In Situ Observation of Cellular Structure Changes in and Chain Segregations of Anabaena sp. PCC 7120 on TiO2 Films under a Photocatalytic Device
Abstract
:1. Introduction
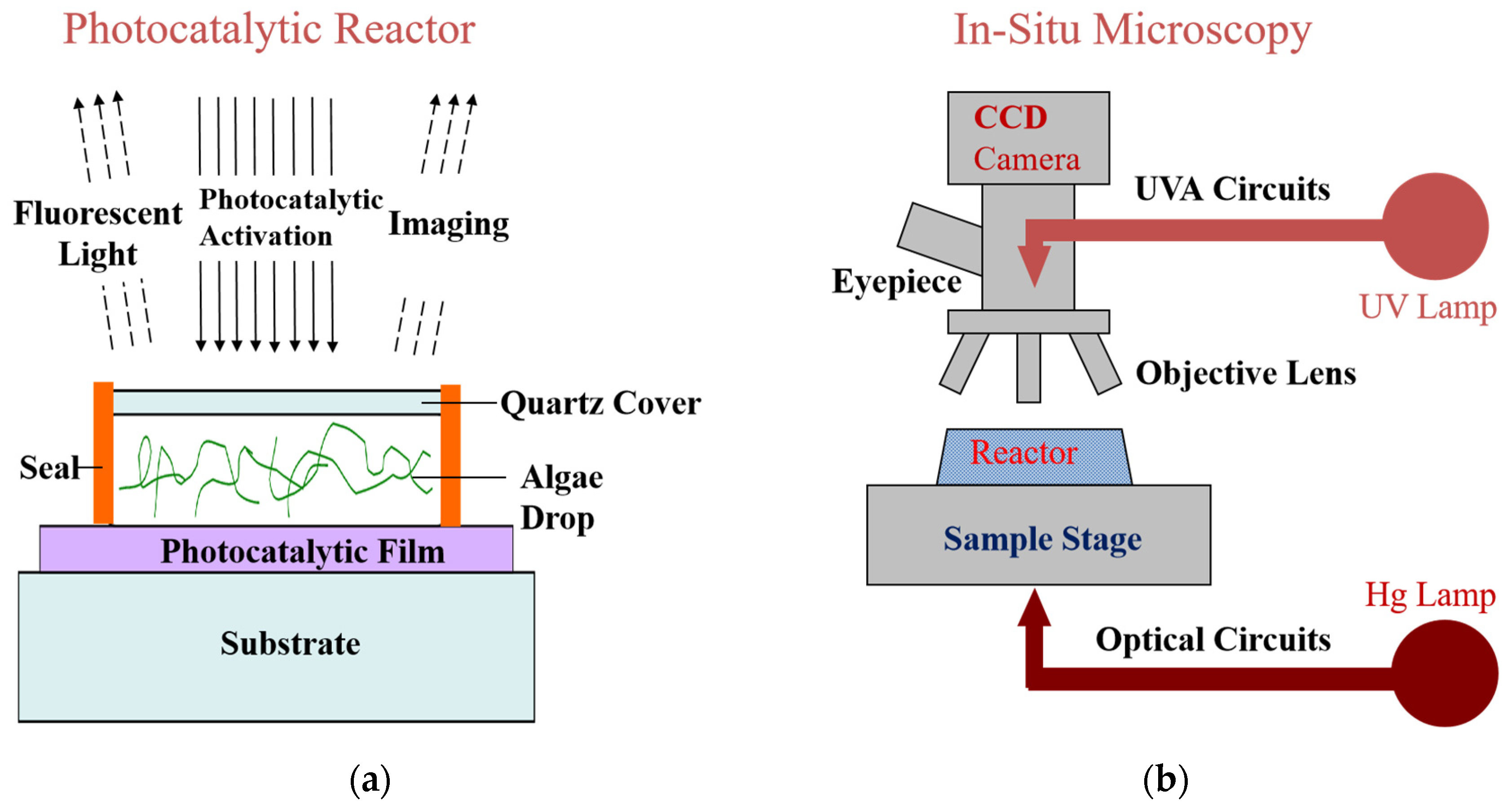
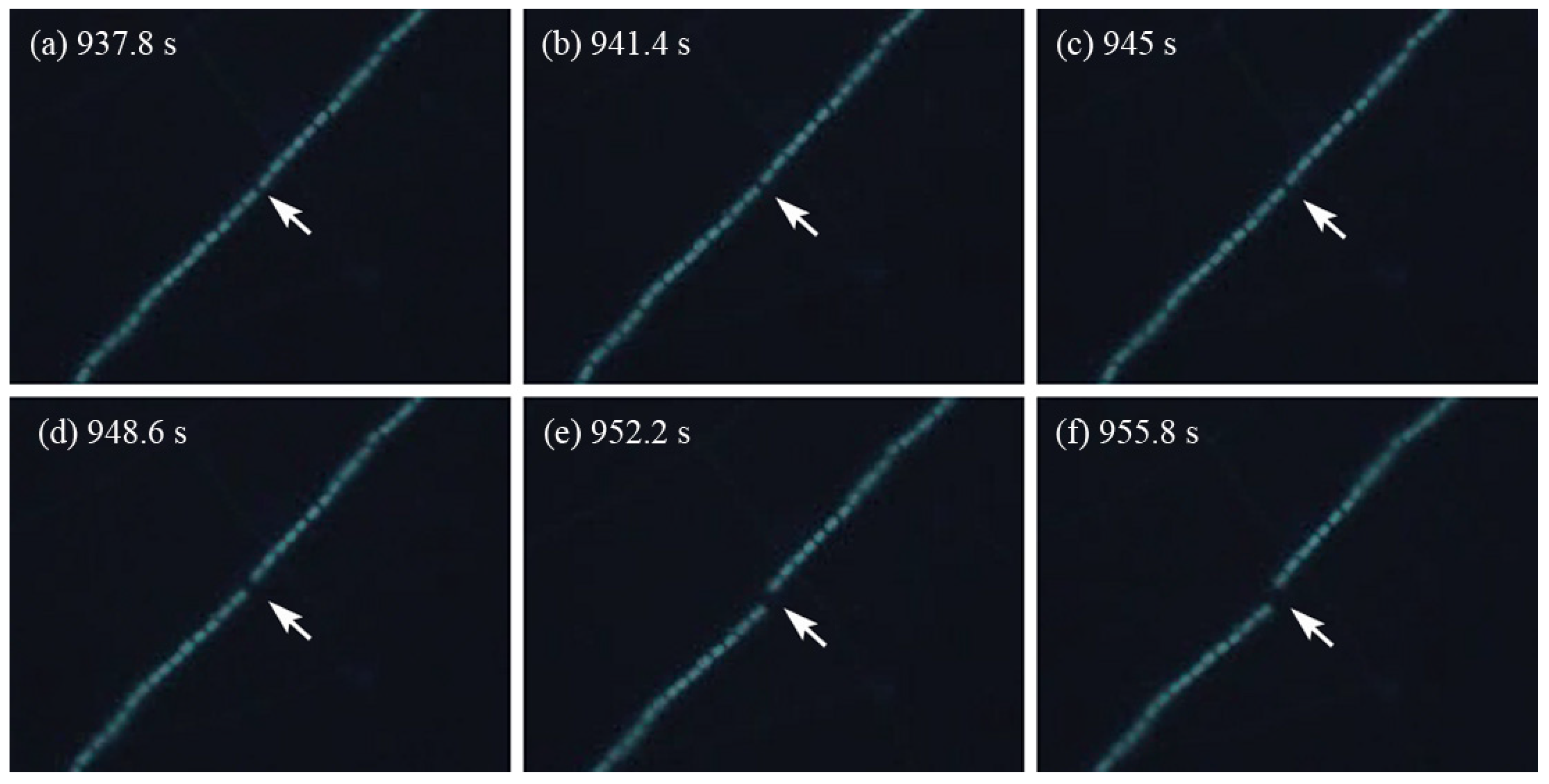
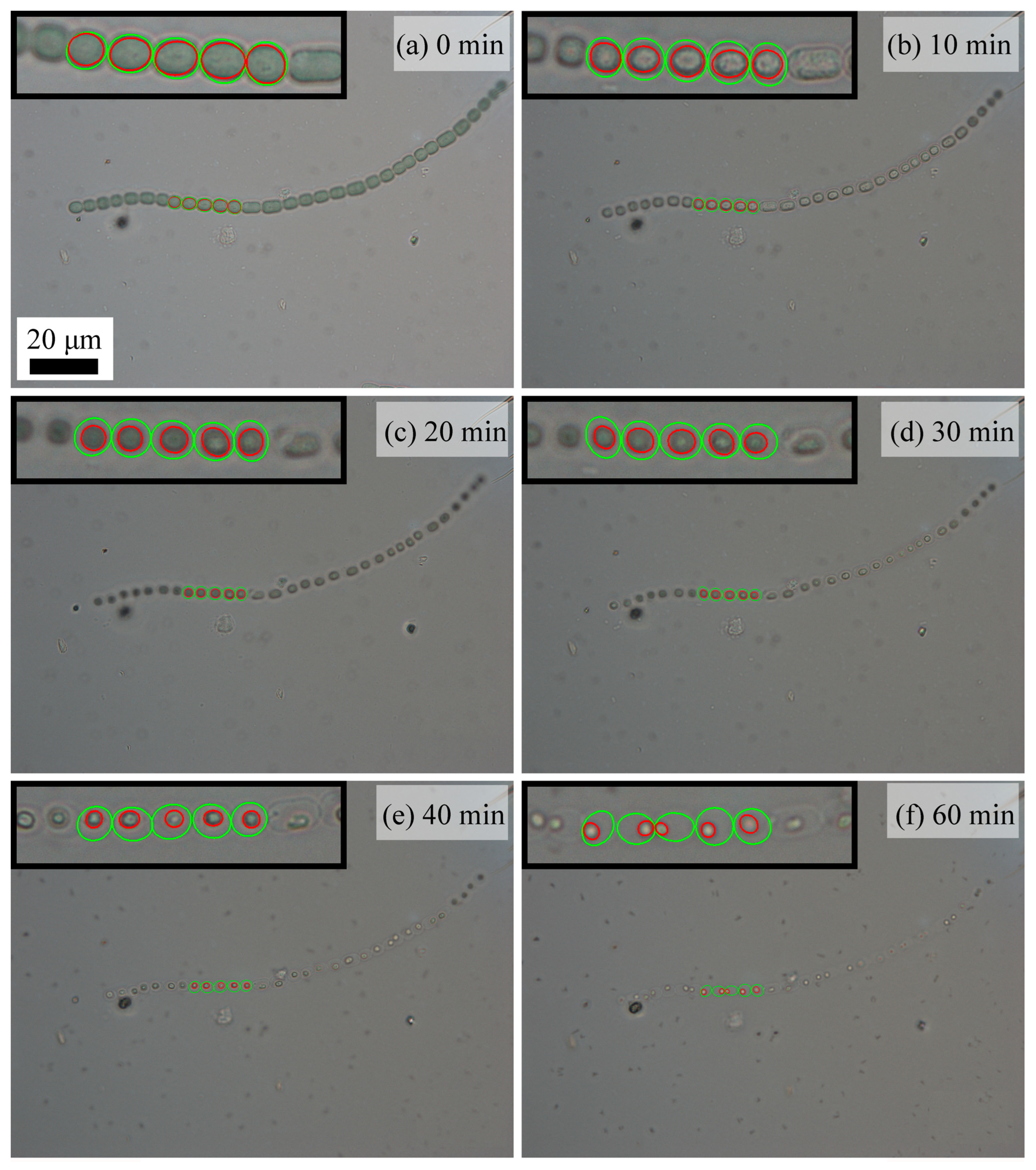
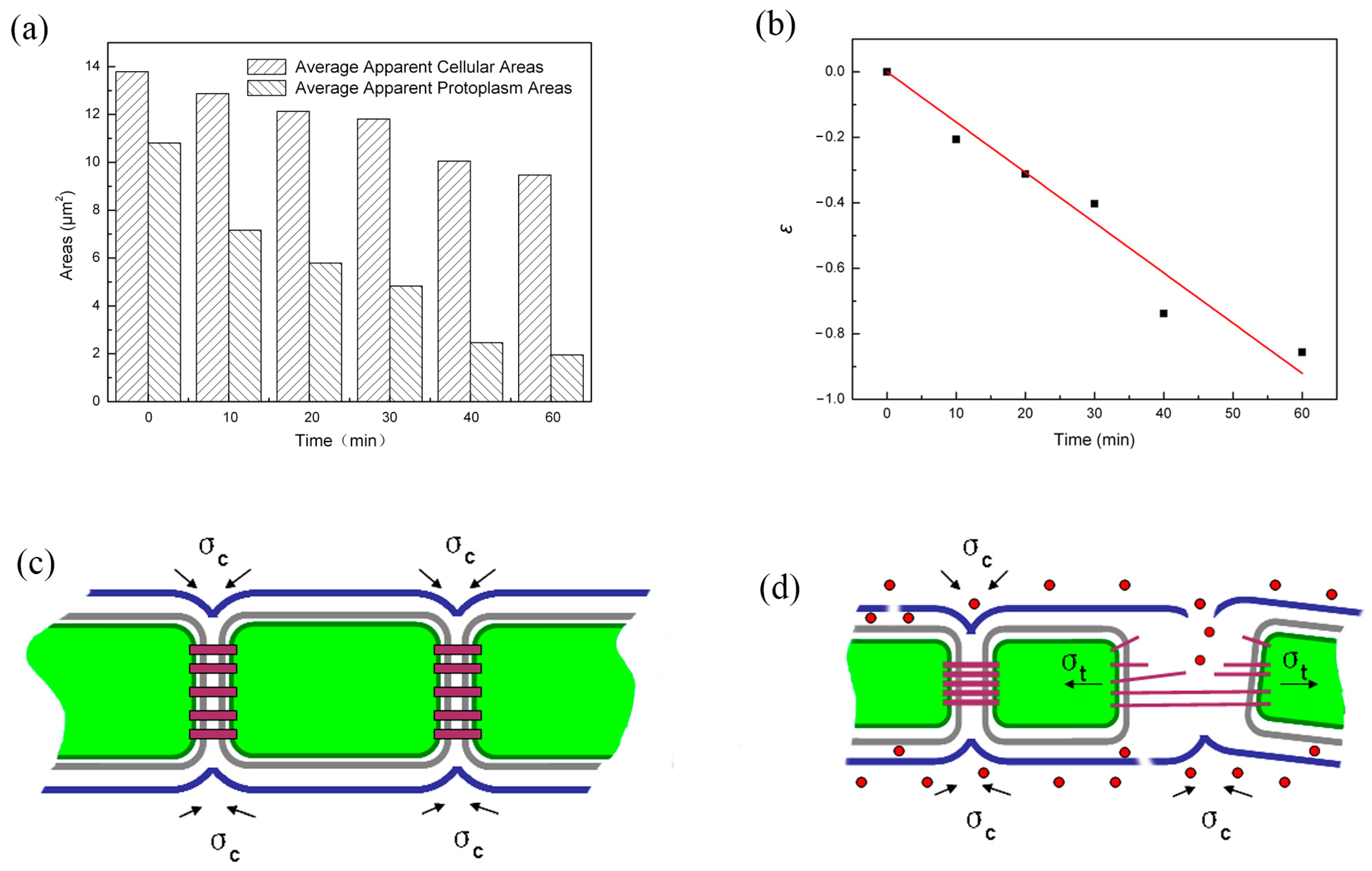
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, M.; Wang, Y.; Wu, X.; Kang, Z.; Zhang, D.; Pan, X. Potential of ozone micro-bombs in simultaneously fast removing bloom-forming cyanobacteria and in situ degrading microcystins. Chem. Eng. J. 2021, 407, 127186. [Google Scholar] [CrossRef]
- McKay, R.M.L.; Tuttle, T.; Reitz, L.A.; Bullerjahn, G.S.; Cody, W.R.; McDowell, A.J.; Davis, T.W. Early onset of a microcystin-producing cyanobacterial bloom in an agriculturally-influenced Great Lakes tributary. J. Oceanol. Limnol. 2018, 36, 1112–1125. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Kraak, M.H.S.; van Teulingen, C.; Kuijt, S.; van Herk, M.J.; Sigon, C.A.M.; Piel, T.; Sandrini, G.; Leon-Grooters, M.; de Baat, M.L.; et al. Sensitivity of phytoplankton, zooplankton and macroinvertebrates to hydrogen peroxide treatments of cyanobacterial blooms. Water. Res. 2022, 225, 119169. