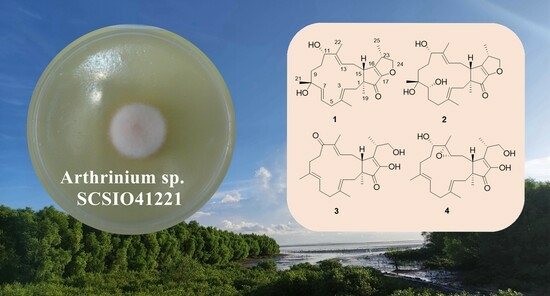
Arthproliferins A–D, Four New Sesterterpenes from the Mangrove-Sediment-Derived Fungus Arthrinium sp. SCSIO41221
Abstract
1. Introduction
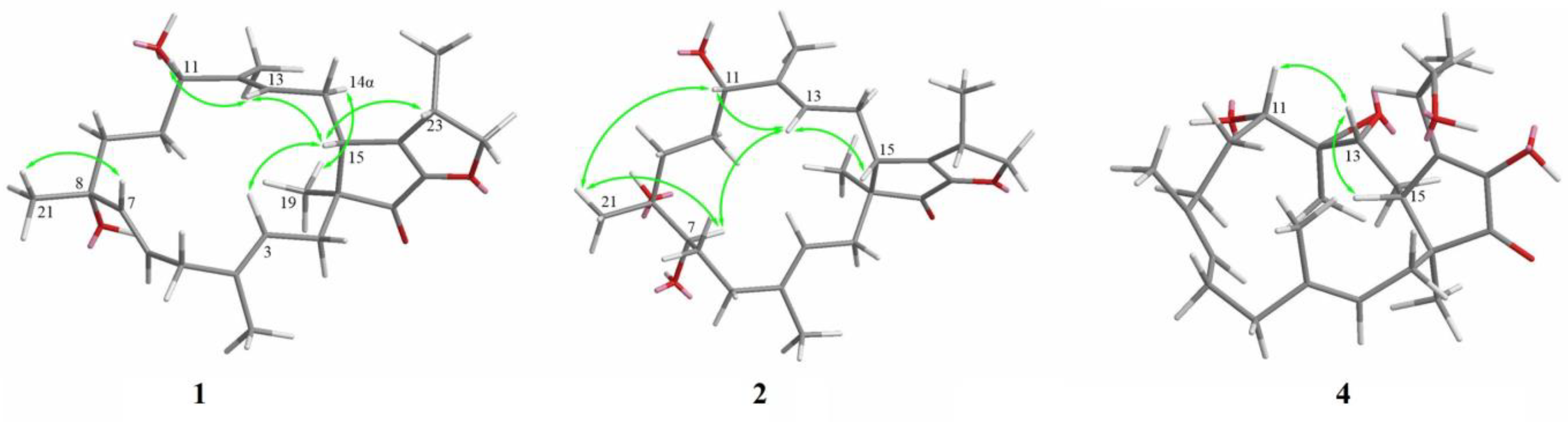
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Compound Characterization
- Arthproliferin A (1): Pale yellow amorphous solid; [α + 5.9 (MeOH; c 0.08); CD cm2mol−1: Δε 323 +2.72, Δε 201 −48.51 (MeOH; c 0.5); 1H and 13C NMR data: see Table 1 and Table 2; HRESIMS in m/z: 423.2516 [M + Na]+ (calcd for C25H36NaO4 423.2506), m/z: 823.5128 [2M + Na]+ (calcd for C50H72NaO8 823.5119).
- Arthproliferin B (2): Pale yellow oil; [α + 33.5 (MeOH; c 1.2); CD cm2mol−1: Δε 324 +2.13, Δε 264 −7.91, Δε 200 −39.28 (MeOH; c 0.47); 1H and 13C NMR data: see Table 1 and Table 2; HRESIMS in m/z: 441.2623 [M + Na]+ (calcd for C25H38NaO5 441.2611), m/z: 859.5351 [2M + Na]+ (calcd for C50H76NaO10 859.5331).
