Novel MAGL Inhibitors Alleviate LPS-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammatory Vesicles, Modulating Intestinal Flora, Repairing the Intestinal Barrier, and Interfering with Serum Metabolism
Abstract
:1. Introduction
2. Results
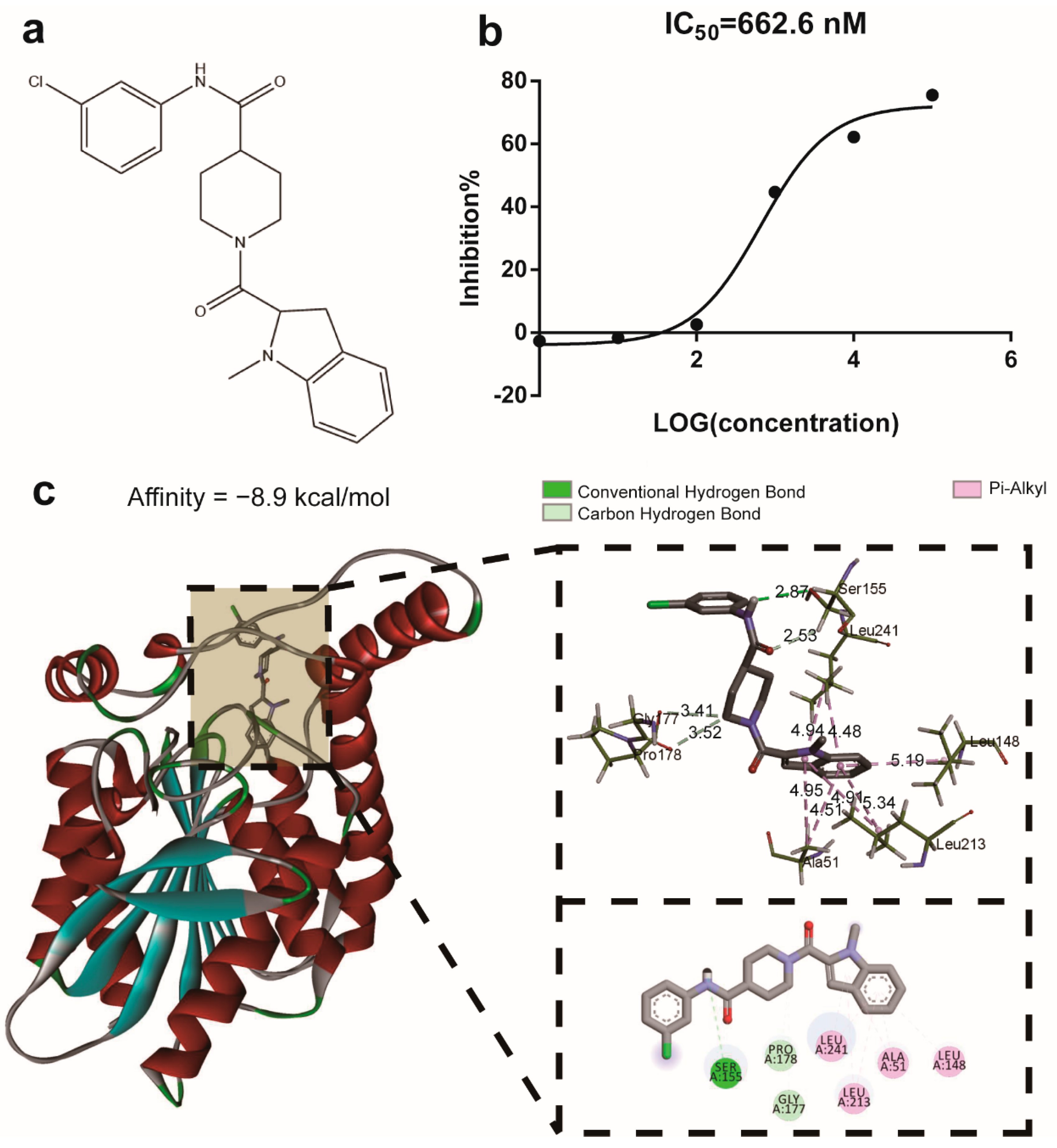
2.1. The Compound M-18C Functions as an Inhibitor of the Enzyme MAGL
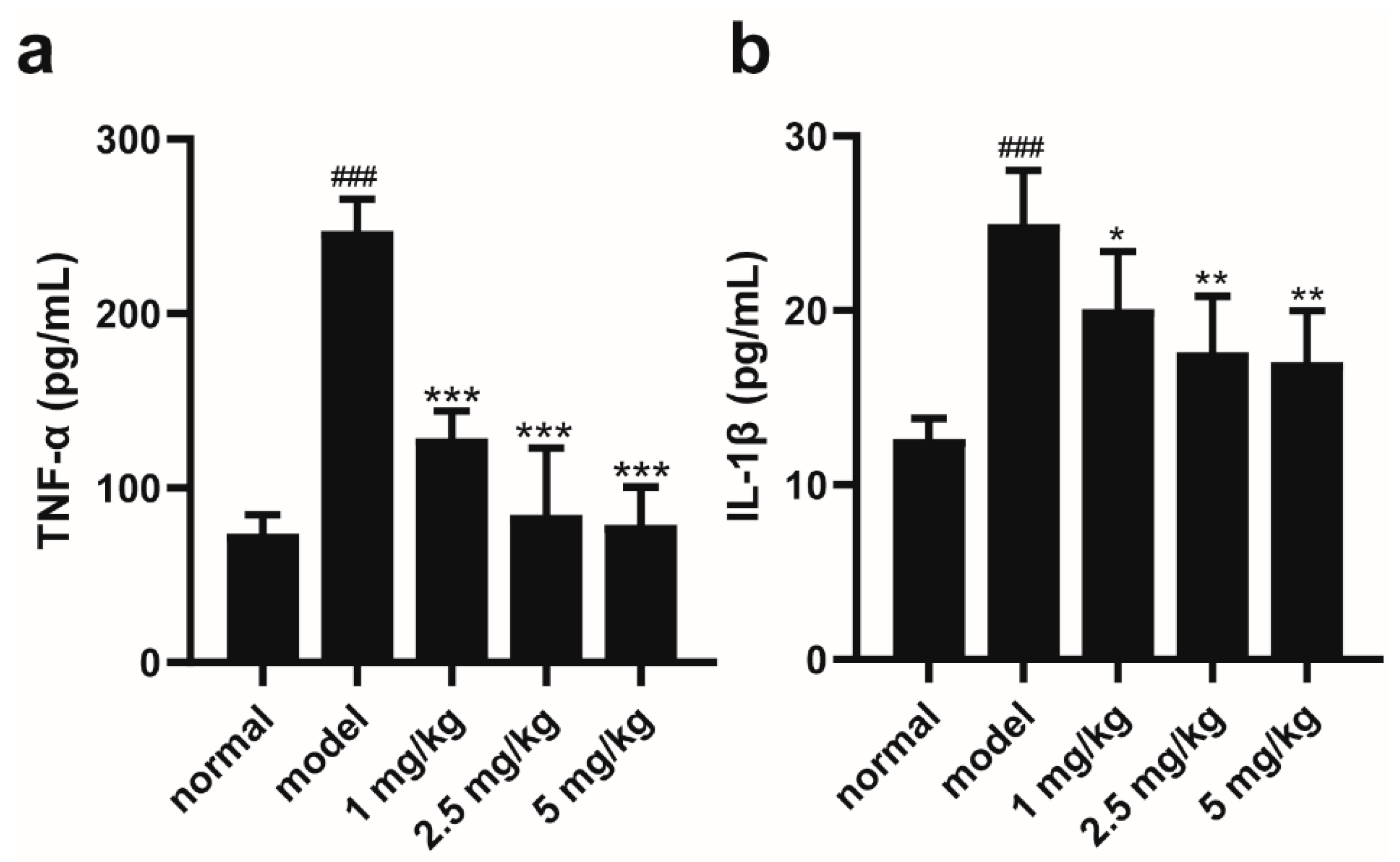
2.2. M-18C Reduces the Levels of TNF-α and IL-1β in the Serum of Mice Caused by LPS
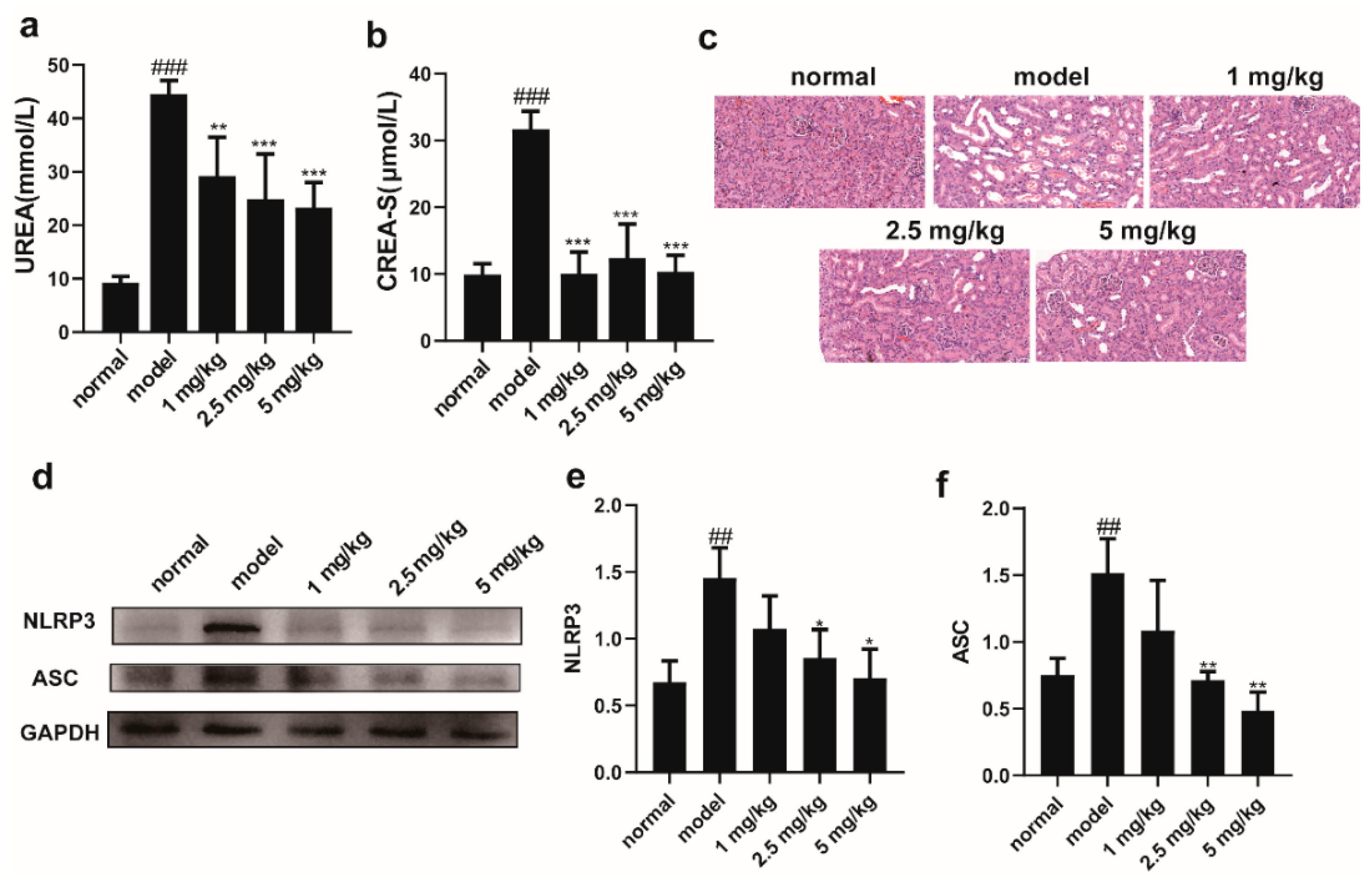
2.3. M-18C Alleviates LPS-Induced AKI in Mice by Inhibiting NLRP3-Mediated Inflammation
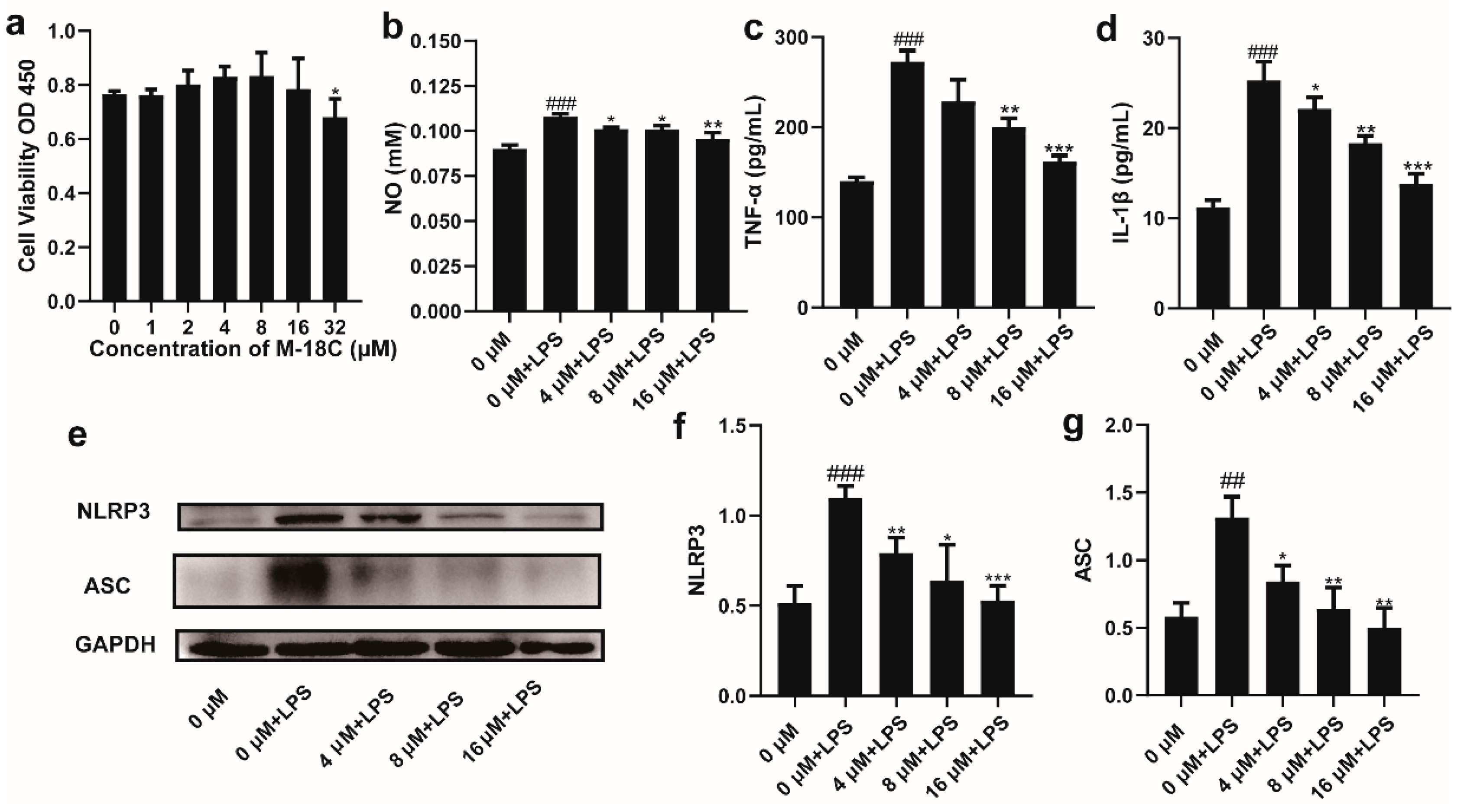
2.4. The Administration of M-18C Significantly Reduced NO, TNF-α, and IL-1β Levels and Downregulated NLRP3 and ASC Expression in LPS-induced RAW264.7
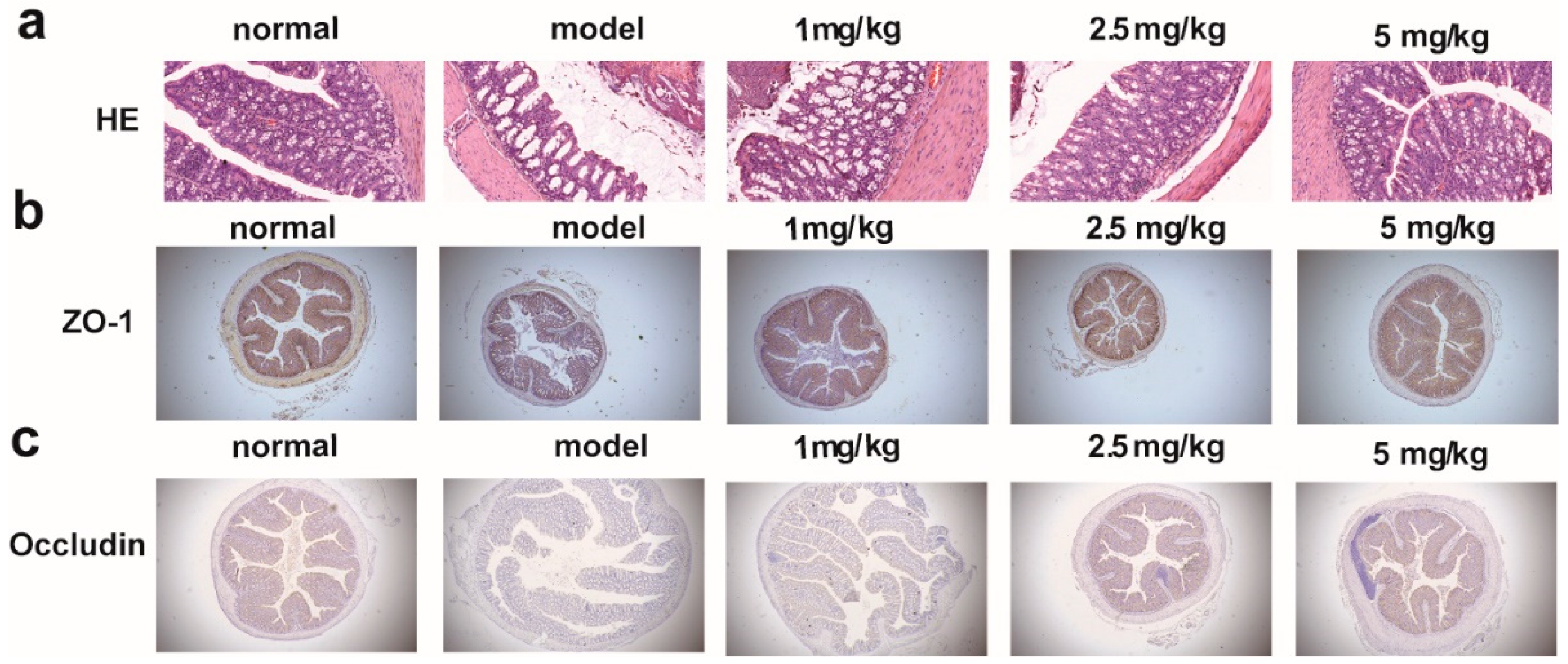
2.5. M-18C Protects the Intestinal Barrier Caused by LPS
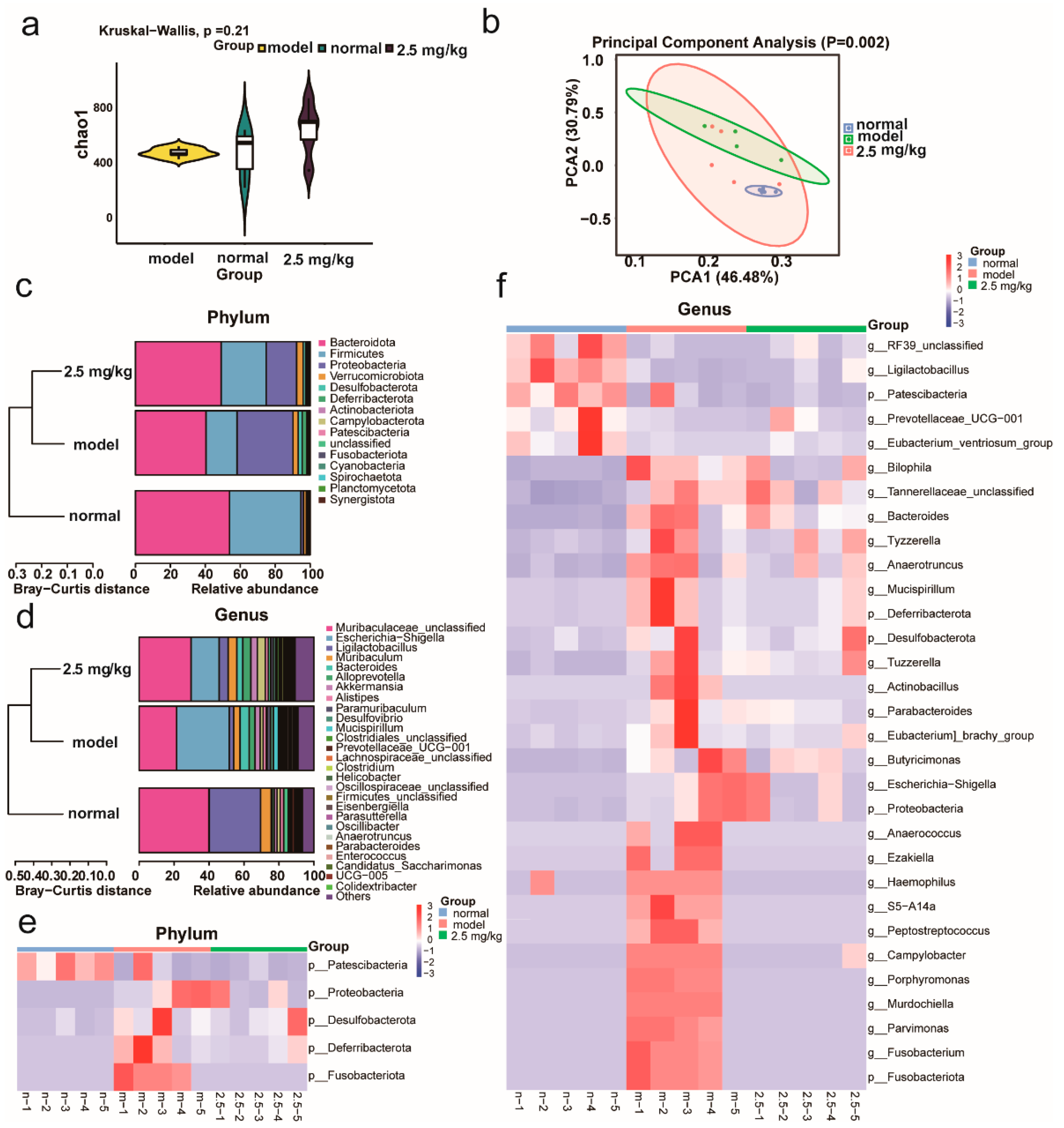
2.6. M-18C Regulates Imbalance of Gut Microbiota Caused by LPS
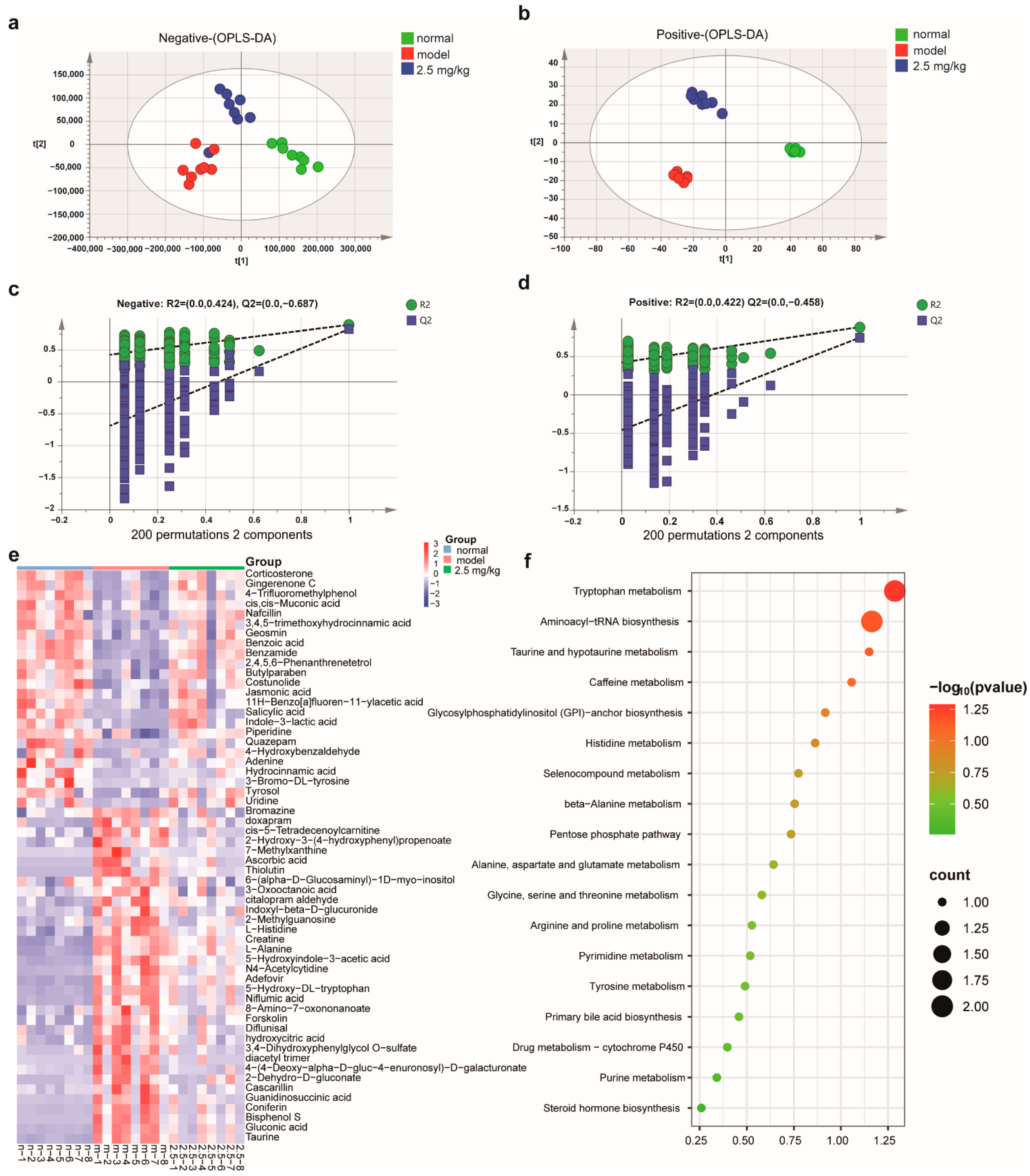
2.7. M-18C Regulates Serum Metabolism Caused by LPS
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Establishment of a Mouse Model of LPS-Induced AKI
4.3. IC50 Determination of MAGL Inhibitor
4.4. Molecular Docking
4.5. Cell Lines and Cell Culture
4.6. Cell Viability Assessment
4.7. Determination of NO
4.8. Western Blotting Assay
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Blood Biochemistry
4.11. Hematoxylin and Eosin (HE) Staining
4.12. Immunohistochemistry (IHC)
4.13. Examining the Influence of M-18C on Serum Metabolism Caused by LPS via Unbiased LC-MS Analysis
4.14. 16S rRNA Gene Sequencing