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Liu, J.; Li, C.; Lin, Q.; Zhang, T.; Zhang, K.; Sharma, V.K. Ferrate(VI) pre-treatment and subsequent chlorination of blue-green algae: Quantification of disinfection byproducts. Environ. Int. 2019, 133, 105195. [Google Scholar] [CrossRef] [PubMed]
- Coral, L.A.; Zamyadi, A.; Barbeau, B.; Bassetti, F.J.; Lapolli, F.R.; Prevost, M. Oxidation of Microcystis aeruginosa and Anabaena flos-aquae by ozone: Impacts on cell integrity and chlorination by-product formation. Water Res. 2013, 47, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, M.; Koh, K.Y.; Neo, W.; Ong, C.N.; Chen, J.P. An optimized CaO2-functionalized alginate bead for simultaneous and efficient removal of phosphorous and harmful cyanobacteria. Sci. Total Environ. 2022, 806, 150382. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Shao, L.; Liu, H.; Gao, Q.; Li, B.; Fu, H.; Fu, S.; Ye, H.; Zhao, F.; et al. Defective engineering in graphitic carbon nitride nanosheet for efficient photocatalytic pathogenic bacteria disinfection. Appl. Catal. B Environ. 2020, 261, 118201. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.P.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Mukherjee, K.; Acharya, K.; Biswas, A.; Jana, N.R. TiO2 Nanoparticles Co-doped with Nitrogen and Fluorine as Visible-Light-Activated Antifungal Agents. ACS Appl. Nano Mater. 