Abstract
Glucosinolates (GSLs) in different plant parts of broccoli (Brassica oleracea var. italic) and rocket (Eruca vesicaria) were analyzed qualitatively and quantitatively before and after treatment with sodium selenate (2 and 5 mM), by their desulfo-counterparts using the UHPLC-DAD-MS/MS technique. Twelve GSLs were detected in broccoli (five aliphatic, one arylaliphatic, and six indolic), where 4-(methylsulfanyl)butyl GSL (glucoerucin) was the main one in the roots (4.88–9.89 µmol/g DW), 4-(methylsulfinyl)butyl GSL (glucoraphanin) in stems (0.44–1.11 µmol/g DW), and 4-hydroxyindol-3-ylmethyl GSL (4-hydroxyglucobrassicin) in leaves (0.51–0.60 µmol/g DW). No GSL containing selenium was detected in the treated broccoli. Ten GSLs were detected in rocket (seven aliphatic and three indolic), where 4-(methylsulfanyl)butyl GSL (glucoerucin) was the main one in the roots (4.50–20.59 µmol/g DW) and 4-methoxyindol-3-ylmethyl GSL (4-methoxyglucobrassicin) in the aerial part (0.57–5.69 µmol/g DW). As a result of induced stress by selenium fertilization, the total GSL content generally increased in both plants. In contrast to broccoli, the roots and the aerial part of the rocket treated with a high concentration of sodium selenate contained 4-(methylseleno)butyl GSL (glucoselenoerucin) (0.36–4.48 µmol/g DW). Although methionine-derived GSLs are the most abundant in both plants, the plants’ ability to tolerate selenate and its regulation by selenoglucosinolate production is species- and growth-stage-dependent.
1. Introduction
Selenium (Se) is an important micronutrient required by most living organisms. The difference between deficiency and toxicity is incredibly narrow compared to other micronutrients, with toxic dosages of >400 μg/day and a dietary deficiency of 40 μg/day, which is an emerging global problem [1,2,3]. Plants are important sources of organic Se, as they have the capacity to accumulate inorganic Se or metabolites and store them as organic Se forms. Selenates (SeO42−) and selenites (SeO32−) are the most significant inorganic forms of Se that are easily taken by plants [4]. Selenate is the most common and fastest-assimilating form of bioavailable Se in alkaline soils with high oxygen content, whereas selenite is more prevalent in anoxic settings with higher acidity and humidity [4]. Plants usually intake selenate, which is reduced, incorporated into organic compounds (selenomethionine, selenocysteine) and further transported through the food chain, and finally decomposed and excreted from the organism, thus completing the biogeochemical cycle of Se [5]. Se levels in soil generally reflect its presence in foods and, therefore, its availability to humans. With an estimated mean Se content in human soft tissues of 110 μg/kg, it was found that the Se intake status in Europe was low, which means that the Se levels in European soils are not sufficient [1,3]. Therefore, biofortification is an important strategy to increase Se in the edible parts of plants.
Based on their ability to accumulate Se, plants can be categorized into three main groups: non-accumulators (accumulate less than 100 mg Se/kg dry weight, DW), secondary accumulators or accumulators (accumulate up to 1000 mg Se/kg DW), and hyperaccumulators (accumulate over 1000 mg Se/kg DW) [6]. The ability to hyperaccumulate Se appears to have evolved within the Asteraceae, Brassicaceae, and Fabaceae families [6]. Broccoli (Brassica oleracea var. italica) and rocket (Eruca vesicaria) are members of Brassicaceae family, which is the largest family in the order Brassicales. Some known Se secondary accumulators include several plants of the Brassica genus, such as Brassica juncea, Brassica napus, and Brassica oleracea var. italic, whereas Se-hyperaccumulators include plants of the Cardamine genus (Cardamine hupingshanensis, Cardamine violofolia) and Stanleya genus (Stanleya pinnata, Stanleya bipinnata) [6,7].
Glucosinolates (GSLs) are well-defined specialized plant metabolites containing sulfur and nitrogen which represent the molecular tags of plants from the Brassicaceae family. Generally, they are found in 16 families of the order Brassicales, as well as several plants outside of this order, such as the genus Drypetes (family Putranjivaceae, order Malpighiales) and Rinorea genus (family Violaceae, order Malpighiales) [8,9]. GSLs can be divided into three classes based on the structure of various amino acid precursors: aliphatic GSLs derived from methionine, isoleucine, leucine, or valine; arylaliphatic GSLs derived from phenylalanine or tyrosine; and indole GSLs derived from tryptophan. Only 90 of the 139 GSLs discovered in the plant kingdom have been thoroughly described by relevant spectroscopy techniques (MS, NMR) to date [10,11,12]. Se-biofortification influences GSL production with possible exchange positions of sulfur (S) by selenium (Se) in three places in the side chain, in thioglucose, and in the sulfate group (Figure 1) [7].
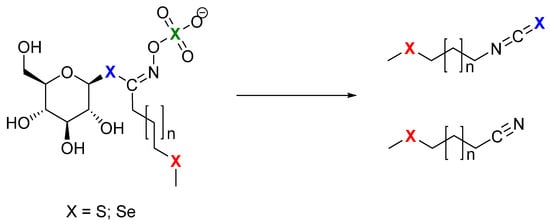
Figure 1.