- Arthproliferin C (3): Pale yellow amorphous solid; [α − 62.8 (MeOH; c 0.29); CD cm2mol−1: Δε 238 −2.59, Δε 200 −41.24 (MeOH; c 0.47); 1H and 13C NMR data: see Table 1 and Table 2; HRESIMS in m/z: 423.2511 [M + Na]+ (calcd for C25H36NaO4 423.2506), m/z: 823.5124 [2M + Na]+ (calcd for C50H72NaO8 823.5119).
- Arthproliferin D (4): Pale yellow amorphous solid; [α + 4.9 (MeOH; c 0.05); CD cm2mol−1: Δε 322 +1.88, Δε 262 −2.53, Δε 200 −42.46 (MeOH; c 0.47); 1H and 13C NMR data: see Table 1 and Table 2; HRESIMS in m/z: 441.2617 [M + Na]+ (calcd for C25H38NaO5 441.2611), m/z: 859.5332 [2M + Na]+ (calcd for C50H76NaO10 859.5331).
3.5. Bioassay
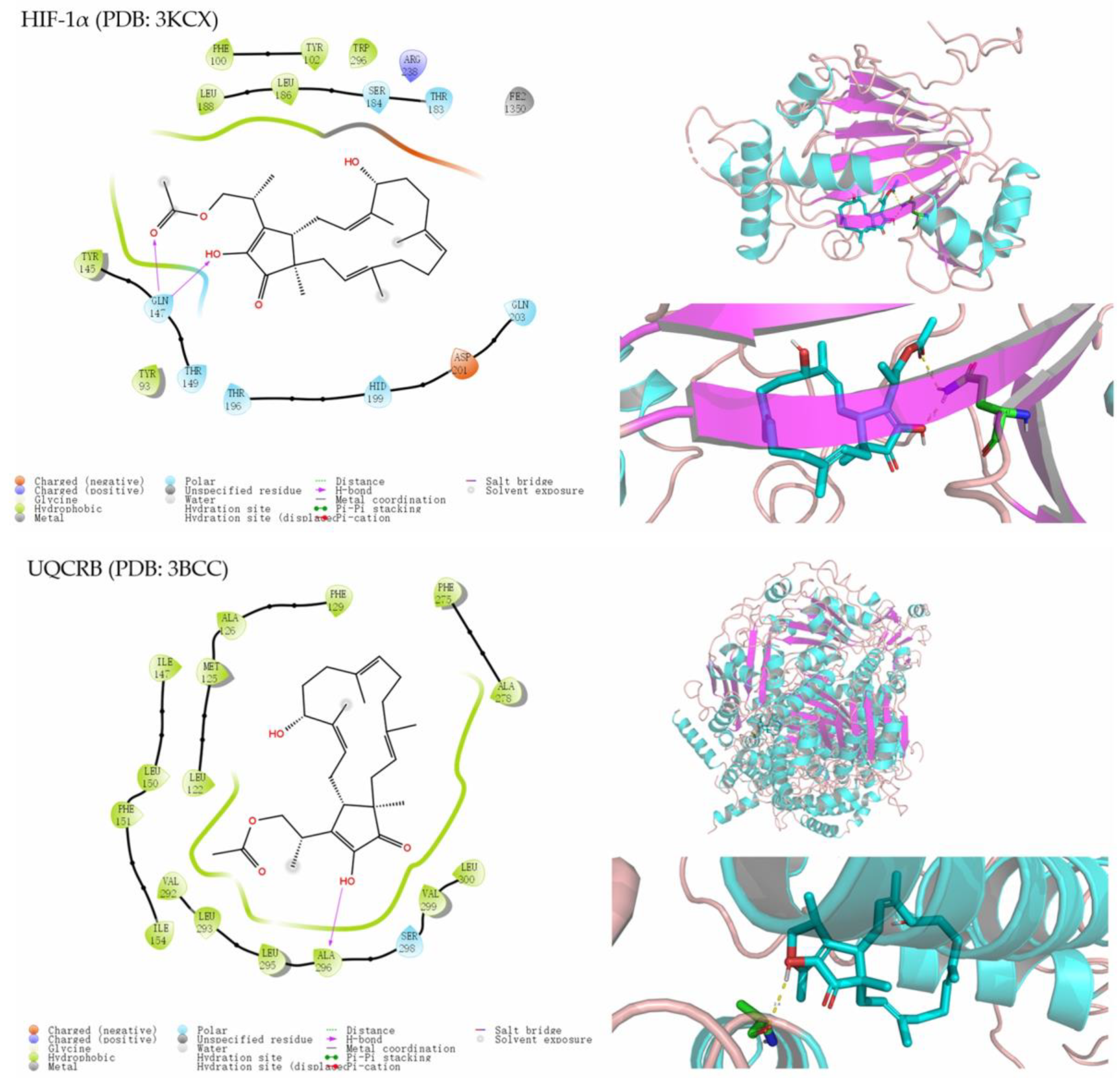
3.6. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf S. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Euro. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a New Sesterterpenoid as an Inhibitor of Mycobacterium tuberculosis Protein Tyrosine Phosphatase B from the Culture of Aspergillus sp 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, C.; Lu, Z.; Zhu, T.; Hong, K.; Zhu, W. New Isochromane Derivatives from the Mangrove Fungus Aspergillus ustus 094102. Nat. Prod. Comms. 2015, 10, 2123–2126. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, X.; He, Y.; Lin, X.; Zhou, X.; Liu, Y.; Yang, B. Secondary metabolites from coral-associated fungi: Source, chemistry and bioactivities. J. Fungi 2022, 8, 1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qi, J.; Duan, Y.; Gao, J.-M.; Liu, C. Research progress on fungal sesterterpenoids biosynthesis. J. Fungi 2022, 8, 1080. [Google Scholar] [CrossRef]
- Maximo, P.; Lourenco, A. Marine sesterterpenes: An overview. Cur. Org. Chem. 2018, 22, 2381–2393. [Google Scholar] [CrossRef]
- Oka, M.; Iimura, S.; Tenmyo, O.; Sawada, Y.; Sugawara, M.; Ohkusa, N.; Yamamoto, H.; Kawano, K.; Hu, S.L.; Fukagawa, Y.; et al. Terpestacin, a new syncytium formation inhibitor from Arthrinium sp. J. Antibiot. 1993, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Iimura, S.; Narita, Y.; Furumai, T.; Konishi, M.; Oki, T.; Gao, Q.; Kakisawa, H. Stereochemistry and biosynthesis of terpestacin, a new syncytium formation inhibitor. J. Org. Chem. 1993, 58, 1875–1881. [Google Scholar] [CrossRef]
- Iimura, S.; Oka, M.; Narita, Y.; Konishi, M.; Kakisawa, H.; Gao, Q.; Oki, T. Terpestacin, a novel syncytium formation inhibitor, isolated from Arthrinium species. Tetrahedron Lett. 1993, 34, 493–496. [Google Scholar] [CrossRef]