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Wu, B.; Chen, Q.; Min, S. Parecoxib ameliorates renal toxicity and injury in sepsis-induced mouse model and LPS-induced HK-2 cells. Drug Dev. Res. 2022, 83, 659–668. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery from Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.L.; Ix, J.H. Is Tubular Dysfunction a Risk Factor for AKI? Nephron 2020, 144, 680–682. [Google Scholar] [CrossRef]
- Duncan, C.F.; Youngstein, T.; Kirrane, M.D.; Lonsdale, D.O. Diagnostic Challenges in Sepsis. Curr. Infect. Dis. Rep. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Adib-Conquy, M.; Cavaillon, J.M. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007, 581, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Krivan, S.; Kapelouzou, A.; Vagios, S.; Tsilimigras, D.I.; Katsimpoulas, M.; Moris, D.; Aravanis, C.V.; Demesticha, T.D.; Schizas, D.; Mavroidis, M.; et al. Increased expression of Toll-like receptors 2, 3, 4 and 7 mRNA in the kidney and intestine of a septic mouse model. Sci. Rep. 2019, 9, 4010. [Google Scholar] [CrossRef]
- Chao, L.K.; Lin, C.H.; Chiu, H.W.; Wong, W.T.; Chiu, H.W.; Tasi, Y.L.; Kuo, Y.H.; Chiu, Y.C.; Liu, M.L.; Ho, C.L.; et al. Peroxyauraptenol Inhibits Inflammation and NLRP3 Inflammasome Activation by Inhibiting Reactive Oxygen Species Generation and Preserving Mitochondrial Integrity. J. Agric. Food Chem. 2015, 63, 1210–1219. [Google Scholar] [CrossRef]
- Zhang, J.; Ankawi, G.; Sun, J.; Digvijay, K.; Yin, Y.; Rosner, M.H.; Ronco, C. Gut-kidney crosstalk in septic acute kidney injury. Crit. Care 2018, 22, 117. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.G.; Kim, D.J.; Park, S.H.; Jeong, K.H.; Lee, Y.H.; Lim, S.J.; Lee, S.H.; Moon, J.Y. Inflammasome-Independent Role of NLRP3 Mediates Mitochondrial Regulation in Renal Injury. Front. Immunol. 2018, 9, 2563. [Google Scholar] [CrossRef]
- Dinh, T.P.; Freund, T.F.; Piomelli, D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem. Phys. Lipids 2002, 121, 149–158. [Google Scholar] [CrossRef]
