Abstract
A Sonogashira coupling of meta-iodocalix[4]arene with various terminal acetylenes confirmed that the meta position of calixarene is well addressable, and that both thermal and microwave protocols led to good yields of alkynylcalixarenes. Alkynes thus obtained were subjected to the ferric chloride and diphenyl diselenide-promoted electrophilic closure. It turns out that the calix[4]arenes give completely different bridging products than those described for the non-macrocyclic starting compounds. This can be demonstrated not only by the isolation of products with a six-membered ring (6-exo-dig), but mainly by the smooth formation of the 5-endo-dig cyclization, which has never been observed in the aliphatic series. An attempt at electrocyclization led to a high yield of the 1,2-diketone (oxidation of the starting alkyne), again in contrast to the reaction described for the acyclic derivatives. The structures of the unexpected products were unequivocally established by X-ray analysis and clearly demonstrate how the preorganized macrocyclic skeleton favors a completely different regioselectivity of cyclization reactions compared to common aliphatic compounds.
1. Introduction
Calixarenes [1,2] have become an integral part of modern supramolecular chemistry, where they have found wide application due to their unique properties. Their use as building blocks or molecular scaffolds with the possibility of purposefully changing the 3D structure of the basic skeleton (e.g., immobilization of four different conformations (atropisomers) in the case of calix[4]arene) makes them an ideal choice for the design and synthesis of new receptors and similar functional molecular systems [3,4].
Calix[4]arenes show an almost unlimited possibility of derivatization of the basic skeleton, enabling the introduction of desired functional groups at virtually any place in the macrocycle. Currently, many strategies are known leading to regio- or stereo-selective derivatization of basic calix[4]arene, usually based on a combination of alkylation reactions on the lower rim (OH groups) and electrophilic substitution of the upper rim (aromatic part). In this context, however, we come across a limitation given by the directing effects of the substituents present in the macrocycle (OH, OR), where electrophilic substitution takes place preferentially in the para position to these groups. For this reason, in calixarene chemistry, meta substitution [5] represents a synthetic challenge, and the meta substituted derivatives appear only rarely.
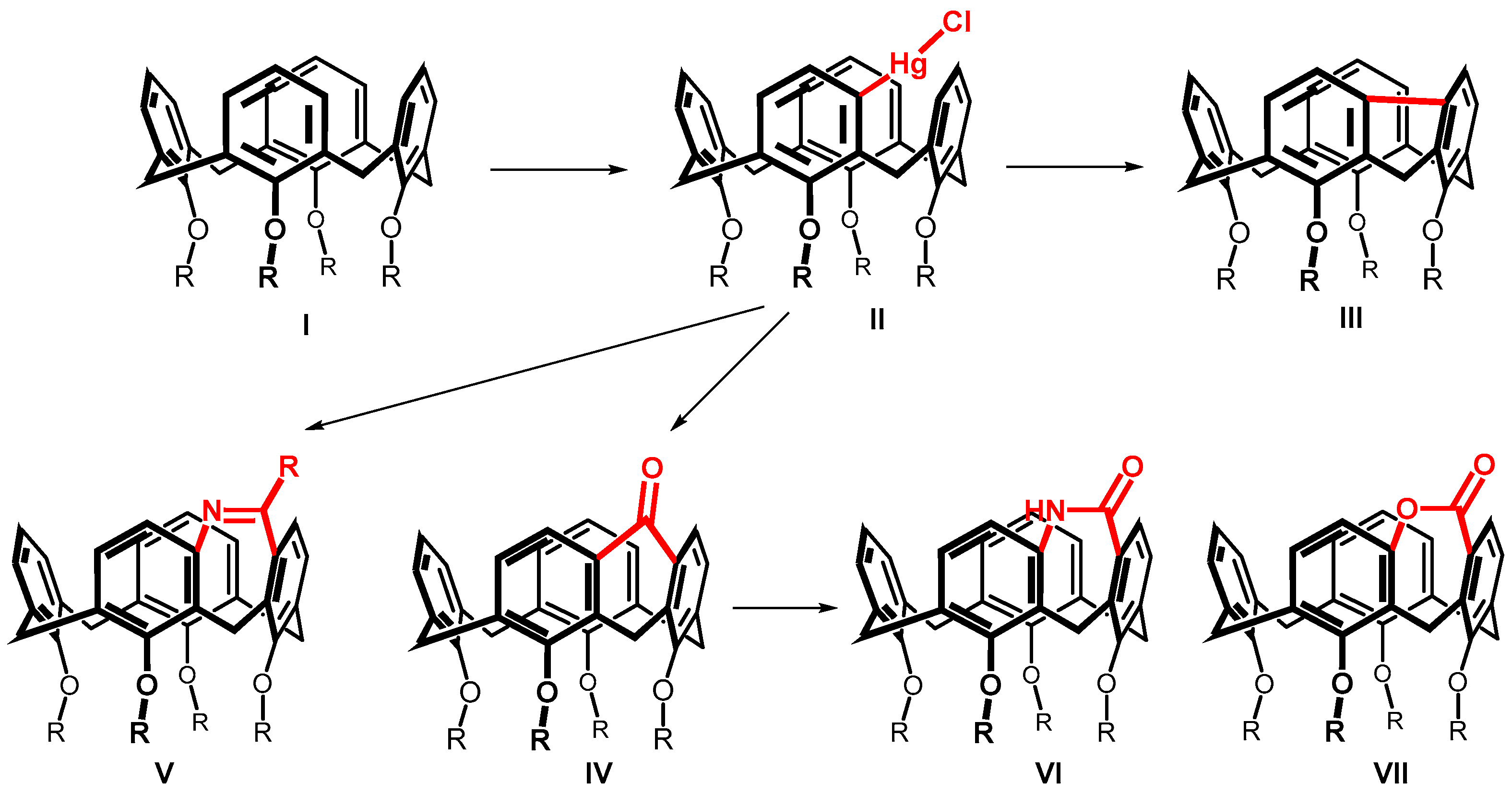
Systematic studies on the calix[4]arene I reactivity surprisingly revealed that the reaction with mercury trifluoroacetate (Hg(TFA)2) leads to a direct substitution [6] of the hitherto practically inaccessible meta position (Scheme 1). The formation of an organomercury compound II then enabled the preparation of new types of calixarenes with a meta-meta bridged upper rim [7]. These compounds are not only interesting because of their highly increased rigidity (see e.g., III and IV), but often also represent inherently chiral systems potentially suitable for the design of chiral receptors (V–VII) [8,9].
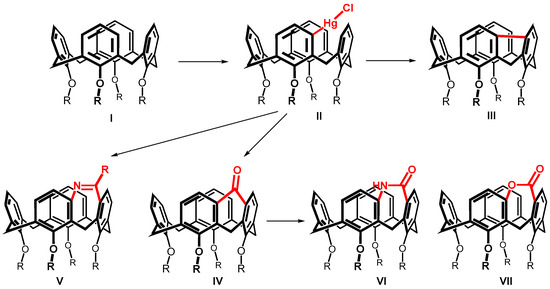
Scheme 1.
The upper rim-bridged calix[4]arenes bearing five-membered, six-membered and seven-membered rings within the molecules.
The bridging of two adjacent aromatic nuclei in the meta position leads to the formation of condensed tricyclic systems having a five-, six-, or seven-membered ring (Scheme 1). While a number of systems having a five- or six-membered ring have been prepared, only three seven-membered systems (V–VII) have been described so far [8,9]. Thus, compounds of type V were obtained by cyclization of the corresponding meta-amides, and compounds VI and VII were obtained from ketone IV using a ring extension via Baeyer-Villiger or Beckmann rearrangements.
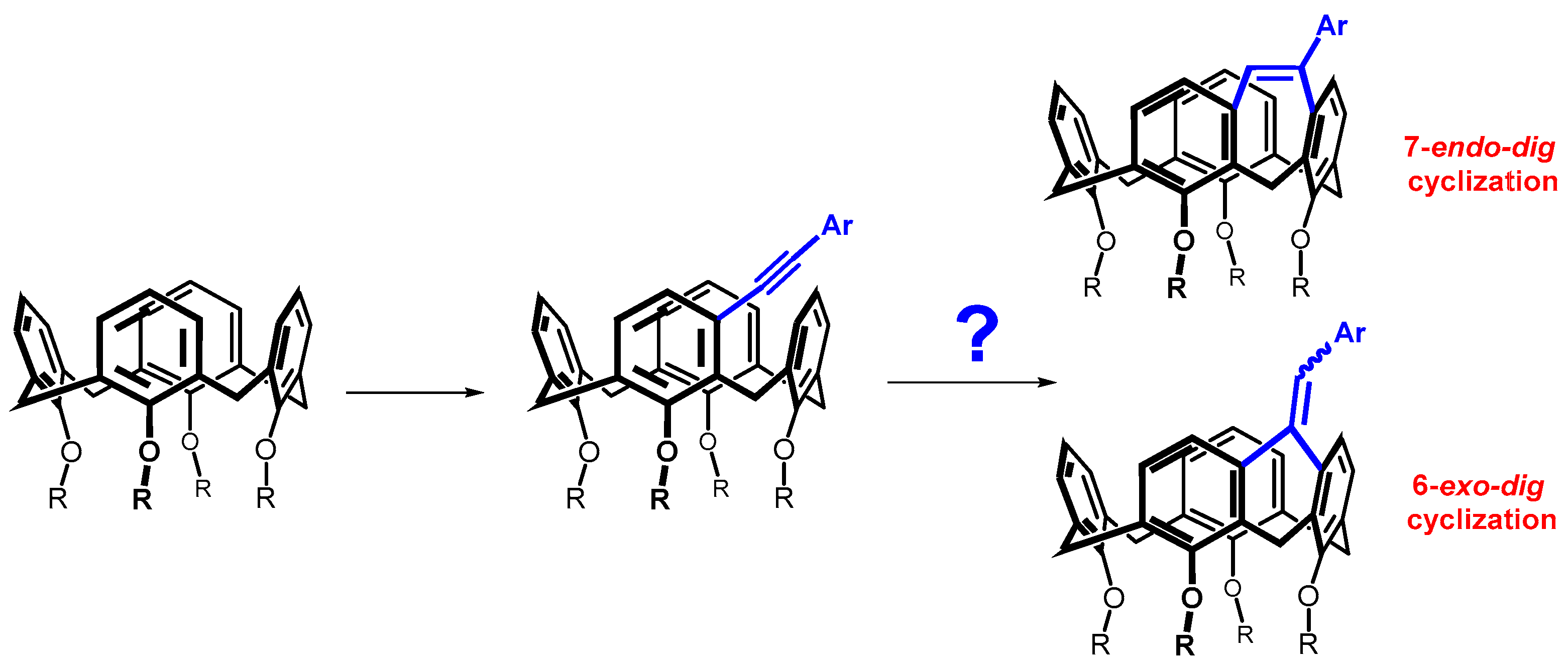
Since different cycle sizes introduce different internal strains into the macrocyclic system, we thought it interesting to apply a bridging reaction that could provide different sizes of cyclic moieties. Such a reaction could be the cyclization of alkynes proceeding either by the exo-dig or endo-dig mechanism (Baldwin’s rules), where both processes are allowed (Scheme 2) [10]. This paper describes an effort to prepare the above systems, which show a surprising preference of calix[4]arene skeleton compared to acyclic molecules.
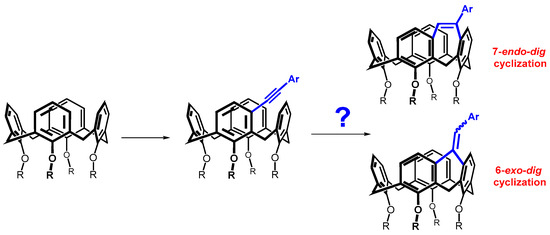
Scheme 2.
meta-Alkynylcalix[4]arenes and possible regioisomers formed by their cyclization.
