Analysis of Volatile Compounds in Processed Cream Cheese Models for the Prediction of “Fresh Cream” Aroma Perception
Abstract
1. Introduction
2. Results and Discussion
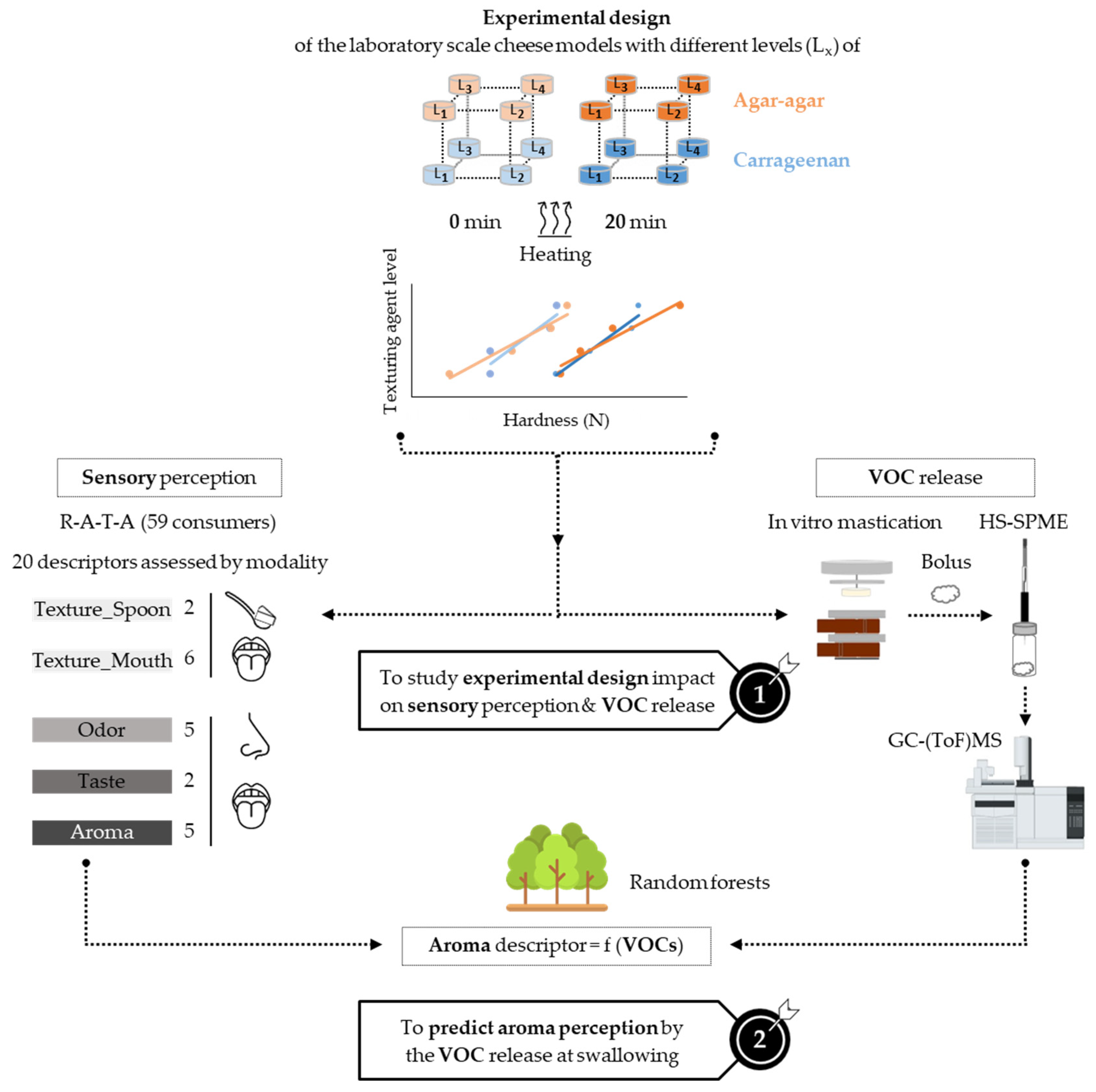
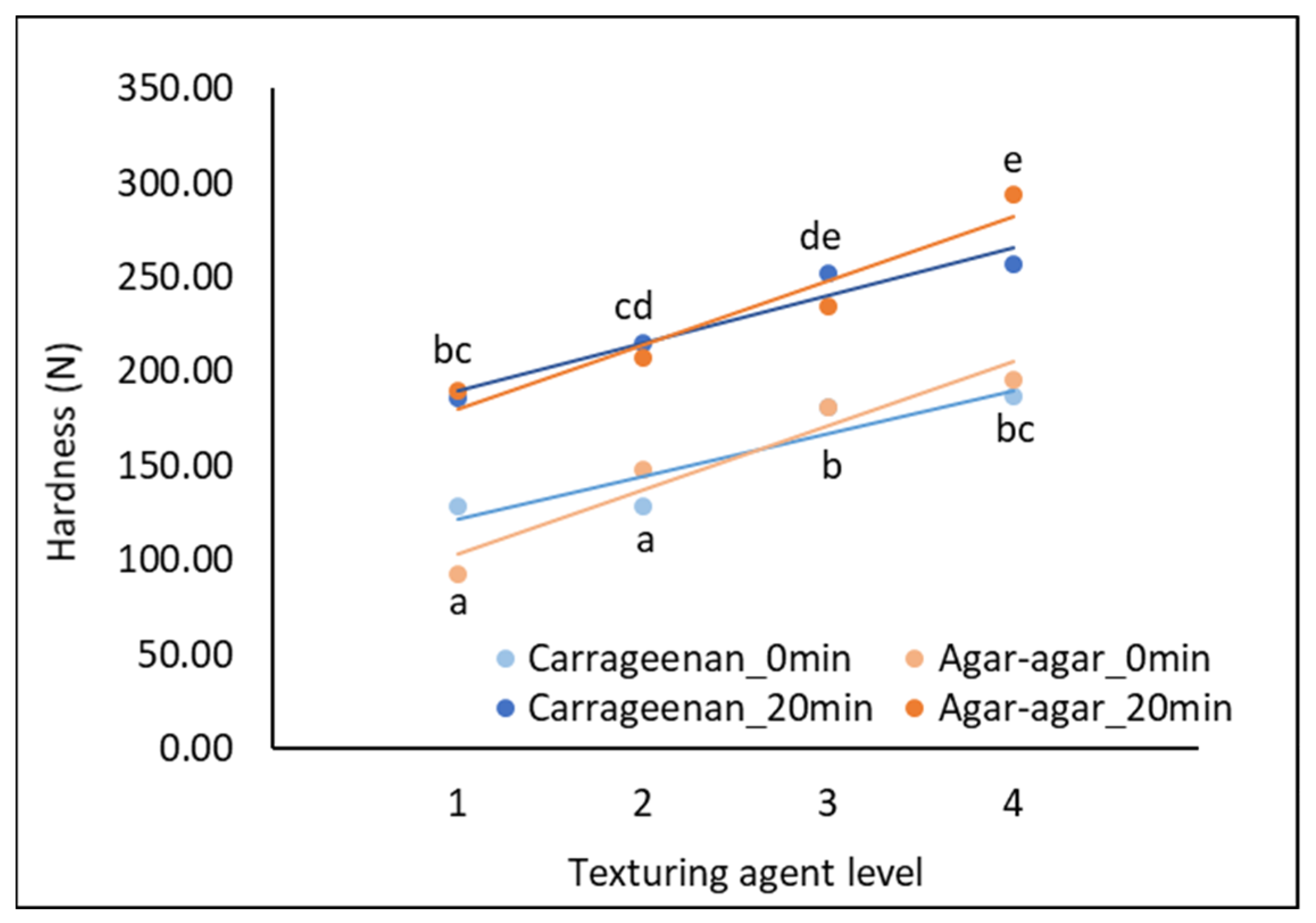
2.1. Laboratory-Scale Production of Cheese Models with the Same Hardness, Regardless of the Texturing Agent Type
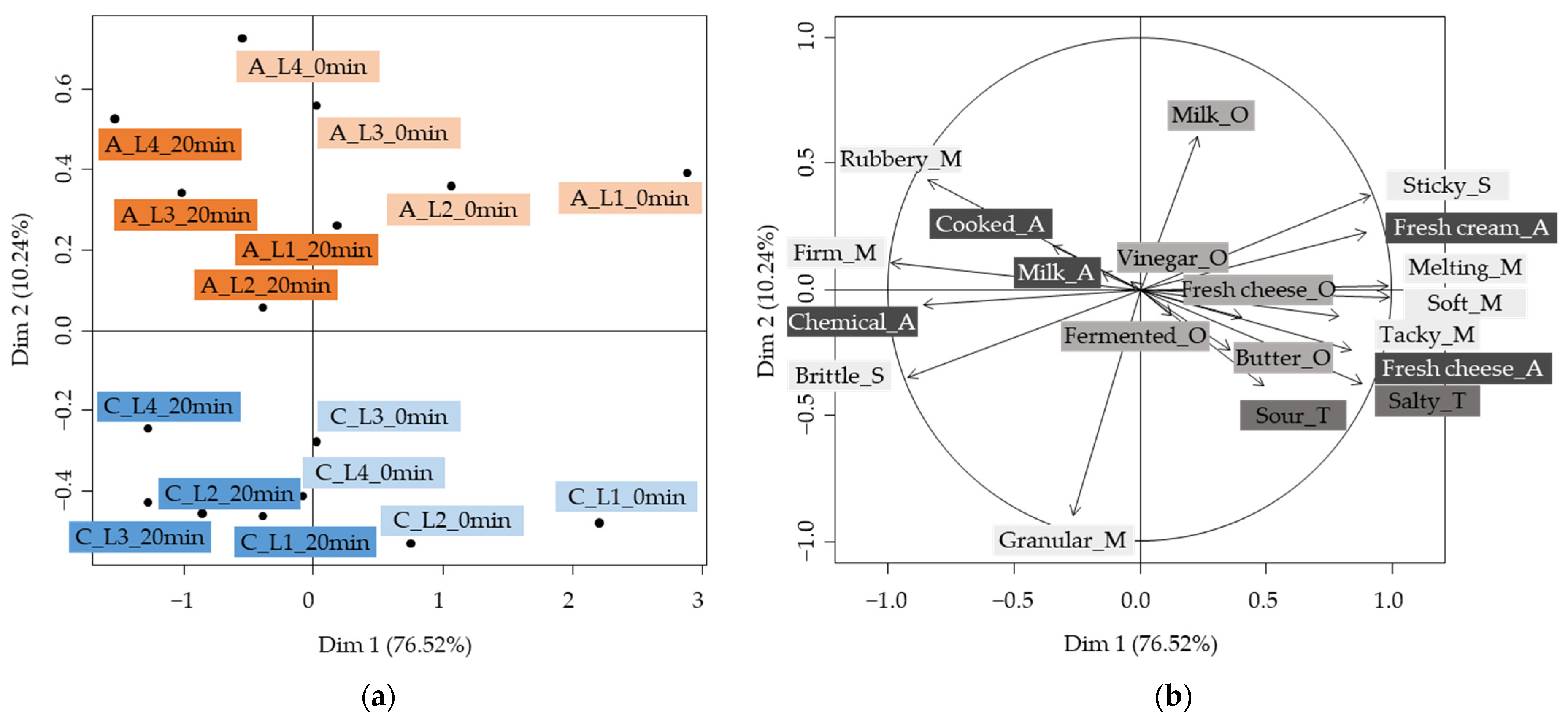
2.2. Impact of the Processed Cream Cheese Model Composition and Process on the Sensory Perception
2.3. Release of Volatile Compounds
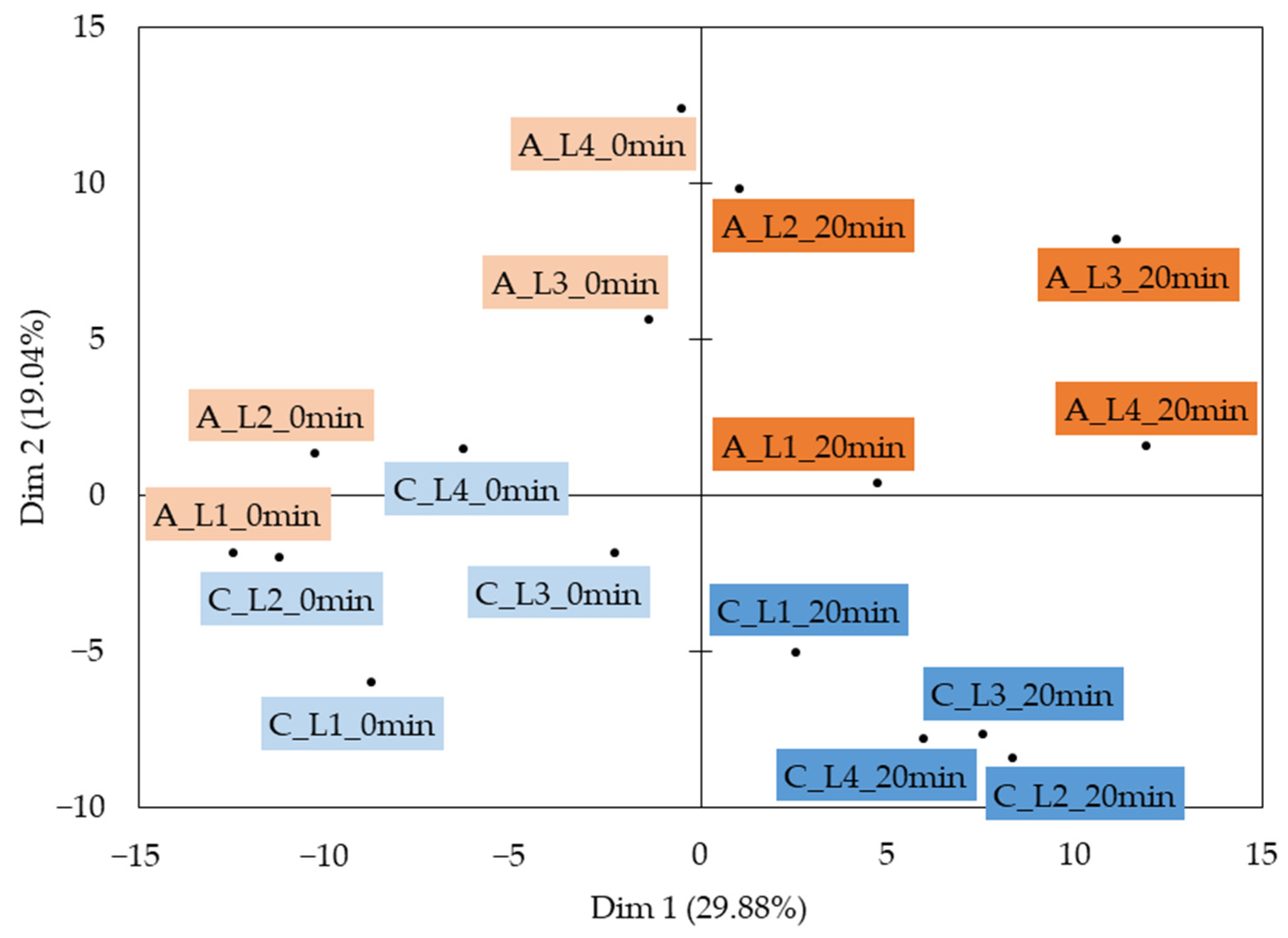
2.3.1. VOCs from the Processed Cream Cheese Models
2.3.2. Impact of the Processed Cream Cheese Model Composition and Process on the VOC Release during Swallowing
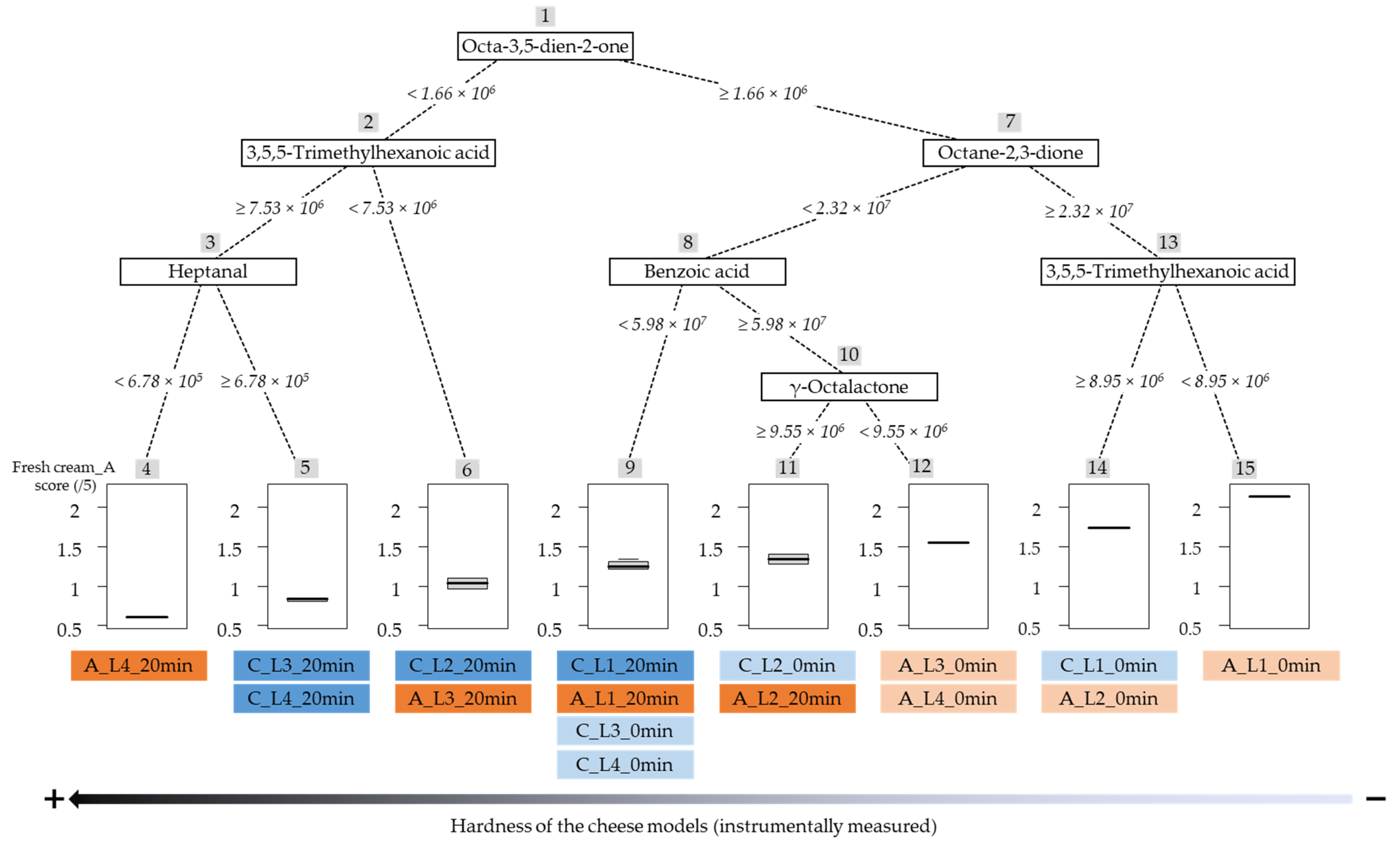
2.4. Prediction of the “Fresh Cream” Aroma Descriptor by the VOC Composition
3. Materials and Methods
3.1. Materials
3.2. Experimental Design at Laboratory Scale
- (1)
- (2)
- Texturing agent level (4 levels): for κ-carrageenan cheese models: 1 = 0.20% (w/w), 2 = 0.35% (w/w), 3 = 0.50% (w/w), 4 = 0.65% (w/w); for agar-agar cheese models: 1 = 0.60% (w/w), 2 = 0.90% (w/w), 3 = 1.20% (w/w), 4 = 1.50% (w/w). Different contents were selected to produce processed cream cheese models comparable in hardness (instrumentally measured), regardless of the texturing agent type, for both unheated and heated samples (Figure 2). As κ-carrageenan is known to be more viscous (240 ± 2.0 mPa·s) than agar-agar (29 ± 2.0 mPa·s) [62], highest agar-agar quantities were needed to obtain cheese models with the same hardness as κ-carrageenan.
- (3)
- Heating time (2 levels): 0 and 20 min.
3.3. In Vitro Mastication
3.4. Texture Analysis
3.5. Sensory Analysis
3.5.1. Ethics
3.5.2. Organization
3.6. Volatile Compound Analysis
3.7. Statistical Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Foegeding, E.; Drake, M. Invited Review: Sensory and Mechanical Properties of Cheese Texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Gierczynski, I.; Guichard, E.; Laboure, H. Aroma perception in dairy products: The roles of texture, aroma release and consumer physiology. A review. Flavour Fragr. J. 2011, 26, 141–152. [Google Scholar] [CrossRef]
- Toro, E.E.B.; Valencia, J.U.S.; Molina, D.A.R. Characterization of a processed cheese spread produced from fresh cheese (quesito antioqueño). Rev. Fac. Nac. Agron. Medellín 2016, 69, 8015–8022. [Google Scholar] [CrossRef]
- Kohama-Kubouchi, A.; Isogai, T.; Kobayashi, F.; Odake, S.; Shiota, M. The effect of mixing temperature on the flavour expression of processed cream cheese. Int. Dairy J. 2020, 111, 104842. [Google Scholar] [CrossRef]
- Vollmer, A.H.; Kieferle, I.; Youssef, N.N.; Kulozik, U. Mechanisms of structure formation underlying the creaming reaction in a processed cheese model system as revealed by light and transmission electron microscopy. J. Dairy Sci. 2021, 104, 9505–9520. [Google Scholar] [CrossRef]
- Weel, K.G.C.; Boelrijk, A.E.M.; Alting, A.C.; van Mil, P.J.J.M.; Burger, J.J.; Gruppen, H.; Voragen, A.G.J.; Smit, G. Flavor Release and Perception of Flavored Whey Protein Gels: Perception Is Determined by Texture Rather than by Release. J. Agric. Food Chem. 2002, 50, 5149–5155. [Google Scholar] [CrossRef]