- Pagano, E.; Borrelli, F.; Orlando, P.; Romano, B.; Monti, M.; Morbidelli, L.; Aviello, G.; Imperatore, R.; Capasso, R.; Piscitelli, F.; et al. Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol. Res. 2017, 119, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Karwad, M.A.; Couch, D.G.; Theophilidou, E.; Sarmad, S.; Barrett, D.A.; Larvin, M.; Wright, K.L.; Lund, J.N.; O’Sullivan, S.E. The role of CB(1) in intestinal permeability and inflammation. FASEB J. 2017, 31, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Oveisi, F.; Khanifar, E.; Moreno-Sanz, G.; Vaziri, N.D.; Piomelli, D. Increased Renal 2-Arachidonoylglycerol Level Is Associated with Improved Renal Function in a Mouse Model of Acute Kidney Injury. Cannabis Cannabinoid Res. 2016, 1, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Çakır, M.; Aydın, A.; Tekin, S. Protective effect of fatty acid amide hydrolase inhibitor URB597 and monoacylglycerol lipase inhibitor KML29 on renal ischemia–reperfusion injury via toll-like receptor 4/nuclear factor-kappa B pathway. Int. Immunopharmacol. 2023, 114, 109586. [Google Scholar] [CrossRef]
- Chen, R.; Xu, H.; Guo, Z.; Zhang, P.; Chen, J.; Chen, Z. CID16020046, a GPR55 antagonist, attenuates sepsis-induced acute kidney injury. Mol. Med. Rep. 2022, 25, 155. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Ide, S.; Chen, Y.; Kitai, H.; Ren, J.; Fradin, H.; Lu, X.; Souma, T.; Crowley, S.D. A macrophage-endothelial immunoregulatory axis ameliorates septic acute kidney injury. Kidney Int. 2023, 103, 514–528. [Google Scholar] [CrossRef]
- Wang, C.Q.; Su, Z.; Dai, C.G.; Song, J.L.; Qian, B. Multi-omics analysis reveals BDE47 induces depression-like behaviors in mice by interfering with the 2-arachidonoyl glycerol-associated microbiota-gut-brain axis. Ecotoxicol. Environ. Saf. 2023, 259, 115041. [Google Scholar] [CrossRef]