2. Results and Discussion
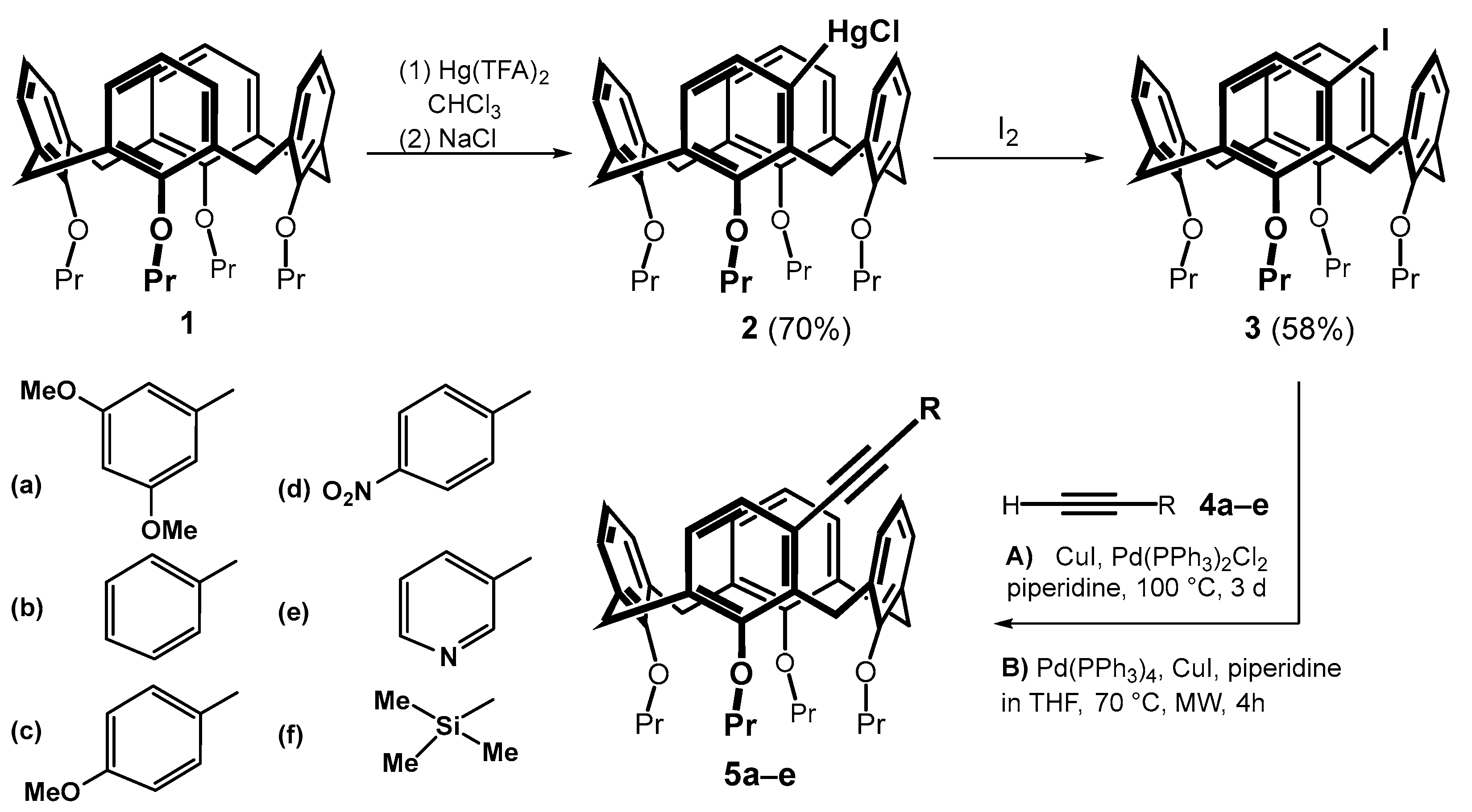
Starting calix[4]arene 1 immobilized in the cone conformation was successively reacted with mercury(II) trifluoroacetate (Hg(TFA)2) and sodium chloride using a literature procedure [6]. The corresponding meta-substituted chloromercurio derivative 2 was obtained regioselectively in good yield (70%). A subsequent reaction with iodine provided the meta-iodo derivative 3 in 58% yield (Scheme 3) [8]. The introduction of triple bond into the calixarene skeleton was carried out by Sonogashira coupling [11,12], which is well known for terminal alkynes. This reaction has already been successfully used several times in calixarene chemistry, but it involved either the para position of the aromatic subunit [13,14,15] or the transformation of the lower rim of the macrocycle [16,17]. The reactivity of the halogen in the meta position was therefore unknown. To study this cross-coupling of compound 3, a series of substituted aromatic acetylenes 4a–e completed with a silyl- derivative 4f was selected.
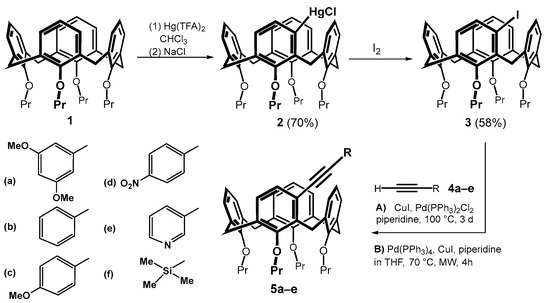
Scheme 3.
Sonogashira coupling of meta-substituted calixarenes.
Based on the literature search [11], we applied two different implementations of the Sonogashira reaction: (i) classical heating on a magnetic stirrer and (ii) application of microwave reactor. Thus, heating compound 3 with 4a and Pd(PPh3)2Cl2/CuI in piperidine at 100 °C for 3 days gave the expected product 5a in 89% yield. The reaction carried out in a microwave reactor (Pd(PPh3)4/CuI, piperidine in THF) at 70 °C for 4 h provided the same product in 74% yield. A similar trend, i.e., a higher yield upon classical heating, was observed for all electron-donating substituents (5a–c) and the silyl-substituted derivative 5f, see Table 1. In contrast, both derivatives bearing electron-withdrawing substituents 5d and 5e were obtained in a higher yield upon microwave reaction (compare 33% vs. 47% for 5e).

Table 1.
The yields of the Sonogashira cross-coupling of calixarene 3 with acetylenes 4a–4f.
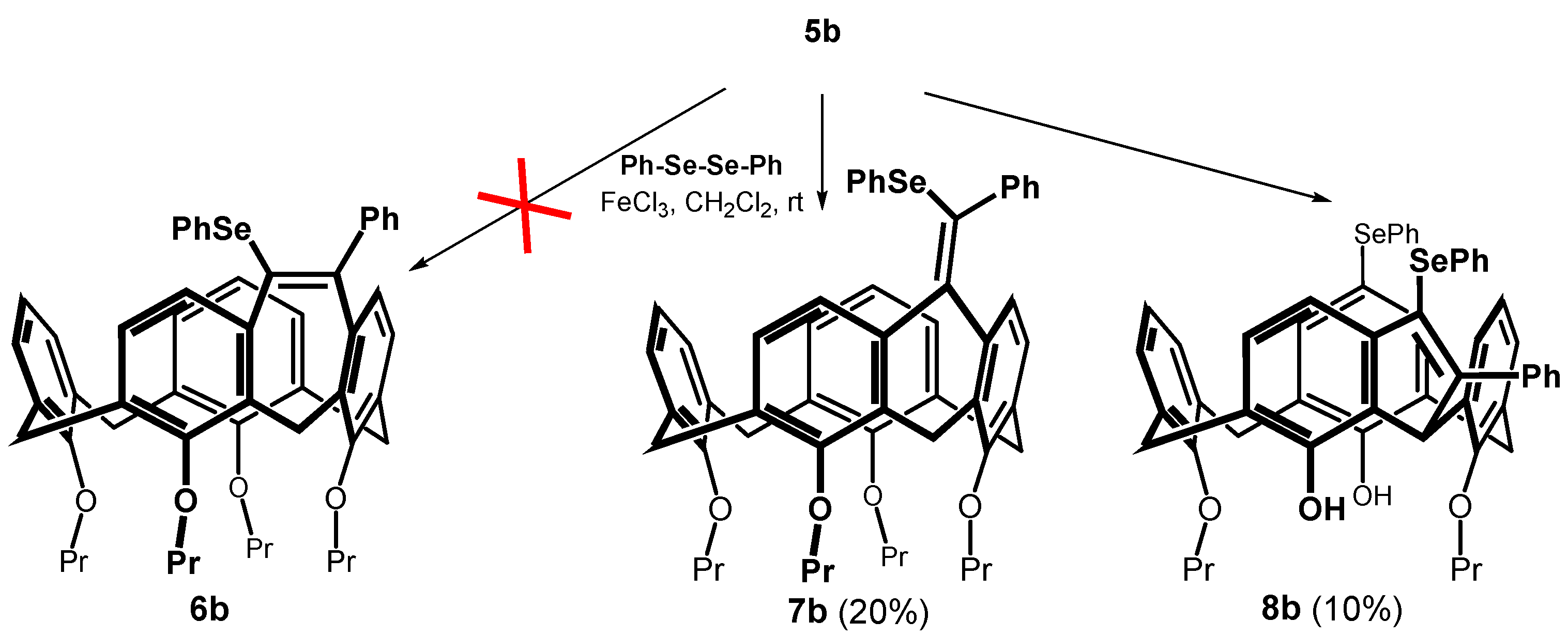
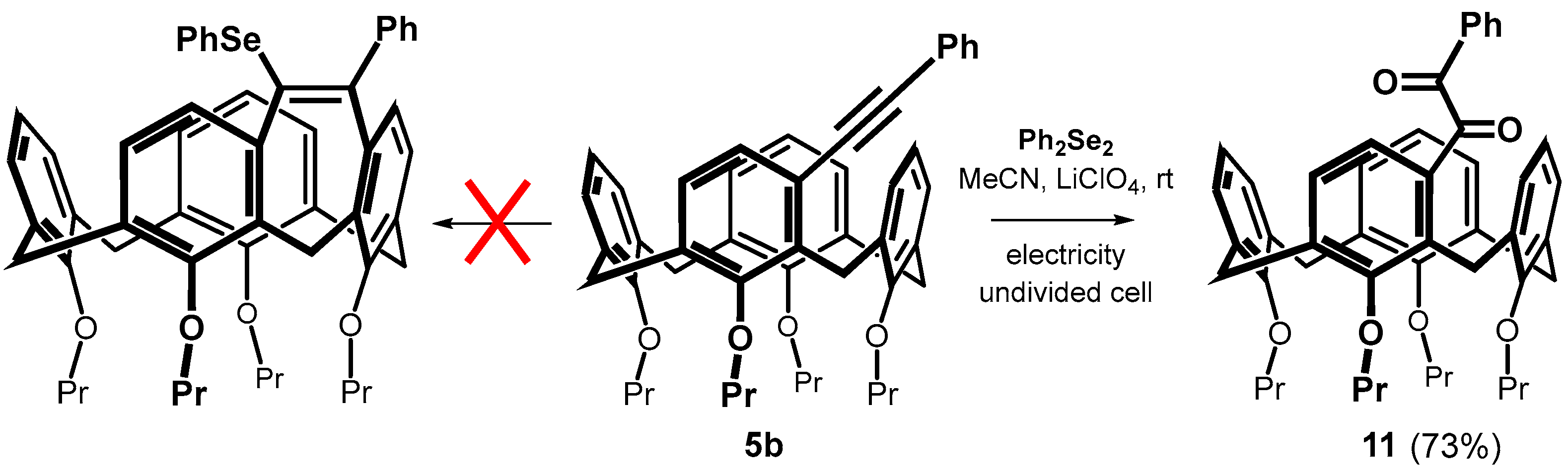
Alkynes thus obtained were subjected to the recently described iron(III) chloride and diorganyl diselenide-promoted electrophilic closure [18,19,20], giving selectively the seven-membered cyclization products [18]. The reaction of 5b carried out under the conditions described in the literature (alkyne + Ph2Se2 (0.55 equiv.) + FeCl3 (1.0 equiv.) in CH2Cl2 at RT) gave practically unreacted starting compounds. In order to achieve higher conversion of the calixarene derivatives, we increased the number of equivalents of both reagents, and the reaction was carried out at a higher temperature (Scheme 4). Thus, heating of 5b with 2.5 equivs. of iron(III) chloride and 2 equivs. of Ph2Se2 in dichloromethane at 30 °C for 4 h gave a crude reaction mixture with 42% of unreacted starting compound. The column chromatography on silica gel provided an expected cyclized product (20% yield) as indicated by the HRMS (ESI+) peak at m/z = 871.3240 well corresponding to the theoretical value for C54H56O4SeNa+ ion (m/z = 871.3245). Other fractions contained derivatives that were apparently bridged but lacked varying numbers of propyl groups (1H NMR), indicating a concurrent dealkylation reaction. The 1H NMR spectrum of the product (CDCl3, 400 MHz) showed eight doublets with typical geminal coupling constants (13–15 Hz) corresponding to the signals of the methylene bridges (3.00 to 4.7 ppm) in the cone conformation. Unfortunately, despite our efforts, it was not possible to decide between the two possible structures 6b and 7b using NMR spectroscopy.
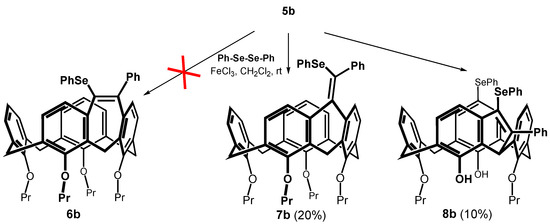
Scheme 4.
An attempted cyclization of 5b with Ph2Se2/FeCl3 system.
