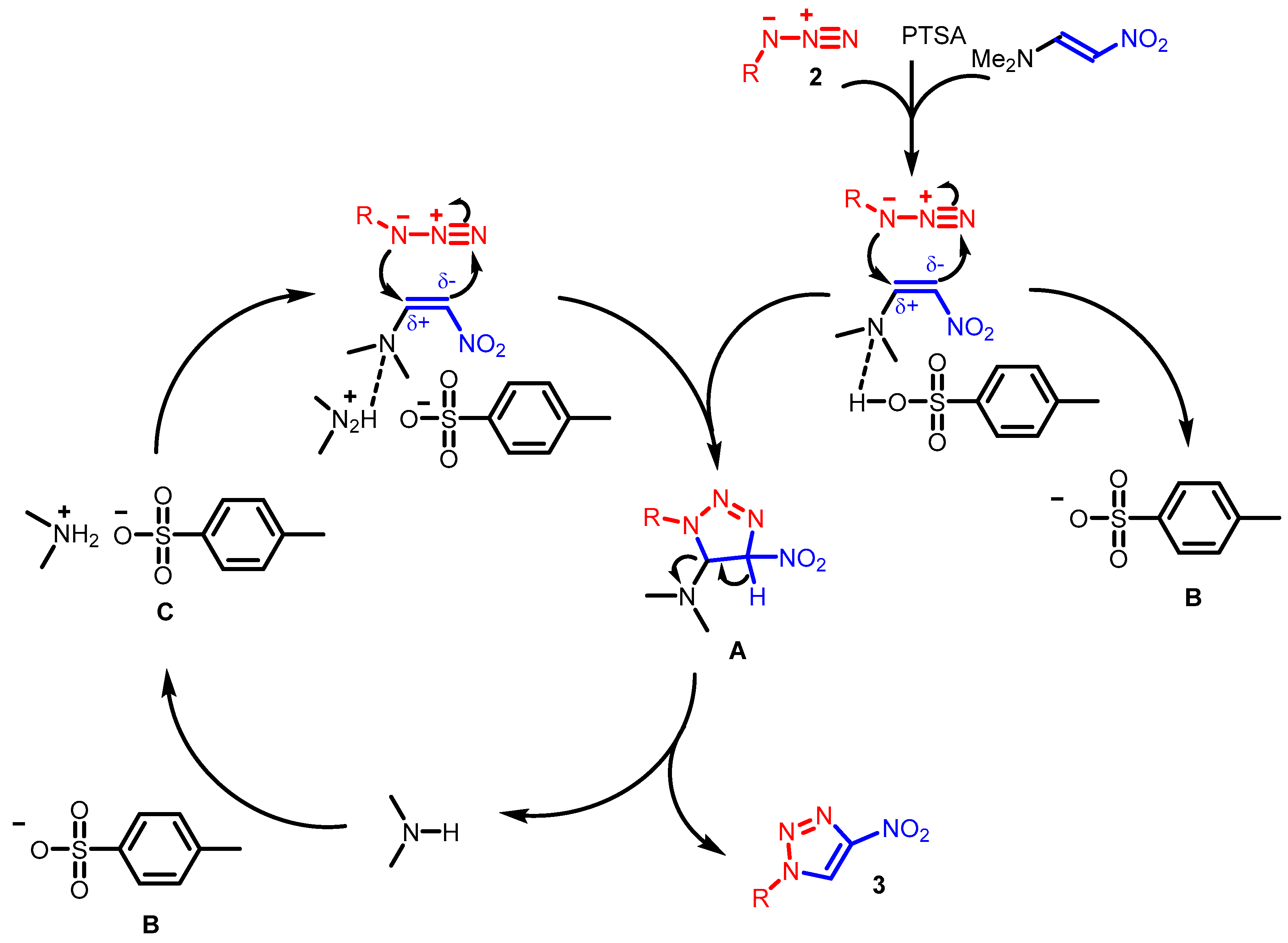
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Methods
- CAUTION! Although we have encountered no difficulties during preparation and handling of azides 1a–w described in this paper, they are potentially explosive and may be sensitive to impact and friction. Mechanical actions of these species, involving scratching or scraping, must be avoided. Any manipulations must be carried out by using appropriate standard safety precautions.
4.2. X-ray Crystallography
4.3. Synthetic Procedures
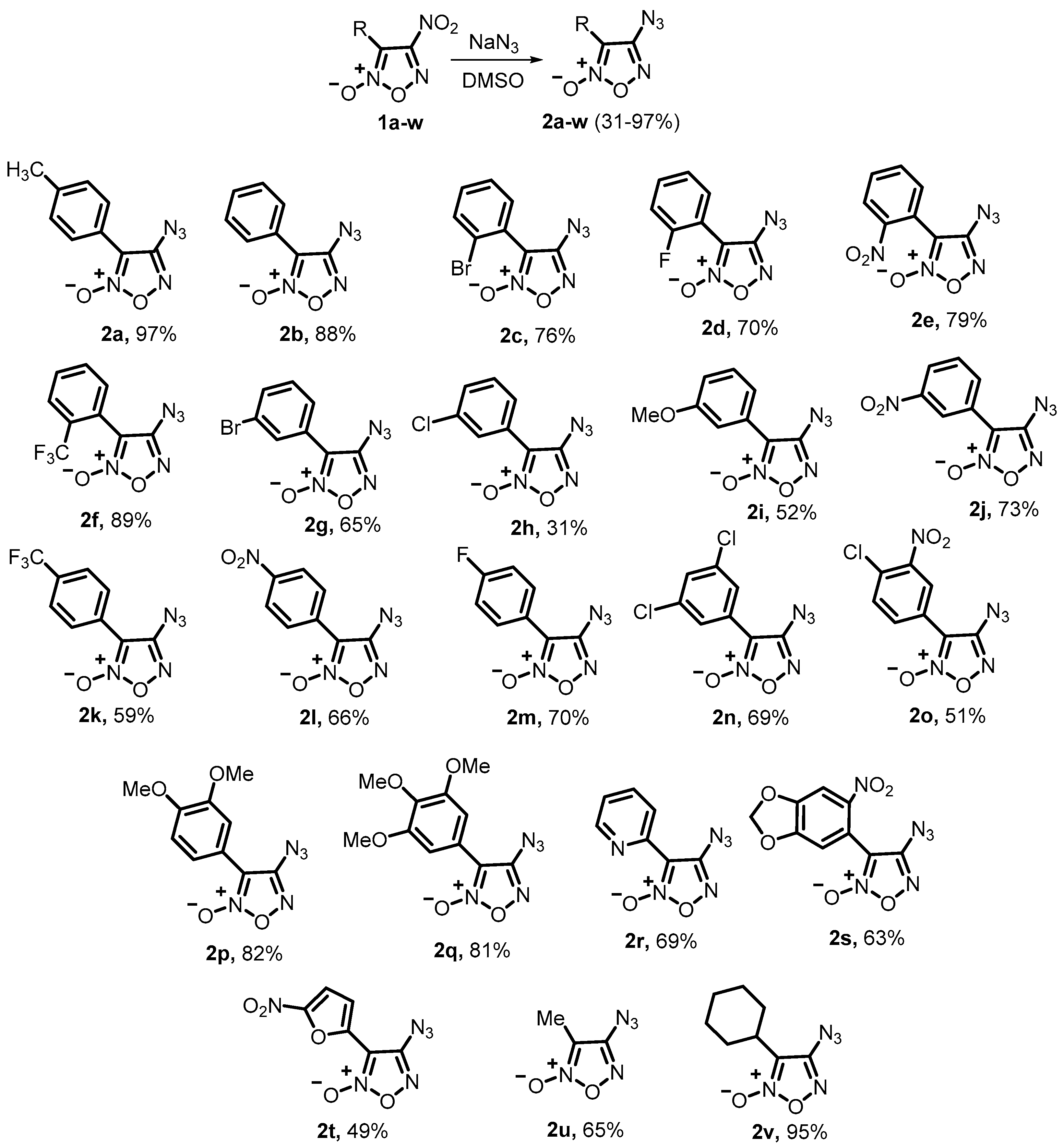
4.3.1. General Procedure for the Synthesis of 4-Azidofuroxans 2a–v
- 4-Azido-3-(p-tolyl)furoxan (2a): yield 1.72 g (97%), light yellow solid; mp 100–101 °C, Rf (CH2Cl2) = 0.85. 1H NMR (300 MHz, DMSO-[d6]) δ, ppm: 7.85 (d, 2H, J = 8.4 Hz, Ar), 7.41 (d, 2H, J = 8.4 Hz, Ar), 2.39 (s, 3H, CH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.7, 141.5, 129.7, 126.5, 118.5, 108.9, 21.6; 14N NMR (21.7 MHz, DMSO-[d6]): δ = −145.8 (br s, N3). IR (KBr), ν: 2918, 2148, 1922, 1592, 1518, 1450, 1404, 1331, 1317, 1289, 1238, 1111, 1066, 971, 854, 821, 735 cm−1. HRMS (ESI) calcd. for C9H8N5NaO2+: 240.0492. Found: 240.0485 [M+Na]+.