Possible sites (X) for selenium to replace sulfur: green—incorporated in sulfate group; blue—incorporated in thioglucose, and then it is selenoGSL, which produces isoselenocyanides; red—incorporated in side chain, and then it is selenoMet-derived GSL, as breakdown results in isothiocyanates.
Several crop species have been biofortified with Se in field or greenhouse experiments where various Se sources and techniques were used. According to Stewart et al. (1974), sinigrin, which was isolated from horseradish (Armoracia rusticana) after receiving Na275SeO4, was shown to contain Se in its structure [13]. After three weeks at high Se concentrations (100 ppm) in water-cultured specimens of the Se-tolerant species desert princes’ plume (Stanleya pinnata), Bertelsen et al. (1988) detected trace quantities of GSL containing Se named as but-3-enylselenoglucosinolate, whereas those grown in environments with lower, more typical Se amounts did not contain this compound [14]. Additionally, no such selenoglucosinolates were found in garden cress (Lepidium sativum) and horseradish (Armoracia rusticana) [14]. This selenoglucosinolate, originally defined as a compound in which the Se atom is replaced by an S atom in the anomeric position in the GSL structure, is converted into isoselenocyanates by enzymatic hydrolysis [14]. Since the formation of isoselenocyanates rather than isothiocyanates significantly departs from the characteristics of GSLs, they are, by definition, not GSLs (Figure 1).
Matich et al. (2012) treated broccoli (B. oleracea var. italica), cauliflower (B. oleracea L.), and forage rape (B. napus) with elevated sodium selenate and examined them for the presence of organoselenides. Based on the analysis of broccoli and cauliflower florets, and the roots of forage rape, the discovery of putative GSL hydrolysis products using synthesized standards confirmed by MS and NMR, as well as by LC-MS analysis, three Se-Met artificially derived GSLs of the general type (methylseleno)alkyl GSL are accepted: 3-(methylseleno)propyl GSL (glucoselenoibervirin [144]), 4-(methylseleno)butyl GSL (glucoselenoerucin, [145]), and 5-(methylseleno)pentyl GSL (glucoselenoberteroin, [146]) [15]. This analysis showed that Se is preferentially incorporated into the methylselenyl moiety rather than the sulfate or β-thioglucose groups. A further Se-biofortification study by Matich et al. (2015) using HPLC-MS/MS tentatively indicated glucoselenoraphanin in broccoli (B. oleracea var. italica) florets with Se envisioned in the side chain and glucoselenonasturtiin in forage rape (B. napus) roots, but with Se, that could not be envisioned to be on the side chain, suggesting a selenoGSL sensu Bertelsen et al. [14,16].
Black mustard (Brassica nigra) seeds grown on naturally Se-rich soils in the Punjab region of India, where the Se soil content ranges from 2 to 7 mg/kg, were studied by Ouerdane et al. (2013) [17]. Using HPLC ESI Orbitrap MS(/MS) and GC APCI TQ MS/MS for the extract analysis, among over 30 Se species, they detected the presence of “selenoglucosinolates”, namely glucoselenoibervirin and glucoselenoerucin, as well as their corresponding degradation products 3-(methylseleno)propylisothiocyanate and 4-(methylseleno)butanenitrile, i.e., 4-(methylseleno)butylisothiocyanate and 5-(methylseleno)pentanenitrile, respectively. To avoid confusion with selenoglucosinolates according to Bertelsen et al. [14], GSLs with Se in the side chain are suggested to be referred to as selenoMet-derived GSLs [8]. The presence of Se in the side chains is fully compatible with the definition of GSLs because they form isothiocyanates and not isoselenocyanates as hydrolysis products (Figure 1).
The effects of Se-fertilization on GSL production in a radish (Raphanus sativus) have been studied by McKenzie et al. (2019) throughout a period of five developmental phases (from seed to fully developed salad greens). With the double bond geometry still unclear, they used tandem mass spectrometry to determine the existence of a novel Se-containing GSL, 4-(methylseleno)but-3-enyl GSL (also named selenoglucoraphenin) [18]. By using GC-MS, two similar isothiocyanates of 4-(methylseleno)but-3-enyl isothiocyanate were tentatively identified as (E/Z?) isomers. Se-biofertilization of mature radish led to the presence of selenoglucosinolates in the seed [18].
The aim of this study is to investigate the influence of Se intake and its metabolism, with special emphasis on the identification and quantification of GSLs and selenoMet-derived GSLs. For this purpose, cultivated broccoli (Brassica olaracea var. italic) and rocket (Eruca vesicaria) were watered with sodium selenate salt solutions (Na2SeO4) of different concentrations for 35 days and GSLs were analyzed by UHPLC-DAD-MS/MS according to the ISO 9167-1 official method [19].