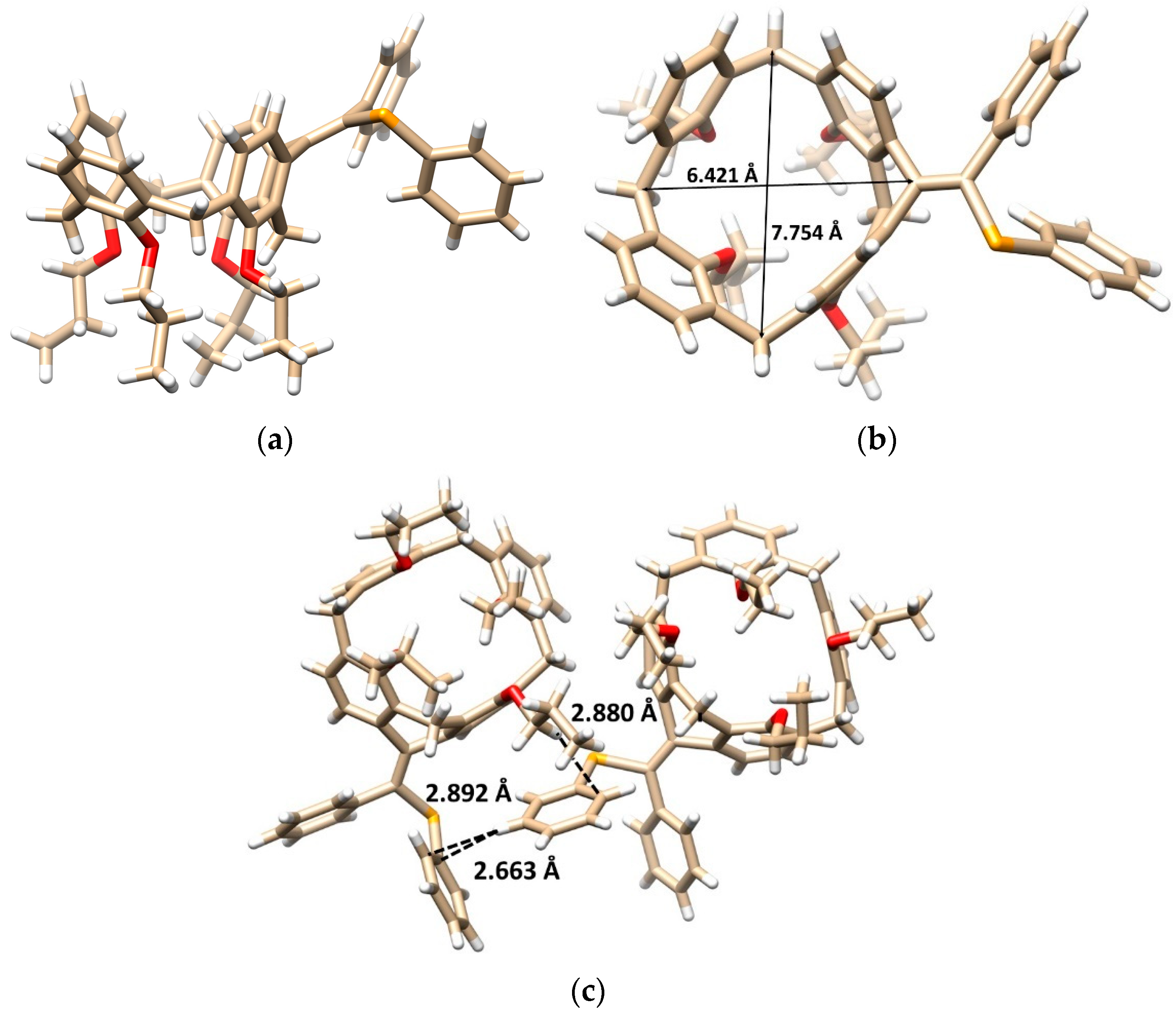
The final proof of the product structure was performed using single crystal X-ray diffraction analysis. The product is in the form of a six-membered ring structure 7b crystallized in a triclinic crystal system, space group P-1, and adopted the cone conformation (Figure 1a). The alkylidene bridge results not only in the rigidification of the calixarene skeleton, but also in the partial flattening of the originally square shape of the cavity. This can be demonstrated on different lengths of the main diagonals (the distance between two opposite Ar-CH2-Ar carbons, compare 7.754 vs. 6.421 Å, Figure 1b).
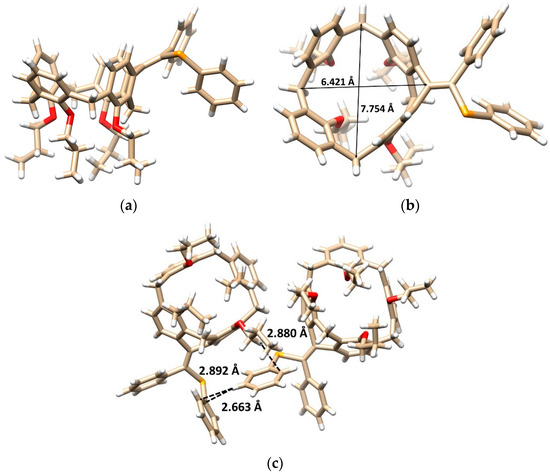
Figure 1.
Single crystal X-ray structure of 7b: (a) the side view, (b) top view showing the main diagonals, (c) dimeric motif within the crystal packing based on the CH··· π and the π-π (T-shaped) interactions (red = O, orange = Se, white = H, beige = C atoms).
The presence of two different substituents on the double bond makes the molecule inherently chiral, with both enantiomers included in the unit cell as the independent molecules (racemic mixture). The opposite enantiomers are held together throughout the crystal packing of 7b (Figure 1c) by the CH-π interactions between the O-CH2- group and the phenyl selenyl moiety (CH···CAr distance = 2.880 Å). At the same time, the close contacts between PhSe groups can be found, based on the T-shaped π-π interactions [21,22] between phenyl moieties (Figure 1c).
Compound 5a also afforded the cyclization product 7a in low yield, which, however, could not be sufficiently purified for analysis. Nevertheless, the structure was determined by comparing its 1H NMR spectrum with that of 7b. At the same time, the HRMS (ESI+) showed a molecular peak at m/z = 931.3453 (compared with the theoretical value 931.3447 for the [M + Na]+ ion). Under the same conditions, nitro compound 5d gave only a trace amount of product (TLC, HRMS), indicating a negative influence of the electron-withdrawing group on the course of the reaction (conversion ca 1% (1H NMR)). Similar conclusions can be drawn for compound 5e.
Since all cyclization attempts exhibited low conversion of the starting compound, we tried to increase the reaction temperature by using DCE as a solvent. Reaction of 5b with Ph2Se2 and FeCl3 (both 2.5 equiv) under reflux (85 °C) gave a very complex reaction mixture. As it was proved by means of model reactions, FeCl3 is a strong dealkylating agent under these conditions, so that a partial non-selective dealkylation of both starting substances and products took place. The isolation by preparative TLC gave the bridged product 8b in low yield (Scheme 4). The 1H NMR spectra (400 MHz, CDCl3) measured at room temperature showed two singlets at 8.21 and 8.31 ppm, indicating the presence of free phenolic groups. The aliphatic part of the spectrum contains signals of only two propyl groups, which proves the partial dealkylation of the starting macrocycle. Interestingly, instead of 8 doublets with a geminal coupling constants, only two sets of three doublets (J = 12.5–13.7 Hz) were seen for the methylene linkages (3.20, 3.28 and 3.37 ppm for the equatorial and 4.06, 4.36 and 4.44 ppm for axial CH bonds). The last bridge showed only singlet at 5.42 ppm corresponding to one hydrogen. This suggests that one methylene group was transformed during the reaction, but the actual structure remained unknown.
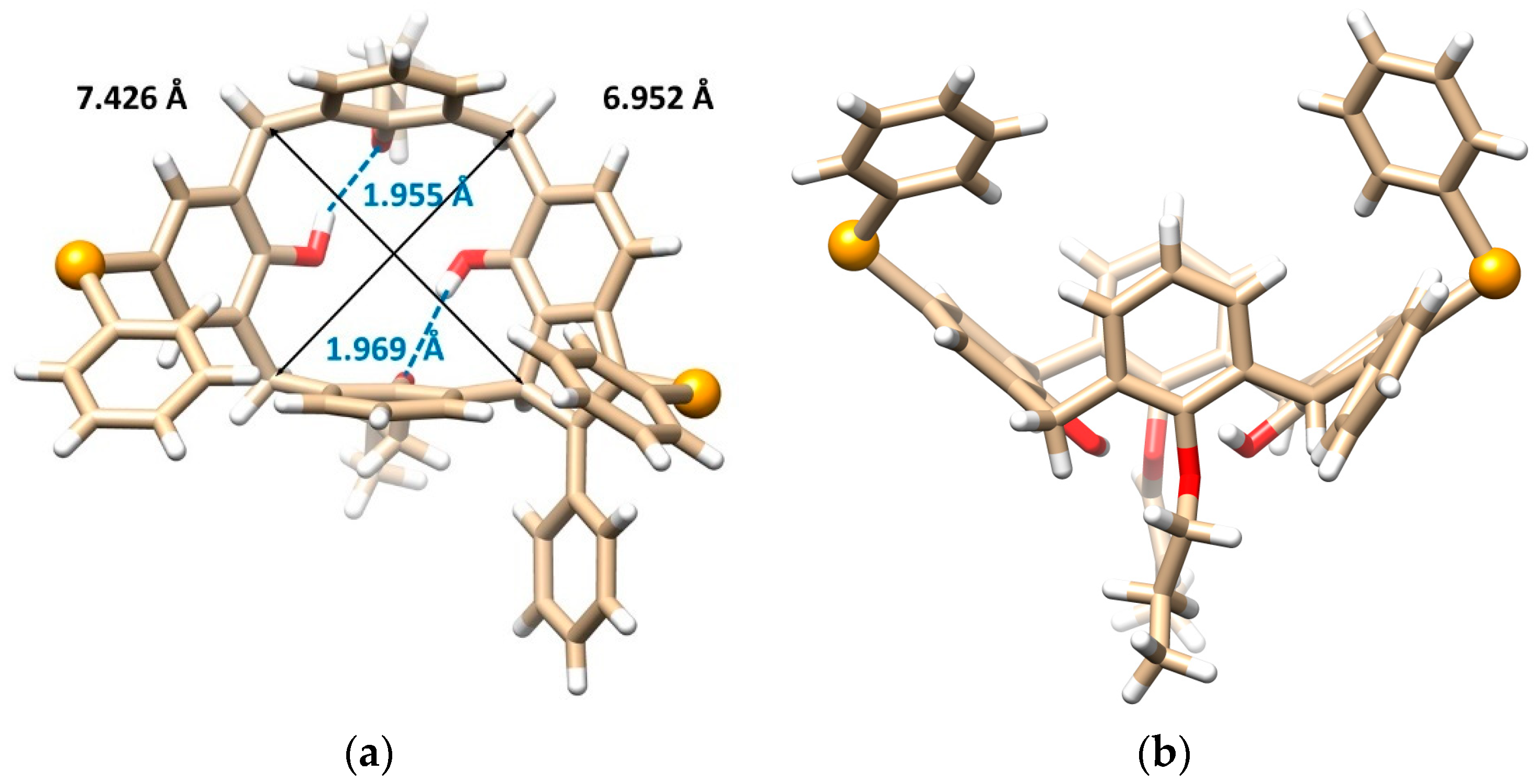
This problem was unequivocally solved by single crystal X-ray diffraction analysis. Compound 8b crystallizes in a monoclinic crystal system, space group P21/n, and forms a CH2Cl2 solvate with 1:1 stoichiometry. As shown in Figure 2a,b, calixarene adopts the cone conformation containing the five-membered ring formed by the addition of the methylene bridge to the alkyne moiety. The unexpected cyclization is accompanied by the introduction of a second PhSe- group into the para position of the opposite aromatic subunit. Both phenols bearing the substitution are dealkylated at the same time. The cone conformation is held by hydrogen bonds between the free OH groups and the oxygens of the neighbor propoxy functions (OH···O distance = 1.955 and 1.969 Å). The presence of the indene moiety within the backbone has surprisingly little effect on the shape of the cavity, which can be demonstrated by its approximately square shape (the length of the main diagonals is 6.952 vs. 7.426 Å), see Figure 2a.
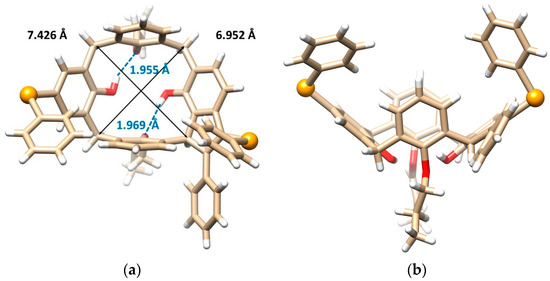
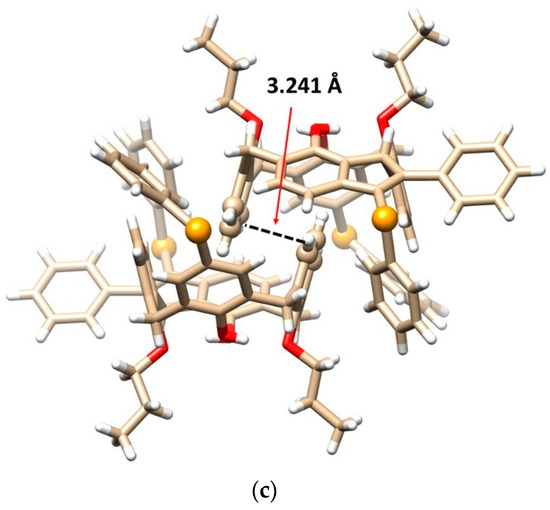
Figure 2.