- 4-Azido-3-(2-bromophenyl)furoxan (2c): yield 1.75 g (76%), yellow oil; Rf (CHCl3/CCl4, 4:1) = 0.79. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.72–7.75 (m, 1H, Ar), 7.36–7.51 (m, 3H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 148.5, 128.9, 128.1, 127.3, 123.2, 119.4, 117.7, 105.2; 14N NMR (21.7 MHz, CDCl3): δ = −152.3 (s, N3). IR (KBr), ν: 2144, 1608, 1497, 1438, 1331, 1259, 1133, 1071, 969, 837, 759 cm−1. MS (70 eV, m/z (%)): 225 (1) [M–NO]+, 195 (3) [M−2NO]+, 281 (4) [M]+, 144 (10) [M–Br–N3–O]+, 114 (19) [M–Br–N3–NO2]+, 30 (100) [NO]+. Calcd. for C8H4BrN5O2 (%): C, 34.07; H, 1.43; N, 24.83. Found (%): C, 33.89; H, 1.59; N, 24.59.
- 4-Azido-3-(2-fluorophenyl)furoxan (2d): yield 1.27 g (70%), yellow solid; mp 122–124 °C, Rf (CH2Cl2) = 0.93. 1H NMR (300 MHz, DMSO-[d6]) δ, ppm: 7.66–7.75 (m, 2H, Ar), 7.42–7.51 (m, 2H, Ar); 13C NMR (75.5 MHz, DMSO-[d6]) δ, ppm: 159.9 (d, J = 253.3 Hz), 158.2, 134.6 (d, J = 8.5 Hz), 131.7 (d, J = 1.9 Hz), 125.6 (d, J = 3.5 Hz), 117.0 (d, J = 20.0 Hz), 109.1 (d, J = 14.2 Hz), 107.1; 14N NMR (21.7 MHz, DMSO-[d6]): δ = −148.9 (s, N3). IR (KBr), ν: 2924, 2141, 1805, 1725, 1599, 1550, 1502, 1453, 1404, 1326, 1271, 1092, 970, 846, 753 cm−1. Calcd. for C8H4FN5O2 (%): C, 43.45; H, 1.82; N, 31.67. Found (%): C, 43.52; H, 1.93; N, 31.50.
- 4-Azido-3-(2-nitrophenyl)furoxan (2e): yield 1.60 g (79%), orange solid; mp 100–102 °C, Rf (CH2Cl2) = 0.88. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.27 (d, 1H, J = 8.1 Hz, Ar), 7.87–7.68 (m, 3H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.8, 147.5, 134.1, 132.3, 131.6, 125.8, 116.1, 107.9; 14N NMR (21.7 MHz, CDCl3): δ = −13.5 (s, NO2), δ = −147.8 (s, N3). IR (KBr), ν: 2976, 2152, 1611, 1553, 1517, 1481, 1383, 1323, 1207, 1128, 1049, 964, 847, 727 cm−1. HRMS (ESI) calcd. for C8H8N7O4+: 266.0625. Found: 266.0632 [M+NH4]+. HRMS (ESI) calc. for C8H4N6NaO4+: 271.0181. Found: 271.0186 [M+Na]+.
- 4-Azido-3-(2-(trifluoromethyl)phenyl)furoxan (2f): yield 1.97 g (89%), white solid; mp 116–118 °C, Rf (CH2Cl2) = 0.83. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.86–7.89 (m, 1H, Ar), 7.71–7.78 (m, 2H, Ar), 7.43–7.49 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.4, 132.7, 132.3, 131.9, 130.8, 127.5 (q, J = 4.6 Hz), 124.9, 119.5 (q, J = 92.7 Hz), 108.1; 14N NMR (21.7 MHz, CDCl3): δ = −147.3 (s, N3). IR (KBr), ν: 2978, 2148, 1606, 1566, 1484, 1422, 1316, 1273, 1226, 1128, 1051, 967, 844, 786 cm−1. HRMS (ESI) calcd. for C9H4F3NaN5O2+: 294.0221. Found: 294.0209 [M+Na]+.
- 4-Azido-3-(3-bromophenyl)furoxan (2g): yield 1.50 g (65%), beige solid; mp 124–126 °C, Rf (CHCl3/CCl4, 4:1) = 0.85. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.22 (t, 1H, J = 1.9 Hz, Ar), 8.01–8.05 (m, 1H, Ar), 7.63–7.67 (m, 1H, Ar), 7.41 (t, 1H, J = 8.0 Hz, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.5, 133.9, 130.5, 129.2, 125.0, 123.4, 123.1, 107.8; 14N NMR (21.7 MHz, CDCl3): δ = −147.4 (s, N3). IR (KBr), ν: 2922, 2153, 1593, 1485, 1393, 1334, 1272, 1211, 1078, 985, 857, 751 cm−1. HRMS (ESI) calcd. for C8H479BrN5NaO2+: 303.9432. Found: 303.9441 [M+Na]+.
- 4-Azido-3-(3-chlorophenyl)furoxan (2h): yield 0.60 g (31%), white solid; mp 65–66 °C, Rf (CH2Cl2) = 0.92. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.06–8.10 (m, 1H, Ar), 7.95–8.02 (m, 1H, Ar), 7.44–7.51 (m, 2H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.5, 135.2, 131.0, 130.3, 126.4, 124.5, 123.2, 107.8; 14N NMR (21.7 MHz, CDCl3): δ = −146.0 (s, N3). IR (KBr), ν: 2973, 2923, 2852, 2150, 1642, 1562, 1486, 1395, 1335, 1274, 1220, 1122, 1055, 899, 752 cm−1. HRMS (ESI) calcd. for C8H4ClN5NaO2+: 259.9957 (35Cl), 261.9924 (37Cl). Found: 259.9946 (35Cl), 261.9917 (37Cl) [M+Na]+.
- 4-Azido-3-(3-methoxyphenyl)furoxan (2i): yield 0.99 g (52%), white solid; mp 116–118 °C, Rf (CH2Cl2) = 0.84. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.62–7.65 (m, 2H, Ar), 7.41–7.46 (m, 1H, Ar), 7.03–7.07 (m, 1H, Ar), 3.88 (s, 3H, OCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 159.8, 152.8, 130.1, 122.6, 119.0, 116.9, 111.7, 108.8; 14N NMR (21.7 MHz, CDCl3): δ = −146.3 (s, N3). IR (KBr), ν: 2947, 2918, 2144, 1631, 1547, 1503, 1481, 1383, 1322, 1274, 1134, 1028, 962, 873, 777 cm−1. Calcd. for C9H7N5O3 (%): C, 46.36; H, 3.03; N, 30.03. Found (%): C, 46.18; H, 3.09; N, 29.88.
- 4-Azido-3-(3-nitrophenyl)furoxan (2j): yield 1.48 g (73%), yellow solid; mp 120–122 °C, Rf (CHCl3/CCl4, 4:1) = 0.83. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.95–8.96 (m, 1H, Ar), 8.46–8.49 (m, 1H, Ar), 8.35–8.38 (m, 1H, Ar), 7.73–7.79 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.4, 148.5, 131.7, 130.3, 125.3, 123.4, 121.5, 107.5; 14N NMR (21.7 MHz, CDCl3): δ = −14.3 (s, NO2), δ = −148.2 (s, N3). IR (KBr), ν: 2925, 2150, 1659, 1593, 1530, 1492, 1467, 1350, 1286, 1216, 1080, 999, 876, 794 cm−1. HRMS (ESI) calcd. for C8H4N6NaO4+: 271.0184. Found: 271.0186 [M+Na]+.
- 4-Azido-3-(4-(trifluoromethyl)phenyl)furoxan (2k): yield 1.31 g (59%), light yellow solid; mp 122–124 °C, Rf (CH2Cl2) = 0.88. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.23 (d, 2H, J = 8.3 Hz, Ar), 7.79 (d, 2H, J = 8.3 Hz, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.6, 132.5 (d, J = 33.0 Hz), 128.3 (d, J = 52.0 Hz), 126.9, 125.9 (q, J = 3.7 Hz), 125.1, 108.0; 14N NMR (21.7 MHz, CDCl3): δ = −147.8 (s, N3). IR (KBr), ν: 2976, 2921, 2152, 1629, 1563, 1514, 1485, 1390, 1323, 1237, 1173, 1054, 955, 846, 771 cm−1. Calcd. for C9H4F3N5O2 (%): C, 39.87; H, 1.49; N, 25.83. Found (%): C, 40.04; H, 1.33; N, 25.69.