2. Results and Discussion
2.1. Effect of Selenium Treatment on Broccoli
Brassica oleracea var. italica, a young plant which, after 18 days, had no developed flowers or florets, was exposed to Na2SeO4 concentrations of 2 mM and 5 mM over a period of 35 days. During that time period, the plants did not exhibit any signs of decay of the existing plant parts throughout cultivation. The detrimental influence of selenate solution on the aerial part’s growth was notable when compared to the reference plant’s height, which was 33 cm, and in comparison to 25 cm of the plant exposed to 5 mM selenate solution (Figure S1, Table S1). Se-biofertilization had a detrimental effect on root growth, as evidenced by the fact that the reference plant’s measured roots were 24 cm long compared to 6 cm length of roots watered with the highest Na2SeO4 concentration (Figure S1, Table S1). The symptom of Se exposure is a reduction in root elongation. Selenate treatment was shown to decrease cytokinin oxidase transcript levels. Cytokinin oxidases in Arabidopsis are known to catalyze the irreversible degradation of the hormone cytokinin, in which accumulation in the root tips may partly be responsible for the root meristem shortening [20,21,22]. A previous report by Adamopoulou et al. included the study of Se toxicity on broccoli florets grown hydroponically in a greenhouse for 12 weeks [23,24]. The plants were fortified with two different concentrations of sodium selenate (1.5 mM and 3.0 mM) between the 5th and 10th week. Although broccoli florets grown in the presence and absence of sulfur had the same weight, the absence of sulfur also led to increased Se toxicity, which reduced weight by up to 65%. Also, the leaves’ optical characteristics revealed no visible signs of the biofortification [23,24]. Tian et al. reported the influence of selenium treatment on broccoli growth and showed that when S nutrition was low, Se was particularly harmful to plants and dramatically reduced plant sizes. They proposed that the Se toxicity could be counteracted by increasing sulfate supplementation, which would likely occur via the decreasing non-specific integration of Se into proteins and altering the redox system [25].
GSLs were qualitatively and quantitatively analyzed via desulfated forms using UHPLC-DAD-MS/MS, and the results are presented in Table 1 and Figure 2, as well as in the Supplementary Materials (Figures S3–S6).

Table 1.
Glucosinolate content (µmol/g DW) in Brassica oleracea var. italica.
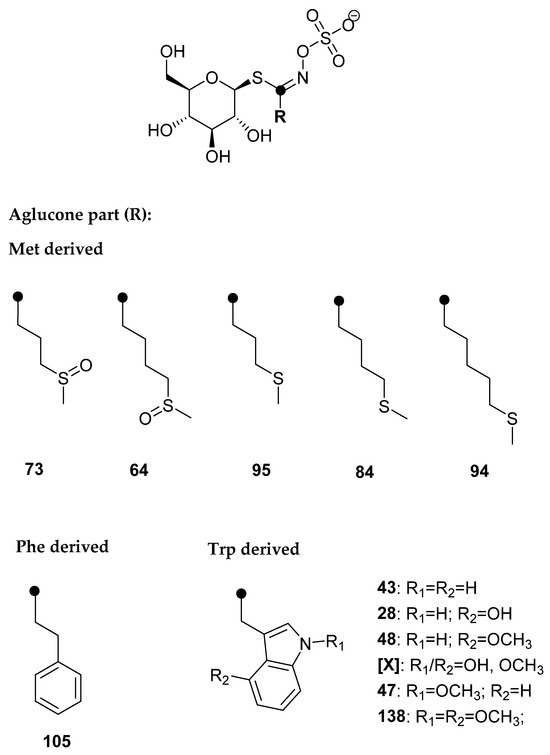
Five Met-derived desulfoGSLs (dGSLs) with sulfur in the side chain, one Phe-derived dGSL, and six Trp-derived dGSLs were identified. Based on the obtained [M+Na]+, no GSLs with Se in the structure were detected. At tR = 8.30 min, [M + Na]+ = 437 was observed, which was assumed to be a hitherto unidentified dGSL, and was labeled [X]. The UV spectrum of dGSL indicated that it is an indole GSL, and the MS2 spectrum was recorded in order to identify the characteristic fragments (Figure S6). Fragments m/z 185 (“a”, Na+ adduct of C6H10O5) and m/z 274 (“c”, loss of C6H10O5) are observed, which suggested that it is a GSL. The fragment m/z 389 indicated the loss of the methoxy and hydroxyl groups. Based on this information, hydroxymethoxyglucobrassicin was proposed, since it is known that hydroxylation and the subsequent methylation of the indole ring takes place at positions 1 and 4 (R1 and R2, respectively, Figure 2) [26]. Namely, the key enzymes are cytochrome P450 monooxygenases of the CYP81F subfamily, which carry out hydroxylation reactions at position 4 or 1 of the indole ring. IGMT enzymes, which belong to the family of plant O-methyltransferases, convert hydroxy intermediates into methoxylated products, and the enzyme IGMT5 specific for position 1 of the indole ring was found, i.e., IGMT1-4 enzymes specific for position 4 [27].
The aliphatic and indole GSL type represent the major GSL found in the analyzed plant parts, with the roots having the highest GSL content. The total content of GSLs in the roots increased for both solution concentrations added (2 mM and 5 mM) when compared to the control and differed significantly (p ≤ 0.05). Glucoerucin (84), as the main GSL in roots, significantly increased from 4.88 µmol/g DW to 9.05 and 9.89 µmol/g DW, respectively.