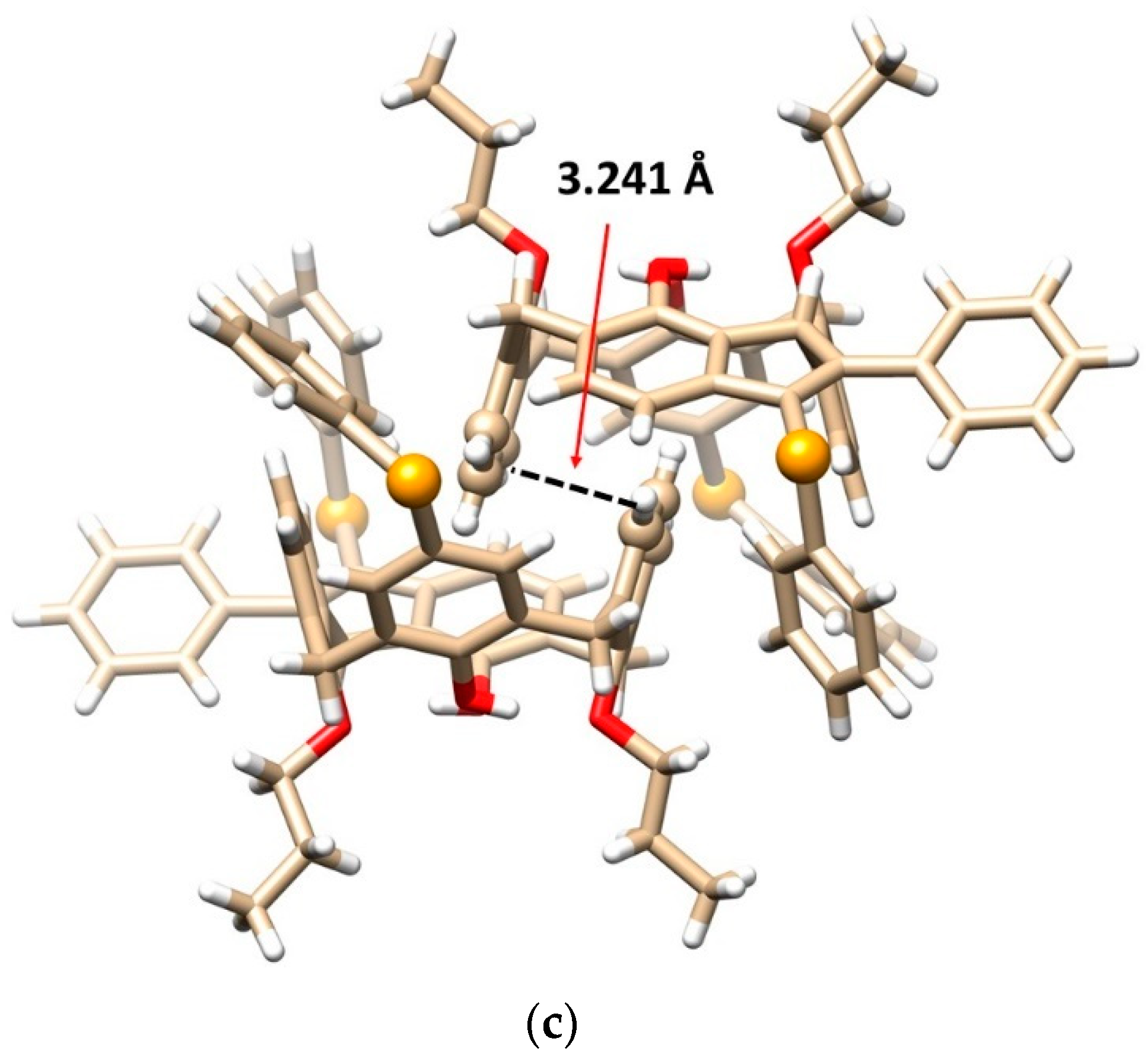
Single crystal X-ray structures of compound 8b: (a) the top view and (b) the side view, showing the cone conformation with the corresponding hydrogen bods (dashed lines) and the main diagonals (solid lines), (c) dimeric self-inclusion motif within the crystal packing based on the π-π interactions (dashed line) between opposite enantiomers (Se atoms and interacting aromatic units shown as balls, the CH2Cl2 solvate molecules removed for better clarity), (red = O, orange = Se, white = H, beige = C atoms).
Since molecule 8b is inherently chiral, the crystal contains both enantiomers (racemic mixture), which form an interesting dimeric motif (Figure 2c). The aromatic subunits of opposite enantiomers bearing the propoxy groups are nested into each other’s adjacent cavity to form self-included dimers. The aromatic subunits are ideally coplanar interacting via the π-π interactions with each other in the so-called parallel stacked arrangement. The interplanar spacing of these aromatic subunits is exceptionally short (only 3.241 Å, while the usual distance is around 3.5 Å), indicating unusually strong π-π interactions.
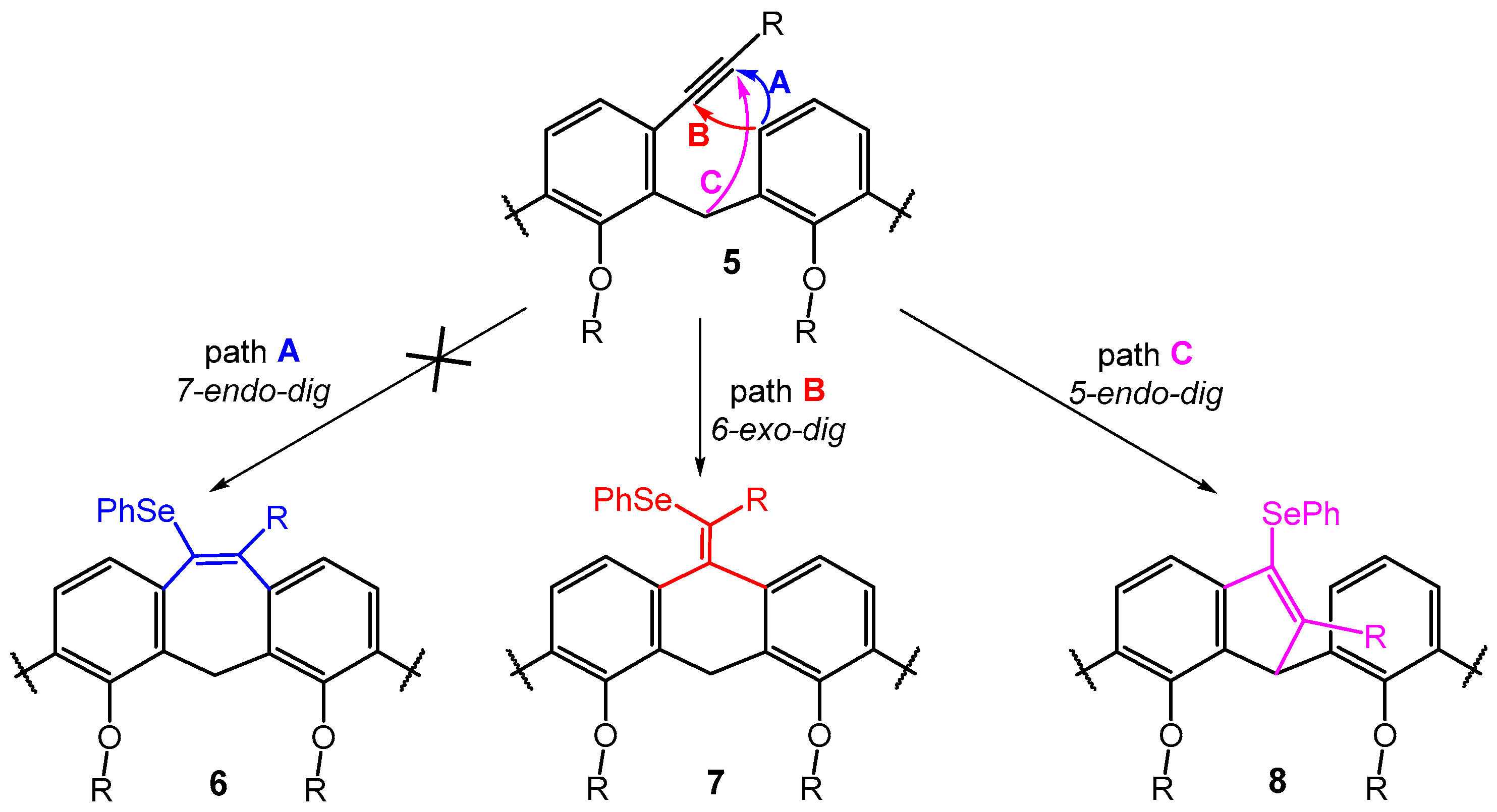
The formation of product 8b is completely unexpected and to the best of our knowledge, there is no example of similar structures in the literature. The selenium-mediated electrophilic cyclization reaction is well known in the art and has been used to construct a variety of structures [19,23,24,25]. On the other hand, although the FeCl3/Ph2Se2 promoted cyclization of o-alkynyl substituted diaryl methanes (a structural fragment within a calixarene moiety) has been investigated in detail, [18] the formation of a five-membered product of type 8 has never been observed (Scheme 5). The formation of indene structures [26] from o-substituted aryl alkynes is known, but it is based on direct C(sp3)-H activation using Au(I) [27] or Pt(II) [28] catalysts. A similar reaction was also achieved by the intramolecular addition of triarylmethanes [29] to the alkynyl group in the ortho position induced by the presence of a base (t-BuOK), but it was never reported for selenium-induced cyclization.
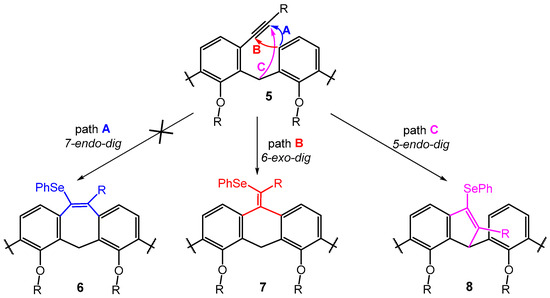
Scheme 5.
The cyclization of meta-alkynylcalix[4]arenes the three different arrow colors show the three possible reaction pathways leading to the corresponding products.
Furthermore, it is noteworthy that the aforementioned FeCl3/Ph2Se2 cyclization studies describe endo-dig cyclization leading to products of type 6 as the sole exclusive reaction pathway (path A, Scheme 5) [18,19]. In this respect, the calix[4]arene skeleton represents a unique system, since we did not observe the formation of a seven-membered cycle in any case. A possible explanation lies in the fact that the relative position of the neighboring aromatic nuclei imposed by the macrocyclic structure favors 6-exo-dig (type 7) or 5-endo-dig (type 8) over 7-endo-dig (type 6) cyclization, i.e., reaction pathways that are unknown for common acyclic derivatives (Scheme 5).
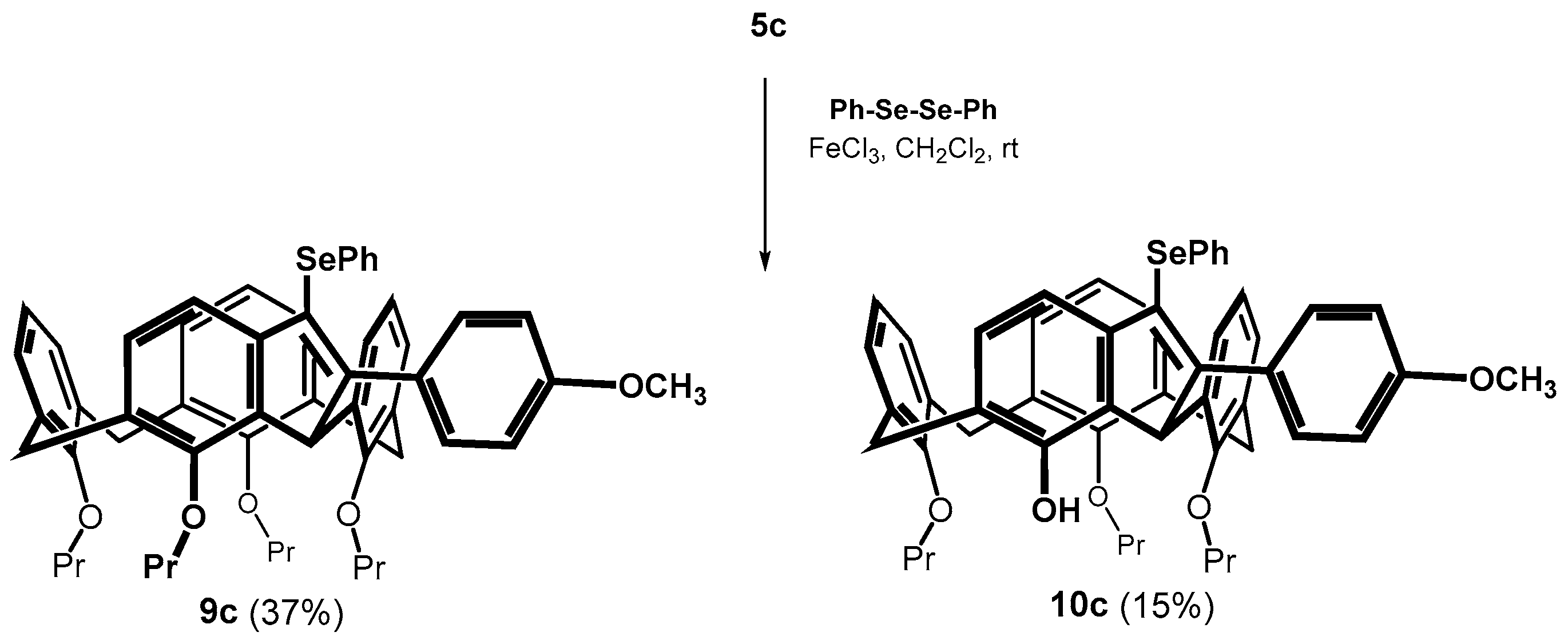
From this point of view, the reaction of the methoxy derivative 5c was very surprising. The cyclization of the alkyne proceeded smoothly already at 30 °C (100% conversion), whereby two products were isolated from the reaction mixture (Scheme 6). The HRMS (ESI+) of the main product 9c (37% yield) showed a molecular peak at m/z = 901.3345 which corresponded to the expected mass of the cyclized product m/z = 901.3342 ([M + Na]+ ion). The 1H NMR (CDCl3, 298 K, 400 MHz) comprised two sets of three doublets for the methylene bridges (J ≈ 13.3 Hz), together with a singlet at 5.70 ppm (1H). This splitting pattern is very similar to that of compound 8b and refers to cyclization via a single methylene bridge. The second isolated product was assigned as the same type of structure, but lacking one propyl unit. Based on two-dimensional NMR experiments (HSQC, HMBC) it appears most likely that this derivative has the structure 10c with the OH group in the indene fragment (Scheme 6). Thus, the result again reflects the remarkable regioselectivity of the reaction, where the cyclization proceeds only through the adjacent methylene bridge, while the expected bridging of the adjacent aromatic subunit was not observed at all.
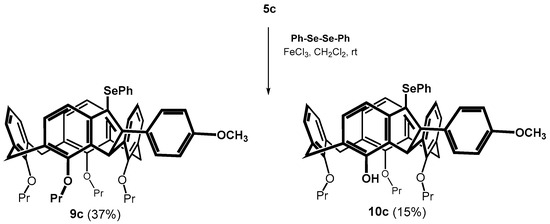
Scheme 6.
Cyclization of methoxy derivative 5c (5-endo dig).