- 4-Azido-3-(4-nitrophenyl)furoxan (2l): yield 1.34 g (66%), yellow solid; mp 116–118 °C, Rf (CH2Cl2) = 0.78. 1H NMR (300 MHz, DMSO-[d6]) δ, ppm: 8.44 (d, 2H, J = 9.0 Hz, Ar), 8.22 (d, 2H, J = 9.0 Hz, Ar); 13C NMR (75.5 MHz, DMSO-[d6]) δ, ppm: 153.7, 148.6, 128.5, 128.1, 124.6, 109.2; 14N NMR (21.7 MHz, DMSO-[d6]): δ = −9.3 (s, NO2), −146.5 (s, N3). IR (KBr): 2924, 2138, 1709, 1598, 1520, 1457, 1404, 1330, 1196, 1065, 971, 847, 752 cm−1. Calcd. for C8H4N6O4 (%): C, 38.72; H, 1.62; N, 33.87. Found (%): C, 38.85; H, 1.87; N, 33.63.
- 4-Azido-3-(4-fluorophenyl)furoxan (2m): yield 1.52 g (84%), light yellow solid; mp 103–105 °C, Rf (CH2Cl2) = 0.90. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.07–8.12 (m, 2H, Ar), 7.20–7.28 (m, 2H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 162.4 (d, J = 253.7 Hz), 152.5, 128.9 (d, J = 8.6 Hz), 117.6 (d, J = 3.4 Hz), 116.3 (d, J = 22.1 Hz), 108.3; 14N NMR (21.7 MHz, CDCl3): δ = −146.6 (s, N3). IR (KBr), ν: 2971, 2150, 1594, 1553, 1476, 1384, 1325, 1231, 1170, 1066, 964, 845, 771 cm−1. Calcd. for C8H4FN5O2 (%): C, 43.45; H, 1.82; N, 31.67. Found (%): C, 43.60; H, 1.93; N, 31.48.
- 4-Azido-3-(2,4-dichlorophenyl)furoxan (2n): yield 1.53 g (69%), light yellow solid; mp 56–58 °C, Rf (CH2Cl2) = 0.95. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.59 (d, 1H, J = 2.0 Hz, Ar), 7.42–7.45 (m, 1H, Ar), 7.28–7.35 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.1, 138.5, 135.7, 132.4, 130.6, 127.9, 118.9, 107.9; 14N NMR (21.7 MHz, CDCl3): δ = −147.0 (s, N3). IR (KBr), ν: 2926, 2150, 1725, 1586, 1490, 1370, 1259, 1104, 1052, 955, 855, 777 cm−1. Calcd. for C8H3Cl2N5O2 (%): C, 35.32; H, 1.11; N, 25.74. Found (%): C, 35.14; H, 1.18; N, 25.48.
- 4-Azido-3-(3-chloro-4-nitrophenyl)furoxan (2o): yield 1.18 g (51%), orange solid; mp 122–124 °C, Rf (CH2Cl2) = 0.88. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 8.62 (d, 1H, J = 2.2 Hz, Ar), 8.37–8.41 (m, 1H, Ar), 7.83 (d, 1H, J = 8.7 Hz, Ar); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 153.1, 140.8, 136.4, 131.4, 123.4, 122.4, 118.7, 107.8; 14N NMR (21.7 MHz, acetone-[d6]): δ = −14.0 (s, NO2), −147.5 (s, N3). IR (KBr), ν: 2970, 2922, 2117, 1741, 1592, 1529, 1467, 1402, 1337, 1282, 1205, 1066, 997, 906, 860, 829 cm−1. Calcd. for C8H3ClN6O4 (%): C, 34.00; H, 1.07; N, 29.74. Found (%): C, 34.23; H, 0.93; N, 29.56.
- 4-Azido-3-(3,4-dimethoxyphenyl)furoxan (2p): yield 1.76 g (82%), orange solid; mp 131–133 °C, Rf (CH2Cl2) = 0.90. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.66–7.69 (m, 2H, Ar), 6.97–7.00 (d, 1H, J = 8.7 Hz, Ar), 3.96 (s, 6H, 2xOCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.6, 151.0, 149.1, 120.4, 113.7, 111.1, 109.8, 109.0, 56.1, 56.0; 14N NMR (21.7 MHz, CDCl3): δ = −148.7 (s, N3). IR (KBr), ν: 2974, 2923, 2851, 2167, 1658, 1612, 1582, 1517, 1483, 1396, 1267, 1217, 1150, 1018, 921, 883, 807 cm−1. HRMS (ESI) calc. for C10H10N5O4+: 264.0727. Found: 264.0730 [M+H]+.
- 4-Azido-3-(3,4,5-trimethoxyphenyl)furoxan (2q): yield 1.94 g (81%), beige solid; mp 120–121 °C. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.55 (s, 1H, Ar), 7.19 (s, 1H, Ar), 3.94 (s, 1H, OCH3), 3.93 (s, 1H, OCH3), 3.90 (s, 1H, OCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.3, 150.0, 147.5, 145.9, 144.9, 125.2, 121.0, 104.6, 61.2, 56.1; 14N NMR (21.7 MHz, CDCl3): δ = −146.9 (s, N3). IR (KBr), ν: 2975, 2922, 2199, 1680, 1566, 1485, 1396, 1353, 1295, 1243, 1172, 1061, 970, 826, 713 cm−1. Calcd. for C11H11N5O5 (%): C, 45.06; H, 3.78; N, 23.88. Found (%): C, 44.89; H, 3.90; N, 23.62.
- 4-Azido-3-(pyridin-2-yl)furoxan (2r): yield 1.15 g (69%), light yellow solid; mp 81–83 °C, Rf (CH2Cl2) = 0.91. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.81 (d, 1H, J = 4.9 Hz, Py), 8.22–8.25 (m, 1H, Py), 7.88–7.94 (m, 1H, Py), 7.41–7.45 (m, 1H, Py); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.0, 150.1, 142.7, 137.2, 125.0, 122.4, 118.9; 14N NMR (21.7 MHz, CDCl3): δ = −146.8 (s, N3). IR (KBr), ν: 2979, 2135, 1592, 1483, 1418, 1341, 1296, 1213, 1139, 1049, 993, 854 cm−1. HRMS (ESI) calc. for C7H5N6O2+: 205.0468. Found: 205.0474 [M+H]+.
- 4-Azido-3-(6-nitro-1,3-benzodioxol-5-yl)furoxan (2s): yield 1.72 g (72%), brick solid; mp 166–168 °C, Rf (CH2Cl2) = 0.75. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.73 (s, 1H, Ar), 6.99 (s, 1H, Ar), 6.26 (s, 2H, CH2); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.9, 152.3, 150.4, 111.4, 109.8, 108.2, 106.7, 104.1; 14N NMR (21.7 MHz, CDCl3): δ = −12.3 (s, NO2), −145.6 (s, N3). IR (KBr), ν: 2924, 2157, 1612, 1529, 1468, 1418, 1360, 1328, 1263, 1213, 1161, 1026, 919, 883, 783, 743 cm−1. HRMS (ESI) calc. for C9H5N6O6+: 293.0265. Found: 293.0259 [M+H]+.
- 4-Azido-3-(5-nitrofuran-2-yl)furoxan (2t): yield 0.95 g (49%), beige solid; mp 121–123 °C, Rf (CH2Cl2) = 0.80. 1H NMR (300 MHz, CDCl3) δ, ppm: 7.48 (d, 1H, J = 3.9 Hz, Furan), 7.39 (d, 1H, J = 3.9 Hz, Furan); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.5, 151.0, 139.1, 114.6, 112.5, 103.5; 14N NMR (21.7 MHz, CDCl3): δ = −31.7 (s, NO2), −148.3 (s, N3). IR (KBr), ν: 2927, 2141, 1725, 1587, 1503, 1459, 1404, 1332, 1246, 1194, 1147, 1087, 1023, 996, 848, 812, 792, 752 cm−1. Calcd. for C6H2N6O5 (%): C, 30.26; H, 0.85; N, 35.29. Found (%): C, 30.12; H, 0.98; N, 35.03.
- 4-Azido-3-methylfuroxan (2u): yield 0.75 g (65%), yellow oil; Rf (CHCl3) = 0.88. 1H NMR (300 MHz, CDCl3) δ, ppm: 2.08 (s, 3H, CH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 154.0, 107.3, 6.8. IR (KBr), ν: 2930, 2140, 1625, 1508, 1381, 1306, 1237, 1108, 1023, 880, 801 cm−1. Calcd. for C3H3N5O2 (%): C, 25.54; H, 2.14; N, 49.64. Found (%): C, 25.32; H, 2.31; N, 49.42.