Several studies have reported the existence of Se-GSLs in certain plant parts, such as florets and roots, after the Se-biofertilization of GSL-containing plants. Matich et al. (2012, 2015) showed the presence of Se-GSLs in biofortified broccoli florets [15,16]. However, the same plant in our study did not reach this stage of development (no florets) and no Se-GSLs were detected. According to Tian et al., the total GSL contents of broccoli sprouts determined by glucose from GSL hydrolysis were not significantly changed by the impacts of Se treatments (100 µmol/L selenite and selenate) [25]. However, since the analysis was not conducted in accordance with the ISO 9167 standard method [19], this should only be accepted cautiously.
2.2. Effect of Selenium Treatment on Rocket
Eruca vesicaria (rocket), a 24-day old plant, was also exposed to Na2SeO4 concentrations of 2 mM and 5 mM over a period of 35 days. A similar detrimental influence of Se-biofortification on morphological characteristics was observed for rocket as in broccoli. When sodium selenate (5 mM) was applied, the plant’s height decreased from ca. 23 cm to 8 cm and its root length from ca. 15 cm to 10 cm. In this case, the aerial part biomass decreased, and the beginning of decay was observed for the highest concentration (Figure S2, Table S2).
GSLs were qualitatively and quantitatively analyzed via desulfated forms using UHPLC-DAD-MS/MS, and the results are presented in Table 2 and Figure 3 and Figure 4, as well as in the Supplementary Materials (Figures S7–S12).

Table 2.
Glucosinolate content (µmol/g DW) in cultivated Eruca vesicaria.
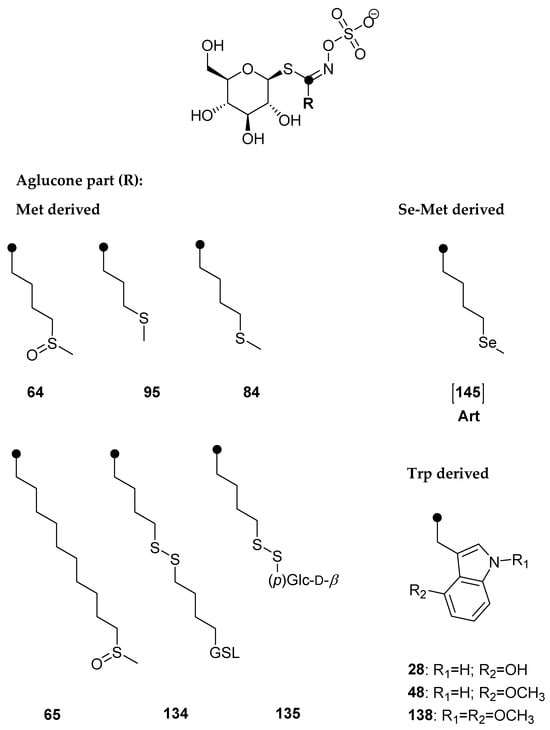
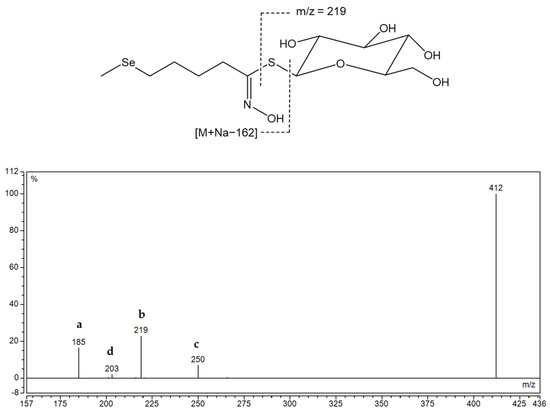
Figure 4.
Structure of desulfoglucoselenoerucin d[145] and MS2 spectra of its sodium adduct. Fragment types observed, alone or in combination, in MS2 spectra desulfoglucosinolates in positive mode: a—Na+ adduct of anhydroglucose, C6H10O5 (at m/z 185) or an acyl derivative; b—Na+ adduct of thioglucose, C6H11O5SH (at m/z 219) or an acyl derivative; c—Loss of anhydroglucose (m/z 162) or an acyl derivative; d—Na+ adduct of glucose, C6H12O6 (at m/z 203) [28].
Six methionine-derived GSLs and three indole ones were identified in the rocket. By comparing the chromatograms obtained for the root and aerial component, it was discovered that Se-treated samples exhibit a signal that is not present in the reference plant, particularly in the root. Its [M+Na]+ was 412 at tR = 7.6 min, which did not correspond to previously known GSL of that mass. It was assumed that it is selenomethionine-derived selenoglucosinolate, namely glucoselenoerucin ([145]), whose desulfo form structure is given in Figure 4, since the obtained mass corresponds to this dGSL when sulfur is replaced by selenium.
In the MS2 spectrum, fragments marked “a”, “b”, “c”, and “d” are present, indicating that Se was not found in the thioglucose part, but in the side chain derived from selenomethionine (Se-Met). Right next to 412, 410 appears, which is assumed to be the isotope of selenium 78, so MS2 was obtained for this [M + Na]+ and fragment m/z 185 (a) was also observed (Figure S12). According to previous literature, this selenoglucosinolate was identified for the first time through its desulfated form and its MS2 was shown for the first time.