2020, 3, 2016–2025. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Sun, W.; Yang, W.; Cao, J.; Li, Q.; Peng, Y.; Shang, J.K. Anti-algal activity of palladium oxide-modified nitrogen-doped titanium oxide photocatalyst on Anabaena sp. PCC 7120 and its photocatalytic degradation on Microcystin LR under visible light illumination. Chem. Eng. J. 2015, 264, 437–444. [Google Scholar] [CrossRef]
- Madany, P.; Xia, C.; Bhattacharjee, L.; Khan, N.; Li, R.; Liu, J. Antibacterial activity of γFe2O3 /TiO2 nanoparticles on toxic cyanobacteria from a lake in Southern Illinois. Water Environ. Res. 2021, 93, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, M.; Zhang, X.; Cui, N.; Chen, G.; Zou, G.-y. In-situ nitrogen-doped black TiO2 with enhanced visible-light-driven photocatalytic inactivation of Microcystis aeruginosa cells: Synthesization, performance and mechanism. Appl. Catal. B Environ. 2020, 272, 119019. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Kanth, N.; Xu, W.; Prasad, U.; Ravichandran, D.; Kannan, A.M.; Song, K. PMMA-TiO2 Fibers for the Photocatalytic Degradation of Water Pollutants. Nanomaterials 2020, 10, 1279. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Han, Z.; Wang, R.; He, J.; Zhang, C.; Que, W. Industrially Scalable and Refreshable Photocatalytic Foam. Adv. Sustain. Syst. 2023, 7, 2300041. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, T.; Que, W. Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment. Materials 2022, 15, 2466. [Google Scholar] [CrossRef]
- Koksharova, O.; Wolk, C. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar] [CrossRef]
- Priyalakshmi Devi, K.; Goswami, P.; Chaturvedi, H. Fabrication of nanocrystalline TiO2 thin films using Sol-Gel spin coating technology and investigation of its structural, morphology and optical characteristics. Appl. Surf. Sci. 2022, 591, 153226. [Google Scholar] [CrossRef]
- Fung, Y.C. Microrheology and constitutive equation of soft tissue. Biorheology 1988, 25, 261. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer: New York, NY, USA, 1994; pp. 32–33. [Google Scholar] [CrossRef]
- Wilk, L.; Strauss, M.; Rudolf, M.; Nicolaisen, K.; Flores, E.; Kuhlbrandt, W.; Schleiff, E. Outer membrane continuity and septosome formation between vegetative cells in the filaments of Anabaena sp. PCC 7120. Cell Microbiol. 2011, 13, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Haselkorn, R. Heterocysts. Ann. Rev. Plant Physiol. 1978, 29, 319–344. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Generation of OH radicals and oxidation mechanism in photocatalysis of WO3 and BiVO4 powders. J. Photochem. Photobiol. A Chem. 2015, 303, 53–58. [Google Scholar] [CrossRef]