- Saranya, G.R.; Viswanathan, P. Gut microbiota dysbiosis in AKI to CKD transition. Biomed. Pharmacother. 2023, 161, 114447. [Google Scholar] [CrossRef]
- He, Y.; Xu, M.; Lu, S.; Zou, W.; Wang, Y.; Fakhar, E.A.K.M.; Iqbal, M.; Li, K. Seaweed polysaccharides treatment alleviates injury of inflammatory responses and gut barrier in LPS-induced mice. Microb. Pathog. 2023, 180, 106159. [Google Scholar] [CrossRef]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, X.; Zhu, Y.; Xu, J.; Mao, H.; Li, J.; Zhang, J.; Zhu, X. Contribution of gut microbiota toward renal function in sepsis. Front. Microbiol. 2022, 13, 985283. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwata, Y.; Nakade, Y.; Wada, T. Significance of the Gut Microbiota in Acute Kidney Injury. Toxins 2021, 13, 369. [Google Scholar] [CrossRef]
- Zmora, N.; Levy, M.; Pevsner-Fishcer, M.; Elinav, E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 2017, 10, 865–883. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Huo, J.; Yan, Y. Sesamolin Attenuates Kidney Injury, Intestinal Barrier Dysfunction, and Gut Microbiota Imbalance in High-Fat and High-Fructose Diet-Fed Mice. J. Agric. Food Chem. 2023, 71, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, T.; Kang, J.; Xu, J.; Dengzhang, Y.; Zhao, Z.; Yang, C.; Wu, M.; Xu, X.; Zhang, G.; et al. Synergistic Effect of Huangqin Decoction Combined Treatment with Radix Actinidiae chinensis on DSS and AOM-Induced Colorectal Cancer. Front. Pharmacol. 2022, 13, 933070. [Google Scholar] [CrossRef]
- Herp, S.; Durai Raj, A.C.; Salvado Silva, M.; Woelfel, S.; Stecher, B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Med. Microbiol. Immunol. 2021, 210, 173–179. [Google Scholar] [CrossRef]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota. mSystems 2017, 2, e00171-16. [Google Scholar] [CrossRef]