- 4-Azido-3-cyclohexylfuroxan (2v): yield 1.62 g (95%), yellow solid; mp 71–73 °C, Rf (CH2Cl2) = 0.94. 1H NMR (300 MHz, CDCl3) δ, ppm: 2.55–2.61 (m, 1H, Cy), 1.12–1.75 (m, 10H, Cy); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 154.2, 113.6, 33.1, 27.6, 25.6, 25.4; 14N NMR (21.7 MHz, CDCl3): δ = −146.6 (s, N3). IR (KBr), ν: 2927, 2856, 2142, 1708, 1599, 1496, 1451, 1367, 1306, 1251, 1201, 1048, 952, 883, 823, 761 cm−1. Calcd. for C8H11N5O2 (%): C, 45.93; H, 5.30; N, 33.48. Found (%): C, 45.64; H, 5.49; N, 33.16.
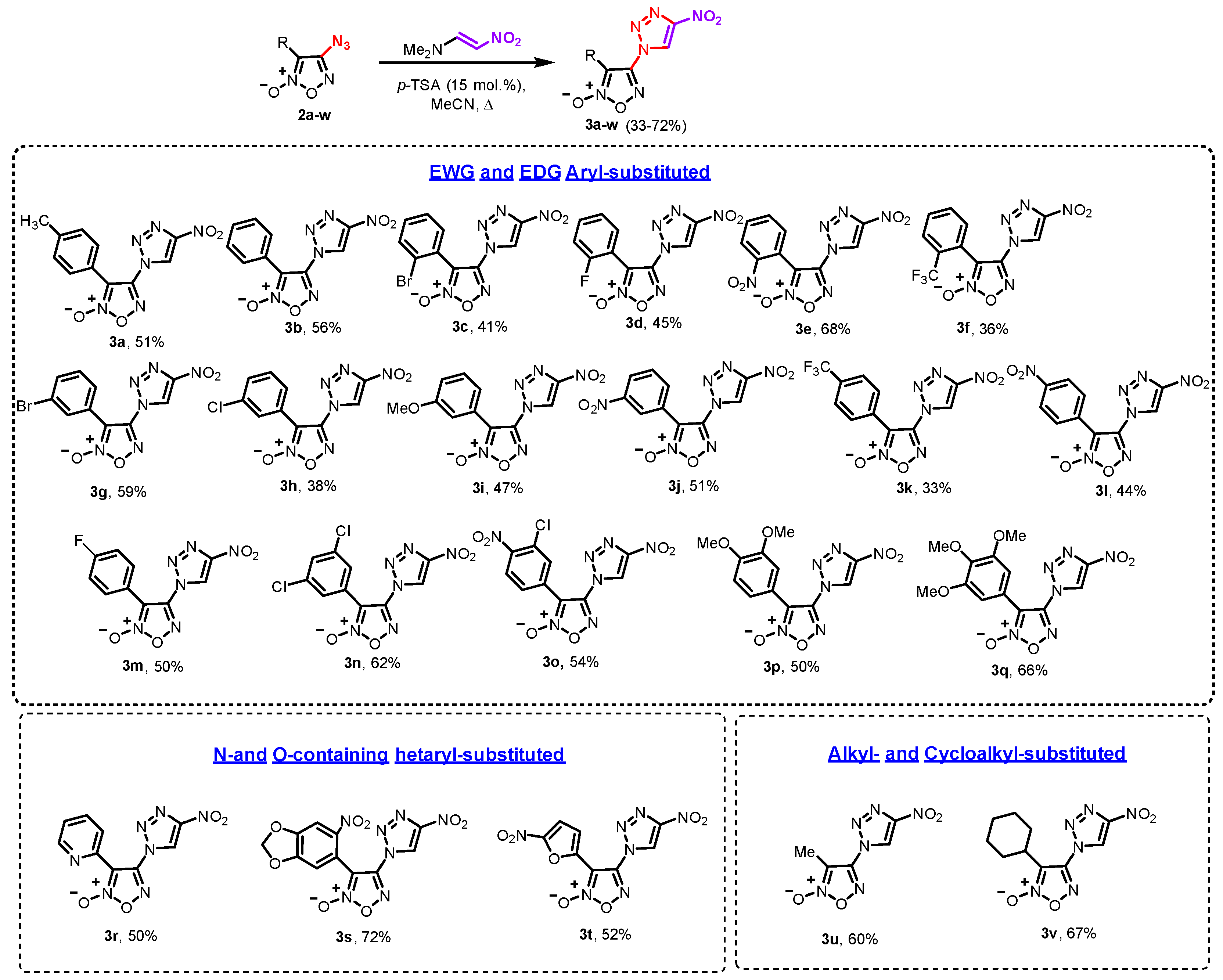
4.3.2. General Procedure for the Synthesis of 4-(4-Nitro-1H-1,2,3-triazol-1-yl)furoxans 3a–v
- 3-(4-p-Tolyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3a): yield 161 mg (56%), light yellow solid; mp 132–134 °C, Rf (CH2Cl2) = 0.7. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.68 (s, 1H, CH), 7.51 (d, 2H, J = 8.1 Hz, Ar), 7.37 (d, 2H, J = 8.1 Hz, Ar), 2.41 (s, 3H, CH3); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 154.0, 149.3, 142.1, 129.8, 128.1, 125.9, 117.5, 111.1, 20.6. IR (KBr), ν: 2922, 1623, 1549, 1523, 1486, 1389, 1325, 1273, 1137, 1112, 1027, 993, 955, 850, 786, 753 cm−1. HRMS (ESI) calcd. for C11H12N7O4+: 306.0945. Found: 306.0954 [M+NH4]+.
- 4-(4-Nitro-1H-1,2,3-triazol-1-yl)-3-phenylfuroxan (3b): yield 140 mg (51%), white solid; mp 132–134 °C, Rf (CH2Cl2) = 0.7. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.69 (s, 1H, CH), 7.56–7.65 (m, 5H, Ar); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 155.0, 149.7, 131.9, 129.6, 128.7, 126.3, 121.1, 111.6. IR (KBr), ν: 2922, 1620, 1551, 1513, 1483, 1388, 1345, 1273, 1238, 1137, 1111, 1075, 1027, 1003, 955, 852, 825, 771 cm−1. HRMS (ESI) calcd. for C10H6N6NaO4+: 297.0343. Found: 297.0346 [M+Na]+.
- 3-(2-Bromophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3c): yield 144 mg (41%), yellow crystals; mp 122–124 °C, Rf (CHCl3/CCl4, 4:1) = 0.33. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.82 (s, 1H, CH), 7.86–7.89 (m, 1H, Ar), 7.81–7.84 (m, 1H, Ar), 7.63–7.66 (m, 2H, Ar); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 154.3, 149.8, 134.1, 133.8, 133.4, 128.9, 124.5, 124.4, 123.1, 111.3. IR (KBr), ν: 2923, 1612, 1554, 1502, 1468, 1386, 1326, 1277, 1182, 1138, 1048, 1026, 950, 864, 825, 771 cm−1. HRMS (ESI) calcd. for C10H5BrN6NaO4: 374.9448 (79Br), 376.9428 (81Br). Found: 374.9441 (79Br), 376.9423 (81Br) [M+Na]+.
- 3-(2-Fluorophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3d): yield 131 mg (45%), white solid; mp 148–150 °C, Rf (CH2Cl2) = 0.70. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.79 (s, 1H, CH), 7.81–7.87 (m, 1H, Ar), 7.70–7.78 (m, 1H, Ar), 7.45–7.50 (m, 1H, Ar), 7.34–7.40 (m, 1H, Ar); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 160.1 (d, J = 240.0 Hz), 153.8, 149.4, 134.4 (d, J = 8.6 Hz), 131.2,(d, J = 1.4 Hz), 125.3 (d, J = 3.6 Hz), 124.5, 116.3 (d, J = 21.4 Hz), 109.2 (d, J = 14.2 Hz), 107.3. IR (KBr), ν: 2924, 2855, 1725, 1710, 1597, 1550, 1502, 1453, 1404, 1326, 1214, 1197, 1066, 1026, 971, 847, 793 cm−1. Calcd. for C10H5FN6O4 (%): C, 41.11; H, 1.72; N, 28.76. Found (%): C, 41.34; H, 1.64; N, 28.52.
- 3-(2-Nitrophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3e): yield 217 mg (68%), yellow solid; mp 121–123 °C, Rf (CH2Cl2) = 0.70. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.11 (s, 1H, CH), 8.42–8.45 (m, 1H, Ar), 7.85–7.97 (m, 2H, Ar) 7.74–7.79 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.5, 148.7, 147.3, 135.0, 133.3, 132.6, 126.2, 121.2, 116.5, 108.5. IR (KBr), ν: 2922, 1620, 1553, 1485, 1388, 1322, 1170, 1133, 1067, 1027, 953, 824, 754 cm−1. HRMS (ESI) calcd. for C10H5N7NaO6+: 342.0194. Found: 342.0192 [M+Na]+.