- Romero-Morán, A.; Sánchez-Salas, J.L.; Molina-Reyes, J. Influence of selected reactive oxygen species on the photocatalytic activity of TiO2/SiO2 composite coatings processed at low temperature. Appl. Catal. B Environ. 2021, 291, 119685. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, J.; Li, X.; Guo, K.; Liu, W.; Fang, J. Multiple roles of UV/KMnO4 in cyanobacteria containing water treatment: Cell inactivation & removal, and microcystin degradation. J. Hazard. Mater. 2023, 457, 131772. [Google Scholar] [CrossRef]
- Holzinger, A.; Lütz, C. Algae and UV irradiation: Effects on ultrastructure and related metabolic functions. Micron 2006, 37, 190–207. [Google Scholar] [CrossRef]
- He, Y.Y.; Hader, D.P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: Protective effects of ascorbic acid and N-acetyl--cysteine. J. Photochem. Photobiol. B Biol. 2002, 66, 115–124. [Google Scholar] [CrossRef]
- Allen, M.M. Simple Conditions for Growth of unicellular Blue-Green Algae on Plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Arıer, Ü.Ö.A.; Tepehan, F.Z. Influence of heat treatment on the particle size of nanobrookite TiO2 thin films produced by sol-gel method. Surf. Coat. Technol. 2011, 206, 37–42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, J.; Li, Q.; Jia, R.; Qiao, M.; Cui, W. In Situ Observation of Cellular Structure Changes in and Chain Segregations of Anabaena sp. PCC 7120 on TiO2 Films under a Photocatalytic Device. Molecules 2023, 28, 7200. https://doi.org/10.3390/molecules28207200
Wang X, Zhang J, Li Q, Jia R, Qiao M, Cui W. In Situ Observation of Cellular Structure Changes in and Chain Segregations of Anabaena sp. PCC 7120 on TiO2 Films under a Photocatalytic Device. Molecules. 2023; 28(20):7200. https://doi.org/10.3390/molecules28207200
Chicago/Turabian StyleWang, Xiaoxin, Jingtao Zhang, Qi Li, Ran Jia, Mei Qiao, and Wanling Cui. 2023. "In Situ Observation of Cellular Structure Changes in and Chain Segregations of Anabaena sp. PCC 7120 on TiO2 Films under a Photocatalytic Device" Molecules 28, no. 20: 7200. https://doi.org/10.3390/molecules28207200
APA StyleWang, X., Zhang, J., Li, Q., Jia, R., Qiao, M., & Cui, W. (2023). In Situ Observation of Cellular Structure Changes in and Chain Segregations of Anabaena sp. PCC 7120 on TiO2 Films under a Photocatalytic Device. Molecules, 28(20), 7200. https://doi.org/10.3390/molecules28207200