- Lethuaut, L.; Brossard, C.; Rousseau, F.; Bousseau, B.; Genot, C. Sweetness–texture interactions in model dairy desserts: Effect of sucrose concentration and the carrageenan type. Int. Dairy J. 2003, 13, 631–641. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Michon, C.; Launay, B.; Parker, A.; De Kruif, C. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocoll. 2000, 14, 273–280. [Google Scholar] [CrossRef]
- Benjamin, O.; Davidovich-Pinhas, M.; Shpigelman, A.; Rytwo, G. Utilization of polysaccharides to modify salt release and texture of a fresh semi hard model cheese. Food Hydrocoll. 2018, 75, 95–106. [Google Scholar] [CrossRef]
- Hoefler, A.C. Hydrocolloids; Eagan Press, Ed.; American Association of Cereal Chemist: St. Paul, MI, USA, 2004; pp. 27–41. [Google Scholar]
- Spagnuolo, P.; Dalgleish, D.; Goff, H.; Morris, E. Kappa-carrageenan interactions in systems containing casein micelles and polysaccharide stabilizers. Food Hydrocoll. 2005, 19, 371–377. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, M.; Bi, J.; Sun, D.; Li, H.; Yang, H. Effects of kappa-carrageenan on egg white ovalbumin for enhancing the gelation and rheological properties via electrostatic interactions. Food Hydrocoll. 2023, 134, 108031. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, C.; Cui, C.; Xin, Z.; Xia, Y.; Xue, Z. Seaweed Fiber Fabricated with Agar Alkali-Free Extracted from Gracilaria Lemaneiformis. J. Renew. Mater. 2023, 11, 1199–1208. [Google Scholar] [CrossRef]
- Glenn, T.; Daubert, C.; Farkas, B.; Stefanski, L. A statistical analysis of creaming variables impacting process cheese melt quality. J. Food Qual. 2003, 26, 299–321. [Google Scholar] [CrossRef]
- Černíková, M.; Salek, R.N.; Kozáčková, D.; Běhalová, H.; Luňáková, L.; Buňka, F. The effect of selected processing parameters on viscoelastic properties of model processed cheese spreads. Int. Dairy J. 2017, 66, 84–90. [Google Scholar] [CrossRef]
- Lee, S.K.; Buwalda, R.J.; Euston, S.R.; Foegeding, E.A.; McKenna, A.B. Changes in the rheology and microstructure of processed cheese during cooking. LWT-Food Sci. Technol. 2003, 36, 339–345. [Google Scholar] [CrossRef]
- Lesme, H.; Rannou, C.; Loisel, C.; Famelart, M.-H.; Bouhallab, S.; Prost, C. Controlled whey protein aggregates to modulate the texture of fat-free set-type yoghurts. Int. Dairy J. 2019, 92, 28–36. [Google Scholar] [CrossRef]
- Marshall, S.G.; Vaisey, M. Sweetness perception in relation to some textural characteristics of hydrocolloid gels. J. Texture Stud. 1972, 3, 173–185. [Google Scholar] [CrossRef]
- Carr, J.; Baloga, D.; Guinard, J.-X.; Lawter, L.; Marty, C.; Squire, C. The effect of gelling agent type and concentration on flavor release in model systems. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 633. [Google Scholar] [CrossRef][Green Version]
- Eggink, P.; Maliepaard, C.; Tikunov, Y.; Haanstra, J.; Bovy, A.; Visser, R. A taste of sweet pepper: Volatile and non-volatile chemical composition of fresh sweet pepper (Capsicum annuum) in relation to sensory evaluation of taste. Food Chem. 2012, 132, 301–310. [Google Scholar] [CrossRef]
- Castada, H.Z.; Hanas, K.; Barringer, S.A. Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference. Foods 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.E.; Brown, W.E. Influence of food matrix structure and oral breakdown during mastication on temporal perception of flavor. J. Sens. Stud. 1997, 12, 69–86. [Google Scholar] [CrossRef]
