Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy
Abstract
:1. Introduction
2. How to Pair Strains for Co-Culture?
2.1. Based on the Ecological Niches
2.2. Pairing with Pathogens
2.3. Pairing with Sub-Inhibitory Concentration Antibiotics
2.4. Pairing with Phototrophs
2.5. Pairing with Mycolic Acid-Containing Bacteria
2.6. Pairing with Microorganisms Containing Halogen Peroxidase-Related Gene Cluster
3. How to Select the Co-Culture Conditions?
3.1. Culture Parameters
3.1.1. Scale of Fermentation
3.1.2. Optimization of Culture Parameters
3.2. Culture Apparatus
4. How to Screen the Chemical Diversity of Co-Culture Extracts?
4.1. Morphological Interactions
4.2. TLC and HPLC Fingerprints
4.3. MS and Other Methods
5. Activation of Biosynthetic Gene Clusters in Co-Cultured Strains
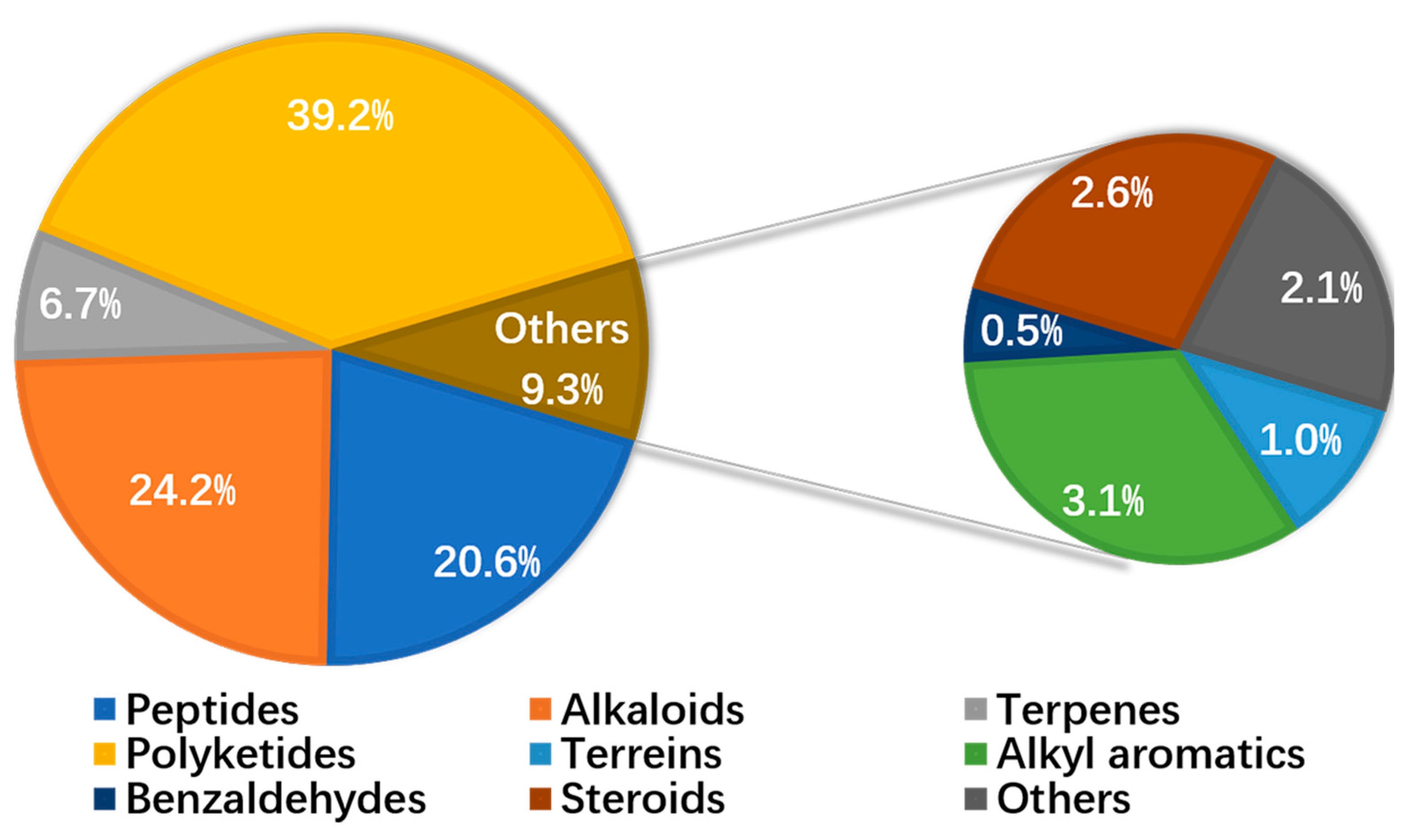
6. Chemical and Activity Diversity of Secondary Metabolites
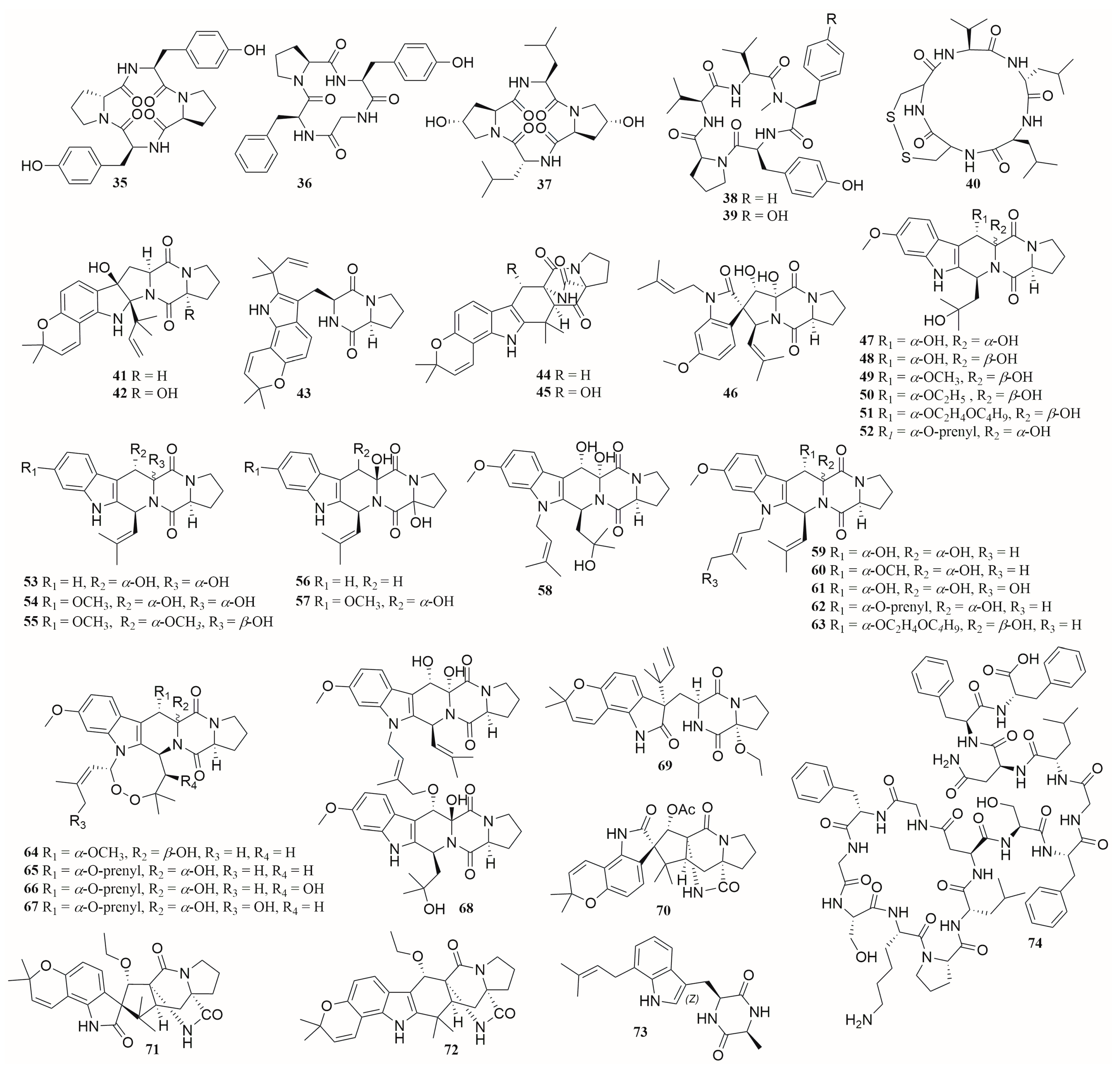
6.1. Peptides
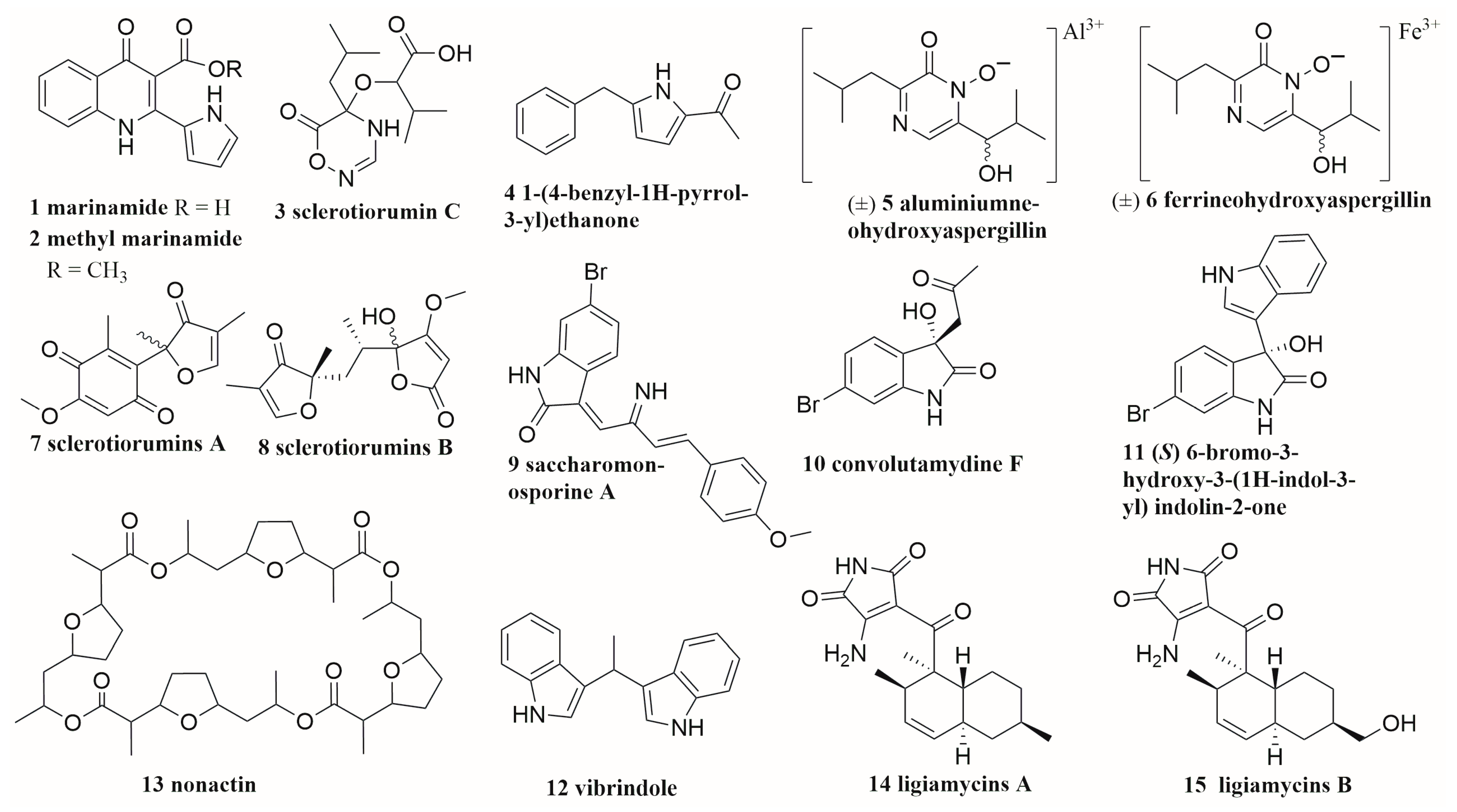
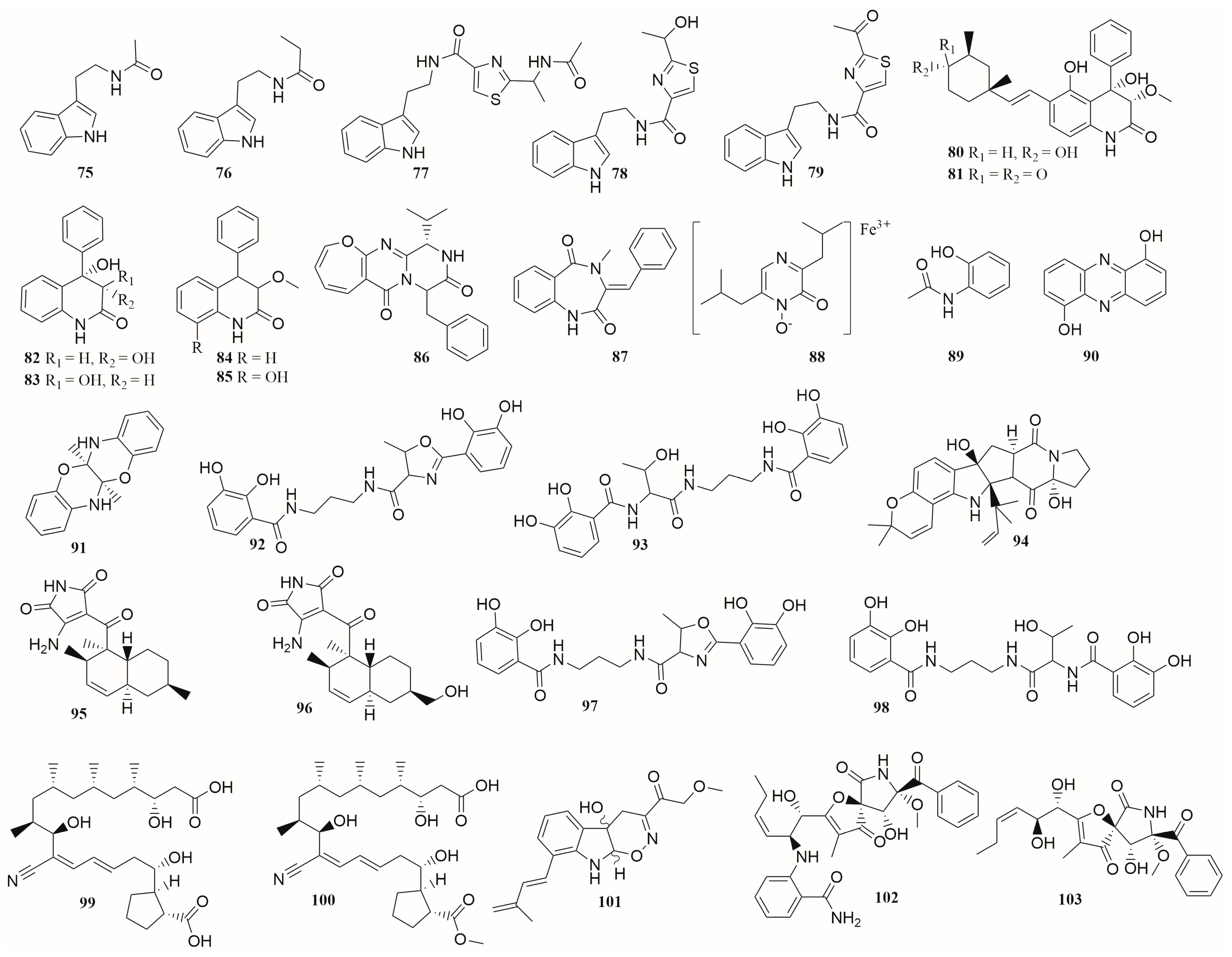
6.2. Alkaloids
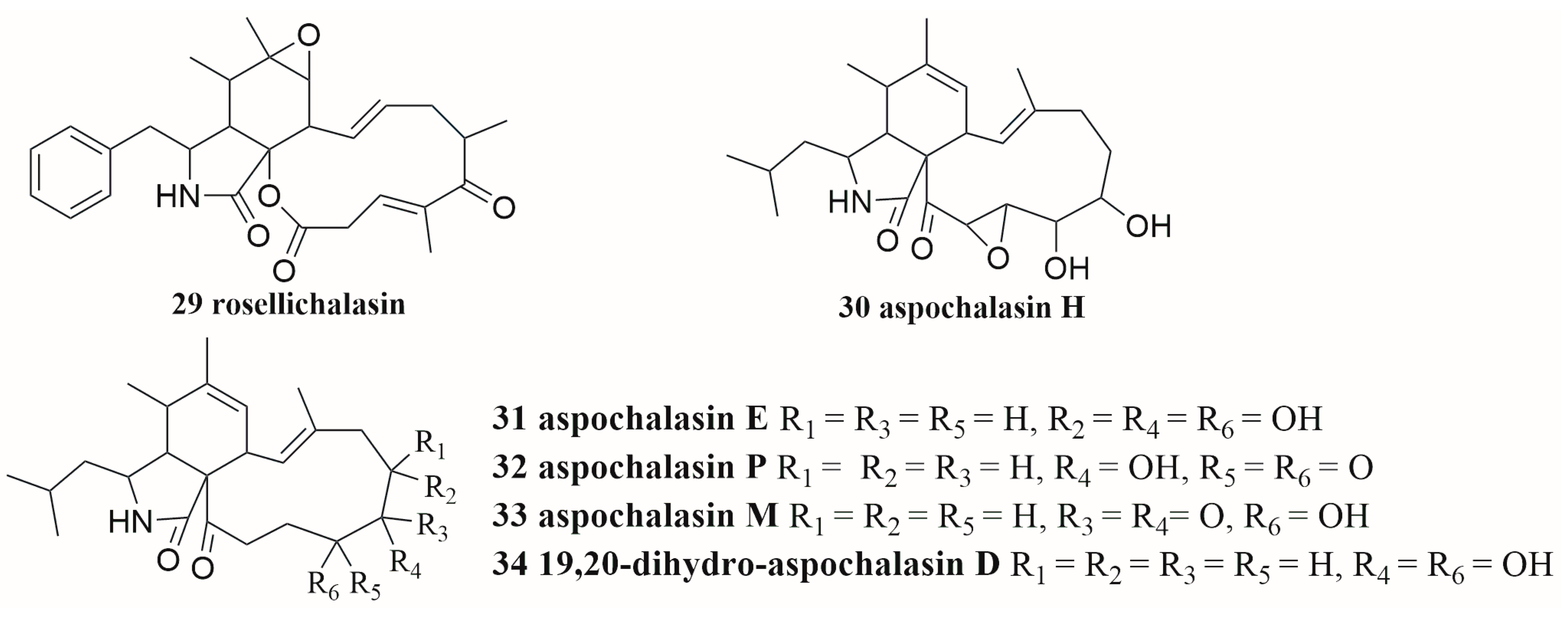
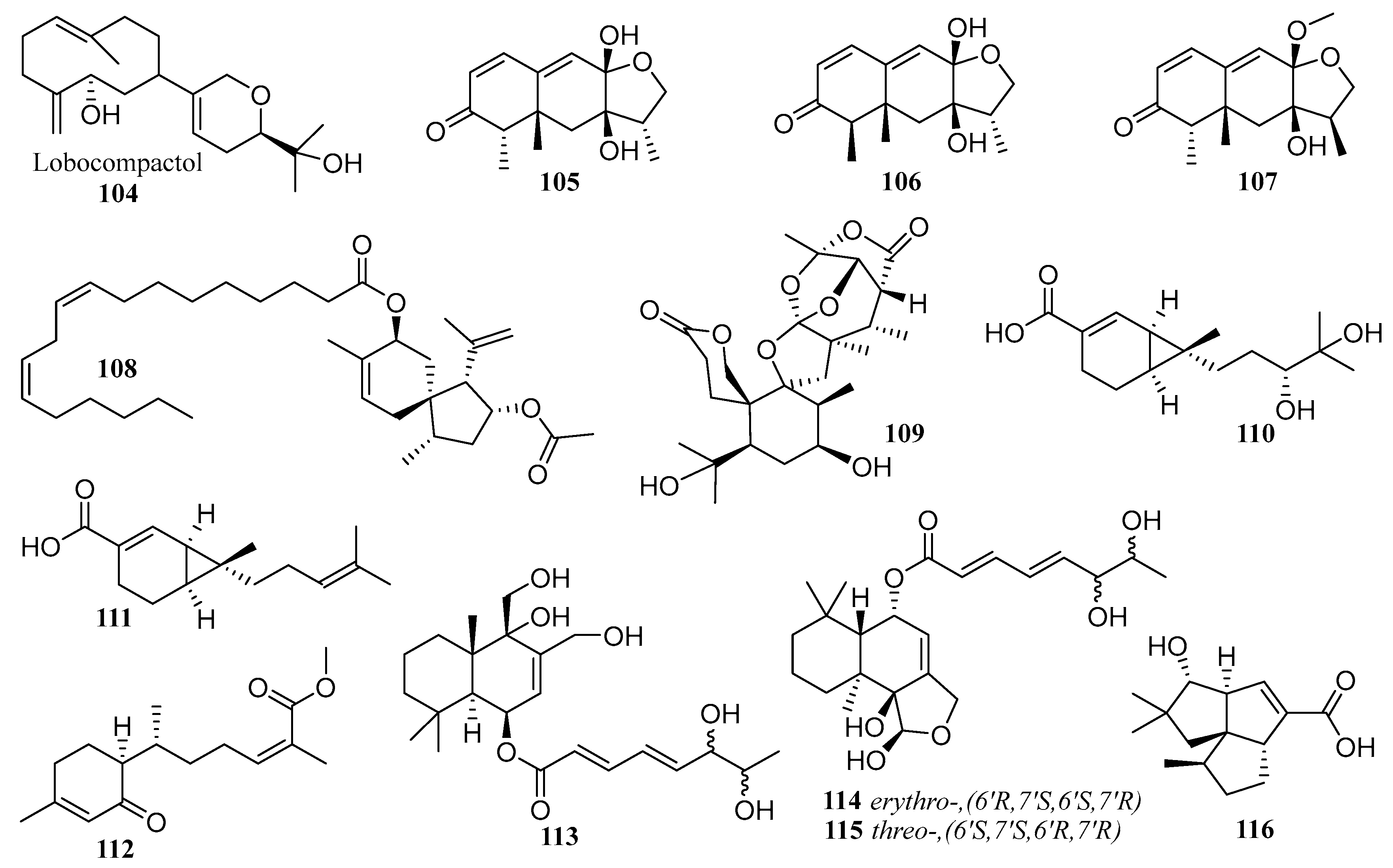
6.3. Terpenes
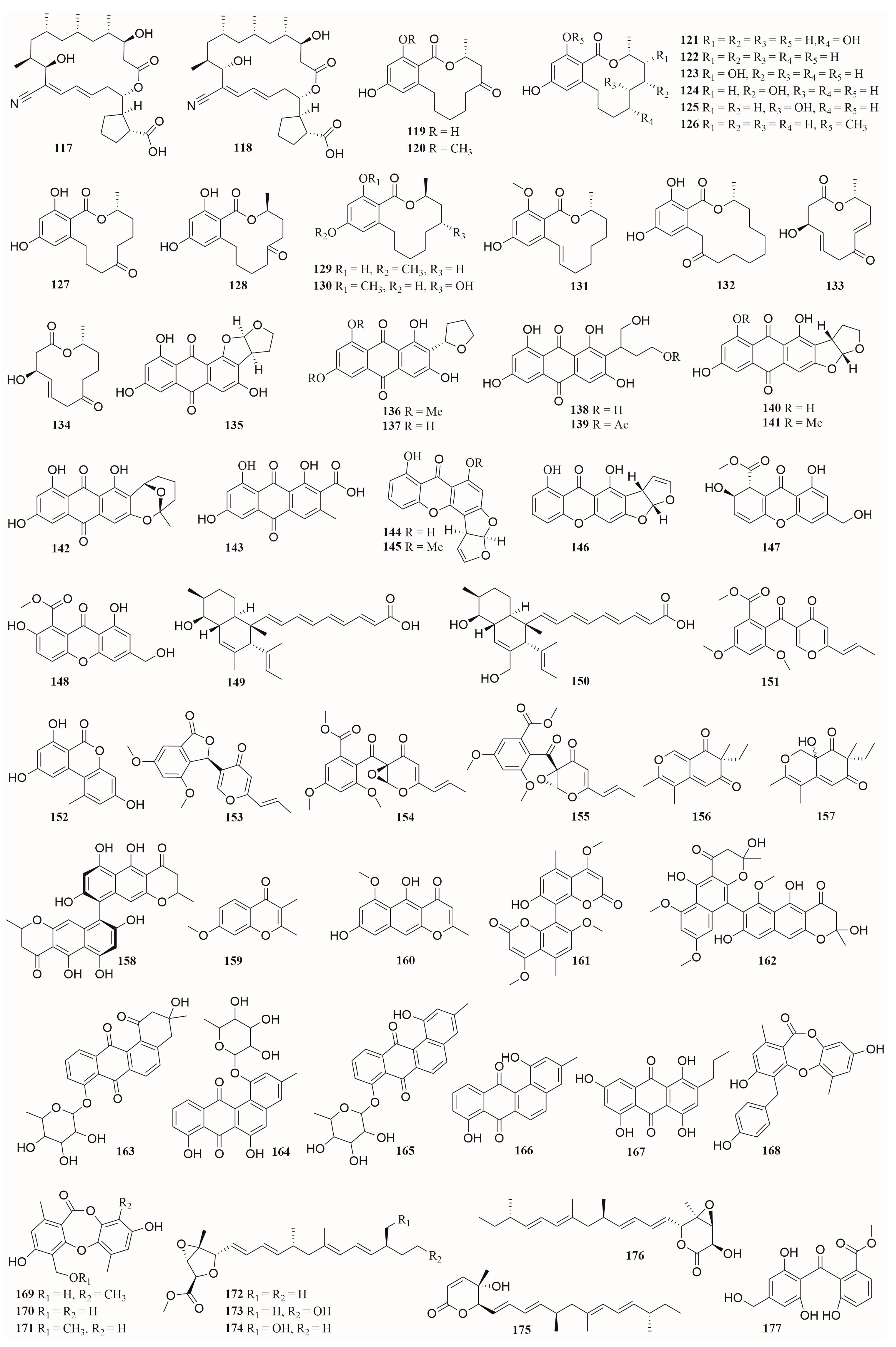
6.4. Polyketides
6.5. Others
7. Challenges and Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| UV | Ultraviolet |
| OSMAC | One strain-many compound |
| SA | Sabouraud medium |
| PDA | Potato dextrose agar medium |
| MIC | Minimal inhibitory concentration |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| HRESIMS | High resolution electrospray ionization mass spectroscopy |
| LC | Liquid chromatography |
| PVDF | Polyvinylidene fluoride |
| HPLC | High-performance liquid chromatography |
| UPLC | Ultra-high-performance liquid chromatography |
| QTOF | Quadrupole time of flight |
| MS/MS | Tandem mass spectrometry |
| TLC | Thin-layer chromatography |
| MS | Mass spectrometer |
| PCA | Principal component analysis |
| MALDI-IMS | Matrix-assisted laser desorption/ionization imaging mass spectrometry |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ikhimiukor, O.O.; Odih, E.E.; Donado-Godoy, P.; Okeke, I.N. A bottom-up view of antimicrobial resistance transmission in developing countries. Nat. Microbiol. 2022, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, A.F.; Zhang, Y.; Adil, M.; Deng, Y. Antibiotic discovery: Combining isolation chip (iChip) technology and co-culture technique. Appl. Microbiol. Biotechnol. 2018, 102, 7333–7341. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Saldivar-Gonzalez, F.I.; Aldas-Bulos, V.D.; Medina-Franco, J.L.; Plisson, F. Natural product drug discovery in the artificial intelligence era. Chem. Sci. 2022, 13, 1526–1546. [Google Scholar] [CrossRef]