- Suthiphasilp, V.; Raksat, A.; Maneerat, T.; Hadsadee, S.; Jungsuttiwong, S.; Pyne, S.G.; Chomnunti, P.; Jaidee, W.; Charoensup, R.; Laphookhieo, S. Cytotoxicity and nitric oxide production inhibitory activities of compounds isolated from the plant Pathogenic fungus Curvularia sp. J. Fungi 2021, 7, 408. [Google Scholar] [CrossRef]
- Ritieni, A.; Fogliano, V.; Randazzo, G.; Scarallo, A.; Logrieco, A.; Moretti, A.; Mannina, L.; Bottalico, A. Isolation and characterization of fusaproliferin, a new toxic metabolite from Fusarium Proliferatum. Nat. Toxins 1995, 3, 17–20. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.-M.; Li, C.-S.; Wang, B.-G. Sesterterpenes and 2h-pyran-2-ones (=alpha-pyrones) from the mangrove-derived endophytic fungus Fusarium proliferatum MA-84. Hel. Chim. Acta 2013, 96, 437–444. [Google Scholar] [CrossRef]
- Yang, D.; Micalizio, G.C. Stereochemical lability of azatitanacyclopropanes: Dynamic kinetic resolution in reductive cross-coupling reactions with allylic alcohols. Chem. Commun. 2013, 49, 8857–8859. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Meca, G.; Uhlig, S.; Ritieni, A. Fusaproliferin, beauvericin and enniatins: Occurrence in food —A review. World Mycotoxin J. 2012, 5, 71–81. [Google Scholar] [CrossRef]
- Liao, S.; Yuk, N.; Kim, Y.J.; Xu, H.; Li, X.; Wang, L.; Liu, Y.; Jung, H.J. Novel terpestacin derivatives with l-amino acid residue as anticancer agents against U87MG-derived glioblastoma stem cells. Bioorg. Chem. 2023, 132, 106392. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, K.; Masuda, N.; Nishida, H. The first total synthesis of (+/−)-terpestacin, HIV syncytium formation inhibitor. Tetrahedron Lett. 1998, 39, 83–86. [Google Scholar] [CrossRef]
- Trost, B.M.; Dong, G.; Vance, J.A. Cyclic 1,2-diketones as core building blocks: A strategy for the total synthesis of (−)-terpestacin. Chem- Eur. J. 2010, 16, 6265–6277. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Dong, G.; Vance, J.A. A diosphenol-based strategy for the total synthesis of (−)-terpestacin. J. Am. Chem Soc. 2007, 129, 4540–4541. [Google Scholar] [CrossRef]
- Li, K.-L.; Dai, Y.; She, J.-L.; Zeng, Y.-B.; Dai, H.-F.; Ou, S.-L.; Zhou, X.-F.; Liu, Y.-H. Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp. J. Asian Nat. Prod. Res. 2021, 24, 88–95. [Google Scholar] [CrossRef]