- Wendin, K.; Langton, M.; Caous, L.; Hall, G. Dynamic analyses of sensory and microstructural properties of cream cheese. Food Chem. 2000, 71, 363–378. [Google Scholar] [CrossRef]
- Ares, G.; Bruzzone, F.; Vidal, L.; Cadena, R.S.; Giménez, A.; Pineau, B.; Hunter, D.C.; Paisley, A.G.; Jaeger, S.R. Evaluation of a rating-based variant of check-all-that-apply questions: Rate-all-that-apply (RATA). Food Qual. Prefer. 2014, 36, 87–95. [Google Scholar] [CrossRef]
- Oppermann, A.; de Graaf, C.; Scholten, E.; Stieger, M.; Piqueras-Fiszman, B. Comparison of Rate-All-That-Apply (RATA) and Descriptive sensory Analysis (DA) of model double emulsions with subtle perceptual differences. Food Qual. Prefer. 2017, 56, 55–68. [Google Scholar] [CrossRef]
- Saint-Eve, A. Compréhension de la Libération et de la Perception des Composés D’arômes en Condition de Consommation: Cas Du Yaourt Brassé Aromatisé. Ph.D. Thesis, Institut National Agronomique Paris-Grignon, Paris, France, 2006. [Google Scholar]
- Sonmezdag, A.S.; Cataneo, C.; Rannou, C.; Selli, S.; Prost, C. Elucidation of retro- and orthonasal aroma differences in biscuits (panis biscoctus) using artificial masticator. J. Food Process. Preserv. 2021, 46, e16088. [Google Scholar] [CrossRef]
- Bertrand, E.; Machado-Maturana, E.; Chevarin, C.; Portanguen, S.; Mercier, F.; Tournayre, P.; Abouelkaram, S.; Guillard, A.-S.; Kondjoyan, A.; Berdagué, J.-L. Heat-induced volatiles and odour-active compounds in a model cheese. Int. Dairy J. 2011, 21, 806–814. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R. Supercritical CO2 Extraction of Narcissus poeticus L. Flowers for the Isolation of Volatile Fragrance Compounds. Molecules 2022, 27, 353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Torri, L.; Aprea, E.; Piochi, M.; Cabrino, G.; Endrizzi, I.; Colaianni, A.; Gasperi, F. Relationship between Sensory Attributes, (Dis) Liking and Volatile Organic Composition of Gorgonzola PDO Cheese. Foods 2021, 10, 2791. [Google Scholar] [CrossRef]
- Granitto, P.; Gasperi, F.; Biasioli, F.; Trainotti, E.; Furlanello, C. Modern data mining tools in descriptive sensory analysis: A case study with a Random forest approach. Food Qual. Prefer. 2007, 18, 681–689. [Google Scholar] [CrossRef]
- Brillante, L.; Gaiotti, F.; Lovat, L.; Vincenzi, S.; Giacosa, S.; Torchio, F.; Segade, S.R.; Rolle, L.; Tomasi, D. Investigating the use of gradient boosting machine, random forest and their ensemble to predict skin flavonoid content from berry physical–mechanical characteristics in wine grapes. Comput. Electron. Agric. 2015, 117, 186–193. [Google Scholar] [CrossRef]
- Rocha, R.S.; Calvalcanti, R.N.; Silva, R.; Guimarães, J.T.; Balthazar, C.F.; Pimentel, T.C.; Esmerino, E.A.; Freitas, M.Q.; Granato, D.; Costa, R.G.; et al. Consumer acceptance and sensory drivers of liking of Minas Frescal Minas cheese manufactured using milk subjected to ohmic heating: Performance of machine learning methods. LWT 2020, 126, 109342. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Černíková, M.; Dimitreli, G.; Nebesářová, J.; Exarhopoulos, S.; Thomareis, A.S.; Salek, R.N.; Buňka, F. Influence of the melt holding time on fat droplet size and the viscoelastic properties of model spreadable processed cheeses with different compositions. Int. Dairy J. 2020, 113, 104880. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 240–250. [Google Scholar] [CrossRef]