The FeCl3-mediated electrophilic selanylation/annulation of meta-substituted calixarenes showed very unexpected results; however, the use of a Lewis acid led to relatively low yields due to dealkylation of the lower rim of the calixarene. To avoid this, we decided to apply the recently described method based on the action of diphenyl diselenide under electrochemical conditions [30,31]. Thus, the reaction of 5b with 0.6 equiv. of Ph2Se2 in MeCN in the presence of LiClO4 as an electrolyte was carried out in an undivided cell under constant current electrolysis (CCE) at 10 mA for 3 h under aerobic atmosphere. Although the original publications promise a virtually quantitative bridging yield, we were unable to demonstrate any cyclization. The only resulting product 11 was isolated in 73% yield (Scheme 7), and its 1,2-diketone [32] structure was confirmed by a combination of HRMS and NMR spectroscopy (see typical carbonyl signals at 197.92 and 195.89 ppm in 13C NMR spectrum). Altering the reaction parameters, such as using a different electrode material (Pt vs. glassy carbon), did not lead to any changes. We attempted to eliminate the unwanted oxidation by performing electrolysis at a constant potential (+1.5 V), which was chosen from appropriate cyclic voltammogram (Figure S78) as the first irreversible step of Ph2Se2. Unfortunately, compound 11 was still the only isolated product. Finally, carrying out the reaction in a degassed solvent (purged with a stream of argon) prevented oxidation but gave no reaction. It indicates that the mentioned methodology is not suitable for the electrocyclization of meta-alkynyl calixarenes.
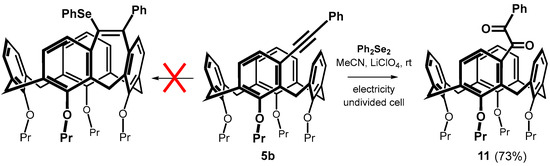
Scheme 7.
Attempted electrocyclization of phenyl derivative 5b under aerobic conditions.
3. Materials and Methods
3.1. General Experimental Procedures
All commercially obtained chemicals were purchased from commercial sources and used as received without further purification. Solvents were dried and distilled using conventional methods. NMR spectra were performed on Agilent 400-MR DDR2, JEOL-ECZL400G (1H: 400 MHz, 13C: 100 MHz) and Bruker Avance DRX 500 (1H: 500 MHz, 13C: 125 MHz) spectrometers at 298 K. Deuterated solvents used are indicated in each case. Chemical shifts (δ) are reported in parts per million (ppm) and were referenced to residual peak of the solvent or TMS as an internal standard; coupling constants (J) are expressed in Hz. NMR data were processed and displayed using MestReNova (Version: 14.3.3) and TopSpin (Version 3.6.5) softwares. The IR spectra were measured on an FT–IR spectrometer Nicolet iS50 (Thermo-Nicolet, Waltham, MA, USA) with a heatable Golden Gate Diamante ATR–Unit GladiATR (Specac, Orpington, UK). 64 scans for one spectrum were co-added at a spectral resolution of 4 cm–1. Electrospray ionization mass spectra (ESI-MS) were recorded using a LTQ Orbitrap Velos—hybrid ion-trap-orbitrap (Thermo Scientific, Waltham, MA, USA). The purity of the substances and courses of the reactions were monitored by thin layer chromatography (TLC) using silica gel 60 F254 on aluminum-backed sheets (Merck, Lowe, NJ, USA) and analyzed at 254 nm. The column chromatography was conducted on silica gel 60 with particle size 0.063–0.200 mm (Merck). For melting point studies, Heiztisch Mikroskop—Polytherm A (Wagner & Munz, Munich, Germany) was used.
3.2. Synthetic Procedures
3.2.1. Preparation of Alkynylcalixarenes 5a–f
General Procedure for the Synthesis of Alkynylcalixarenes 5a–f (Method A)
Starting calix[4]arene 3 (1 equiv), CuI (0.1 equiv) and Pd(PPh3)2Cl2 (0.05 equiv) were dissolved in 3–4 mL of dry piperidine under an Ar atmosphere. Then, appropriate alkyne 4a–f (1.1 equiv) was added, and the reaction mixture was stirred and heated at 100 °C for 3 days. The mixture was cooled to RT, and the solvent was evaporated under reduced pressure. The crude product was extracted (3 × 30 mL) with dichloromethane (DCM), the organic phase was washed with water, dried over MgSO4, filtered and evaporated to dryness. The residue was purified by column chromatography on silica gel (eluent: DCM: cyclohexane) to afford the title compounds.
General Procedure for the Synthesis of Alkynylcalixarenes 5a–f (Method B)
A mixture of starting calix[4]arene 3 (1 equiv), alkyne 4a–f (4 equiv), piperidine (4 equiv), CuI (0.2 equiv), Pd(PPh3)4 (0.1 equiv) and dry THF was stirred under argon atmosphere at 70 °C for 4 h in the microwave reactor. The mixture was then evaporated to dryness, the residue was extracted with DCM (3 × 30 mL), washed with water and dried over MgSO4. The purification of the crude product was done in the same way as described in Method A.
Alkynylcalixarene 5a
Method A: Calixarene 3 (0.209 mmol, 150 mg), 3,5-dimethoxyphenylacetylene (0.230 mmol, 37 mg), CuI (0.0209 mmol, 4 mg) and Pd(PPh3)2Cl2 (0.0104 mmol, 7 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to afford 5a in 89% yield (140 mg) as a white solid.
Method B: Calixarene 3 (0.139 mmol, 104 mg), 3,5-dimethoxyphenylacetylene (0.556 mmol, 90 mg), CuI (0.028 mmol, 5 mg), piperidine (0.417 mmol, 35 µL) and Pd(PPh3)4 (0.0139 mmol, 16 mg) were used under standard conditions. The product 5a (77 mg, 74%) was obtained by column chromatography on silica gel (DCM/cyclohexane 60:40) as a white solid.
Data for compound 5a: M.p. = 105–107 °C. 1H NMR (CDCl3, 500 MHz, 298 K) δ 7.19 (d, J = 7.7 Hz, 1H, Ar-H), 7.08 (d, J = 7.7 Hz, 2H, Ar-H), 7.06 (d, J = 7.7 Hz, 1H, Ar-H), 6.89 (t, J = 7.5 Hz, 1H, Ar-H), 6.67 (d, J = 2.3 Hz, 2H, Ar-H), 6.43 (t, J = 2.3 Hz, 1H, Ar-H), 6.33 (dd, J = 7.5 Hz, J = 1.1 Hz, 1H, Ar-H), 6.26 (t, J = 7.5 Hz, 1H, Ar-H), 6.24 (t, J = 7.5 Hz, 1H, Ar-H), 6.15 (d, J = 7.5 Hz, 2H, Ar-H), 6.13 (d, J = 7.5 Hz, 1H, Ar-H), 4.47 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 4.46 (d, J = 2.8 Hz, 1H, Ar-CH2-Ar), 4.44 (d, J = 2.8 Hz, 1H, Ar-CH2-Ar), 4.36 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 4.01 (m, 4H, -O-CH2-), 3.95 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 3.79 (s, 6H, -O-CH3), 3.73 (m, 2H, -O-CH2-), 3.69 (m, 2H, -O-CH2-), 3.16 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 3.15 (d, J = 13.2 Hz, 2H, Ar-CH2-Ar), 1.98 (m, overlap, 4H, -CH2-CH3), 1.88 (m, 4H, -CH2-CH3), 1.10 (t, J = 7.3 Hz, 6H, -O-(CH2)2-CH3), 0.91 (t, J = 7.4 Hz, 3H, -CH3), 0.90 (t, J = 7.4 Hz, 3H, -CH3). 13C NMR (CDCl3, 126 MHz, 298 K) δ 160.47, 157.98, 157.88, 155.17, 155.16, 139.31, 137.99, 137.01, 136.89, 133.47, 133.22, 132.92, 132.67, 128.84, 128.78, 128.51, 127.57, 127.41, 127.33, 127.24, 126.21, 125.01, 122.10 (2C), 121.82, 121.78, 109.33, 101.42, 91.1, 88.83, 76.94, 76.90, 76.58, 76.44, 55.43 (2C), 31.04, 30.97, 30.96, 28.11, 23.50 (2C), 22.99, 22.97, 10.79, 10.78, 9.86, 9.84 ppm. HRMS (ESI+) calcd for C50H56O6 775.3969 [M + Na]+, found m/z 775.3971 [M + Na]+. IR (KBr) ν 2959, 2932, 2873, 1587, 1453, 1418, 1205, 1193, 1154, 1060, 965, 756 cm−1.
Alkynylcalixarene 5b
Method A: Calixarene 3 (0.315 mmol, 226 mg), phenylacetylene (0.3465 mmol, 40 µL), CuI (0.0315 mmol, 6 mg) and Pd(PPh3)2Cl2 (0.0157 mmol, 11 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to afford 5b in 96% yield (209 mg) as a yellow oil.
Method B: Calixarene 3 (0.139 mmol, 103 mg), phenylacetylene (0.556 mmol, 60 µL), CuI (0.028 mmol, 5 mg), piperidine (0.417 mmol, 40 µL) and Pd(PPh3)4 (0.0139 mmol, 16 mg) were used under standard conditions. The product 5b (59 mg, 62%) was obtained by column chromatography on silica gel (DCM/cyclohexane 70:30) as a yellow oil.
Data for compound 5b: 1H NMR (CDCl3, 500 MHz, 298 K) δ 7.51 (dd, J = 7.7 Hz, J = 1.6 Hz, 2H, Ar-H), 7.27–7.35 (m, 3H, Ar-H) 7.19 (d, J = 7.7 Hz, 1H, Ar-H), 7.08 (d, J = 7.4 Hz, 2H, Ar-H), 7.06 (d, J = 7.7 Hz, 1H, Ar-H), 6.90 (t, J = 7.7 Hz, 1H, Ar-H), 6.34 (d, J = 7.7 Hz, 1H, Ar-H), 6.26 (d, J = 7.7 Hz, 1H, Ar-H), 6.22 (d, J = 7.7 Hz, 1H, Ar-H), 6.14 (d, J = 8.2 Hz, 2H, Ar-H), 6.12 (d, J = 7.7 Hz, 1H, Ar-H), 4.46 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.45 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.44 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.35 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.95–4.07 (m, 1H (Ar-CH2-Ar) + 4H (-OCH2-)), 3.63–3.76 (m, 4H, -OCH2-), 3.16 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.15 (d, J = 13.4 Hz, 2H, Ar-CH2-Ar), 1.93–2.03 (m, 4H, -CH2-CH3), 1.82–1.92 (m, 4H, -CH2-CH3), 1.10 (t, J = 7.3 Hz, 6H, -CH3), 0.91 (t, J = 7.3 Hz, 3H, -CH3), 0.89 (t, J = 7.3 Hz, 6H, -CH3). 13C NMR (CDCl3, 125 MHz, 298 K) δ 160.47, 157.98, 157.88, 155.17, 155.16, 139.31, 137.99, 137.01, 136.89, 133.47, 133.22, 132.92, 132.67, 128.84, 128.78, 128.51, 127.57, 127.41, 127.33, 127.24, 126.21, 125.01, 122.10 (2C), 121.82, 121.78, 109.33, 101.42, 91.1, 88.83, 76.94, 76.90, 76.58, 76.44, 55.43 (2C), 31.04, 30.97, 30.96, 28.11, 23.50 (2C), 22.99, 22.97, 10.79, 10.78, 9.86, 9.84 ppm. HRMS (ESI+) calcd for C48H52O4 715.3757 [M + Na]+, found m/z 715.3764 [M + Na]+. IR (KBr) ν 2960, 2931, 2873, 1493, 1454, 1382, 1208, 1192, 1087, 1044, 965, 754, 689 cm−1.
Alkynylcalixarene 5c
Method A: Calixarene 3 (0.209 mmol, 150 mg), 4-ethynylanisole (0.230 mmol, 30 µL), CuI (0.0209 mmol, 4 mg) and Pd(PPh3)2Cl2 (0.011 mmol, 7 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to afford 5c in 64% yield (97 mg) as a white solid.
Method B: Calixarene 3 (0.139 mmol, 106 mg), 4-ethynylanisole (0.556 mmol, 70 µL), CuI (0.028 mmol, 5 mg), piperidine (0.417 mmol, 40 µL) and Pd(PPh3)4 (0.0139 mmol, 16 mg) were used under standard conditions. The product 5c (58 mg, 58%) was obtained by column chromatography on silica gel (DCM/cyclohexane 50:50) as a white solid.
Data for compound 5c: M.p. = 146–149 °C. 1H NMR (CDCl3, 400 MHz, 298 K) δ 7.49 (d, 2H, J = 8.4 Hz, Ar-H) 7.22 (d, J = 7.7 Hz, 1H, Ar-H) 7.13 (d, J = 7.4 Hz, 2H, Ar-H) 7.10 (d, J = 7.8 Hz, 1H, Ar-H) 6.94 (t, J = 7.6 Hz, 1H, Ar-H) 6.88 (d, J = 8.4 Hz, 2H, Ar-H) 6.39 (d, J = 7.8 Hz, 1H, Ar-H), 6.29 (t, J = 7.6 Hz, 1H, Ar-H), 6.27 (t, J = 7.6 Hz, 1H, Ar-H), 6.18 (br d, J = 7.4 Hz, 2H, Ar-H), 6.17 (br d, J = 7.4 Hz, 1H, Ar-H), 4.50 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.49 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.48 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.38 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.12–4.02 (m, 4H, -OCH2-), 4.02 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.83 (s, 3H, -OCH3), 3.79–3.64 (m, 4H, -OCH2-), 3.19 (d, J = 13.4 Hz, 3H, Ar-CH2-Ar), 2.09–1.96 (m, 4H, -CH2-CH3), 1.96–1.84 (m, 4H, -CH2-CH3), 1.13 (t, J = 7.4 Hz, 6H, -CH3), 0.93 (t, J = 7.4 Hz, 3H, -CH3), 0.92 (t, J = 7.4 Hz, 3H, -CH3). 13C NMR (CDCl3, 125 MHz, 298 K) δ 160.35, 158.97, 158.99, 156.10 (2C), 140.02, 138.43, 137.93, 137.80, 135.25, 134.01, 133.89, 133.78 (2C), 133.58, 129.69, 129.62, 129.29, 128.35, 128.19, 128.11, 128.08, 126.76, 123.12, 122.92, 122.89, 122.58, 116.60, 114.64 (2C), 91.78, 88.45, 77.49, 77.46, 77.12, 76.99, 55.70, 31.34, 31.27 (2C), 28.30, 23.79 (2C), 23.27, 23.23, 11.03, 11.01, 10.06, 10.04 ppm. HRMS (ESI+) calcd for C49H54O5 745.3863 [M + Na]+, found m/z 775.3871 [M + Na]+. IR (KBr) ν 2960, 2932, 2920, 2874, 1511, 1454, 1249, 1208, 1192, 1105, 964, 830, 794, 775, 756 cm−1.