- Wei, B.; Ren, P.; Xue, C.; Wang, Y.; Tang, Q. Guluronate oligosaccharides exerts beneficial effects on hyperuricemia and regulation of gut microbiota in mice. Food Biosci. 2023, 54, 102855. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Ma, X.; Xu, B.; Chen, L.; Chen, C.; Liu, W.; Liu, Y.; Xiang, Z. Therapeutic effect of Patrinia villosa on TNBS-induced ulcerative colitis via metabolism, vitamin D receptor and NF-kappaB signaling pathways. J. Ethnopharmacol. 2022, 288, 114989. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Zhang, Z.; Tian, X.; Liang, X.; Lu, Y.; Shi, Y.; Kuerman, M.; Wang, R.; Yu, Z.; Gong, P.; et al. Bifidobacterium bifidum Ameliorates DSS-Induced Colitis in Mice by Regulating AHR/NRF2/NLRP3 Inflammasome Pathways through Indole-3-lactic Acid Production. J. Agric. Food Chem. 2023, 71, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, J.; Chen, P.; Li, W.; Shao, J.; Hong, S.; Wang, Y.; Chen, L.; Luo, W.; Liang, G. Costunolide covalently targets NACHT domain of NLRP3 to inhibit inflammasome activation and alleviate NLRP3-driven inflammatory diseases. Acta Pharm. Sin. B 2023, 13, 678–693. [Google Scholar] [CrossRef] [PubMed]
| No. | Name | Formula | RT (min) | Ion Mod | VIP | Model vs. Normal | 2.5 mg/kg vs. Model |
|---|---|---|---|---|---|---|---|
| 1 | 11H-Benzo[a]fluoren-11-ylacetic acid | C19H14O2 | 4.714 | ESI− | 1.23099 | ↓## | ↑* |
| 2 | 2,4,5,6-Phenanthrenetetrol | C14H10O4 | 8.463 | ESI− | 1.50203 | ↓#### | ↑* |
| 3 | 2-Dehydro-D-gluconate | C6H10O7 | 1.202 | ESI− | 1.33805 | ↑### | ↓* |
| 4 | 2-Hydroxy-3-(4-hydroxyphenyl-propenoate | C9H8O4 | 1.882 | ESI− | 1.59186 | ↑## | ↓** |
| 5 | 2-Methylguanosine | C11H15N5O5 | 4.949 | ESI− | 1.57437 | ↑## | ↓** |
| 6 | 3,4,5-trimethoxyhydrocinnamic acid | C12H16O5 | 10.617 | ESI− | 1.45642 | ↓#### | ↑* |