- Bergmann, S.; Schumann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Bok, J.W.; Hoffmeister, D.; Maggio-Hall, L.A.; Murillo, R.; Glasner, J.D.; Keller, N.P. Genomic mining for aspergillus natural products. Chem. Biol. 2006, 13, 31–37. [Google Scholar] [CrossRef]
- Leonard, C.A.; Brown, S.D.; Hayman, J.R. Random mutagenesis of the aspergillus oryzae genome results in fungal antibacterial activity. Int. J. Microbiol. 2013, 2013, 901697. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chung, Y.-M.; Wu, Y.-C.; Hunyadi, A.; Wang, C.C.C.; Chang, F.-R. Natural products development under epigenetic modulation in fungi. Phytochem. Rev. 2020, 19, 1323–1340. [Google Scholar] [CrossRef]
- Goss, R.J.; Shankar, S.; Fayad, A.A. The generation of “unnatural” products: Synthetic biology meets synthetic chemistry. Nat. Prod. Rep. 2012, 29, 870–889. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, O.; Murakami, K.; Namikoshi, M. Induced production of a new unprecedented epitrithiodiketopiperazine, chlorotrithiobrevamide, by a culture of the marine-derived trichoderma cf. brevicompactum with dimethyl sulfoxide. Tetrahedron Lett. 2015, 56, 6262–6265. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Buijs, Y.; Isbrandt, T.; Zhang, S.D.; Larsen, T.O.; Gram, L. The antibiotic andrimid produced by vibrio coralliilyticus increases expression of biosynthetic gene clusters and antibiotic production in photobacterium galatheae. Front. Microbiol. 2020, 11, 622055. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The Potential Use of Fungal Co-Culture Strategy for Discovery of New Secondary Metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From axenic to mixed cultures: Technological advances accelerating a paradigm shift in microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in microbial culturing conditions to activate silent biosynthetic gene clusters for novel metabolite production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1381–1400. [Google Scholar] [CrossRef]
- Liu, C.; Kakeya, H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem. Asian J. 2020, 15, 327–337. [Google Scholar] [CrossRef]
- Boruta, T. A bioprocess perspective on the production of secondary metabolites by Streptomyces in submerged co-cultures. World J. Microb. Biot. 2021, 37, 171. [Google Scholar] [CrossRef]