Alkynylcalixarene 5d
Method A: Calixarene 3 (0.209 mmol, 150 mg), 1-ethynyl-4-nitrobenzene (0.230 mmol, 34 mg), CuI (0.0209 mmol, 4 mg) and Pd(PPh3)2Cl2 (0.011 mmol, 7 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to afford 5d in 27% yield (42 mg) as a yellow oil.
Method B: Calixarene 3 (0.097 mmol, 70 mg), 1-ethynyl-4-nitrobenzene (0.388 mmol, 62 mg), CuI (0.019 mmol, 4 mg), piperidine (0.291 mmol, 30 µL) and Pd(PPh3)4 (0.0097 mmol, 11 mg) were used under standard conditions. The product 5d (28 mg, 39%) was obtained by column chromatography on silica gel (DCM/cyclohexane 50:50) as a yellow oil.
Data for compound 5d: 1H NMR (CDCl3, 500 MHz, 298 K) δ 8.19 (d, J = 8.9 Hz, 2H, Ar-H), 7.63 (d, J = 8.9 Hz, 2H, Ar-H), 7.21 (d, J = 7.7 Hz, 1H, Ar-H), 7.08 (d, J = 7.7 Hz, 1H, Ar-H), 7.07 (d, J = 7.5 Hz, 1H, Ar-H), 6.89 (t, J = 7.5 Hz, 1H, Ar-H), 6.31 (dd, J = 7.7 Hz, J = 1.1 Hz, 1H, Ar-H), 6.26 (td, J = 7.5 Hz, J = 3.2 Hz, 2H, Ar-H), 6.19 (t, J = 6.2 Hz, 2H, Ar-H), 6.15 (d, J = 7.5 Hz, 1H, Ar-H), 4.49 (d, J = 13.3 Hz, 2H, Ar-CH2-Ar) 4.46 (dd, J = 13.3 Hz, J = 3.3 Hz 2H, Ar-CH2-Ar), 4.41 (d, J =13.3 Hz, 1H, Ar-CH2-Ar), 4.08–3.96 (m, 4H, -O-CH2-), 3.90 (d, 1H, J = 13.3 Hz, Ar-CH2-Ar), 3.78–3.65 (m, 4H, -O-CH2-), 3.19 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 3.16 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 3.73 (m, 2H, -O-CH2-), 1.99 (sextet, J = 7.7 Hz, 4H, -CH2-CH3), 1.89 (sextet, J = 7.7 Hz, 4H, -CH2-CH3), 1.11 (t, J = 7.5 Hz, 3H, -CH3), 1.10 (t, J = 7.5 Hz, 3H, -CH3), 0.92 (t, J = 7.5 Hz, 3H, -CH3), 0.91 (t, J = 7.5 Hz, 3H, -CH3).13C NMR (CDCl3, 125 MHz, 298 K) δ 158.02, 157.78, 155.24 (2C), 146.70, 139.56, 139.05, 136.87, 136.78, 133.62, 133.54, 132.59, 132.47, 132.10 (2C), 130.73, 128.80 (2C), 128.72, 127.71, 127.67, 127.21, 127.16, 126.46, 123.60 (2C), 122.11, 122.10, 121.85, 120.80, 95.05, 89.52, 76.97, 76.92, 76.68, 76.46, 31.10, 30.97 (2C), 28.20, 23.48, 23.47, 23.02, 22.98, 10.77, 10.76, 9.86, 9.85 ppm. HRMS (ESI+) calcd for C48H51NO6 760.3609 [M + Na]+, found m/z 760.3607 [M + Na]+. IR (KBr) ν 2960, 2929, 2874, 1589, 1454, 1382, 1340, 1208, 1192, 1087, 1004, 966, 756 cm−1.
Alkynylcalixarene 5e
Method A: Calixarene 3 (0.280 mmol, 205 mg), 3-ethynylpyridine (0.421 mmol, 40 µL), CuI (0.028 mmol, 5 mg) and Pd(PPh3)2Cl2 (0.014 mmol, 10 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 2:1) to afford 5e in 33% yield (64 mg) as a yellow oil.
Method B: Calixarene 3 (0.280 mmol, 204 mg), 3-ethynylpyridine (0.88 mmol, 90 µL), CuI (0.056 mmol, 11 mg), piperidine (0.841 mmol, 80 µL) and Pd(PPh3)4 (0.028 mmol, 32 mg) were used under standard conditions. The product 5e (91 mg, 47%) was obtained by column chromatography on silica gel (DCM/cyclohexane 2:1) as a yellow oil.
Data for compound 5e: 1H NMR (CDCl3, 500 MHz, 298 K) δ 8.61 (br s, 1H, Ar-H) 7.64 (td, J = 7.7 Hz, J = 1.5 Hz, 1H, Ar-H) 7.49 (d, J = 7.8 Hz, 1H, Ar-H) 7.27 (d, J = 7.8 Hz, 1H, Ar-H) 7.20 (dd, J = 7.8 Hz, J = 4.8 Hz, 1H, Ar-H) 7.08 (d, J = 7.4 Hz, 3H, Ar-H) 6.89 (t, J = 7.4 Hz, 1H, Ar-H), 6.33 (d, J = 7.3 Hz, 1H, Ar-H), 6.21–6.28 (m, 2H, Ar-H), 6.10–6.19 (m, 3H, Ar-H), 4.48 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 4.46 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 4.45 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 4.38 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 3.94–4.10 (m, 1H (Ar-CH2-Ar) + 4H (-OCH2-)), 3.62–3.79 (m, 4H, -OCH2-), 3.18 (d, 1H, J = 13.0 Hz, 1H, Ar-CH2-Ar), 3.15 (d, 1H, J = 13.0 Hz, 2H, Ar-CH2-Ar), 1.93–2.03 (m, 4H, -CH2-CH3), 1.84–1.92 (m, 4H, -CH2-CH3), 1.10 (t, J = 7.3 Hz, 6H, -CH3), 0.91 (t, J = 7.3 Hz, 6H, -CH3), 0.90 (t, J = 7.3 Hz, 6H, -CH3).13C NMR (CDCl3, 125 MHz, 298 K) δ 157.98, 157.89, 155.19, 150.02, 143.88, 139.80, 138.70, 137.02, 136.90, 136.04, 133.50, 133.24, 132.79, 132.57, 128.86, 128.80, 128.61, 127.62, 127.45, 127.38, 127.26, 126.70, 122.46, 122.14, 122.12, 121.80, 121.04, 90.56, 89.46, 76.97, 76.90, 76.64, 76.47, 31.10, 31.00, 30.98, 23.51 (2C), 23.01, 23.99, 10.81, 10.79, 9.87, 9.86 ppm. HRMS (ESI+) calcd for C47H51NO4 716.3710 [M + Na]+, found m/z 716.3710 [M + Na]+. IR (KBr) ν 2960, 2931, 2873, 1580, 1455, 1383, 1209, 1192, 1087, 1066, 1005, 956, 756 cm−1.
Alkynylcalixarene 5f
Method A: Calixarene 3 (0.349 mmol, 250 mg), trimethylsilylacetylene (0.418 mmol, 60 µL), CuI (0.035 mmol, 7 mg) and Pd(PPh3)2Cl2 (0.017 mmol, 12 mg) were used under standard conditions; the product was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to afford 5f in 83% yield (199 mg) as a yellow oil.
Method B: Calixarene 3 (0.138 mmol, 104 mg), trimethylsilylacetylene (0.552 mmol, 78 µL), CuI (0.028 mmol, 5 mg), piperidine (0.417 mmol, 40 µL) and Pd(PPh3)4 (0.014 mmol, 16 mg) were used under standard conditions. The product 5f (71 mg, 75%) was obtained by column chromatography on silica gel (DCM/cyclohexane 1:1) as a yellow oil.
Data for compound 5f: 1H NMR (CDCl3, 500 MHz, 298 K) δ 7.14 (d, 1H, J = 7.8 Hz, Ar-H) 7.10 (m, 2H, Ar-H) 7.03 (d, J = 7.8 Hz, 2H, Ar-H) 6.91 (t, J = 7.4 Hz, 1H, Ar-H) 6.27 (dd, J = 7.7 Hz, J = 1.7 Hz, 1H, Ar-H) 6.25 (t, J = 7.4 Hz, 1H, Ar-H) 6.20 (t, J = 7.4 Hz, 1H, Ar-H), 6.15–6.10 (m, 2H, Ar-H), 6.07 (dd, J = 7.4 Hz, J = 1.7 Hz, 1H, Ar-H), 4.43 (d, J = 13.5 Hz, 1H, Ar-CH2-Ar), 4.44 (d, J = 13.5 Hz, 2H, Ar-CH2-Ar), 4.28 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.04–3.95 (m, 4H, -OCH2), 4.48 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.38 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.12–4.02 (m, 4H, -OCH2-), 3.90 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.76–3.60 (m, 4H, -OCH2-), 3.14 (d, J = 13.4 Hz, 2H, Ar-CH2-Ar), 3.13 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 2.02–1.90 (m, 4H, -CH2-CH3), 1.91–1.80 (m, 4H, -CH2-CH3), 1.09 (t, J = 7.4 Hz, 3H, -CH3), 1.08 (t, J = 7.4 Hz, 3H, -CH3), 0.87 (t, J = 7.4 Hz, 6H, -CH3), 0.21 (s, 9H, -CH3) 13C NMR (CDCl3, 100 MHz, 298 K) δ 158.40, 158.36, 155.55, 155.50, 140.07, 138.56, 137.43, 137.35, 133.70, 133.47, 133.28, 132.93, 129.22, 129.12, 128.71, 127.84, 127.65, 127.54, 127.50, 126.79, 122.41, 122.36, 122.20, 122.06, 105.20, 96.13, 76.98 (2C), 76.57, 76.49, 30.82, 30.77, 30.74, 27.64, 23.28 (2C), 22.71 (2C), 10.52 (2C), 9.51 (2C), 0.28 (3C).ppm. HRMS (ESI+) calcd for C45H56SiO4 711.3840 [M + Na]+, found m/z 711.3847 [M + Na]+. IR (KBr) ν 2960, 2932, 2874, 1455, 1383, 1247, 1207, 1192, 1088, 1006, 966, 841, 756 cm−1.
3.2.2. Preparation of Bridged Calixarene 7b
Diphenyl diselenide (0.609 mmol, 190 mg) and FeCl3 (0.752 mmol, 122 mg) were dissolved in 4 mL of dry dichloromethane in a Schlenk flask under an argon atmosphere. The resulting solution was stirred for 15 min at room temperature. After this time, the solution changed color to brown-red. Calixarene 5b (0.301 mmol, 209 mg) dissolved in 2 mL of dry dichloromethane was added to the above solution, and the resulting mixture was heated at 30 °C for 4 h. Dichloromethane (20 mL) was then added to the reaction mixture, the organic layer was washed with a saturated solution of NH4Cl, dried with MgSO4 and concentrated under vacuum. The residue was purified by column chromatography on silica gel (DCM/cyclohexane 1:1) to provide the respective product 7b in 20% yield (51 mg) as a yellow solid.
Data for compound 7b: M.p. = 117–120 °C. 1H NMR (CDCl3, 400 MHz, 298 K) δ 7.24 (dd, J = 8.0 Hz, J = 1.8 Hz, 2H, Ar-H), 7.19 (dd, J = 8.0 Hz, J = 1.8 Hz, 2H, Ar-H), 7.03–6.95 (m, 7H, Ar-H) 6.93 (d, J = 7.3 Hz, 1H, Ar-H), 6.89 (d, J = 8.0 Hz, 1H, Ar-H), 6.87 (dd, J = 8.0 Hz, J = 1.5 Hz, 1H, Ar-H), 6.82 (dd, J = 7.8 Hz, J = 1.5 Hz, 1H, Ar-H), 6.75 (d, J = 9.0 Hz, 1H, Ar-H), 6.73 (t, J = 7.6 Hz, 1H, Ar-H), 6.61 (t, J = 7.4 Hz, 1H, Ar-H), 6.43 (d, J = 7.8 Hz, 1H, Ar-H), 5.98 (d, J = 7.8 Hz, 1H, Ar-H), 4.70 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.50 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 4.41 (d, J = 12.0 Hz, 1H, Ar-CH2-Ar), 4.33 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 4.04–3.79 (m, 6H, -OCH2-), 3.64–3.54 (m, 2H, -OCH2-), 3.28 (d, J = 13.2 Hz, 1H, Ar-CH2-Ar), 3.27 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 3.11 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 3.07 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 2.21 (sextet, J = 7.7 Hz, 2H, -CH2-CH3), 2.13–2.01 (m, 2H, -CH2-CH3), 2.01–1.83 (m, 4H, -CH2-CH3), 1.20 (t, J = 7.4 Hz, 3H, -CH3), 1.19 (t, J = 7.4 Hz, 3H, -CH3), 1.00 (t, J = 7.4 Hz, 3H, -CH3), 0.99 (t, J = 7.4 Hz, 3H, -CH3). 13C NMR (CDCl3, 100 MHz, 298 K) δ 156.32, 155.80, 154.80, 154.77, 140.82, 140.37, 140.36, 140.08, 136.54, 136.23, 135.04, 134.42, 134.18, 132.98, 132.71, 132.70, 132.21, 131.16, 130.97 (2C), 130.39, 129.27, 128.86, 128.41 (2C), 128.09, 127.40, 127.37 (2C), 126.89, 126.58, 126.48, 126.44, 123.27, 123.05, 123.01, 122.94, 77.48, 77.45, 76.99, 76.70, 31.79, 31.56, 29.19, 24.82, 23.77, 23.73, 23.12, 22.99, 11.10 (2C), 10.29, 10.15 ppm. HRMS (ESI+) calcd for C54H56SeO4 871.3236 [M + Na]+, found m/z 871.3246 [M + Na]+. IR (KBr) ν 2959, 2924, 2872, 1455, 1250, 1208, 1066, 1007, 960, 734, 690 cm−1.