| 7 | 3,4-Dihydroxyphenylglycol O-sulfate | C8H10O7S | 1.612 | ESI− | 1.42525 | ↑## | ↓* |
| 8 | 3-Bromo-DL-tyrosine | C9H10BrNO3 | 5.655 | ESI− | 1.02757 | ↓# | ↑* |
| 9 | 3-Oxooctanoic acid | C8H14O3 | 5.061 | ESI− | 1.02523 | ↑## | ↓* |
| 10 | 4-(4-Deoxy-alpha-D-gluc-4-enuronosyl)-D-galacturonate | C12H16O12 | 1.167 | ESI− | 1.40788 | ↑## | ↓* |
| 11 | 4-Hydroxybenzaldehyde | C7H6O2 | 4.433 | ESI− | 1.01709 | ↓# | ↑* |
| 12 | 4-Trifluoromethylphenol | C7H5F3O | 3.941 | ESI− | 1.45253 | ↓#### | ↑* |
| 13 | 5-Hydroxy-DL-tryptophan | C11H12N2O3 | 3.802 | ESI− | 1.46735 | ↑#### | ↓* |
| 14 | 5-Hydroxyindole-3-acetic acid | C10H9NO3 | 4.407 | ESI− | 1.47610 | ↑#### | ↓* |
| 15 | 6-(alpha-D-Glucosaminyl)-1D-myo-inositol | C12H23NO10 | 1.378 | ESI− | 1.47230 | ↑# | ↓** |
| 16 | 7-Methylxanthine | C6H6N4O2 | 1.854 | ESI− | 1.31582 | ↑# | ↓* |
| 17 | 8-Amino-7-oxononanoate | C9H17NO3 | 6.475 | ESI− | 1.17585 | ↑## | ↓* |
| 18 | Adefovir | C8H12N5O4P | 6.24 | ESI− | 1.52359 | ↑#### | ↓* |
| 19 | Adenine | C5H5N5 | 6.998 | ESI− | 1.05053 | ↓# | ↑* |
| 20 | Bisphenol S | C12H10O4S | 1.316 | ESI− | 1.44627 | ↑## | ↓* |
| 21 | Bromazine | C17H20BrNO | 5.243 | ESI− | 1.31946 | ↑# | ↓* |
| 22 | Butylparaben | C11H14O3 | 10.001 | ESI− | 1.62456 | ↓## | ↑** |
| 23 | Cascarillin | C22H32O7 | 8.64 | ESI− | 1.27469 | ↑# | ↓* |
| 24 | cis,cis-Muconic acid | C6H6O4 | 6.379 | ESI− | 1.35960 | ↓## | ↑* |
| 25 | cis-5-Tetradecenoylcarnitine | C21H39NO4 | 12.571 | ESI− | 1.67272 | ↑### | ↓*** |
| 26 | Citalopram aldehyde | C18H14FNO2 | 7.431 | ESI− | 1.13161 | ↑## | ↓* |
| 27 | Coniferin | C16H22O8 | 7.579 | ESI− | 1.26308 | ↑### | ↓* |
| 28 | Corticosterone | C21H30O4 | 11.773 | ESI− | 1.50496 | ↓#### | ↑** |
| 29 | Costunolide | C15H20O2 | 11.187 | ESI− | 1.31224 | ↓## | ↑* |
| 30 | Creatine | C4H9N3O2 | 1.427 | ESI− | 1.70032 | ↑#### | ↓**** |