- Alanzi, A.; Elhawary, E.A.; Ashour, M.L.; Moussa, A.Y. Aspergillus co-cultures: A recent insight into their secondary metabolites and microbial interactions. Arch. Pharm. Res. 2023, 46, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of co-culture on the metabolism of marine microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-Y.; Wu, J.-T.; Shao, C.-L.; Li, Z.-Y.; Chen, M.; Wang, C.-Y. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2021, 3, 363–374. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Ye, X.; Wei, B.; Emam, M.; Zhang, H.; Wang, H. The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019. Mar. Drugs 2020, 18, 449. [Google Scholar] [CrossRef]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 2021, 7, e06362. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.; Wu, J.; Pan, J. Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi. Nat. Prod. Res. 2013, 27, 1960–1964. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi aspergillus sclerotiorum and penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Khanfar, M.A.; Rateb, M.E.; Mohammed, T.A.; Hajjar, D.; Hassan, H.M.; Gulder, T.A.M.; Abdelmohsen, U.R. New pim-1 kinase inhibitor from the co-culture of two sponge-associated actinomycetes. Front. Chem. 2018, 6, 538. [Google Scholar] [CrossRef]
- Lim, H.J.; An, J.S.; Bae, E.S.; Cho, E.; Hwang, S.; Nam, S.J.; Oh, K.B.; Lee, S.K.; Oh, D.C. Ligiamycins A and B, decalin-amino-maleimides from the co-culture of Streptomyces sp. and Achromobacter sp. isolated from the marine Wharf Roach, Ligia exotica. Mar. Drugs 2022, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal-fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Schink, B. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek 2002, 81, 257–261. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Parrot, D.; Blumel, M.; Labes, A.; Tasdemir, D. Molecular networking-based metabolome and bioactivity analyses of marine-adapted fungi co-cultivated with phytopathogens. Front. Microbiol. 2018, 9, 2072. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Blumel, M.; Scarpato, S.; Mangoni, A.; Tasdemir, D. Induction of isochromanones by co-cultivation of the marine fungus Cosmospora sp. and the phytopathogen magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 782. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Budnicka, P.; Blumel, M.; Tasdemir, D. Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar. Drugs 2020, 18, 73. [Google Scholar] [CrossRef]