The total GSL content is related mostly to the content of aliphatic GSLs, with the highest found in the roots. The occurrence of this GSL derived from Se-Met can be related to the high content of its sulfur analogue glucoerucin (84), and the highest content was obtained in the roots of the plant treated with 2 mM solution, 4.48 µmol/g DW. With the addition of 5 mM sodium solution, rocket showed the signs of decay and the total GSL content decreased, having 14.21 and 2.47 µmol/g DW in the roots and aerial parts, in comparison to the control having 32.34 and 13.24 µmol/g DW, respectively, and differed significantly (p ≤ 0.05). Consequently, the content of GSL [145] decreased. Dall’Acqua et al. studied the Se-biofortification of two rocket species (Eruca sativa = E. vesicaria and Diplotaxis tenuifolia) grown at 0–40 µM Na2SeO4 in hydroponics and also showed a GSL decrease [29]. Contrary to our investigation, selenoglucosinolates have not been reported in these species. Also, they showed that selenium treatment reduced cysteine and methionine content, and glutathione (GSH), whose precursor is cysteine, also showed a trend toward reduction; thus, a decrease in GSLs, also observed in this study, may be explained by the negative effects of selenate on aminoacids biosynthesis, during which selenoamino acids can be formed and be incorporated into proteins and/or form selenoGSLs [30].
3. Materials and Methods
3.1. Materials and Reagents
4-(Methylsulfinyl)butyl GSL (glucoraphanin, 64), 4-(methylsulfanyl)butyl GSL (glucoerucin, 84), 5-(methylsulfanyl)pentyl GSL (glucoberteroin, 94), 9-(methylsulfinyl)nonyl GSL (glucoarabin, 68), 10-(methylsulfinyl)decyl GSL (glucocamelinin, 65), 2-phenylethyl GSL (gluconasturtiin, 105), indol-3-ylmethyl GSL (glucobrassicin, 43), 4-hydroxyindol-3-ylmethyl GSL (4-hydroxyglucobrassicin, 28), 4-methoxyindol-3-ylmethyl GSL (4-methoxyglucobrassicin, 48), and N-methoxyindol-3-ylmethyl GSL (neoglucobrassicin, 47) were purchased from Phytoplan Diehm & Neuberger GmbH (Heidelberg, Germany). 4-Hydroxybenzyl GSL (glucosinalbin, 23) was isolated from Sinapis alba seeds and 3-(methylsulfinyl)propyl GSL (glucoiberin, 73) was isolated from Anastatica hierochuntica, both confirmed by NMR. All other chemicals and reagents were of analytical grade.
3.2. Plant Growth and Harvesting
Young plants of Brassica oleracea var. italica (broccoli), one month old and with a total height of 18 cm (root 4 cm), were transplanted into pots (one plant per pot) (dimensions, (w × d × h) 12 × 12 × 10 cm) with soil Potgrond H (Potgrond H 70, Klasmann-Deilmann GmbH, Geeste, Germany) (pH = 6.0) and watered with tap water during the next fifteen days in order to adapt. On the fifteenth day after transplantation, the height of the above-ground part was measured, which was 18 cm, and the height of the stem was 10 cm. The reference plant was watered only with water, whereas the remaining two plants were watered with prepared solutions of sodium selenate with a concentration of 2 mM and 5 mM (three repetition per experiment). During cultivation, the plants were watered with tap water for 35 days, and they were biofortified twice a week with a 20 mL selenate solution. We estimate this selenium application to be equivalent to ca. 22 and 55 kg/ha, respectively, which is considerably higher than the 3–76 g/ha applied to agricultural and forage crops [15,16]. The growth and development of the above-ground part were recorded throughout the period, and on the 35th day from the beginning of watering, the length of the roots and the height of the above-ground part were measured.
Eruca vesicaria (rocket) was grown from the seeds of the Italian distributor Impex s.r.l. cement, obtained from the Sjeme store, Split, Croatia. Seeds (100 mg) were placed in each cultivation pot (n = 3) with soil Potgrond H and germination began on the 3rd day after watering. On the 10th day, the plants were transplanted into three larger pots (dimensions, (w × d × h) 12 × 12 × 10 cm) and on the 24th day after sowing, watering with sodium selenate solutions was started. The height of the aerial part of the plants before watering was 7.5 cm, and the length of the roots was 3 cm.
After collection, all plants were washed with tap water and lyophilized. They were divided into the root and aerial part for the purpose of the qualitative and quantitative analysis of GSLs. The lengths of plant parts were measured by a ruler with an accuracy of 0.1 cm, for the control and each treatment (n = 3).
3.3. Isolation and Chemical Analysis
3.3.1. Isolation of Desulfoglucosinolates
GSLs were extracted as previously reported [31]. Freeze-dried plant parts were ground to a fine powder, from which 100 mg was extracted for 5 min at 80 °C in 2 × 1 mL MeOH/H2O (70:30 v/v) to inactivate the endogenous myrosinase. Each extract (1 mL) was loaded onto a mini-column filled with 0.5 mL of DEAE-Sephadex A-25 anion-exchange resin (GE Healthcare) conditioned with 25 mM acetate buffer (pH 5.6). After washing the column with 70% MeOH and 1 mL of ultrapure water, the optimal conditions for desulfation were set by adding a buffer solution. Each mini-column was loaded with 20 μL (0.35 U/mL) of purified sulfatase and left to stand for 18 h at room temperature. The desulfoGSLs were then eluted with 1.5 mL of ultra-pure H2O, lyophilized, and diluted to 1 mL. The samples were stored at −20 °C until further analysis by UHPLC-DAD-MS/MS.