- 4-(4-Nitro-1H-1,2,3-triazol-1-yl)-3-(2-(trifluoromethyl)phenyl)furoxan (3f): yield 123 mg (36%), light yellow solid; mp 100–102 °C, Rf (CH2Cl2) = 0.62. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.02 (s, 1H, CH), 7.79–7.89 (m, 3H, Ar), 7.64–7.68 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.5, 148.7, 133.1, 132.7, 132.5, 130.6, 127.4 (q, J = 4.7 Hz), 124.9, 121.5, 118.6 (d, J = 2.0 Hz), 108.0. IR (KBr), ν: 2923, 2851, 1620, 1585, 1553, 1485, 1439, 1389, 1319, 1172, 1121, 1033, 951, 867, 824, 771 cm−1. Calcd. for C11H5F3N6O4 (%): C, 38.61; H, 1.47; N, 24.56. Found (%): C, 38.47; H, 1.64; N, 24.30.
- 3-(3-Bromophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3g): yield 208 mg (59%), yellow solid; mp 110–112 °C, Rf (CH2Cl2) = 0.65. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.97 (s, 1H, CH), 7.83 (t, 1H, J = 1.8 Hz, Ar), 7.70–7.74 (m, 1H, Ar), 7.36–7.50 (m, 2H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 156.3, 153.3, 135.1, 131.3, 130.8, 127.1, 123.4, 123.0, 121.6, 109.2. IR (KBr), ν: 2921, 1610, 1529, 1427, 1383, 1351, 1325, 1271, 1231, 1179, 1133, 1075, 1026, 960, 852, 826, 786 cm−1. HRMS (ESI) calcd. for C10H579BrN6NaO4: 374.9448. Found: 374.9456 [M+Na]+.
- 3-(3-Chlorophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3h): yield 117 mg (38%), light yellow solid; mp 1118–120 °C, Rf (CH2Cl2) = 0.54. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.96 (s, 1H, CH), 7.78 (t, 1H, J = 1.9 Hz, Ar), 7.56–7.63 (m, 2H, Ar), 7.49 (t, 1H, J = 7.9 Hz, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 144.4, 135.5, 132.2, 130.6, 129.3, 127.2, 124.0, 123.8, 116.6. IR (KBr), ν: 1620, 1547, 1504, 1477, 1387, 1322, 1302, 1278, 1176, 1135, 1066, 1025, 952, 840 cm−1. Calcd. for C10H5ClN6O4 (%): C, 38.92; H, 1.63; N, 27.23. Found (%): C, 39.09; H, 1.51; N, 26.99.
- 3-(3-Methoxyphenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3i): yield 142 mg (47%), white solid; mp 116–118 °C, Rf (CH2Cl2) = 0.61. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.90 (s, 1H, CH), 7.41 (t, 1H, J = 8.1 Hz, Ar), 7.16 (t, 1H, J = 2.1 Hz, Ar), 7.06–7.10 (m, 1H, Ar), 7.00–7.04 (m, 1H, Ar), 3.83 (s, 3H, OCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 160.1, 148.2, 130.6, 123.6, 120.5, 120.2, 117.8, 113.6, 110.2, 55.5. IR (KBr), ν: 2918, 2846, 1631, 1603, 1548, 1503, 1430, 1383, 1323, 1274, 1112, 1047, 1004, 977, 824, 784 cm−1. Calcd. for C11H8N6O5 (%): C, 43.43; H, 2.65; N, 27.63. Found (%): C, 43.29; H, 2.76; N, 27.41.
- 3-(3-Nitrophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3j): yield 163 mg (51%), light yellow solid; mp 121–123 °C, Rf (CH2Cl2) = 0.86. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.44 (s, 1H, CH), 8.52 (t, 1H, J = 2.0 Hz, Ar), 8.35–8.38 (m, 1H, Ar), 7.91–7.95 (m, 1H, Ar), 7.72–7.77 (m, 1H, Ar); 13C NMR (75.5 MHz, acetone-[d6]+CDCl3) δ, ppm: 154.0, 148.5, 148.4, 134.5, 130.6, 126.1, 124.6, 123.8, 122.3, 109.6. IR (KBr), ν: 2921, 1626, 1551, 1502, 1440, 1383, 1326, 1291, 1170, 1142, 1091, 1023, 960, 902, 844, 808 cm−1. Calcd. for C10H5N7O6 (%): C, 37.63; H, 1.58; N, 30.72. Found (%): C, 37.82; H, 1.42; N, 30.48.
- 4-(4-Nitro-1H-1,2,3-triazol-1-yl)-3-(4-(trifluoromethyl)phenyl)furoxan (3k): yield 112 mg (33%), light yellow solid; mp 145–147 °C, Rf (CH2Cl2) = 0.84. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.01 (s, 1H, CH), 7.75–7.84 (m, 4H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 148.0, 133.4, 129.8, 129.1, 126.4 (q, J = 3.6 Hz, CF3), 125.0, 123.8, 123.4, 123.1, 121.4, 109.5. IR (KBr), ν: 2922, 1620, 1551, 1519, 1486, 1388, 1322, 1164, 1111, 1066, 1027, 953, 823, 754 cm−1. HRMS (ESI) calcd. for C11H5F3N6NaO4+: 365.0217. Found: 365.0207 [M+Na]+.
- 3-(4-Nitrophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3l): yield 150 mg (47%), pale orange solid; mp 119–121 °C, Rf (CH2Cl2) = 0.55. 1H NMR (300 MHz, acetone-[d6]+CDCl3) δ, ppm: 9.77 (s, 1H, CH), 8.38–8.44 (m, 2H, Ar), 7.96–8.00 (m, 2H, Ar); 13C NMR (75.5 MHz, acetone-[d6]+CDCl3) δ, ppm: 149.2, 130.1, 127.8, 127.1, 125.6, 124.1, 123.43, 110.4. IR (KBr), ν: 1602, 1553, 1517, 1390, 1325, 1113, 1031, 987, 950, 854, 752 cm−1. Calcd. for C10H5N7O6 (%): C, 37.63; H, 1.58; N, 30.72. Found (%): C, 37.47; H, 1.67; N, 30.49.
- 3-(4-Fluorophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)-furoxan (3m): yield 146 mg (50%), light yellow solid; mp 120–122 °C, Rf (CH2Cl2) = 0.74. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.97 (s, 1H, CH), 7.61–7.65 (m, 2H, Ar), 8.25–8.29 (m, 2H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 164.4 (d, J = 255.5 Hz), 148.2, 130.8 (d, J = 9.0 Hz), 123.2, 117.0 (d, J = 22.5 Hz), 115.6 (d, J = 3.6 Hz), 109.8, 105.8. IR (KBr), ν: 2974, 2923, 1627, 1551, 1516, 1486, 1392, 1355, 1324, 1238, 1203, 1112, 1016, 954, 839, 752 cm−1. Calcd. for C10H5FN6O4 (%): C, 41.11; H, 1.72; N, 28.76. Found (%): C, 40.95; H, 1.90; N, 28.49.
- 3-(2,4-Dichlorophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3n): yield 212 mg (62%), pale yellow solid; mp 117–119 °C, Rf (CH2Cl2) = 0.84. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.06 (s, 1H, CH), 7.56–7.61 (m, 2H, Ar), 7.50–7.53 (m, 1H, Ar); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.7, 148.6, 139.4, 135.5, 132.7, 130.5, 128.5, 121.5, 118.4, 108.0. IR (KBr), ν: 2923, 2853, 1726, 1628, 1551, 1510, 1474, 1407, 1322, 1277, 1180, 1137, 1053, 950, 884, 788 cm−1. Calcd. for C10H4Cl2N6O4 (%): C, 35.01; H, 1.18; N, 24.50. Found (%): C, 34.83; H, 1.30; N, 24.32.
- 3-(4-Chloro-3-nitrophenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3o): yield 191 mg (54%), yellow solid; mp 134–136 °C, Rf (CH2Cl2) = 0.45. 1H NMR (300 MHz, acetone-[d6]) δ, ppm: 9.10 (s, 1H, CH), 8.06 (br. s, 1H, Ar), 7.69 (br. s, 1H, Ar), 7.28 (s, 1H, Ar); 13C NMR (75.5 MHz, acetone-[d6]) δ, ppm: 156.5, 149.1, 149.0, 146.6, 138.0, 125.4, 117.7, 113.7, 104.9. IR (KBr), ν: 2978, 2901, 1613, 1592, 1553, 1480, 1384, 1278, 1080, 1023, 993, 908, 805, 725 cm−1. Calcd. for C10H4ClN7O6 (%): C, 33.96; H, 1.14; N, 27.73. Found (%): C, 34.13; H, 1.01; N, 27.51.