3.2.3. Preparation of Bridged Calixarene 8b
Diphenyl diselenide (0.28 mmol, 90 mg) and FeCl3 (0.351 mmol, 57 mg) were dissolved in 3 mL of dry DCE in a Schlenk flask under an argon atmosphere, and the resulting solution was stirred for 15 min at room temperature. After this time, the color of the solution turned to brown-red. Calixarene 5b (0.144 mmol, 100 mg) dissolved in 1 mL of dry DCE was added, and the reaction mixture was heated to 85 °C for 4 h. The eluent was then evaporated under reduced pressure, dichloromethane (20 mL) was added to the crude product, washed with a saturated solution of NH4Cl, dried with MgSO4 and then concentrated under vacuum. The residue was purified by preparative thin layer chromatography on silica gel (DCM/cyclohexane 1:1) to provide the methylene bridged product 8b in 10% yield (12 mg) as a white solid.
Data for compound 8b: M.p. = 101–104 °C. 1H NMR (CDCl3, 400 MHz, 298 K) δ 8.38 (s, 1H, -OH), 8.25 (s, 1H, -OH), 7.45 (d, J = 7.9 Hz, 2H, Ar-H), 7.39 (d, J = 1.4 Hz, 1H, Ar-H), 7.34 (t, J = 7.0 Hz, 3H, Ar-H), 7.30–7.11 (m, 10H, Ar-H) 7.07 (d, J = 7.3 Hz, 1H, Ar-H), 6.87 (d, J = 7.3 Hz, 1H, Ar-H), 6.83 (d, J = 7.3 Hz, 2H, Ar-H), 6.74 (d, J = 7.5 Hz, 1H, Ar-H), 6.73 (t, J = 7.4 Hz, 1H, Ar-H), 6.59 (t, J = 7.7 Hz, 1H, Ar-H), 6.50 (d, J = 7.2 Hz, 1H, Ar-H), 5.49 (s, 1H, Ar-CH(R)-Ar), 4.51 (d, J = 12.4 Hz, 1H, Ar-CH2-Ar), 4.43 (d, J = 12.5 Hz, 1H, Ar-CH2-Ar), 4.18–4.04 (m, 2H, -OCH2-), 4.03–3.90 (m, 2H, -OCH2-), 3.43 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 3.35 (d, J = 13.0 Hz, 1H, Ar-CH2-Ar), 3.26 (d, J = 12.4 Hz, 1H, Ar-CH2-Ar), 2.19–1.96 (m, 4H, -CH2-CH3), 1.37 (t, J = 7.3 Hz, 3H, -CH3), 1.30 (t, J = 7.4 Hz, 3H, -CH3). 13C NMR (CDCl3, 125 MHz, 298 K) δ 154.14, 153.59, 153.13, 152.32, 151.24, 144.49, 135.99, 134.72, 134.00, 133.82, 132.04, 131.70, 130.81, 130.35, 130.24 (2C), 130.24, 130.06, 130.02, 129.72 (2C), 129.46 (2C), 129.32 (2C), 129.21, 129.17, 128.68, 128.56, 128.23 (2C), 128.01, 127.57, 126.25, 125.94, 125.76, 125.50, 125.22, 116.72, 113.61, 78.29, 78.21, 50.51, 32.11, 31.45, 30.09, 23.88, 23.69, 11.18, 11.06 ppm. HRMS (ESI+) calcd for C54H48Se2O4 943.1775 [M + Na]+, found m/z 943.1785 [M + Na]+.
3.2.4. Preparation of Bridged Calixarenes 9c and 10c
Diphenyl diselenide (0.170 mmol, 53 mg) and FeCl3 (0.213 mmol, 34 mg) were dissolved in 3 mL of dry dichloromethane in a Schlenk flask under an argon atmosphere. The resulting solution was stirred for 15 min at room temperature. After this time, the solution changed color to brown-red. Calixarene 5c (0.065 mmol, 85 mg) dissolved in 1 mL of dry dichloromethane was added, and the reaction mixture was heated at 30 °C for 4 h. Dichloromethane (20 mL) was added to the mixture, the organic layer was washed with a saturated solution of NH4Cl, dried over MgSO4, and concentrated under vacuum. The residue was purified by column chromatography on a silica gel (DCM/cyclohexane 1:1) to provide the respective products 9c in 37% yield (27 mg) and 10c in 15% yield (11 mg) as yellow oils.
Data for compound 9c: 1H NMR (CDCl3, 400 MHz, 298 K) δ 7.41 (dd, J = 7.4 Hz, J = 1.5 Hz, 2H, Ar-H), 7.31 (d, J = 8.4 Hz, 2H, Ar-H), 7.23–7.15 (m, 4H, Ar-H) 7.13 (dd, J = 7.5 Hz, J = 1.4 Hz, 1H, Ar-H), 7.07 (d, J = 7.5 Hz, 1H, Ar-H), 6.95 (t, J = 7.3 Hz, 1H, Ar-H), 6.87 (d, J = 7.4 Hz, 1H, Ar-H), 6.77 (d, J = 8.9 Hz, 2H, Ar-H), 6.20 (t, J = 7.4 Hz, 1H, Ar-H), 6.14–6.07 (m, 3H, Ar-H), 6.02 (dd, J = 7.5 Hz, J = 1.3 Hz, 1H, Ar-H), 5.80 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, Ar-H), 5.70 (s, 1H, Ar-CH(R)-Ar), 4.49 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 4.44 (d, J = 13.3 Hz, 2H, Ar-CH2-Ar), 4.34 (td, J = 11.0 Hz, J = 5.3 Hz, 1H, -OCH2-), 4.23–4.10 (m, 2H, -OCH2-), 4.04–3.94 (m, 1H, -OCH2-), 3.74 (s, 3H, -OCH3), 3.71–3.64 (m, 3H, -OCH2-), 3.54–3.46 (m, 1H, -OCH2-), 3.19 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 3.15 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 3.13 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 2.04–1.81 (m, 8H, -CH2-CH3), 1.14 (t, J = 7.5 Hz, 3H, -CH3), 1.10 (t, J = 7.4 Hz, 3H, -CH3), 0.91 (t, J = 7.5 Hz, 3H, -CH3), 0.89 (t, J = 7.4 Hz, 3H, -CH3). We cannot acquire reliable 13C NMR spectra due to degradation of compound. HRMS (ESI+) calcd for C55H58SeO5 901.3341 [M + Na]+, found m/z 901.3345 [M + Na]+.
Data for compound 10c: 1H NMR (CDCl3, 400 MHz, 298 K) δ 7.40 (dd, J = 7.4 Hz, J = 1.5 Hz, 2H, Ar-H), 7.37 (d, J = 8.6 Hz, 2H, Ar-H), 7.22–7.14 (m, 3H, Ar-H), 7.10 (d, J = 7.6 Hz, 3H, Ar-H), 6.93 (d, J = 7.6 Hz, 1H, Ar-H), 6.81 (d, J = 7.6 Hz, 1H, Ar-H), 6.77 (d, J = 8.6 Hz, 2H, Ar-H), 6.46–6.34 (m, 3H, Ar-H), 6.27 (~dd, J = 7.6 Hz, J = 1.5 Hz, 1H, Ar-H), 6.22 (t, J = 7.6 Hz, 1H, Ar-H), 5.98 (dd, J = 7.6 Hz, J = 1.5 Hz, 1H, Ar-H), 5.74 (s, 1H, Ar-CH(R)-Ar), 4.42 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 4.40 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 4.37 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 4.28–4.19 (m, 1H, -OCH2-), 4.00–3.92 (m, 1H, -OCH2-), 3.77–3.72 (m, 3H + 3H, -OCH2-, -OCH3), 3.65–3.59 (m, 1H, -OCH2-), 3.33 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 3.23 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 3.22 (d, J = 13.3 Hz, 1H, Ar-CH2-Ar), 2.26–2.20 (m, 1H, -CH2-CH3), 2.15–2.07 (m, 1H, -CH2-CH3), 1.97–1.84 (m, 4H, -CH2-CH3), 1.15 (t, J = 7.3 Hz, 3H, -CH3), 1.10 (t, J = 7.3 Hz, 3H, -CH3), 0.93 (t, J = 7.4 Hz, 3H, -CH3). We cannot acquire 13C NMR spectra due to degradation of compound. HRMS (ESI+) calcd for C52H52SeO5 859.2881 [M + Na]+, found m/z 859.2881 [M + Na]+.
3.2.5. Electrochemical Experiments
Batch Electrolysis Setup
The electrolysis was performed in a 20 mL undivided cell filled with 15 mL of MeCN (HPLC gradient grade ≥99.9%, Merck), containing 0.02 M LiClO4·H2O (BDH Chemicals Ltd., Poole, England) used as a supporting electrolyte. Additionally, the solution contained compound 5b with 0.6 equiv. of Ph2Se2. As working electrodes, two different materials were tested: Pt sheet (5 mm × 30 mm), or glassy carbon (GC, diameter 3 mm). In the case of using GC as the working electrode, GC was conducted by Motorcontroller for Rotator, and 100 RPM rotation speed was used. As the reference electrode, a saturated calomel electrode (SCE) separated from the investigated sample by a salt-bridge filled by the blank (MeCN electrolyte solution) was used, and as the counter (auxiliary) electrode, a Pt sheet (5 mm × 30 mm) or GC (diameter 3 mm) were applied. The reaction mixture was stirred and electrolyzed at room temperature during (a) constant current conditions (10 mA was applied) (b) controlled-potential electrolysis (potential was set to +1.5 V). In the cases when electrolysis was performed under inert atmosphere, oxygen was removed from the solution by passing a stream of argon (99.998%, Messer, Bad Soden, Germany). Measurements were carried out using the computer-driven digital potentiostat PGSTAT204 (Autolab-Metrohm, Herisau, Switzerland) controlled by software NOVA 1.11.
Attempted Electrolytic Cyclization of 5b
Calixarene 5b (25 mg, 0.036 mmol) with 0.6 equiv. of diphenyl diselenide (6.76 mg, 0.022 mmol) was dissolved in 15 mL of solution of a supporting electrolyte (0.02 M LiClO4·H2O). The solution was poured into an undivided cell, and then the Pt plate (used as working electrode), SCE (reference electrode), and Pt plate (auxiliary electrode) were placed in solution (Figure S79). The reaction mixture was stirred by a magnetic bar at room temperature during constant current (set to 10 mA) under aerobic condition. The progress of the reaction was monitored by TLC until the full conversion of 5b was detected (3.5 h).
After completion, the crude reaction mixture was collected and evaporated to dryness. The solid was partitioned between DCM and water; the organic layer was washed with water and dried over MgSO4. Crude evaporated product was purified using preparative TLC on silica gel (DCM/cyclohexane 1:1) to provide product 11 (19 mg, 73%) as a white solid.
Data for compound 11: M.p. = 137–141 °C. 1H NMR (CDCl3, 400 MHz, 298 K) δ 7.92 (d, J = 7.4 Hz, 2H, Ar-H), 7.65 (t, J = 7.8 Hz, 1H, Ar-H), 7.51 (t, J = 7.1 Hz, 2H, Ar-H), 7.15 (d, J = 8.2 Hz, 1H, Ar-H), 6.97 (d, J = 7.5 Hz, 1H, Ar-H), 6.91 (t, J = 7.1 Hz, 2H, Ar-H), 6.70 (t, J = 7.0 Hz, 1H, Ar-H), 6.39–6.27 (m, 5H, Ar-H), 6.23 (d, J = 7.4 Hz, 1H, Ar-H), 4.55 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.52 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.45 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.44 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.41 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 4.04–3.91 (m, 4H, -OCH2-), 3.82–3.65 (m, 4H, -OCH2-), 3.20 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.17 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 3.15 (d, J = 13.4 Hz, 1H, Ar-CH2-Ar), 2.06–1.82 (m, 8H, -CH2-CH3), 1.06 (t, J = 7.5 Hz, 3H, -CH3), 1.05 (t, J = 7.4 Hz, 3H, -CH3), 0.95 (t, J = 7.5 Hz, 3H, -CH3), 0.93 (t, J = 7.4 Hz, 3H, -CH3). 13C NMR (CDCl3, 100 MHz, 298 K) δ 197.92, 195.89, 160.23, 158.43, 156.69, 156.58, 144.17, 140.08, 137.42, 137.02, 135.35, 135.17, 134.68, 134.09, 134.01, 132.94, 132.51, 130.79 (2C), 129.71 (2C), 129.56, 129.49, 129.42, 129.28, 129.07, 129.02, 128.92, 128.83, 128.72, 128.39, 128.22, 128.12, 122.97, 122.76, 122.59, 77.51, 77.49, 77.09, 77.05, 31.47, 31.37, 31.29, 25.94, 23.64, 23.48, 23.33, 23.30, 10.81, 10.76, 10.18, 10.12 ppm. HRMS (ESI+) calcd for C48H52O6 747.3656 [M + Na]+, found m/z 747.3662 [M + Na]+. IR (KBr) ν 2961, 2924, 2872, 1674, 1665, 1586, 1454, 1384, 1290, 1245, 1228, 1105, 964, 785, 756, 719 cm−1.