3.3.2. UHPLC-MS/MS Analysis
Analysis was performed on UHPLC-MS/MS (Ultimate 3000RS with TSQ Quantis MS/MS detector, Thermo Fisher Scientific, Waltham, MA, USA) using a Hypersil GOLD column (3.0 µm, 3.0 × 100 mm, Thermo Fisher Scientific, Waltham, MA, USA). A gradient consisting of solvent A (50 μM NaCl in H2O) and solvent B (acetonitrile:H2O 30:70 v/v) was applied at a flow rate of 0.5 mL/min as follows: 0.14 min 96% A; 7.84 min 14% A; 8.96 min 14% A; 9.52 min 5% A; 13.16 min 5% A; 13.44 min 96% A; 15.68 min 96% A. The column temperature was held at 15 °C for E. vesicaria dGSLs and 25 °C for B. oleracea var. italica dGSLs and the injection volume was 5 µL. The electrospray interface was an H-ESI source operating with a capillary voltage of 3.5 kV at 350 °C. The system was operated in the positive ion electrospray mode.
Qualitative analysis was performed by the manual examination of chromatograms and MS2 spectra of each peak of desulfoglucosinolate as sodium adduct ([M+Na]+). The amount of GSLs was quantified using a calibration curve of pure desulfosinigrin solution (range from 0.14 to 1.4 mM) and RPFs for each individual desulfoGSL [31]. The RPF values for quantification of desulfoGSLs were as follows: RPF 1.07 for 3-(methylsulfinyl)propyl GSL (glucoiberin, 73) and 4-(methylsulfinyl)butyl GSL (glucoraphanin, 64); 0.80 for 3-(methylsulfanyl)propyl GSL (glucoibervirin, 95); 1.04 for 4-(methylsulfanyl)butyl GSL (glucoerucin, 84); 0.50 for glucosinalbin (23); 0.95 for gluconasturtiin (105); 0.29 for glucobrassicin (43); 0.28 for 4-hydroxyglucobrassicin (28); 0.25 for 4-methoxyglucobrassicin (48); 0.20 for N-methoxyglucobrassicin (47) and 1,4-dimethoxyindol-3-ylmethyl GSL (138) [32,33]; arbitrary RPF 0.25 for hydroxymethoxyglucobrassicin ([X]), and 1.0 for 5-(methylsulfanyl)pentyl GSL (glucoberteroin, 94), 9-(methylsulfinyl)nonyl GSL (glucoarabin, 68), 9-(methylsulfonyl)nonyl GSL (79), 10-(methylsulfinyl)decyl GSL (65), 4-(methylseleno)butyl GSL [145], 4-(β-d-glucopyranosyldisulfanyl)butyl GSL (135), and dimeric 4-mercaptobutyl GSL (134). The bold numbers are related to the GSL number given in the review paper by Blažević et al. [10].
3.4. Statistical Analysis
One-way analysis of variance (ANOVA) was performed with SPSS software, version 25.0 (IBM Corporation, New York, NY, USA), in order to determine the difference between treatments. First, the data were tested for normal distribution and log-transformed for further analysis. One-way ANOVA was performed if the requirement of homogeneity of variance was fulfilled; otherwise, Welch correction was performed first. If there was a statistically significant effect between different treatments, Tukey’s honest significant test was performed with a significance level of p ≤ 0.05.
4. Conclusions
The morphological changes and changes in GSL content in broccoli and rocket were used to track the stress caused by selenate. Morphological alterations were detected in the form of decreased root development, which can be explained by the competing action of sulfate and selenate. Both plants’ principal GSLs were produced from methionine biosynthesis, with the major GSL being 4-(methylsulfanyl)butyl GSL (glucoerucin, 84). However, only rocket biosynthesized the Se analogue of 84, 4-(methylseleno)butyl GSL (glucoselenoerucin, [145]). Se was determined in the side chain based on distinctive fragmentations in the MS2 spectrum, which was recorded and analyzed for the first time for this desulfoselenoglucosinolate. Although other Met-derived GSLs with sulfur in the side chain have been identified, no Se analogues have been found. This study emphasizes the importance of plant tolerance to selenate and the growth stage, such as in broccoli, where biofortification should begin as soon as the florets begin to emerge from the meristerm and not before.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28207203/s1, Figure S1. Broccoli (Brassica oleracea var. italic) watered with water (reference) and different concentrations of sodium selenate solution; Table S1. The effect of selenate treatments on B. oleracea var. italic; Figure S2. Rocket (Eruca vesicaria) watered with water (reference) and different concentrations of sodium selenate solution.; Table S2. The effect of selenate treatments on E. vesicaria; Figure S3. Chromatograms of desulfoglucosinolates (column temperature 25 °C) obtained from the roots of Brassicca oleracea var. italica before and after the treatment with Na2SeO4; Figure S4. Chromatograms of desulfoglucosinolates (column temperature 25 °C) obtained from the stem of Brassicca oleracea var. italica before and after the treatment with Na2SeO4; Figure S5. Chromatograms of desulfoglucosinolates (column temperature 25 °C) obtained from the leaf of Brassicca oleracea var. italica before and after the treatment with Na2SeO4; Figure S6. MS2 spectra at 15V and 20V of desulfo-4-hydroxy-N-methoxyglucobrassicin; Figure S7. Chromatograms of desulfoglucosinolates (column temperature 15 °C) obtained from the roots of Eruca vesicaria before and after the treatment with Na2SeO4; Figure S8. Chromatogram of desulfoglucosinolates (column temperature 15 °C) obtained from the aerial part of Eruca vesicaria before and after the treatment with Na2SeO4; Figure S9. Chromatograms of desulfoglucosinolate (dGSL) standards.; Figure S10. MS2 spectra of desulfoglucosinolates.; Figure S11. MS and UV spectra of detected desulfoglucoselenoerucin. Figure S12. MS2 spectra at 15V of desulfoglucoselenoerucin formed during biofortification (two isotopes of Se—m/z 410 and 412).