- 3-(3,4-Dimethoxyphenyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3p): yield 167 mg (50%), white solid; mp 115–117 °C, Rf (CH2Cl2) = 0.86. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.92 (s, 1H, CH), 7.20 (d, 1H, J = 2.0 Hz, Ar), 7.02 (dd, 1H, J = 8.5, 2.0 Hz, Ar), 6.92–6.94 (d, 1H, J = 8.5 Hz, Ar), 3.92 (s, 3H, OCH3), 3.85 (s, 3H, OCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.7, 151.9, 149.5, 148.3, 123.9, 121.7, 111.5, 111.1, 110.5, 110.4, 56.2, 56.1. IR (KBr), ν: 2921, 1580, 1517, 1484, 1433, 1387, 1324, 1221, 1149, 1115, 1012, 953, 860, 769 cm−1. Calcd. for C12H10N6O6 (%): C, 43.12; H, 3.02; N, 25.14. Found (%): C, 42.95; H, 3.15; N, 24.92.
- 4-(4-Nitro-1H-1,2,3-triazol-1-yl)-3-(3,4,5-trimethoxyphenyl)furoxan (3q): yield 240 mg (66%), white solid; mp 138–140 °C, Rf (CHCl3/EtOAc, 15:1) = 0.38. 1H NMR (300 MHz, CDCl3) δ, ppm: 8.56 (s, 1H, CH), 7.27 (s, 2H, Ar), 3.91–3.95 (m, 9H, 3 OCH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 152.3, 150.0, 147.7, 145.4, 127.7, 124.2, 121.5, 111.5, 105.0, 61.3, 61.2, 56.3. IR (KBr), ν: 2924, 1591, 1565, 1488, 1426, 1352, 1296, 1201, 1110, 1061, 1013, 923, 825, 763 cm−1. Calcd. for C13H12N6O7 (%): C, 42.86; H, 3.32; N, 23.07. Found (%): C, 43.09; H, 3.16; N, 22.88.
- 4-(4-Nitro-1H-1,2,3-triazol-1-yl)-3-(pyridin-2-yl)furoxan (3r): yield 137 mg (50%), pale yellow solid; mp 139–141 °C, Rf (CH2Cl2) = 0.86. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.13 (s, 1H, CH), 8.51–8.54 (m, 1H, Py), 8.25–8.29 (m, 1H, Py), 7.94–8.00 (m, 1H, Py), 7.43–7.48 (m, 1H, Py); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 150.2, 148.1, 141.2, 137.8, 125.9, 125.7, 122.5, 110.0. IR (KBr), ν: 2922, 1625, 1544, 1466, 1389, 1302, 1272, 1157, 1091, 1033, 989, 866, 785 cm−1. HRMS (ESI) calcd. for C9H5N7NaO4+: 298.0295. Found: 298.0306 [M+Na]+.
- 3-(6-Nitro-1,3-benzodioxol-5-yl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3s): yield 260 mg (72%), brown solid; mp 138–140 °C, Rf (CH2Cl2) = 0.86. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.08 (s, 1H, CH), 7.84 (s, 1H, Ar), 7.06 (s, 1H, Ar), 6.32 (s, 2H, CH2); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.1, 151.2, 137.1, 131.6, 121.2, 118.5, 110.5, 110.2, 106.9, 104.5. IR (KBr), ν: 2923, 1712, 1625, 1588, 1503, 1481, 1391, 1331, 1265, 1110, 1024, 972, 881, 822 cm−1. HRMS (ESI) calcd. for C11H5N7NaO8+: 386.0081. Found: 386.0092 [M+Na]+.
- 3-(5-Nitrofuran)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3t): yield 152 mg (52%), white solid; mp 139–141 °C, Rf (CH2Cl2) = 0.50. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.85 (s, 1H, CH Triazole), 7.77 (d, 1H, J = 4.0 Hz, CH Furan), 7.60 (d, 1H, J = 4.0 Hz, CH Furan); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 155.4, 153.9, 146.9, 137.9, 126.9, 116.2, 112.7, 105.9. IR (KBr), ν: 2925, 1796, 1725, 1630, 1503, 1475, 1394, 1310, 1244, 1155, 1025, 912, 856, 755 cm−1. Calcd. for C8H3N7O7 (%): C, 31.08; H, 0.98; N, 31.72. Found (%): C, 30.89; H, 1.11; N, 31.56.
- 3-Metyl-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3u): yield 127 mg (60%), pale yellow solid; mp 129–131 °C, Rf (CH2Cl2) = 0.45. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.10 (s, 1H, CH), 2.63 (s, 3H, CH3); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.7, 149.8, 121.0, 107.5, 9.3. IR (KBr), ν: 2961, 2924, 2854, 1725, 1658, 1592, 1501, 1461, 1388, 1313, 1261, 1179, 1026, 950, 823, 752 cm−1. Calcd. for C5H4N6O4 (%): C, 28.31; H, 1.90; N, 39.62. Found (%): C, 28.09; H, 1.98; N, 39.39.
- 3-(Cyclohexyl)-4-(4-nitro-1H-1,2,3-triazol-1-yl)furoxan (3v): yield 187 mg (67%), beige solid; mp 121–123 °C, Rf (CH2Cl2) = 0.76. 1H NMR (300 MHz, CDCl3) δ, ppm: 9.03 (s, 1H, CH), 3.17–3.24 (m, 1H), 1.25–1.96 (m, 10H, Cy); 13C NMR (75.5 MHz, CDCl3) δ, ppm: 153.7, 148.8, 122.2, 113.4, 33.4, 26.4, 25.6, 25.0. IR (KBr), ν: 2929, 2854, 1726, 1608, 1547, 1491, 1449, 1387, 1313, 1270, 1180, 1105, 1049, 987, 881, 823 cm−1. Calcd. for C10H12N6O4 (%): C, 42.86; H, 4.32; N, 29.99. Found (%): C, 43.09; H, 4.18; N, 29.72.
4.3.3. NO Release Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Smith, J.M.; Dixon, J.A.; deGruyter, J.N.; Baran, P.S. Alkyl Sulfinates: Radical Precursors Enabling Drug Discovery Miniperspective. J. Med. Chem. 2019, 62, 2256–2264. [Google Scholar] [CrossRef]
- Krska, S.W.; DiRocco, D.A.; Dreher, S.D.; Shevlin, M. The Evolution of Chemical High-Throughput Experimentation To Address Challenging Problems in Pharmaceutical Synthesis. Acc. Chem. Res. 2017, 50, 2976–2985. [Google Scholar] [CrossRef]
- Hansen, E.C.; Pedro, D.J.; Wotal, A.C.; Gower, N.J.; Nelson, J.D.; Caron, S.; Weix, D.J. New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem. 2016, 8, 1126–1130. [Google Scholar] [CrossRef]
- Hilton, M.C.; Zhang, X.; Boyle, B.T.; Alegre-Requena, J.V.; Paton, R.S.; McNally, A. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science 2018, 362, 799–804. [Google Scholar] [CrossRef]
- Das, P.; Delost, M.D.; Qureshi, M.H.; Smith, D.T.; Njardarson, J.T. A Survey of the Structures of US FDA Approved Combination Drugs. J. Med. Chem. 2019, 62, 4265–4311. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Kawamata, Y.; Vantourout, J.C.; Hickey, D.P.; Bai, P.; Chen, L.; Hou, Q.; Qiao, W.; Barman, K.; Edwards, M.A.; Garrido-Castro, A.F.; et al. Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2019, 141, 6392–6402. [Google Scholar] [CrossRef]
- Zhang, X.; McNally, A. Cobalt-Catalyzed Alkylation of Drug-Like Molecules and Pharmaceuticals Using Heterocyclic Phosphonium Salts. ACS Catal. 2019, 9, 4862–4866. [Google Scholar] [CrossRef]