- Kealey, C.; Creaven, C.A.; Murphy, C.D.; Brady, C.B. New approaches to antibiotic discovery. Biotechnol. Lett. 2017, 39, 805–817. [Google Scholar] [CrossRef]
- Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and identification of antibiotic activity of a marine-derived Streptomyces sp. via co-cultures with human pathogens. Mar. Drugs 2017, 15, 250. [Google Scholar] [CrossRef]
- Shakeri Moghaddam, H.; Shahnavaz, B.; Makhdoumi, A.; Iranshahy, M. Evaluating the effect of various bacterial consortia on antibacterial activity of marine Streptomyces sp. AC117. Biocontrol Sci. Technol. 2021, 31, 1248–1266. [Google Scholar] [CrossRef]
- Özkaya, F.C.; Peker, Z.; Camas, M.; Sazak Camas, A.; Altunok, M. Marine fungi against aquaculture pathogens and induction of the activity via co-culture. CLEAN Soil Air Water 2017, 45, 1700238. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef]
- Hodson, S.; Croft, M.; Deery, E.; Smith, A.; Warren, M. Algae acquire Vitamin B12 through a symbiotic relationship with bacteria. Comp. Biochem. Phys. 2007, 146, 25. [Google Scholar] [CrossRef]
- Mouget, J.L.; Dakhama, A.; Lavoie, M.C.; Noue, J.d.l. Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved? FEMS Microbiol. Ecol. 1995, 18, 35–44. [Google Scholar] [CrossRef]
- Chhun, A.; Sousoni, D.; Aguilo-Ferretjans, M.D.M.; Song, L.; Corre, C.; Christie-Oleza, J.A. Phytoplankton trigger the production of cryptic metabolites in the marine actinobacterium salinispora tropica. Microb. Biotechnol. 2021, 14, 291–306. [Google Scholar] [CrossRef]
- Shamikh, Y.I.; El Shamy, A.A.; Gaber, Y.; Abdelmohsen, U.R.; Madkour, H.A.; Horn, H.; Hassan, H.M.; Elmaidomy, A.H.; Alkhalifah, D.H.M.; Hozzein, W.N. Actinomycetes from the Red Sea Sponge Coscinoderma mathewsi: Isolation, Diversity, and Potential for Bioactive Compounds Discovery. Microorganisms 2020, 8, 783. [Google Scholar] [CrossRef]
- Onaka, H.; Mori, Y.; Igarashi, Y.; Furumai, T. Mycolic acid-containing bacteria induce natural-product biosynthesis in streptomyces species. Appl. Environ. Microbiol. 2011, 77, 400–406. [Google Scholar] [CrossRef]
- Jomori, T.; Hara, Y.; Sasaoka, M.; Harada, K.; Setiawan, A.; Hirata, K.; Kimishima, A.; Arai, M. Mycobacterium smegmatis alters the production of secondary metabolites by marine-derived aspergillus niger. J. Nat. Med. 2020, 74, 76–82. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef]
- Maglangit, F.; Fang, Q.; Kyeremeh, K.; Sternberg, J.M.; Ebel, R.; Deng, H. A co-culturing approach enables discovery and biosynthesis of a bioactive indole alkaloid metabolite. Molecules 2020, 25, 256. [Google Scholar] [CrossRef] [PubMed]