Author Contributions
Conceptualization, I.B.; Methodology, A.Đ., L.K.M. and I.B.; Formal analysis, A.Đ., K.U. and I.B.; Investigation, A.Đ., L.K.M. and I.B.; Data curation, A.Đ., K.U. and I.B.; Writing—original draft, I.B.; Writing—review & editing, A.Đ., K.U., L.K.M. and I.B.; Supervision, I.B.; Project administration, I.B.; Funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been fully supported by the Croatian Science Foundation (Grant IP-2016-06-1316).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are also thankful for the scientific research equipment financed by EU grant “Functional integration of the University of Split, PMF-ST, PFST and KTFST through the development of the scientific and research infrastructure” (KK.01.1.1.02.0018).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Revised Edition; Selinus, O., Ed.; British Geological Survey: Nottingham, UK, 2013; pp. 375–419. [Google Scholar] [CrossRef]
- dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. In Selenium in Plants; Plant Ecophysiology; Pilon-Smits, E., Winkel, L., Lin, Z.Q., Eds.; Springer: Cham, Switzerland, 2017; Volume 11. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, H.A.; Banuelos, G.S.; Mayland, H.F. Selenium uptake by plants from soils amended with inorganic and organic materials. J. Environ. Qual. 1998, 27, 1218–1227. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in Brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel sources and biological potential. In Glucosinolates, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–60. [Google Scholar] [CrossRef]
- Montaut, S.; De Nicola, G.R.; Agnaniet, H.; Issembe, Y.; Rollin, P.; Menut, C. Probing for the presence of glucosinolates in three Drypetes spp. (Drypetes euryodes (Hiern) Hutch., Drypetes gossweileri S. Moore, Drypetes laciniata Hutch.) and two Rinorea spp. (Rinorea subintegrifolia O. Ktze and Rinorea woermanniana (Büttner) Engl.) from Gabon. Nat. Prod. Res. 2017, 31, 308–313. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Montaut, S.; Read, S.; Blažević, I.; Nuzillard, J.-M.; Roje, M.; Harakat, D.; Rollin, P. Investigation of the glucosinolates in Hesperis matronalis L. and Hesperis laciniata All.: Unveiling 4′-O-β-D-apiofuranosylglucomatronalin. Carbohydr. Res. 2020, 488, 107898. [Google Scholar] [CrossRef] [PubMed]
- Trabelcy, B.; Chinkov, N.; Samuni-Blank, M.; Merav, M.; Izhaki, I.; Carmeli, S.; Gerchman, Y. Investigation of glucosinolates in the desert plant Ochradenus baccatus (Brassicales: Resedaceae). Unveiling glucoochradenin, a new arabinosylated glucosinolate. Phytochemistry 2021, 187, 112760. [Google Scholar] [CrossRef]
- Stewart, J.M.; Nigiam, S.N.; McConnel, W.B. Metabolism of Na275SeO4 in horseradish: Formation of selenosinigrin. Can. J. Biochem. 1974, 52, 144–145. [Google Scholar] [CrossRef]
- Bertelsen, F.; Gissel-Nielsen, G.; Kjær, A.; Skrydstrup, T. Selenoglucosinolates in nature: Fact or myth? Phytochemistry 1988, 27, 3743–3749. [Google Scholar] [CrossRef]
- Matich, A.J.; McKenzie, M.J.; Lill, R.E.; Brummell, D.A.; McGhie, T.K.; Chen, R.K.-Y.; Rowan, D.D. Selenoglucosinolates and their metabolites produced in Brassica spp. fertilised with sodium selenate. Phytochemistry 2012, 75, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Matich, A.J.; McKenzie, M.J.; Lill, R.E.; McGhie, T.K.; Chen, R.K.; Rowan, D.D. Distribution of selenoglucosinolates and their metabolites in Brassica treated with sodium selenate. J. Agric. Food Chem. 2015, 63, 1896–1905. [Google Scholar] [CrossRef]
- Ouerdane, L.; Aureli, F.; Flis, P.; Bierla, K.; Preud’homme, H.; Cubadda, F.; Szpunar, J. Comprehensive speciation of low-molecular weight selenium metabolites in mustard seeds using HPLC-electrospray linear trap/Orbitrap tandem mass spectrometry. Metallomics 2013, 5, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Matich, A.; Hunter, D.; Esfandiari, A.; Trolove, S.; Chen, R.; Lill, R. Selenium application during radish (Raphanus sativus) plant development alters glucosinolate metabolic gene expression and results in the production of 4-(methylseleno)but-3-enyl glucosinolate. Plants 2019, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- European Economic Community, Commisssion Regulation. Oilseeds—determination of glucosinolates high performance liquid chromatography. Off. J. Eur. Comm. 1990, L170, 27–34. [Google Scholar]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilon-Smits, E.A.H. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant. 2008, 132, 236–253. [Google Scholar] [CrossRef]