| 31 | Diacetyl trimer | C12H18O6 | 1.653 | ESI− | 1.28257 | ↑## | ↓* |
| 32 | Diflunisal | C13H8F2O3 | 1.809 | ESI− | 1.27618 | ↑## | ↓* |
| 33 | Doxapram | C24H30N2O2 | 7.239 | ESI− | 1.31590 | ↑## | ↓* |
| 34 | Forskolin | C22H34O7 | 8.29 | ESI− | 1.34054 | ↑### | ↓* |
| 35 | Geosmin | C12H22O | 11.826 | ESI− | 1.27614 | ↓## | ↑* |
| 36 | Gingerenone C | C20H22O4 | 10.646 | ESI− | 1.35761 | ↓### | ↑* |
| 37 | Gluconic acid | C6H12O7 | 1.219 | ESI− | 1.41214 | ↑### | ↓* |
| 38 | Guanidinosuccinic acid | C5H9N3O4 | 1.200 | ESI− | 1.31446 | ↑## | ↓* |
| 39 | Hydrocinnamic acid | C9H10O2 | 7.034 | ESI− | 1.15891 | ↓## | ↑* |
| 40 | Hydroxycitric acid | C6H8O8 | 1.169 | ESI− | 1.41456 | ↑### | ↓** |
| 41 | Indole-3-lactic acid | C11H11NO3 | 4.963 | ESI− | 1.45166 | ↓### | ↑** |
| 42 | Indoxyl-beta-D-glucuronide | C14H15NO7 | 3.785 | ESI− | 1.15719 | ↑## | ↓* |
| 43 | Jasmonic acid | C12H18O3 | 10.665 | ESI− | 1.44216 | ↓### | ↑* |
| 44 | L-Histidine | C6H9N3O2 | 1.448 | ESI− | 1.59545 | ↑#### | ↓** |
| 45 | N4-Acetylcytidine | C11H15N3O6 | 5.189 | ESI− | 1.42193 | ↑### | ↓* |
| 46 | Nafcillin | C21H22N2O5S | 10.423 | ESI− | 1.14129 | ↓# | ↑* |
| 47 | Niflumic acid | C13H9F3N2O2 | 1.507 | ESI− | 1.49980 | ↑#### | ↓* |
| 48 | Quazepam | C17H11ClF4N2S | 1.892 | ESI− | 1.26974 | ↓### | ↑* |
| 49 | Salicylic acid | C7H6O3 | 5.038 | ESI− | 1.32580 | ↓### | ↑* |
| 50 | Taurine | C2H7NO3S | 1.318 | ESI− | 1.46605 | ↑#### | ↓* |
| 51 | Thiolutin | C8H8N2O2S2 | 1.502 | ESI− | 1.27014 | ↑# | ↓* |
| 52 | Tyrosol | C8H10O2 | 8.738 | ESI− | 1.51769 | ↓## | ↑** |
| 53 | Uridine | C9H12N2O6 | 3.061 | ESI− | 1.01969 | ↓# | ↑** |
| 54 | Ascorbic acid | C6H8O6 | 0.910 | ESI+ | 3.63786 | ↑## | ↓* |
| 55 | Benzamide | C7H7NO | 3.990 | ESI+ | 1.23133 | ↓#### | ↑*** |
| 56 | Benzoic acid | C7H6O2 | 4.250 | ESI+ | 9.38332 | ↓#### | ↑*** |
| 57 | Creatine | C4H9N3O2 | 0.916 | ESI+ | 9.34523 | ↑#### | ↓* |