- Adnani, N.; Vazquez-Rivera, E.; Adibhatla, S.N.; Ellis, G.A.; Braun, D.R.; Bugni, T.S. Investigation of interspecies interactions within marine micromonosporaceae using an improved co-culture approach. Mar. Drugs 2015, 13, 6082–6098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Glukhov, E.; He, Y.; Liu, Y.; Zhou, L.; Ma, X.; Hu, X.; Hong, P.; Gerwick, W.H.; Zhang, Y. Secondary metabolite variation and bioactivities of two marine aspergillus strains in static co-culture investigated by molecular network analysis and multiple database mining based on LC-PDA-MS/MS. Antibiotics 2022, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, U.; Dethoup, T. Effects of co-culturing with live and autoclaved Bacillus subtilis on antagonistic activity of marine fungi against plant pathogens. J. Pure Appl. Microbiol. 2020, 14, 1245–1254. [Google Scholar] [CrossRef]
- Buijs, Y.; Zhang, S.D.; Jorgensen, K.M.; Isbrandt, T.; Larsen, T.O.; Gram, L. Enhancement of antibiotic production by co-cultivation of two antibiotic producing marine vibrionaceae strains. FEMS Microbiol. Ecol. 2021, 97, 041. [Google Scholar] [CrossRef]
- Boruta, T.; Scigaczewska, A.; Bizukojc, M. Production of secondary metabolites in stirred tank bioreactor co-cultures of streptomyces noursei and aspergillus terreus. Front. Bioeng. Biotechnol. 2022, 10, 1011220. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual induction of new, icrobial secondary metabolites by fungal bacterial co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Rateb, M.E.; Hallyburton, I.; Houssen, W.E.; Bull, A.T.; Goodfellow, M.; Santhanam, R.; Jaspars, M.; Ebel, R. Induction of diverse secondary metabolites in aspergillus fumigatus by microbial co-culture. RSC Adv. 2013, 3, 14444–14450. [Google Scholar] [CrossRef]
- Yu, L.; Hu, Z.; Ma, Z. Production of bioactive tryptamine derivatives by co-culture of marine streptomyces with bacillus mycoides. Nat. Prod. Res. 2015, 29, 2087–2091. [Google Scholar] [CrossRef]
- Yu, L.; Ding, W.; Wang, Q.; Ma, Z.; Xu, X.; Zhao, X.; Chen, Z. Induction of cryptic bioactive 2,5-diketopiperazines in fungus Penicillium sp. DT-F29 by microbial co-culture. Tetrahedron 2017, 73, 907–914. [Google Scholar] [CrossRef]
- Li, X.; Ge, Y.; Ma, Y.; Wang, S.; Li, S.; Yin, Q.; Liu, X.; Wie, J.; Wu, X.; Wu, B. New cytotoxic secondary metabolites from two deep-sea-derived fungi and the co-culture impact on the secondary metabolic patterns. Chem. Biodivers. 2022, 19, e202200055. [Google Scholar] [CrossRef]