- Peltier, C.; Visalli, M.; Schlich, P. Canonical Variate Analysis of Sensory Profiling Data. J. Sens. Stud. 2015, 30, 316–328. [Google Scholar] [CrossRef]
- Boland, A.B.; Delahunty, C.M.; Vanruth, S. Influence of the texture of gelatin gels and pectin gels on strawberry flavour release and perception. Food Chem. 2006, 96, 452–460. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Martin, N.; Guillemin, H.; Sémon, E.; Guichard, E.; Souchon, I. Flavored Yogurt Complex Viscosity Influences Real-Time Aroma Release in the Mouth and Sensory Properties. J. Agric. Food Chem. 2006, 54, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Saint-Eve, A.; Lauverjat, C.; Magnan, C.; Déléris, I.; Souchon, I. Reducing salt and fat content: Impact of composition, texture and cognitive interactions on the perception of flavoured model cheeses. Food Chem. 2009, 116, 167–175. [Google Scholar] [CrossRef]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of Furan and Methylfuran by Maillard-Type Reactions in Model Systems and Food. J. Agric. Food Chem. 2008, 56, 3639–3647. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.; Barbano, D.; Drake, M. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef]
- Bertrand, E.; Meyer, X.-M.; Machado-Maturana, E.; Berdagué, J.-L.; Kondjoyan, A. Modelling the Maillard reaction during the cooking of a model cheese. Food Chem. 2015, 184, 229–237. [Google Scholar] [CrossRef]
- de Vries, J. Hydrocolloid gelling agents and their applications. In Gums and Stabilisers for the Food Industry 12; Phillips, G.O., Williams, P.A., Eds.; The Royal Society of Chemistry: London, UK, 2004; pp. 23–31. [Google Scholar] [CrossRef]
- Koliandris, A.; Lee, A.; Ferry, A.-L.; Hill, S.; Mitchell, J. Relationship between structure of hydrocolloid gels and solutions and flavour release. Food Hydrocoll. 2008, 22, 623–630. [Google Scholar] [CrossRef]
- da Costa, J.N.; Leal, A.R.; Nascimento, L.G.; Rodrigues, D.C.; Muniz, C.R.; Figueiredo, R.W.; Mata, P.; Noronha, J.P.; de Sousa, P.H.M. Texture, microstructure and volatile profile of structured guava using agar and gellan gum. Int. J. Gastron. Food Sci. 2020, 20, 100207. [Google Scholar] [CrossRef]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, C.; Li, H.; Dong, X.; Zhang, X. Studies on the physicochemical properties, gelling behavior and drug release performance of agar/κ-carrageenan mixed hydrogels. Int. J. Biol. Macromol. 2020, 154, 878–887. [Google Scholar] [CrossRef]
- Chai, E.; Oakenfull, D.G.; McBride, R.L.; Lane, A.G. Sensory perception and rheology of flavoured gels. Food Aust. 1991, 43, 256–261. [Google Scholar]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. Flavour profiles of functional reduced-fat cream cheese: Effects of β-glucan, phytosterols, and probiotic L. rhamnosus. LWT 2019, 105, 16–22. [Google Scholar] [CrossRef]
- Jeon, S.-S.; Lee, S.-J.; Ganesan, P.; Kwak, H.-S. Qualitative and Quantitative Analyses of Volatile Compounds in Cream Cheese and Cholesterol-removed Cream Cheese Made from Whole Milk Powder. Korean J. Food Sci. Anim. Resour. 2011, 31, 879–885. [Google Scholar] [CrossRef]
- Coolbear, T.; Janin, N.; Traill, R.; Shingleton, R. Heat-induced changes in the sensory properties of milk. Int. Dairy J. 2021, 126, 105199. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef]
- The Good Scents Company. Available online: https://www.thegoodscentscompany.com/ (accessed on 20 September 2022).