- Lehotai, N.; Feigl, G.; Koos, A.; Molnar, A.; Ördög, A.; Peto, A.; Erdei, L.; Kolbert, Z.S. Nitric oxide-cytokinin interplay influences selenite sensitivity in Arabidopsis. Plant Cell Rep. 2016, 35, 2181–2195. [Google Scholar] [CrossRef]
- Kolbert, Z.; Lehotai, N.; Molnár, Á.; Feigl, G. “The roots” of selenium toxicity: A new concept. Plant Signal. Behav. 2016, 11, e1241935. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulou, M.; Bouzas, E.A.; Siyiannis, V.; Perouli, M.; Kokotou, M.; Chorianopoulou, S.N.; Constantinou-Kokotou, V.; Bouranis, D.L. Selenium assimilation by broccoli: Effect of Se inputs on the biosynthesis of secondary metabolites under normal or reduced S inputs. In Proceedings of the 28th International Symposium of CIEC, Fertilization and Nutrient Use Efficiency in Mediterranean Environments; Bouranis, D.L., Haneklaus, S.H., Chorianopoulou, S.N., Li, J., De Kok, L.J., Schnug, E., Ji, L., Eds.; Utopia Publishing Ltd.: Athens, Greece, 2020; pp. 169–173. [Google Scholar]
- Bouranis, D.L.; Stylianidis, G.P.; Manta, V.; Karousis, E.N.; Tzanaki, A.; Dimitriadi, D.; Bouzas, E.A.; Siyiannis, V.F.; Constantinou-Kokotou, V.; Chorianopoulou, S.N.; et al. Floret biofortification of broccoli using amino acids coupled with selenium under different surfactants: A case study of cultivating functional foods. Plants 2023, 12, 1272. [Google Scholar] [CrossRef]
- Tian, M.; Hui, M.; Thannhauser, T.W.; Pan, S.; Li, L. Selenium-induced toxicity is counteracted by sulfur in broccoli (Brassica oleracea L. var. italica). Front. Plant Sci. 2017, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.E.; Huang, X.C.; Hansen, C.I.C.; Cipollini, D.; Ørgaard, M.; Matthes, A.; Geu-Flores, F.; Koch, M.A.; Agerbirk, N. Glucosinolate diversity within a phylogenetic framework of the tribe Cardamineae (Brassicaceae) unraveled with HPLC-MS/MS and NMR-based analytical distinction of 70 desulfoglucosinolates. Phytochemistry 2016, 132, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, M.; Mukhaimar, M.; Perreau, F.; Kirk, J.; Hansen, C.I.C.; Olsen, C.E.; Agerbirk, N.; Kroymann, J. Methyl transfer in glucosinolate biosynthesis mediated by indole glucosinolate O-Methyltransferase 5. Plant Physiol. 2016, 172, 2190–2203. [Google Scholar] [CrossRef]
- Agerbirk, N.; Hansen, C.C.; Olsen, C.E.; Kiefer, C.; Hauser, T.P.; Christensen, S.; Jensen, K.R.; Ørgaard, M.; Pattison, D.I.; Lange, C.B.A.; et al. Glucosinolate profiles and phylogeny in Barbarea compared to other tribe Cardamineae (Brassicaceae) and Reseda (Resedaceae), based on a library of ion trap HPLC-MS/MS data of reference desulfoglucosinolates. Phytochemistry 2021, 185, 112658. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium biofortification differentially affects sulfur metabolism and accumulation of phytochemicals in two rocket species (Eruca sativa Mill. and Diplotaxis tenuifolia) grown in hydroponics. Plants 2019, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, S.; Xu, H.; Wu, Q.; Liang, M.; Yuan, Y.; Liu, M.; Huang, K.; Wu, Q. Effects of sulfur and selenium on glucosinolate biosynthesis in cabbage. Plant Mol. Biol. Rep. 2020, 38, 62–74. [Google Scholar] [CrossRef]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Popović, M.; Guillot, X.; Burčul, F.; Rollin, P. Microwave-assisted versus conventional isolation of glucosinolate degradation products from Lunaria annua L. and their cytotoxic activity. Biomolecules 2020, 10, 215. [Google Scholar] [CrossRef]
- Wathelet, J.-P.; Iori, R.; Leoni, O.; Quinsac, A.; Palmieri, S.; Rollin, P. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 2004, 3, 257–344. [Google Scholar]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).