- Boddy, A.J.; Affron, D.P.; Cordier, C.J.; Rivers, E.L.; Spivey, A.C.; Bull, J.A. Rapid Assembly of Saturated Nitrogen Heterocycles in One-Pot: Diazo-Heterocycle “Stitching” by N–H Insertion and Cyclization. Angew. Chem. Int. Ed. 2019, 58, 1458–1462. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug. Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef]
- Bryan, N.S. Natural Product Chemistry for Nitric Oxide Based Therapeutics. Isr. J. Chem. 2019, 59, 414–419. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Sinha, B.K.; Perera, L.; Cannon, R.E. NCX-4040, a Unique Nitric Oxide Donor, Induces Reversal of Drug-Resistance in Both ABCB1- and ABCG2-Expressing Multidrug Human Cancer Cells. Cancers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2020, 176, 113740. [Google Scholar] [CrossRef]
- Zhang, L.; Rong, Y.; Zheng, J.; Yang, C.; Chen, Y.; Wang, J.; Wei, G. Design, synthesis and biological evaluation of novel nitric oxide-donating podophyllotoxin derivatives as potential antiproliferative agents against multi-drug resistant leukemia cells. RSC Adv. 2018, 8, 34266–34274. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef]
- Makhova, N.N.; Fershtat, L.L. Recent advances in the synthesis and functionalization of 1,2,5-oxadiazole 2-oxides. Tetrahedron Lett. 2018, 59, 2317–2326. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Polkovnichenko, M.S.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Novel Arylazo-1,2,5-oxadiazole Photoswitches: Synthesis, Photoisomerization and Nitric Oxide Releasing Properties. ChemPhotoChem 2020, 4, 5346–5354. [Google Scholar] [CrossRef]
- Chaplygin, D.A.; Gorbunov, Y.K.; Fershtat, L.L. Ring Distortion Diversity-Oriented Approach to Fully Substituted Furoxans and Isoxazoles. Asian J. Org. Chem. 2021, 10, 2644–2653. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Teslenko, F.E. Five-Membered Hetarene N-Oxides: Recent Advances in Synthesis and Reactivity. Synthesis 2021, 53, 3673–3682. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Straightforward Access to the Nitric Oxide Donor Azasydnone Scaffold by Cascade Reactions of Amines. Chem. Eur. J. 2019, 25, 14284–14289. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Ustyuzhanina, N.E.; Fershtat, L.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant effects of (1,2,5-oxadiazolyl)azasydnone ring assemblies as novel antiplatelet agents. Chem. Biol. Drug Des. 2022, 100, 1017–1024. [Google Scholar] [CrossRef]
- Shuvaev, A.D.; Zhilin, E.S.; Fershtat, L.L. NOBF4-Mediated Assembly of the Sydnone Imine Scaffold in the Synthesis of Double Nitric Oxide Donors. Synthesis 2023, 55, 1863–1874. [Google Scholar]
- Titenkova, K.; Shuvaev, A.D.; Teslenko, F.E.; Zhilin, E.S.; Fershtat, L.L. Empowering Strategies of Electrochemical N-N Bond Forming Reactions: Direct Access to Previously Neglected 1,2,3-Triazole 1-Oxides. Green Chem. 2023, 25, 6686–6693. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Zhilin, E.S.; Shuvaev, A.D.; Fershtat, L.L.; Makhova, N.N. Design and synthesis of pyrazolo[3,4-d]pyridazine 5,6-dioxides as novel NO-donors. Mendeleev Commun. 2021, 31, 42–45. [Google Scholar] [CrossRef]
- Chugunova, E.; Akylbekov, N.; Bulatova, A.; Gavrilov, N.; Voloshina, A.; Kulik, N.; Zobov, V.; Dobrynin, A.; Syakaev, V.; Burilov, A. Synthesis and biological evaluation of novel structural hybrids of benzofuroxan derivatives and fluoroquinolones. Eur. J. Med. Chem. 2016, 116, 165–172. [Google Scholar] [CrossRef]
- de Souza, P.C.; Fernandes, G.F.S.; Marino, L.B.; Ribeiro, C.M.; da Silva, P.B.; Chorilli, M.; Silva, C.S.P.; Resende, F.A.; Solcia, M.C.; de Grandis, R.A.; et al. Furoxan derivatives demonstrated in vivo efficacy by reducing Mycobacterium tuberculosis to undetectable levels in a mouse model of infection. Biomed. Pharmacother. 2020, 130, 110592. [Google Scholar] [CrossRef]
- Chugunova, E.A.; Smolobochkin, A.V.; Gazizov, A.S.; Burilov, A.R.; Voloshina, A.D.; Lyubina, A.P.; Amerhanova, S.K.; Melnikova, T.A.; Tulesinova, A.I.; Akylbekov, N.I.; et al. Anticancer activity of new benzofuroxan–imidazolone hybrids. Mendeleev Commun. 2021, 31, 865–866. [Google Scholar] [CrossRef]
- Chugunova, E.; Micheletti, G.; Telese, D.; Boga, C.; Islamov, D.; Usachev, K.; Burilov, A.; Tulesinova, A.; Voloshina, A.; Lyubina, A.; et al. Novel Hybrid Compounds Containing Benzofuroxan and Aminothiazole Scaffolds: Synthesis and Evaluation of Their Anticancer Activity. Int. J. Mol. Sci. 2021, 22, 7497. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Matveeva, V.; Tulesinova, A.; Iskanderov, E.; Akylbekov, N.; Dobrynin, A.; Khamatgalimov, A.; Appazov, N.; Boltayeva, L.; Duisembekov, B.; et al. Water-Soluble Salts Based on Benzofuroxan Derivatives—Synthesis and Biological Activity. Int. J. Mol. Sci. 2022, 23, 14902. [Google Scholar] [CrossRef]
- Chugunova, E.; Gibadullina, E.; Matylitsky, K.; Bazarbayev, B.; Neganova, M.; Volcho, K.; Rogachev, A.; Akylbekov, N.; Nguyen, H.B.T.; Voloshina, A.; et al. Diverse Biological Activity of Benzofuroxan/Sterically Hindered Phenols Hybrids. Pharmaceuticals 2023, 16, 499. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L.; Ustyuzhanina, N.E.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New hybrid furoxan structures with antiaggregant activity. Mendeleev Commun. 2018, 28, 595–597. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Yang, J.-J.; Yu, W.-W.; Hu, L.-L.; Liu, W.-J.; Lin, X.-H.; Wang, W.; Zhang, Q.; Wang, P.-L.; Tang, S.-W.; Wang, X.; et al. Discovery and Characterization of 1H-1,2,3-Triazole Derivatives as Novel Prostanoid EP4 Receptor Antagonists for Cancer Immunotherapy. J. Med. Chem. 2020, 63, 569–590. [Google Scholar] [CrossRef]