- Sunesen, L.; Lund, P.; Sørensen, J.; Hølmer, G. Development of Volatile Compounds in Processed Cheese during Storage. LWT-Food Sci. Technol. 2002, 35, 128–134. [Google Scholar] [CrossRef]
- Cerny, C. The Aroma Side of the Maillard Reaction. Ann. N. Y. Acad. Sci. 2008, 1126, 66–71. [Google Scholar] [CrossRef]
- Parker, J.K. The kinetics of thermal generation of flavour: The kinetics of flavour formation. J. Sci. Food Agric. 2013, 93, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of different heat treatments on the Maillard reaction products, volatile compounds and glycation level of milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Valero, E.; Sanz, J.; Martinez-Castro, I. Direct thermal desorption in the analysis of cheese volatiles by gas chromatography and gas chromatography-mass spectrometry: Comparison with simultaneous distillation-extraction and dynamic headspace. J. Chromatogr. Sci. 2001, 39, 222–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meena, R.; Prasad, K.; Siddhanta, A. Development of a stable hydrogel network based on agar–kappa-carrageenan blend cross-linked with genipin. Food Hydrocoll. 2009, 23, 497–509. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Flavor and Extract Manufacturer Association. Available online: https://www.femaflavor.org (accessed on 20 September 2022).
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Chemical and Olfactometric Characterization of Volatile Flavor Compounds in a Fish Oil Enriched Milk Emulsion. J. Agric. Food Chem. 2004, 52, 311–317. [Google Scholar] [CrossRef]
- Rizzo, P.; Del Toro-Gipson, R.; Cadwallader, D.; Drake, M. Identification of aroma-active compounds in Cheddar cheese imparted by wood smoke. J. Dairy Sci. 2022, 105, 5622–5640. [Google Scholar] [CrossRef]
- Bonaïti, C.; Irlinger, F.; Spinnler, H.; Engel, E. An Iterative Sensory Procedure to Select Odor-Active Associations in Complex Consortia of Microorganisms: Application to the Construction of a Cheese Model. J. Dairy Sci. 2005, 88, 1671–1684. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.; Kelly, A.; Delahunty, C. Influence of Starter Culture on Flavor and Headspace Volatile Profiles of Fermented Whey and Whey Produced from Fermented Milk. J. Dairy Sci. 2005, 88, 3745–3753. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Roozen, J.P.; Cozijnsen, J.L.; Posthumus, M.A. Volatile compounds of rehydrated French beans, bell peppers and leeks. Part II. Gas chromatography/sniffing port analysis and sensory evaluation. Food Chem. 1995, 54, 1–7. [Google Scholar] [CrossRef]
- Guilloux, M.; Tarancon, P.; Catanéo, C.; Vigneau, E.; Le Bail, A.; Lethuaut, L.; Prost, C. Efficiency of a new artificial mouth prototype to mimic salt release during food oral processing in order to explain dynamic saltiness perception of pizza varying in salt content. In Proceedings of the 3rd International Conference Food Oral Processing, Wageningen, The Netherlands, 14–17 May 2014. [Google Scholar]
- Vigneau, E.; Courcoux, P.; Symoneaux, R.; Guérin, L.; Villière, A. Random forests: A machine learning methodology to highlight the volatile organic compounds involved in olfactory perception. Food Qual. Prefer. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Cardinal, M.; Chaussy, M.; Donnay-Moreno, C.; Cornet, J.; Rannou, C.; Fillonneau, C.; Prost, C.; Baron, R.; Courcoux, P. Use of random forest methodology to link aroma profiles to volatile compounds: Application to enzymatic hydrolysis of Atlantic salmon (Salmo salar) by-products combined with Maillard reactions. Food Res. Int. 2020, 134, 109254. [Google Scholar] [CrossRef] [PubMed]
- Villière, A.; Le Roy, S.; Fillonneau, C.; Prost, C. InnOscent system: Advancing flavor analysis using an original gas chromatographic analytical device. J. Chromatogr. A 2018, 1535, 129–140. [Google Scholar] [CrossRef] [PubMed]

| Product Name | Texturing Agent Type | Texturing Agent Level | Heating Time (min) |
|---|---|---|---|
| C_L1_0min | Carrageenan | 1 | 0 |
| C_L2_0min | Carrageenan | 2 | 0 |
| C_L3_0min | Carrageenan | 3 | 0 |
| C_L4_0min | Carrageenan | 4 | 0 |
| C_L1_20min | Carrageenan | 1 | 20 |
| C_L2_20min | Carrageenan | 2 | 20 |
| C_L3_20min | Carrageenan | 3 | 20 |
| C_L4_20min | Carrageenan | 4 | 20 |
| A_L1_0min | Agar-agar | 1 | 0 |
| A_L2_0min | Agar-agar | 2 | 0 |
| A_L3_0min | Agar-agar | 3 | 0 |
| A_L4_0min | Agar-agar | 4 | 0 |
| A_L1_20min | Agar-agar | 1 | 20 |
| A_L2_20min | Agar-agar | 2 | 20 |
| A_L3_20min | Agar-agar | 3 | 20 |
| A_L4_20min | Agar-agar | 4 | 20 |
| Descriptor | Evaluation Modality | Fisher | p-Value |
|---|---|---|---|
| Soft_M | Texture (in-mouth) | 43.05 | 0.0000 *** |
| Firm_M | Texture (in-mouth) | 38.83 | 0.0000 *** |
| Melting_M | Texture (in-mouth) | 27.38 | 0.0000 *** |
| Sticky_S | Texture (spoon) | 19.67 | 0.0000 *** |
| Brittle_S | Texture (spoon) | 17.34 | 0.0000 *** |
| Rubbery_M | Texture (in-mouth) | 10.07 | 0.0000 *** |
| Granular_M | Texture (in-mouth) | 9.47 | 0.0000 *** |
| Salty_T | Taste | 5.79 | 0.0000 *** |
| Fresh cream_A | Aroma | 5.77 | 0.0000 *** |
| Tacky_M | Texture (in-mouth) | 4.02 | 0.0000 *** |
| Fresh cheese_A | Aroma | 5.19 | 0.0000 *** |
| Chemical_A | Aroma | 4.92 | 0.0000 *** |
| Sour_T | Taste | 2.44 | 0.0006 *** |
| Fermented_O | Odor | 1.65 | 0.0392 * |
| Vinegar_O | Odor | 1.35 | 0.1440 |
| Fresh cheese_O | Odor | 1.08 | 0.3648 |
| Cooked_A | Aroma | 1.05 | 0.4011 |
| Butter_O | Odor | 1.00 | 0.4537 |
| Milk_O | Odor | 0.65 | 0.8701 |
| Milk_A | Aroma | 0.60 | 0.9079 |
| CAS Number | VOC Name | LRIexp | m/z | Stat. | CAS Number | VOC Name | LRIexp | m/z | Stat. |