Cyclic Voltammetry
Cyclic voltammetry measurements (with scan rates 100, 200, and 500 mV·s−1) were performed in MeCN (HPLC gradient grade ≥99.9%, Merck) using 0.02 M LiClO4·H2O (BDH Chemicals Ltd., Poole, England) as the supporting electrolyte (Figures S77 and S78). Due to low conductivity, the three-electrode systems were applied for all measurements. As the working electrode, a glassy carbon electrode (diameter 3 mm) or Pt disk electrode (diameter 1 mm) was utilized. As the reference electrode, a saturated calomel electrode (SCE) separated from the investigated sample by a bridge filled by the blank (MeCN-electrolyte solution) was applied, and as the auxiliary electrode, a Pt sheet was used. All those experiments were standardly carried out in an undivided 20 mL cell (using 10 mL of the corresponding solution), and before measurements, the solutions were de-aerated by argon (99.998%, Messer). Measurements were carried out using the computer-driven digital potentiostat PGSTAT101 (Autolab-Metrohm) controlled by software NOVA 1.11.
3.3. X-ray Measurements
3.3.1. Crystallographic Data for 7b
M = 848 g·mol−1, triclinic system, space group P-1, a = 11.6678 (4) Å, b = 17.7287(5) Å, c = 21.7145(6) Å, α = 82.4177(12)°, β = 86.9096(13)°, γ = 88.6006(13)°, Z = 4, V = 4445.3(2) Å3, Dc = 1.267 g·cm−3, μ(Cu-Kα) = 1.494 mm−1, crystal dimensions of 0.077 × 0.077 × 0.158 mm. Data were collected at 180 (2) K on a Bruker D8 Venture Photon II 7 diffractometer with Incoatec microfocus sealed tube Cu-Kα radiation. The data were integrated, scaled and corrected for absorption using Apex4 [33]. The structure was solved by SIR92 [34] and anisotropically refined by full matrix least squares on F squared using the CRYSTALS [35] to final value R = 0.048 and wR = 0.124 using 1486 independent reflections (θmax = 72.2°), 1082 parameters and 42 restrains. The hydrogen atoms bonded to carbon atoms were placed in calculated positions and refined with riding constraints, while hydrogen atoms bonded to oxygen were refined using soft restraints. The disordered functional groups positions were found in difference electron density maps and refined with restrained geometry. MCE [36] was used for the visualization of electron density maps. The occupancy of disordered functional groups was constrained to full. The structure was deposited into Cambridge Structural Database under number CCDC 2332078. The full numbering scheme can be found in Supporting information Figures S74 and S75.
3.3.2. Crystallographic Data for 8b
M = 1003.82 g·mol−1, monoclinic system, space group P21/n, a = 13.3272(4) Å, b = 13.0362(4) Å, c = 27.1526(7) Å, β = 94.7510(10)°, Z = 4, V = 4701.2(2) Å3, Dc = 1.418 g·cm−3, μ(Cu-Kα) = 3.38 mm−1, crystal dimensions of 0.048 × 0.126 × 0.426 mm. Data were collected at 180 (2) K on a Bruker D8 Venture Photon II 7 diffractometer with Incoatec microfocus sealed tube Cu-Kα radiation. The data were integrated, scaled and corrected for absorption using Apex4 [33]. The structure was solved by SIR92 [34] and anisotropically refined by full matrix least squares on F squared using the CRYSTALS [35] to final value R = 0.060 and wR = 0.178 using 9241 independent reflections (θmax = 72.1°), 577 parameters and 0 restrains. The hydrogen atoms bonded to carbon atoms were placed in calculated positions and refined with riding constraints, while hydrogen atoms bonded to oxygen were first refined using soft restraints, then refined with riding constraints. The disordered solvent positions were found in difference electron density maps. MCE [36] was used for the visualization of electron density maps. The occupancy of disordered functional groups was first constrained to full, later fixed at values of 2/3 and 1/3, respectively. The structure was deposited into Cambridge Structural Database under number CCDC 2332079. The full numbering scheme can be found in Supporting information Figure S76.
4. Conclusions
A Sonogashira coupling of meta-iodocalix[4]arene with various terminal acetylenes confirmed that the meta position of calixarene is well addressable, and that both thermal and microwave protocols lead to good yields of the corresponding alkynylcalixarenes. Alkynes thus obtained were subjected to the FeCl3/Ph2Se2-promoted electrophilic closure. It turns out that the calix[4]arenes give completely different bridging products than those described for the non-macrocyclic starting compounds. This can be demonstrated not only by the isolation of products with a six-membered ring (6-exo-dig), but mainly by the smooth formation of the 5-endo-dig cyclization, which has never been observed in the aliphatic series. An attempt at electrocyclization led to a high yield of the 1,2-diketone, formed by oxidation of the starting alkyne. The structures of the unexpected products were unequivocally established by X-ray analysis, and clearly demonstrate how the preorganized macrocyclic skeleton favors a completely different regioselectivity of cyclization reactions compared to common aliphatic derivatives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29061237/s1, Spectral characterization of all new compounds (1H NMR, 13C NMR, HRMS, IR), VT NMR experiments, X-ray structures and electrochemical experiments.
Author Contributions
Conceptualization, writing—review and editing, P.L.; experimental work, M.P.; synthesis, spectra analysis, editing, A.S.; X-ray measurement, J.Č.; electrochemical experiments, K.S. and L.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation, grant number 23-07154S (P.L., A.S.), 21-05926X (J.C., A.S.) and by Specific university research (A2_FCHT_2022_073) (A.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All experimental data are provided in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gutsche, C.D. Calixarenes: An Introduction; Royal Society of Chemistry, RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Asfari, Z.; Böhmer, V.; Harrowfield, J.; Vicens, J. Calixarenes 2001; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Neri, P.; Sessler, J.L.; Wang, M.X. Calixarenes and Beyond; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Mandolini, L.; Ungaro, R. Calixarenes in Action; World Scientific Publishing Company: Singapore, 2000. [Google Scholar]
- Lhoták, P. Direct meta substitution of calix[4]arenes. Org. Biomol. Chem. 2022, 20, 7377–7390. [Google Scholar] [CrossRef] [PubMed]
- Slavik, P.; Dudic, M.; Flidrova, K.; Sykora, J.; Cisarova, I.; Bohm, S.; Lhotak, P. Unprecedented Meta-Substitution of Calixarenes: Direct Way to Inherently Chiral Derivatives. Org. Lett. 2012, 14, 3628–3631. [Google Scholar] [CrossRef] [PubMed]
- Flidrova, K.; Slavik, P.; Eigner, V.; Dvorakova, H.; Lhotak, P. meta-Bridged calix[4]arenes: A straightforward synthesis via organomercurial chemistry. Chem. Commun. 2013, 49, 6749–6751. [Google Scholar] [CrossRef] [PubMed]
- Tlusty, M.; Spalovska, D.; Kohout, M.; Eigner, V.; Lhotak, P. Ketone transformation as a pathway to inherently chiral rigidified calix[4]arenes. Chem. Commun. 2020, 56, 12773–12776. [Google Scholar] [CrossRef] [PubMed]
- Tlusty, M.; Slavik, P.; Kohout, M.; Eigner, V.; Lhotak, P. Inherently Chiral Upper-Rim-Bridged Calix[4]arenes Possessing a Seven Membered Ring. Org. Lett. 2017, 19, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, K.; Alabugin, I.V. Cyclizations of Alkynes: Revisiting Baldwin’s Rules for Ring Closure. Chem. Rev. 2011, 111, 6513–6556. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Karak, M.; Barbosa, L.C.; Hargaden, G.C. Recent mechanistic developments and next generation catalysts for the Sonogashira coupling reaction. RSC Adv. 2014, 4, 53442–53466. [Google Scholar] [CrossRef]
- Dyker, G.; Mastalerz, M.; Müller, I.M. Electron-Rich Cavitands via Fourfold Sonogashira Cross-Coupling Reaction of Calix[4]arenes and Bromopyridines—Effect of the Nitrogen Position on Complexation Abilities. Eur. J. Org. Chem. 2005, 2005, 3801–3812. [Google Scholar] [CrossRef]
- Böhmer, V.; Brusko, V.; Rissanen, K. Extended Calix[8]arenes by Sonogashira Cross-Coupling with Ethynylarenes. Synthesis 2002, 2002, 1898–1902. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Yodta, Y.; Niamnont, N.; Sukwattanasinitt, M. Fluorescent phenylethynylene calix[4]arenes for sensing TNT in aqueous media and vapor phase. RSC Adv. 2015, 5, 33306–33311. [Google Scholar] [CrossRef]
- Hennrich, G.; Murillo, M.T.; Prados, P.; Al-Saraierh, H.; El-Dali, A.; Thompson, D.W.; Collins, J.; Georghiou, P.E.; Teshome, A.; Asselberghs, I.; et al. Alkynyl Expanded Donor–Acceptor Calixarenes: Geometry and Second-Order Nonlinear Optical Properties. Chem. Eur. J. 2007, 13, 7753–7761. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraierh, H.; Miller, D.O.; Georghiou, P.E. Narrow-rim functionalization of calix[4]arenes via Sonogashira coupling reactions. J. Org. Chem. 2005, 70, 8273–8280. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, T.; Back, D.F.; Zeni, G. Iron(III) Chloride and Diorganyl Diselenide-Promoted Nucleophilic Closures of 1-Benzyl-2-alkynylbenzenes in the Preparation of 9-(Organoselanyl)-5H-benzo[7]annulenes. Adv. Synth. Catal. 2016, 358, 1119–1129. [Google Scholar] [CrossRef]
- Goulart, T.A.C.; Kazmirski, J.A.G.; Back, D.F.; Zeni, G. Iron(III)-Promoted Synthesis of 3-(Organoselanyl)-1,2-Dihydroquinolines from Diorganyl Diselenides and N-Arylpropargylamines by Sequential Carbon-Carbon and Carbon-Selenium Bond Formation. Adv. Synth. Catal. 2019, 361, 96–104. [Google Scholar] [CrossRef]
- Sperança, A.; Godoi, B.; Pinton, S.; Back, D.F.; Menezes, P.H.; Zeni, G. Regioselective Synthesis of Isochromenones by Iron(III)/PhSeSePh-Mediated Cyclization of 2-Alkynylaryl Esters. J. Org. Chem. 2011, 76, 6789–6797. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Mukherjee, N.; Satyanarayana, A.N.; Singh, P.; Dixit, M.; Chatterjee, T. Recyclable iodine-catalyzed radical selenylative annulation of 2-alkynyl biaryls with diselenides in water: A green approach to selanyl polycyclic aromatic hydrocarbons and polycyclic heteroaromatics. Green Chem. 2022, 24, 7029–7038. [Google Scholar] [CrossRef]
- An, S.; Zhang, Z.; Li, P. Metal-Free Synthesis of Selenodihydronaphthalenes by Selenoxide—Mediated Electrophilic Cyclization of Alkynes. Eur. J. Org. Chem. 2021, 2021, 3059–3070. [Google Scholar] [CrossRef]
- Zhang, X.; Campo, M.A.; Yao, T.; Larock, R.C. Synthesis of Substituted Quinolines by Electrophilic Cyclization of N-(2-Alkynyl)anilines. Org. Lett. 2005, 7, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Veltri, L. Recent Advances in the Synthesis of Indanes and Indenes. Chem. Eur. J. 2016, 22, 5056–5094. [Google Scholar] [CrossRef]
- Nahide, P.D.; Jiménez-Halla, J.O.C.; Wrobel, K.; Solorio-Alvarado, C.R.; Ortiz Alvarado, R.; Yahuaca-Juárez, B. Gold(i)-catalysed high-yielding synthesis of indenes by direct Csp3–H bond activation. Org. Biomol. Chem. 2018, 16, 7330–7335. [Google Scholar] [CrossRef]
- Yang, S.; Li, Z.; Jian, X.; He, C. Platinum(II)-Catalyzed Intramolecular Cyclization of o-Substituted Aryl Alkynes through sp3 C-H Activation. Angew. Chem. Int. Ed. 2009, 48, 3999–4001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Chen, Z.-Y.; Zhang, N.-N.; Chen, J.-H.; Zhang, X.-J.; Yan, M. Intramolecular Addition of Triarylmethanes to Alkynes Promoted by KOtBu/DMF: A Synthetic Approach to Indene Derivatives. Eur. J. Org. Chem. 2016, 2016, 599–606. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Alhumade, H.; Yi, H.; Cai, H.; Lei, A. Electrochemical Radical Selenylation of Alkenes and Arenes via Se–Se Bond Activation. Org. Lett. 2021, 23, 7724–7729. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, T. Highly Atom-Economic and Efficient Electrochemical Selenylative Annulation of 2-Alkynyl Biaryls. Adv. Synth. Catal. 2023, 365, 2255–2263. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, X.-Z.; Dai, J.-J.; Li, C.-G.; Xu, J.; Xu, H.-M.; Xu, H.-J. Electrochemical synthesis of 1,2-diketones from alkynes under transition-metal-catalyst-free conditions. Chem. Commun. 2019, 55, 9208–9211. [Google Scholar] [CrossRef]
- Bruker. APEX4, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2021. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIRPOW.92—A program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Crystallogr. 1994, 27, 435–436. [Google Scholar] [CrossRef]
- Betteridge, P.; Carruthers, J.; Cooper, R.; Prout, K.; Watkin, D. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Rohlicek, J.; Husak, M. MCE2005—A new version of a program for fast interactive visualization of electron and similar density maps optimized for small molecules. J. Appl. Crystallogr. 2007, 40, 600–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).