- Luo, X.-W.; Chen, C.-M.; Li, K.-L.; Lin, X.-P.; Gao, C.-H.; Zhou, X.-F.; Liu, Y.-H. Sesquiterpenoids and meroterpenoids from a mangrove derived fungus Diaporthe sp. SCSIO 41011. Nat. Prod. Res. 2021, 35, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.W.; Lin, X.P.; Tao, H.M.; Wang, J.F.; Li, J.Y.; Yang, B.; Zhou, X.F.; Liu, Y.H. Isochromophilones A-F, Cytotoxic Chloroazaphilones from the Marine Mangrove Endophytic Fungus Diaporthe sp SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-F.; Liang, R.; Liao, S.-R.; Yang, B.; Tu, Z.-C.; Lin, X.-P.; Wang, B.-G.; Liu, Y. Vaccinols J-S, ten new salicyloid derivatives from the marine mangrove-derived endophytic fungus Pestalotiopsis vaccinii. Fitoterapia 2017, 120, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, X.; Gan, X.; Wang, J.; Wei, X.; Lu, X.; Xu, F.; Wan, J.; Lin, X.; Zhou, X.; et al. Eight new polyketide metabolites from the fungus Pestalotiopsis vaccinii endogenous with the mangrove plant Kandelia candel (L.) Druce. Tetrahedron 2014, 70, 9695–9701. [Google Scholar]
- Zhou, Q.; Luo, X.; Yang, B.; Liu, Y.; Ratnasekera, D.; Zhou, X. New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409. Marine Drugs 2023, 21, 32. [Google Scholar]
- Cimmino, A.; Masi, M.; Minkovich, E.; Evidente, M.; Gannibal, P.; Krivorotov, D.; Chisty, L.; Berestetskiy, A.; Evidente, A. Saponaroxins A-C, a new 19-oxa-tricyclohenicosatetraenone and, a new dioxacyclopropacycloundecene-10-carboaldehyde and its 6,7-dihydro derivative, produced by Alternaria saponariae, a pathogen of a medicinal plant Saponaria officinalis. Tetrahedron Lett. 2016, 57, 1702–1705. [Google Scholar] [CrossRef]
- Ye, B.; Ding, W.; Wang, P.-M.; Xu, J. Two new sesterterpenes from marine-derived fungus Arthrinium sp. Chem. Nat. Comp. 2019, 55, 281–284. [Google Scholar] [CrossRef]
- Mukund, V.; Saddala, M.S.; Farran, B.; Mannavarapu, M.; Alam, A.; Nagaraj, G.P. Molecular docking studies of angiogenesis target protein HIF-1 alpha and genistein in breast cancer. Gene 2019, 701, 169–172. [Google Scholar] [CrossRef]