|---|---|---|---|---|---|---|---|---|---|
| Acids | Alkanes | ||||||||
| 00064-19-7 | Acetic acid | 1455 | 43 | u, - | 00109-66-0 | Pentane | 499 | 43 | h, - |
| 00064-18-6 | Formic acid | 1517 | 46 | u, - | 00075-83-2 | 2,2-Dimethylbutane | 516 | 43 | -, - |
| 00079-09-4 | Propanoic acid | 1548 | 45 | u, a | 00107-83-5 | 2-Methylpentane | 550 | 43 | -, - |
| 00079-31-2 | 2-Methylpropanoic acid | 1577 | 43 | u, a | 00096-14-0 | 3-Methylpentane | 577 | 57 | -, - |
| 00075-98-9 | 2,2-Dimethylpropanoic acid | 1587 | 57 | -, - | 00110-54-3 | Hexane | 598 | 57 | h, - |
| 00107-92-6 | Butanoic acid | 1636 | 60 | u, - | 01191-96-4 | Ethylcyclopropane | 628 | 42 | -, - |
| 00503-74-2 | 3-Methylbutanoic acid | 1679 | 60 | u, a | 00096-37-7 | Methylcyclopentane | 682 | 56 | h, - |
| 00116-53-0 | 2-Methylbutanoic acid | 1682 | 74 | u, a | 00142-82-5 | Heptane | 705 | 57 | h, a |
| 00109-52-4 | Pentanoic acid | 1749 | 60 | u, - | 00592-13-2 | 2,5-Dimethylhexane | 715 | 57 | -, a |
| 03724-65-0 | But-2-enoic acid | 1786 | 86 | u, c | 00589-43-5 | 2,4-Dimethylhexane | 719 | 43 | -, a |
| 00142-62-1 | Hexanoic acid | 1856 | 60 | u, - | 00110-82-7 | Cyclohexane | 725 | 56 | -, - |
| 00111-14-8 | Heptanoic acid | 1962 | 60 | -, - | 04516-69-2 | 1,1,3-Trimethylcyclopentane | 732 | 55 | -, a |
| 03302-10-1 | 3,5,5-Trimethylhexanoic acid | 1991 | 57 | -, - | 00592-27-8 | 2-Methylheptane | 750 | 43 | -, a |
| 00124-07-2 | Octanoic acid | 2070 | 60 | u, - | 00589-53-7 | 4-Methylheptane | 758 | 43 | -, a |
| 00110-44-1 | (2E,4E)-Hexa-2,4-dienoic acid | 2150 | 97 | h, c | 00589-81-1 | 3-Methylheptane | 763 | 43 | -, a |
| 00112-05-0 | Nonanoic acid | 2177 | 60 | -, - | 02213-23-2 | 2,4-Dimethylheptane | 805 | 43 | -, a |
| 00334-48-5 | Decanoic acid | 2283 | 60 | u, - | 03074-71-3 | 2,3-Dimethylheptane | 845 | 43 | -, a |
| 14436-32-9 | Dec-9-enoic acid | 2347 | 55 | u, - | 02216-34-4 | 4-Methyloctane | 850 | 43 | -, a |
| 00065-85-0 | Benzoic acid | 2459 | 105 | u, - | 15869-87-1 | 2,2-Dimethyloctane | 894 | 57 | h, a |
| 00143-07-7 | Dodecanoic acid | 2496 | 73 | u, - | 62016-28-8 | 2,2,6-Trimethyloctane | 932 | 57 | h, a |
| Alcohols | 13475-82-6 | 2,2,4,6,6-Pentamethylheptane | 951 | 57 | h, a | ||||
| 00067-63-0 | Propan-2-ol | 943 | 45 | -, c | 62183-74-8 | 2,2,3,3-Tetramethyloctane | 957 | 57 | -, a |
| 00064-17-5 | Ethanol | 949 | 45 | h, - | 17302-14-6 | 2,2-Dimethylnonane | 970 | 57 | -, a |
| 00078-92-2 | Butan-2-ol | 1042 | 45 | -, - | 62016-30-2 | 2,3,3-Trimethyloctane | 978 | 57 | h, a |
| 00071-23-8 | Propan-1-ol | 1057 | 42 | h, c | 62016-19-7 | 6-Ethyl-2-Methyloctane | 1007 | 71 | -, a |
| 00077-74-7 | 3-Methylpentan-3-ol | 1133 | 73 | u, - | 00124-18-5 | Decane | 1003 | 43 | u, a |
| 00071-36-3 | Butan-1-ol | 1166 | 56 | -, - | 01120-21-4 | Undecane | 1098 | 57 | u, a |
| 02566-44-1 | 2-Cyclopropylethanol | 1176 | 67 | u, - | 04390-04-9 | 2,2,4,4,6,8,8-Heptamethylnonane | 1250 | 57 | u, - |
| 00123-51-3 | 3-Methylbutan-1-ol | 1220 | 55 | -, - | Aldehydes | ||||
| 00137-32-6 | 2-Methylbutan-1-ol | 1224 | 57 | -, - | 00075-07-0 | Acetaldehyde | 708 | 44 | h, a |
| 01569-01-3 | 1-Propoxypropan-2-ol | 1258 | 45 | -, a | 00123-38-6 | Propanal | 795 | 58 | u, - |
| 00763-32-6 | 3-Methylbut-3-en-1-ol | 1264 | 41 | -, - | 00078-84-2 | 2-Methylpropanal | 813 | 41 | h, - |
| 00071-41-0 | Pentan-1-ol | 1269 | 42 | -, - | 00123-72-8 | Butanal | 878 | 72 | -, - |
| 01576-96-1 | (E)-Pent-2-en-1-ol | 1361 | 57 | u, a | 00096-17-3 | 2-Methylbutanal | 918 | 41 | h, - |
| 17540-75-9 | 2,6-Bis(1,1-dimethylethyl)-4-(1-methylpropyl)-phenol | 1934 | 233 | -, - | 00590-86-3 | 3-Methylbutanal | 922 | 44 | h, - |
| 00108-95-2 | Phenol | 2022 | 94 | -, - | 00123-73-9 | (E)-But-2-enal | 1051 | 70 | h, - |
| 00096-76-4 | 2,4-Di-t-butylphenol | 2325 | 191 | -, - | 00066-25-1 | Hexanal | 1095 | 44 | u, c |
| Alkenes | 01115-11-3 | 2-Methylbut-2-enal | 1108 | 84 | h, c | ||||
| 00590-18-1 | (Z)-But-2-ene | 511 | 41 | h, a | 01576-87-0 | (E)-Pent-2-enal | 1144 | 55 | h, c |
| 00504-60-9 | Penta-1,3-diene | 653 | 67 | h, a | 00111-71-7 | Heptanal | 1193 | 70 | u, - |
| 04050-45-7 | (E)-Hex-2-ene | 664 | 55 | h, - | 00107-86-8 | 3-Methylbut-2-enal | 1212 | 84 | -, - |
| 00625-27-4 | 2-Methylpent-2-ene | 677 | 69 | -, - | 55136-52-2 | Pent-2-ynal | 1227 | 53 | h, c |
| 00922-62-3 | (Z)-3-Methylpent-2-ene | 715 | 41 | -, - | 06728-26-3 | (E)-Hex-2-enal | 1232 | 41 | -, c |
| 02213-37-8 | 3,4-Dimethylhex-2-ene | 772 | 83 | -, a | 20432-40-0 | (E,E)-Penta-2,4-dienal | 1243 | 81 | h, a |
| 01632-16-2 | 2-Ethylhex-1-ene | 828 | 70 | -, a | 18829-55-5 | (E)-Hept-2-enal | 1339 | 83 | u, - |
| 14919-01-8 | (E)-Oct-3-ene | 840 | 41 | -, a | 00124-19-6 | Nonanal | 1405 | 56 | u, - |
| 07300-03-0 | 3-Methylhept-3-ene | 841 | 83 | -, a | 00498-60-2 | Furan-3-carbaldehyde | 1441 | 96 | -, a |
| 55702-61-9 | 4,4,5-Trimethylhex-2-ene | 858 | 83 | -, a | 02548-87-0 | (E)-Oct-2-enal | 1445 | 55 | -, - |
| 19549-87-2 | 2,4-Dimethylhept-1-ene | 880 | 43 | -, a | 00098-01-1 | Furan-2-carbaldehyde | 1477 | 96 | h, a |
| 74421-06-0 | 5-Ethyl-2,4-dimethylhept-2-ene | 996 | 83 | -, a | 04313-03-5 | (E,E)-Hepta-2,4-dienal | 1513 | 81 | u, a |
| 33933-75-4 | 2,3,7-Trimethyloct-2-ene | 999 | 83 | -, a | 00100-52-7 | Benzaldehyde | 1543 | 77 | u, a |
| 06874-32-4 | (Z) 3,7-Dimethyloct-2-ene | 1020 | 70 | -, a | 00620-02-0 | 5-Methylfuran-2-carbaldehyde | 1591 | 109 | u, - |
| 74421-03-7 | 2,4-Dimethyldec-2-ene | 1078 | 83 | -, a | 00098-03-3 | Thiophene-2-carboxaldehyde | 1721 | 111 | h, - |
| 74630-52-7 | (E)-6-Methylundec-3-ene | 1174 | 57 | -, - | |||||