- Yu, L.; Ding, W.; Ma, Z. Induced production of cytochalasans in co-culture of marine fungus aspergillus flavipes and actinomycete Streptomyces sp. Nat. Prod. Res. 2016, 30, 1718–1723. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.; He, L.-Y.; Xing, Y.; Guo, Q.-F.; Xiu, Z.-L.; Dong, Y.-S. Biosynthetic Profile in the Co-culture of Aspergillus sydowii and Bacillus subtilis to Produce Novel Benzoic Derivatives. Microb. Ecol. 2022, 85, 1288–1299. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Thissera, B.; Hassan, M.H.A.; Behery, F.A.; Ngwa, C.J.; Hassan, H.M.; Pradel, G.; Abdelmohsen, U.R.; Rateb, M.E. Bio-guided isolation of antimalarial metabolites from the coculture of two red sea sponge-derived Actinokineospora and Rhodococcus spp. Mar. Drugs 2021, 19, 109. [Google Scholar] [CrossRef]
- Moree, W.J.; Phelan, V.V.; Wu, C.H.; Bandeira, N.; Cornett, D.S.; Duggan, B.M.; Dorrestein, P.C. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc. Natl. Acad. Sci. USA 2012, 109, 13811–13816. [Google Scholar] [CrossRef]
- Cornforth, D.M.; Foster, K.R. Competition sensing: The social side of bacterial stress responses. Nat. Rev. Microbiol. 2013, 11, 285–293. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Y.; Wei, J.; Ge, Y.; Jiang, W.; He, S.; Wu, X.; Zhang, X.; Wu, B. Comparative metabolomics reveals fungal conversion of co-existing bacterial metabolites within a synthetic aspergillus-streptomyces community. Mar. Drugs 2021, 19, 526. [Google Scholar] [CrossRef]
- Huang, S.; Ding, W.; Li, C.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414. [Google Scholar]
- Li, C.; Wang, J.; Luo, C.; Ding, W.; Cox, D.G. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.M.; Scharf, S.; Ozkaya, F.C.; Kurtan, T.; Mandi, A.; Fouad, M.A.; Kamel, M.S.; Muller, W.E.G.; Kalscheuer, R.; Lin, W.; et al. Induction of secondary metabolites from the marine-derived fungus aspergillus versicolor through co-cultivation with Bacillus subtilis. Planta Med. 2019, 85, 503–512. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Y.; Banakar, S.P.; Liu, L.; Shao, C.; Li, Z.; Wang, C. New metabolites from the co-culture of marine-derived actinomycete streptomyces rochei MB037 and fungus rhinocladiella similis 35. Front. Microbiol. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Z.; Lam, W.; Gullen, E.A.; Yu, Z.; Wei, Y.; Wang, L.; Zeiss, C.; Beck, A.; Cheng, E.C.; et al. Study of malformin C, a gungal source cyclic pentapeptide, as an anti-cancer drug. PLoS ONE 2015, 10, e0140069. [Google Scholar]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.; Jenssen, M.; Isaksson, J.; Hansen, K.O.; Andersen, J.H.; Hansen, E.H. Bioactivity of serratiochelin A, a siderophore isolated from a co-culture of Serratia sp. and Shewanella sp. Microorganisms 2020, 8, 1042. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, M.S. Induction of antifouling diterpene production by streptomyces cinnabarinus PK209 in co-culture with marine-derived Alteromonas sp. KNS-16. Biosci. Biotechnol. Biochem. 2012, 76, 1849–1854. [Google Scholar] [CrossRef]
- Zhang, L.; Niaz, S.; Khan, D.; Wang, Z.; Zhu, Y.; Zhou, H.; Lin, Y.; Li, J.; Liu, L. Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungus Trichoderma sp. (Strain 307) by co-cultivation with acinetobacter johnsonii (Strain B2). Mar. Drugs 2017, 15, 35. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Li, H.L.; Wang, B.G. Chermebilaenes A and B, new bioactive meroterpenoids from co-cultures of marine-derived isolates of penicillium bilaiae MA-267 and penicillium chermesinum EN-480. Mar. Drugs 2020, 18, 339. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic crimane-type sesquiterpenes from co-culture of the marine-derived fungi aspergillus carneus KMM 4638 and beauveria felina (=Isaria felina) KMM 4639. Mar. Drugs 2022, 20, 584. [Google Scholar] [CrossRef]
- Anjum, K.; Sadiq, I.; Chen, L.; Kaleem, S.; Li, X.-C.; Zhang, Z.; Lian, X.-Y. Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett. 2018, 59, 3490–3494. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29-5 and streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niaz, S.I.; Wang, Z.; Zhu, Y.; Lin, Y.; Li, J.; Liu, L. Alpha-glucosidase inhibitory and cytotoxic botryorhodines from mangrove endophytic fungus Trichoderma sp. 307. Nat. Prod. Res. 2018, 32, 2887–2892. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, G.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Inducing secondary metabolite production by combined culture of talaromyces aculeatus and penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef]
- Ebada, S.S.; Fischer, T.; Klassen, S.; Hamacher, A.; Roth, Y.O.; Kassack, M.U.; Roth, E.H. A new cytotoxic steroid from co-fermentation of two marine alga-derived micro-organisms. Nat. Prod. Res. 2014, 28, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Yang, S.Q.; Cao, J.; Li, Y.H.; Wang, B.G. Induced terreins production from marine red algal-derived endophytic fungus aspergillus terreus EN-539 co-cultured with symbiotic fungus paecilomyces lilacinus EN-531. J. Antibiot. 