- Jung, H.J.; Shim, J.S.; Lee, J.; Song, Y.M.; Park, K.C.; Choi, S.H.; Kim, N.D.; Yoon, J.H.; Mungai, P.T.; Schumacker, P.T.; et al. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J. Biol. Chem. 2010, 285, 11584–11595. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Qin, X.; Tian, X.; Liao, L.; Li, K.; Zhou, X.; Yang, X.; Wang, F.; Zhang, T.; et al. Antiviral merosesquiterpenoids produced by the Antarctic fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat.Prod. 2016, 79, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, X.; She, J.; Zhang, H.; Pang, X.; Lin, X.; Zhou, X.; Liu, Y.; Li, Y.; Yang, B. Diversified chaetoglobosins from the marine-derived fungus Emericellopsis sp. SCSIO41202. Molecules 2022, 27, 1823. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Choi, S.H. Effects of endostatin and a new drug terpestacin against human neuroblastoma xenograft and cell lines. Pediatr. Surg. Int. 2013, 29, 1327–1340. [Google Scholar] [CrossRef] [PubMed]



| No. | 1 a | 2 b | 3 c | 4 a |
|---|---|---|---|---|
| 2 | 2.32, dd (9.1, 14.0) | 2.06, m | 2.03 dd (7.0, 16.8) | 2.41, dd (2.8, 13.3) |
| 1.78, m | 1.78 dd (6.3, 13.3) | 1.88, m | ||
| 3 | 5.26, m | 5.50, t (7.5) | 5.15, t (7.7) | 5.39, t (8.4) |
| 5 | 2.72, dd (7.0, 11.2) | 2.25 m | 2.18, m | 2.29, m |
| 1.89, t (11.2) | 2.08, m | |||
| 6 | 5.69, d (15.4) | 2.10 m | 2.13, m | 2.30, m |
| 1.61, m | 2.06, m | 2.24, m | ||
| 7 | 5.82, ddd (7.0, 11.2, 12.6) | 3.60, d (9.5) | 4.94, t (6.3) | 5.14, t (6.3) |
| 9 | 2.08, m | 1.89, m | 2.72, m | 2.21, m |
| 1.81, m | 1.78, m | 2.04, m | ||
| 10 | 2.26, m | 2.24, m | 2.31, m | 1.78, m |
| 1.85, m | 1.79, m | 2.17, m | 1.68, m | |
| 11 | 4.47, t (7.0) | 4.46, d (7.5) | 3.07, t (7.0) | |
| 13 | 5.68, m | 5.61, t (5.5) | 6.79, t (6.3) | 2.91, dd (2.1, 7.7) |
| 14 | 2.57, dd (7.0, 15.4) | 2.51, d (14.0) | 2.63. m | 1.84, m |
| 1.97, ddd (8.4, 11.9, 15.4) | 1.60, ddd (3.5, 9.45, 13.65) | |||
| 15 | 2.46, d (9.8) | 2.58, dd (2.0, 12.0) | 2.76, dd (2.1, 11.2) | 2.85, dd (2.8, 8.4) |
| 19 | 1.01, s | 1.06, s | 0.91, s | 1.04, s |
| 20 | 1.69, s | 1.71, s | 1.55, s | 1.70, s |
| 21 | 1.34, s | 1.15, s | 1.58, s | 1.65, s |
| 22 | 1.67, s | 1.55, s | 1.71, s | 1.27, s |
| 23 | 2.63, q (7.0, 14.0) | 2.63, q (7.0, 16.0) | 2.60, q (7.0, 14.0) | 2.73, q (7.0, 14.0) |
| 24 | 3.86, dd (7.0, 10.5) | 3.86, dd (7.0, 10.5) | 3.63, dd (7.0, 10.5) | 3.87, dd (7.0, 10.5) |
| 3.73, dd (7.0, 10.5) | 3.73, dd (6.5, 10.5) | 3.52, dd (7.0, 10.5) | 3.74, dd (6.3, 10.5) | |
| 25 | 1.30, d (7.0) | 1.26, d (7.0) | 1.20, d (7.0) | 1.27, d (7.0) |
| No. | 1 a | 2 b | 3 c | 4 a |
|---|---|---|---|---|
| 1 | 48.4, C | 50.0, C | 49.3, C | 48.5, C |