| Ketones | Aromatic hydrocarbons | ||||||||
| 00431-03-8 | Butane-2,3-dione | 986 | 43 | u, - | 00071-43-2 | Benzene | 946 | 78 | h, a |
| 01629-58-9 | 1-Penten-3-one | 1030 | 55 | -, c | 00108-88-3 | Toluene | 1050 | 91 | -, a |
| 00600-14-6 | Pentane-2,3-dione | 1073 | 43 | u, a | 00100-41-4 | Ethylbenzene | 1138 | 91 | h, - |
| 00585-25-1 | Octane-2,3-dione | 1332 | 99 | u, - | 00106-42-3 | p-Xylene | 1144 | 91 | -, - |
| 00930-30-3 | Cyclopent-2-en-1-one | 1374 | 82 | -, c | 00095-47-6 | o-Xylene | 1152 | 91 | h, - |
| 05704-20-1 | 2-Hydroxypentan-3-one | 1376 | 45 | u, a | 00622-96-8 | 1-Ethyl-4-Methylbenzene | 1237 | 105 | -, - |
| 01120-73-6 | 2-Methylcyclopent-2-en-1-one | 1389 | 96 | -, - | 00100-42-5 | Styrene | 1270 | 104 | -, - |
| 13679-85-1 | 2-Methylthiolan-3-one | 1551 | 60 | h, - | 00527-84-4 | o-Cymene | 1281 | 119 | -, a |
| 00930-60-9 | Cyclopent-4-ene-1,3-dione | 1605 | 54 | h, - | 00095-63-6 | 1,2,4-Trimethylbenzene | 1294 | 105 | -, - |
| 04505-38-8 | Cyclohex-2-ene-1,4-dione | 1759 | 54 | h, c | 00091-20-3 | Naphtalene | 1769 | 128 | u, - |
| 00557-01-7 | Pyrimidin-2(1H)-one | 1796 | 96 | h, - | Furans | ||||
| 00067-71-0 | Dimethyl sulfone | 1923 | 79 | u, - | 00110-00-9 | Furan | 802 | 68 | h, a |
| Methyl Ketones | 00534-22-5 | 2-Methylfuran | 872 | 82 | h, - | ||||
| 00067-64-1 | Propan-2-one | 816 | 43 | h, a | 00930-27-8 | 3-Methylfuran | 902 | 82 | h, a |
| 00078-93-3 | Butan-2-one | 907 | 43 | h, - | 03208-16-0 | 2-Ethylfuran | 961 | 81 | h, a |
| 00107-87-9 | Pentan-2-one | 984 | 43 | h, - | 03710-43-8 | 2,4-Dimethylfuran | 973 | 96 | h, a |
| 00108-10-1 | 4-Methylpentan-2-one | 1014 | 58 | -, - | 10504-04-8 | 2,3,5-Trimethylfuran | 1068 | 109 | h, a |
| 00591-78-6 | Hexan-2-one | 1094 | 58 | h, - | 04466-24-4 | 2-Butylfuran | 1143 | 81 | h, a |
| 00625-33-2 | Pent-3-en-2-one | 1139 | 69 | -, - | 03777-69-3 | 2-Pentylfuran | 1242 | 81 | h, a |
| 00141-79-7 | 4-Methylpent-3-en-2-one | 1145 | 98 | h, c | 13679-46-4 | 2-(Methoxymethyl)furan | 1248 | 112 | h, a |
| 00110-43-0 | Heptan-2-one | 1194 | 43 | h, - | 13423-15-9 | 3-Methyltetrahydrofuran | 1300 | 41 | -, - |
| 00928-68-7 | 6-Methylheptan-2-one | 1247 | 58 | -, a | 00271-89-6 | Benzofuran | 1525 | 118 | -, - |
| 00111-13-7 | Octan-2-one | 1297 | 58 | h, a | 00098-00-0 | 2-Furanmethanol | 1689 | 98 | -, - |
| 00513-86-0 | 3-Hydroxybutan-2-one | 1302 | 45 | -, - | Nitrogen compounds | ||||
| 00110-93-0 | 6-Methylhept-5-en-2-one | 1350 | 69 | -, - | 00075-05-8 | Acetonitrile | 1008 | 41 | -, - |
| 00821-55-6 | Nonan-2-one | 1401 | 43 | h, - | 00096-54-8 | 1-Methylpyrrole | 1151 | 81 | h, c |
| 01669-44-9 | Oct-3-en-2-one | 1424 | 55 | u, - | 00290-37-9 | Pyrazine | 1228 | 80 | -, a |
| 00693-54-9 | Decan-2-one | 1507 | 58 | h, a | 02516-34-9 | Cyclobutane-1-amine | 1249 | 43 | -, - |
| 01192-62-7 | 1-(Furan-2-yl)ethanone | 1520 | 95 | h, a | 00288-47-1 | 1,3-Thiazole | 1265 | 85 | h, - |
| 38284-27-4 | Octa-3,5-dien-2-one | 1536 | 95 | u, - | 04786-24-7 | 3-Methylbut-2-enenitrile | 1282 | 41 | -, - |
| 00112-12-9 | Undecan-2-one | 1613 | 58 | h, - | 01124-11-4 | 2,3,5,6-Tetramethyl pyrazine | 1499 | 54 | h, a |
| 00098-86-2 | Acetophenone | 1675 | 105 | u, - | 00109-97-7 | 1H-Pyrrole | 1529 | 67 | h, c |
| 00593-08-8 | Tridecan-2-one | 1826 | 58 | -, - | 00100-47-0 | Benzonitrile | 1627 | 103 | -, - |
| Lactones | 04025-37-0 | 2-(Aziridin-1-yl)ethanamine | 1641 | 44 | u, - | ||||
| 00591-12-8 | 5-Methyl-3H-furan-2-one | 1451 | 98 | h, a | Sulfur compounds | ||||
| 00096-48-0 | Butyrolactone | 1654 | 86 | -, - | 00074-93-1 | Methanethiol | 687 | 47 | -, - |
| 00591-11-7 | 2-Methyl-2H-furan-5-one | 1703 | 55 | h, - | 00075-15-0 | Carbon disulfide | 729 | 76 | h, a |
| 00695-06-7 | γ-Hexalactone | 1730 | 85 | u, - | 00075-18-3 | Dimethyl sulfide | 748 | 47 | -, a |
| 00497-23-4 | 5H-Furan-2-one | 1779 | 55 | -, - | 00624-92-0 | Dimethyl disulfide | 1085 | 94 | -, - |
| 00823-22-3 | δ-Hexalactone | 1825 | 42 | u, - | 00554-14-3 | 2-Methylthiophene | 1103 | 97 | h, c |
| 00105-21-5 | γ-Heptalactone | 1836 | 85 | u, - | 03658-80-8 | Dimethyl trisulfide | 1399 | 47 | -, - |
| 00104-50-7 | γ-Octalactone | 1946 | 85 | u, - | Terpenes | ||||
| 00698-76-0 | δ-Octalactone | 2000 | 99 | u, - | 00080-56-8 | α-Pinene | 1026 | 93 | h, a |
| 00705-86-2 | δ-Decalactone | 2233 | 99 | u, - | 00127-91-3 | β-Pinene | 1114 | 93 | - |
| 00713-95-1 | δ-Dodecalactone | 2469 | 99 | u, - | 13466-78-9 | 3-Carene | 1157 | 93 | h, a |
| Esters | 05989-27-5 | Limonene | 1208 | 93 | -, - | ||||
| 00141-78-6 | Ethyl Acetate | 895 | 43 | h, a | Unknown compounds | ||||
| 00105-54-4 | Ethyl butanoate | 1049 | 71 | h, - | - | Unknown | 988 | 57 | -, - |
| 01534-08-3 | S-Methyl ethanethioate | 1059 | 90 | h, a | - | Unknown | 997 | 57 | -, - |
| 00105-66-8 | Propyl butanoate | 1134 | 71 | h, - | - | Unknown | 1253 | 105 | -, - |
| 00123-66-0 | Ethyl hexanoate | 1244 | 88 | -, a | - | Unknown | 2159 | 97 | -, - |
| 04906-24-5 | 3-Oxobutan-2-yl acetate | 1392 | 87 | -, - | |||||
| 03050-69-9 | Vinyl hexanoate | 1723 | 43 | u, - |
| Dim 1 (29.88%) | Dim 2 (19.04%) | ||||||
|---|---|---|---|---|---|---|---|
| CAS Number | VOC Name | VC | Correlation | CAS Number | VOC Name | VC | Correlation |
| 01534-08-3 | S-Methyl ethanethioate | 1.36 | +0.94 | 02216-34-4 | 4-Methyloctane | 2.17 | +0.94 |
| 00695-06-7 | γ-Hexalactone | 1.32 | −0.92 | 74421-06-0 | 5-Ethyl-2,4-dimethylhept-2-ene | 2.17 | +0.94 |
| 00698-76-0 | δ-Octalactone | 1.32 | −0.92 | 74421-03-7 | 2,4-Dimethyldec-2-ene | 2.15 | +0.94 |