| 58 | Gluconic acid | C6H12O7 | 0.772 | ESI+ | 2.19480 | ↑### | ↓* |
| 59 | L-Alanine | C3H7NO2 | 0.917 | ESI+ | 1.63292 | ↑#### | ↓* |
| 60 | Piperidine | C5H11N | 10.42 | ESI+ | 2.02758 | ↓## | ↑* |
| 61 | Taurine | C2H7NO3S | 0.812 | ESI+ | 4.30360 | ↑#### | ↓* |
| 62 | Thiolutin | C8H8N2O2S2 | 0.948 | ESI+ | 1.07162 | ↑#### | ↓** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Wang, Y.; Yang, L.; Liu, M.; Sun, C.; Gu, Y.; Yao, J. Novel MAGL Inhibitors Alleviate LPS-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammatory Vesicles, Modulating Intestinal Flora, Repairing the Intestinal Barrier, and Interfering with Serum Metabolism. Molecules 2023, 28, 7245. https://doi.org/10.3390/molecules28217245
Xiang H, Wang Y, Yang L, Liu M, Sun C, Gu Y, Yao J. Novel MAGL Inhibitors Alleviate LPS-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammatory Vesicles, Modulating Intestinal Flora, Repairing the Intestinal Barrier, and Interfering with Serum Metabolism. Molecules. 2023; 28(21):7245. https://doi.org/10.3390/molecules28217245
Chicago/Turabian StyleXiang, Haixin, Yangui Wang, Lan Yang, Mingfei Liu, Chenghong Sun, Yuchao Gu, and Jingchun Yao. 2023. "Novel MAGL Inhibitors Alleviate LPS-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammatory Vesicles, Modulating Intestinal Flora, Repairing the Intestinal Barrier, and Interfering with Serum Metabolism" Molecules 28, no. 21: 7245. https://doi.org/10.3390/molecules28217245
APA StyleXiang, H., Wang, Y., Yang, L., Liu, M., Sun, C., Gu, Y., & Yao, J. (2023). Novel MAGL Inhibitors Alleviate LPS-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammatory Vesicles, Modulating Intestinal Flora, Repairing the Intestinal Barrier, and Interfering with Serum Metabolism. Molecules, 28(21), 7245. https://doi.org/10.3390/molecules28217245