2020, 73, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Sun, Z.; Peng, J.; Zhu, M.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Secondary metabolites produced by combined culture of penicillium crustosum and a Xylaria sp. J. Nat. Prod. 2019, 82, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, W.; Li, C.; Huang, S.; She, Z.; Lin, Y. A new polysubstituted benzaldehyde from the co-culture broth of two marine fungi (strains nos. E33 and K38). Chem. Nat. Compd. 2013, 49, 799–802. [Google Scholar] [CrossRef]
- Diender, M.; Parera Olm, I.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Sun, T.; Yu, F.; Zhang, K.; Feng, Y.; Xu, C.; Wang, B.; Cheng, L. Synthetic microbial consortia with programmable ecological interactions. Methods Ecol. Evol. 2022, 13, 1608–1621. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Boedeker, C.; Pinto, D.; Vollmers, J.; Rivas-Marin, E.; Kohn, T.; Peeters, S.H.; Heuer, A.; Rast, P.; et al. Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat. Microbiol. 2020, 5, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Gui, M.; Li, H.; Yu, C.; Li, H.; Zeng, Z.; Sun, P. Secondary Metabolites from Marine Micromonospora: Chemistry and Bioactivities. Chem. Biodivers. 2020, 17, e2000024. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, N.; Chai, Z.; Zhou, M.; Xiao, C.; Zhao, S.; Yang, X. Secondary Metabolites from Marine-Derived Bacillus: A Comprehensive Review of Origins, Structures, and Bioactivities. Mar. Drugs 2022, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, R.; Xia, T.; Zhang, S.; Ma, S.; Guo, Z. Natural Products and Biological Activity from Actinomycetes Associated with Marine Algae. Molecules 2023, 28, 5138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, H.; Li, Y.; Liao, S.; Liu, Y. Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy. Molecules 2023, 28, 6371. https://doi.org/10.3390/molecules28176371
Li X, Xu H, Li Y, Liao S, Liu Y. Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy. Molecules. 2023; 28(17):6371. https://doi.org/10.3390/molecules28176371
Chicago/Turabian StyleLi, Xiaolin, Huayan Xu, Yuyue Li, Shengrong Liao, and Yonghong Liu. 2023. "Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy" Molecules 28, no. 17: 6371. https://doi.org/10.3390/molecules28176371
APA StyleLi, X., Xu, H., Li, Y., Liao, S., & Liu, Y. (2023). Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy. Molecules, 28(17), 6371. https://doi.org/10.3390/molecules28176371