| 2 | 38.5, CH2 | 39.9, CH2 | 39.9, CH2 | 37.8, CH2 |
| 3 | 120.8, CH | 120.6, CH | 121.3, CH | 121.1, CH |
| 4 | 137.4, C | 139.3, C | 137.5, C | 138.2, C |
| 5 | 40.0, CH2 | 34.0, CH2 | 40.5, CH2 | 39.7, CH2 |
| 6 | 137.4, CH | 31.0, CH2 | 23.8, CH2 | 23.4, CH2 |
| 7 | 126.2, CH | 77.5, CH | 122.0, CH | 123.3, CH |
| 8 | 83.1, C | 87.4, C | 134, C | 133.4, C |
| 9 | 35.2, CH2 | 36.4, CH2 | 34.6, CH2 | 33.3, CH2 |
| 10 | 30.6, CH2 | 30.4, CH2 | 34.0, CH2 | 30.2, CH2 |
| 11 | 82.4, CH | 83.6, CH | 201.6, C | 75.3, CH |
| 12 | 136.5, C | 137.4, C | 136.6, C | 63.8, C |
| 13 | 121.5, CH | 123.5, CH | 142.7, CH | 62.0, CH |
| 14 | 27.5, CH2 | 29.4, CH2 | 31.7, CH2 | 28.2, CH2 |
| 15 | 50.8, CH | 50.8, CH | 48.7, CH | 47.5, CH |
| 16 | 150.8, C | 152.1, C | 150.3, C | 150.3, C |
| 17 | 147.6, C | 148.9, C | 147.7, C | 147.9, C |
| 18 | 208.8, C | 210.3, C | 207.5, C | 208.1, C |
| 19 | 16.9, CH3 | 16.8, CH3 | 16.3, CH3 | 17.3, CH3 |
| 20 | 15.9, CH3 | 19.5, CH3 | 16.1, CH3 | 14.3, CH3 |
| 21 | 27.0, CH3 | 19.8, CH3 | 17.5, CH3 | 15.1, CH3 |
| 22 | 13.6, CH3 | 14.7, CH3 | 12.0, CH3 | 9.5, CH3 |
| 23 | 37.7, CH | 38.9, CH | 37.8, CH | 37.0, CH |
| 24 | 64.5, CH2 | 65.9, CH2 | 64.3, CH2 | 64.4, CH2 |
| 25 | 13.4, CH3 | 14.5, CH3 | 14.8, CH3 | 13.3, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Li, C.; Chen, Y.; He, Y.; She, J.; Zhou, X.; Tao, H.; Peng, B. Arthproliferins A–D, Four New Sesterterpenes from the Mangrove-Sediment-Derived Fungus Arthrinium sp. SCSIO41221. Molecules 2023, 28, 7246. https://doi.org/10.3390/molecules28217246
Yang B, Li C, Chen Y, He Y, She J, Zhou X, Tao H, Peng B. Arthproliferins A–D, Four New Sesterterpenes from the Mangrove-Sediment-Derived Fungus Arthrinium sp. SCSIO41221. Molecules. 2023; 28(21):7246. https://doi.org/10.3390/molecules28217246
Chicago/Turabian StyleYang, Bin, Cuitian Li, Ying Chen, Yanchun He, Jianglian She, Xuefeng Zhou, Huangming Tao, and Bo Peng. 2023. "Arthproliferins A–D, Four New Sesterterpenes from the Mangrove-Sediment-Derived Fungus Arthrinium sp. SCSIO41221" Molecules 28, no. 21: 7246. https://doi.org/10.3390/molecules28217246
APA StyleYang, B., Li, C., Chen, Y., He, Y., She, J., Zhou, X., Tao, H., & Peng, B. (2023). Arthproliferins A–D, Four New Sesterterpenes from the Mangrove-Sediment-Derived Fungus Arthrinium sp. SCSIO41221. Molecules, 28(21), 7246. https://doi.org/10.3390/molecules28217246