| 00104-50-7 | γ-Octalactone | 1.30 | −0.92 | 02213-23-2 | 2,4-Dimethylheptane | 2.14 | +0.94 |
| 00504-60-9 | Penta-1,3-diene | 1.26 | +0.90 | 03074-71-3 | 2,3-Dimethylheptane | 2.08 | +0.92 |
| 00096-17-3 | 2-Methylbutanal | 1.25 | +0.90 | 33933-75-4 | 2,3,7-Trimethyloct-2-ene | 2.05 | +0.92 |
| 00534-22-5 | 2-Methylfuran | 1.25 | +0.90 | 00589-81-1 | 3-Methylheptane | 2.01 | +0.91 |
| 00823-22-3 | δ-Hexalactone | 1.25 | −0.90 | 55702-61-9 | 4,4,5-Trimethylhex-2-ene | 1.98 | +0.90 |
| 00071-43-2 | Benzene | 1.24 | +0.90 | 00589-53-7 | 4-Methylheptane | 1.98 | +0.90 |
| 00288-47-1 | 1,3-Thiazole | 1.23 | +0.89 | 00592-13-2 | 2,5-Dimethylhexane | 1.96 | +0.90 |
| 00098-86-2 | Acetophenone | 1.23 | −0.89 | 07300-03-0 | 3-Methylhept-3-ene | 1.88 | +0.88 |
| 04313-03-5 | (E,E)-Hepta-2,4-dienal | 1.21 | −0.89 | 00589-43-5 | 2,4-Dimethylhexane | 1.82 | +0.87 |
| 00591-78-6 | Hexan-2-one | 1.20 | +0.88 | 04516-69-2 | 1,1,3-Trimethylcyclopentane | 1.81 | +0.86 |
| 00123-73-9 | (E)-But-2-enal | 1.18 | +0.87 | 02213-37-8 | 3,4-Dimethylhex-2-ene | 1.80 | +0.86 |
| 38284-27-4 | Octa-3,5-dien-2-one | 1.15 | −0.86 | 01632-16-2 | 2-Ethylhex-1-ene | 1.78 | +0.86 |
| 00585-25-1 | Octane-2,3-dione | 1.15 | −0.86 | 14919-01-8 | (E)-Oct-3-ene | 1.76 | +0.85 |
| 03050-69-9 | Vinyl hexanoate | 1.13 | −0.86 | 01120-21-4 | Undecane | 1.74 | +0.85 |
| 00065-85-0 | Benzoic acid | 1.11 | −0.85 | 19549-87-2 | 2,4-Dimethylhept-1-ene | 1.72 | +0.84 |
| 00107-87-9 | Pentan-2-one | 1.11 | +0.85 | 00592-27-8 | 2-Methylheptane | 1.54 | +0.80 |
| 00109-66-0 | Pentane | 1.10 | +0.84 | 00110-00-9 | Furan | 1.47 | +0.78 |
| 00554-14-3 | 2-Methylthiophene | 1.09 | +0.84 | 00498-60-2 | Furan-3-carbaldehyde | 1.41 | +0.76 |
| 00431-03-8 | Butane-2,3-dione | 1.08 | −0.84 | 00124-18-5 | Decane | 1.39 | +0.76 |
| 00110-43-0 | Heptan-2-one | 1.08 | +0.84 | 00930-27-8 | 3-Methylfuran | 1.37 | +0.75 |
| 00064-19-7 | Acetic acid | 1.08 | −0.84 | 01569-01-3 | 1-Propoxypropan-2-ol | 1.31 | +0.73 |
| 00590-18-1 | (Z)-But-2-ene | 1.06 | +0.83 | 00098-01-1 | Furan-2-carbaldehyde | 1.27 | +0.72 |
| 00107-92-6 | Butanoic acid | 1.05 | −0.82 | 13679-46-4 | 2-(Methoxymethyl)furan | 1.26 | +0.72 |
| 04050-45-7 | (E)-Hex-2-ene | 1.05 | +0.82 | 00928-68-7 | 6-Methylheptan-2-one | 1.25 | +0.72 |
| 01576-87-0 | (E)-Pent-2-enal | 1.04 | +0.82 | 00096-14-0 | 3-Methylpentane | 1.16 | +0.69 |
| 00142-82-5 | Heptane | 1.02 | +0.81 | 00141-79-7 | 4-Methylpent-3-ene-2-one | 1.11 | −0.68 |
| 00109-52-4 | Pentanoic acid | 1.00 | −0.81 | 00109-97-7 | 1H-Pyrrole | 1.06 | −0.66 |
| CAS Number | VOC Name | V.I. | Aroma/Flavor Description | Aroma Detection Threshold in Water (ppm) |
|---|---|---|---|---|
| 38284-27-4 | Octa-3,5-dien-2-one | 3.58 | Green 1, sweet 2, cooked 2, creamy 2, coconut 2, milky 2, cheesy 2 | 0.150 2 |
| 00534-22-5 | 2-Methylfuran | 3.13 | Cocoa 3, ethereal 3, green 3, nutty 3, almond 3, coffee 3 | - |
| 00585-25-1 | Octane-2,3-dione | 3.10 | Green 3, cilantro 3, fatty 3, leafy 3, herbal 3 | - |
| 00111-71-7 | Heptanal | 2.97 | Green 3, oily 3, grassy 3, clover 3, cilantro 3 | [0.003; 0.060] 2 |
| 04313-03-5 | (E,E)-Hepta-2,4-dienal | 2.93 | Fatty 3, greasy 3, oily 2, green 2, herbal 2 | - |
| 03302-10-1 | 3,5,5-Trimethylhexanoic acid | 2.51 | - | - |
| 00600-14-6 | Pentane-2,3-dione | 2.33 | Toasted 3, buttery 2, fermented 2, dairy 2, creamy 2 | 0.020 2 |
| 00123-73-9 | (E)-But-2-enal | 2.32 | Plastic 4 | - |
| 00100-41-4 | Ethylbenzene | 2.30 | Metallic 5, phenolic 5, chemical 5 | - |
| 00620-02-0 | 5-Methylfuran-2-carbaldehyde | 2.24 | Brown 3, sweet 3, caramellic 3, grain 3, maple 3 | 6.000 2 |
| 00098-86-2 | Acetophenone | 2.14 | Powdery 3, bitter almond 3, cherry 3 | 0.170 2 |
| 00096-17-3 | 2-Methylbutanal | 2.12 | Fusel 3, nutty 3, caramellic 3, cocoa 3 | - |
| 00065-85-0 | Benzoic acid | 1.82 | Pungent 1, sour 1 | 85.000 2 |
| 00104-50-7 | γ-Octalactone | 1.58 | Lactonic 3, coconut 3, creamy 3, sweet 2, fatty 2 | 0.007 2 |
| 04505-38-8 | Cyclohex-2-ene-1,4-dione | 1.55 | - | - |
| 01115-11-3 | 2-Methylbut-2-enal | 1.50 | Fresh 3, fruity 3, green 3, almond 3, nutty 3 | - |
| 00705-86-2 | δ-Decalactone | 1.46 | Coconut 3, creamy 3, fatty 3, buttery 3, milky 3 | 0.100 2 |
| 00930-60-9 | Cyclopent-4-ene-1,3-dione | 1.44 | Smoky 5, ashy 5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caille, C.; Boukraâ, M.; Rannou, C.; Villière, A.; Catanéo, C.; Lethuaut, L.; Lagadec-Marquez, A.; Bechaux, J.; Prost, C. Analysis of Volatile Compounds in Processed Cream Cheese Models for the Prediction of “Fresh Cream” Aroma Perception. Molecules 2023, 28, 7224. https://doi.org/10.3390/molecules28207224
Caille C, Boukraâ M, Rannou C, Villière A, Catanéo C, Lethuaut L, Lagadec-Marquez A, Bechaux J, Prost C. Analysis of Volatile Compounds in Processed Cream Cheese Models for the Prediction of “Fresh Cream” Aroma Perception. Molecules. 2023; 28(20):7224. https://doi.org/10.3390/molecules28207224
Chicago/Turabian StyleCaille, Coline, Mariem Boukraâ, Cécile Rannou, Angélique Villière, Clément Catanéo, Laurent Lethuaut, Araceli Lagadec-Marquez, Julia Bechaux, and Carole Prost. 2023. "Analysis of Volatile Compounds in Processed Cream Cheese Models for the Prediction of “Fresh Cream” Aroma Perception" Molecules 28, no. 20: 7224. https://doi.org/10.3390/molecules28207224
APA StyleCaille, C., Boukraâ, M., Rannou, C., Villière, A., Catanéo, C., Lethuaut, L., Lagadec-Marquez, A., Bechaux, J., & Prost, C. (2023). Analysis of Volatile Compounds in Processed Cream Cheese Models for the Prediction of “Fresh Cream” Aroma Perception. Molecules, 28(20), 7224. https://doi.org/10.3390/molecules28207224