- Carlucci, R.; di Gresia, G.; Mediavilla, M.G.; Cricco, J.A.; Tekwani, B.L.; Khan, S.I.; Labadie, G.R. Expanding the scope of novel 1,2,3-triazole derivatives as new antiparasitic drug candidates. RSC Med. Chem. 2023, 14, 122–134. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Ding, S. 1,2,3-Triazole-based sequence-defined oligomers and polymers. Polym. Chem. 2021, 12, 2668–2688. [Google Scholar] [CrossRef]
- Niskanen, J.; Tousignant, M.N.; Peltekoff, A.J.; Lessard, B.H. 1,2,3-Triazole based poly(ionic liquids) as solid dielectric materials. Polymer 2021, 212, 123144. [Google Scholar] [CrossRef]
- Kotovshchikov, Y.N.; Sultanov, R.H.; Latyshev, G.V.; Lukashev, N.V.; Beletskaya, I.P. Domino assembly of dithiocarbamates via Cu-catalyzed denitrogenative thiolation of iodotriazole-based diazo precursors. Org. Biomol. Chem. 2022, 20, 5764–5770. [Google Scholar] [CrossRef]
- Voloshkin, V.A.; Kotovshchikov, Y.N.; Latyshev, G.V.; Lukashev, N.V.; Beletskaya, I.P. Annulation-Triggered Denitrogenative Transformations of 2-(5-Iodo-1,2,3-triazolyl)benzoic Acids. J. Org. Chem. 2022, 87, 7064–7075. [Google Scholar] [CrossRef]
- Gevondian, A.G.; Kotovshchikov, Y.N.; Latyshev, G.V.; Lukashev, N.V.; Beletskaya, I.P. Domino Construction of Benzoxazole-Derived Sulfonamides via Metal-Free Denitrogenation of 5-Iodo-1,2,3-triazoles in the Presence of SO2 and Amines. J. Org. Chem. 2021, 86, 5639–5650. [Google Scholar] [CrossRef]
- Pryde, D.C.; Maw, G.N.; Planken, S.; Platts, M.Y.; Sanderson, V.; Corless, M.; Stobie, A.; Barber, C.G.; Russell, R.; Foster, L.; et al. Novel Selective Inhibitors of Neutral Endopeptidase for the Treatment of Female Sexual Arousal Disorder. Synthesis and Activity of Functionalized Glutaramides. J. Med. Chem. 2006, 49, 4409–4424. [Google Scholar] [CrossRef]
- Kumar, A.S.; Ghule, V.D.; Subrahmanyam, S.; Sahoo, A.K. Synthesis of Thermally Stable Energetic 1,2,3-Triazole Derivatives. Chem. Eur. J. 2013, 19, 509–518. [Google Scholar] [CrossRef]
- Thomas, J.; Goyvaerts, V.; Liekens, S.; Dehaen, W. Metal-Free Route for the Synthesis of 4-Acyl-1,2,3-Triazoles from Readily Available Building Blocks. Chem. Eur. J. 2016, 22, 9966–9970. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Ashirbaev, S.S.; Kulikov, A.S.; Kachala, V.V.; Makhova, N.N. Ionic liquid-mediated synthesis of (1H-1,2,3-triazol-1-yl)furoxans by [3 + 2] cycloaddition of azidofuroxans to acetylenes. Mendeleev Commun. 2015, 25, 257–259. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Larin, A.A.; Fershtat, L.L.; Anikina, L.V.; Pukhov, S.A.; Klochkov, S.G.; Struchkova, M.I.; Romanova, A.A.; Ananyev, I.V.; Makhova, N.N. Synthesis, structural characterization and cytotoxic activity of heterocyclic compounds containing the furoxan ring. Arkivoc 2017, iii, 250–268. [Google Scholar] [CrossRef]
- Seregin, I.V.; Batog, L.V.; Makhova, N.N. Synthesis of 1-aryl(hetaryl)-1,2,3-triazoles with the use of ionic liquids. Mendeleev Commun. 2002, 12, 83–84. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Epishina, M.A.; Kulikov, A.S.; Makhova, N.N. Design of hetarylthiofuroxans by nucleophilic substitution of NO2 group in nitrofuroxans. Mendeleev Commun. 2015, 25, 36–38. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Epishina, M.A.; Ovchinnikov, I.V.; Struchkova, M.I.; Romanova, A.A.; Ananyev, I.V.; Makhova, N.N. Side-chain prototropic tautomerism of 4-hydroxyfuroxans in methylation reactions. Tetrahedron Lett. 2016, 57, 5685–5689. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenium microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange withdensity functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]

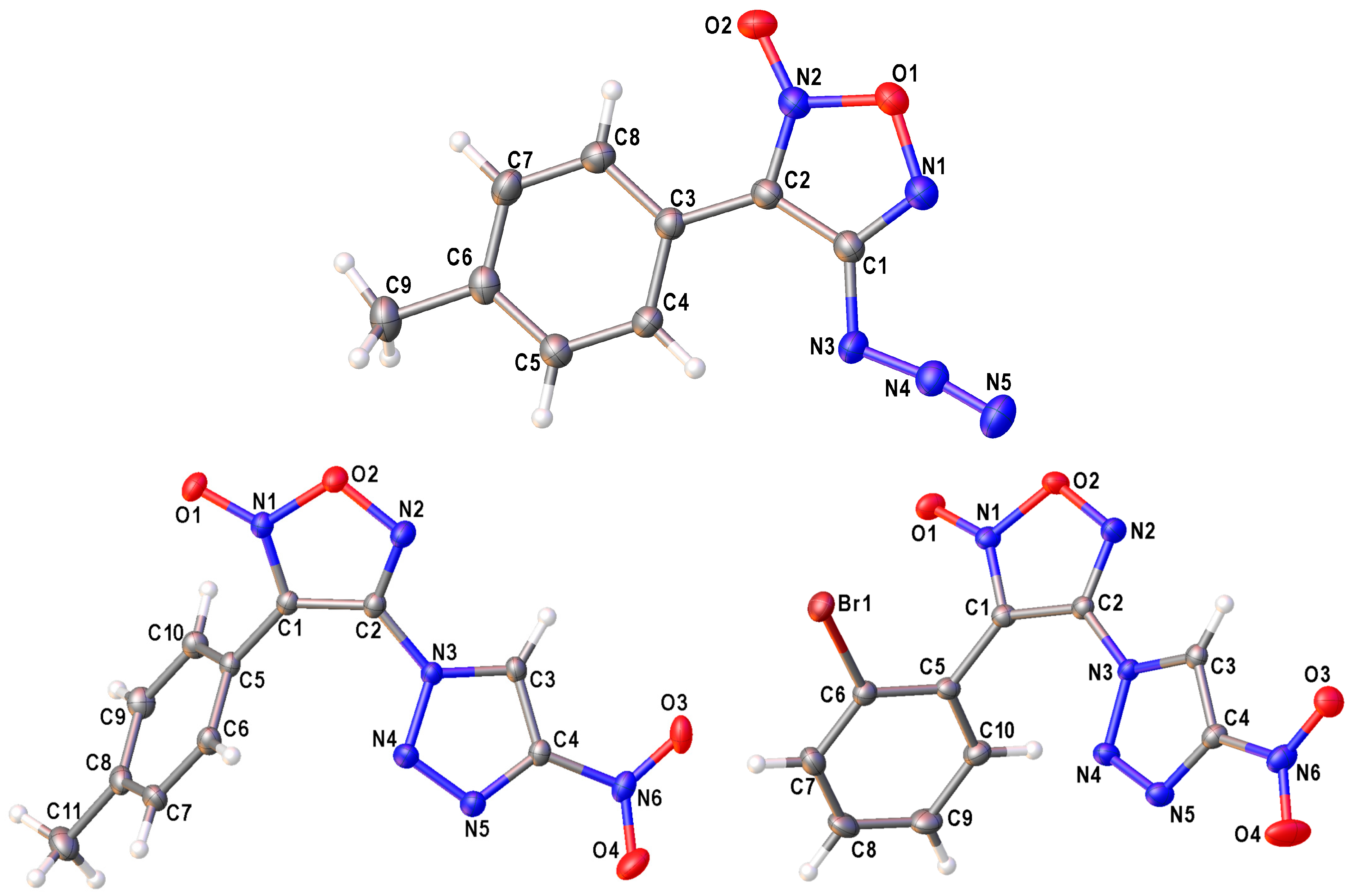

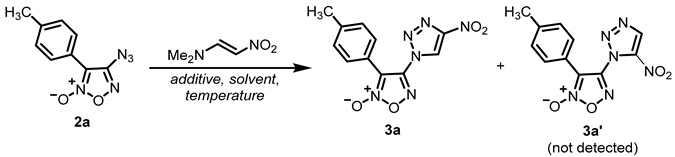 | ||||||
| Entry | Equiv. of Dipolarophile | Additive (mol. %) | Solvent | T, °C | Time, h | Yield of 3a b, % |
| 1 | 1 | — | PhMe | 110 | 72 | — |
| 2 | 2 | — | PhMe | 110 | 72 | traces |
| 3 | 3 | — | PhMe | 110 | 72 | 24 |
| 4 | 5 | — | PhMe | 110 | 72 | 30 |
| 5 | 5 | — | CHCl3 | 60 | 72 | 8 |
| 6 | 5 | — | PhH | 80 | 72 | 32 |
| 7 | 3 | — | dioxane | 102 | 72 | traces |
| 8 | 5 | — | MeCN | 82 | 72 | traces |
| 9 | 3 | BF3·OEt2 (15) | PhMe | 110 | 48 | 23 |
| 10 | 3 | BF3·OEt2 (15) | MeCN | 82 | 72 | 12 |
| 11 | 3 | ZnCl2 (10) | PhMe | 70 | 96 | 18 |
| 12 | 3 | Yb(OTf)3 (15) | PhMe | 110 | 72 | 15 |
| 13 | 3 | Yb(OTf)3 (15) | MeCN | 82 | 72 | traces |
| 14 | 2 | mCPBA (10) | PhMe | 110 | 72 | traces |
| 15 | 5 | p-TSA (15) | PhH | 80 | 72 | 48 |
| 16 | 5 | p- TSA (15) | MeCN | 82 | 72 | 51 |
| 17 | 5 | p-TSA (30) | MeCN | 82 | 72 | 49 |
| 18 | 5 | p-TSA (10) | MeCN | 82 | 72 | 32 |
| 19 | 5 | PPTS (15) | MeCN | 82 | 72 | 36 |
| 20 | 5 | p-TSA (15) | PhMe | 110 | 36 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stebletsova, I.A.; Larin, A.A.; Ananyev, I.V.; Fershtat, L.L. Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition. Molecules 2023, 28, 6969. https://doi.org/10.3390/molecules28196969
Stebletsova IA, Larin AA, Ananyev IV, Fershtat LL. Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition. Molecules. 2023; 28(19):6969. https://doi.org/10.3390/molecules28196969
Chicago/Turabian StyleStebletsova, Irina A., Alexander A. Larin, Ivan V. Ananyev, and Leonid L. Fershtat. 2023. "Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition" Molecules 28, no. 19: 6969. https://doi.org/10.3390/molecules28196969
APA StyleStebletsova, I. A., Larin, A. A., Ananyev, I. V., & Fershtat, L. L. (2023). Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition. Molecules, 28(19), 6969. https://doi.org/10.3390/molecules28196969






