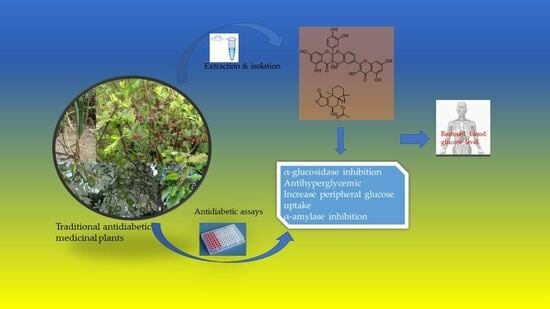
Phytochemical Content and Antidiabetic Properties of Most Commonly Used Antidiabetic Medicinal Plants of Kenya
Abstract
:1. Introduction
2. Diabetes Mellitus Mechanism of Action
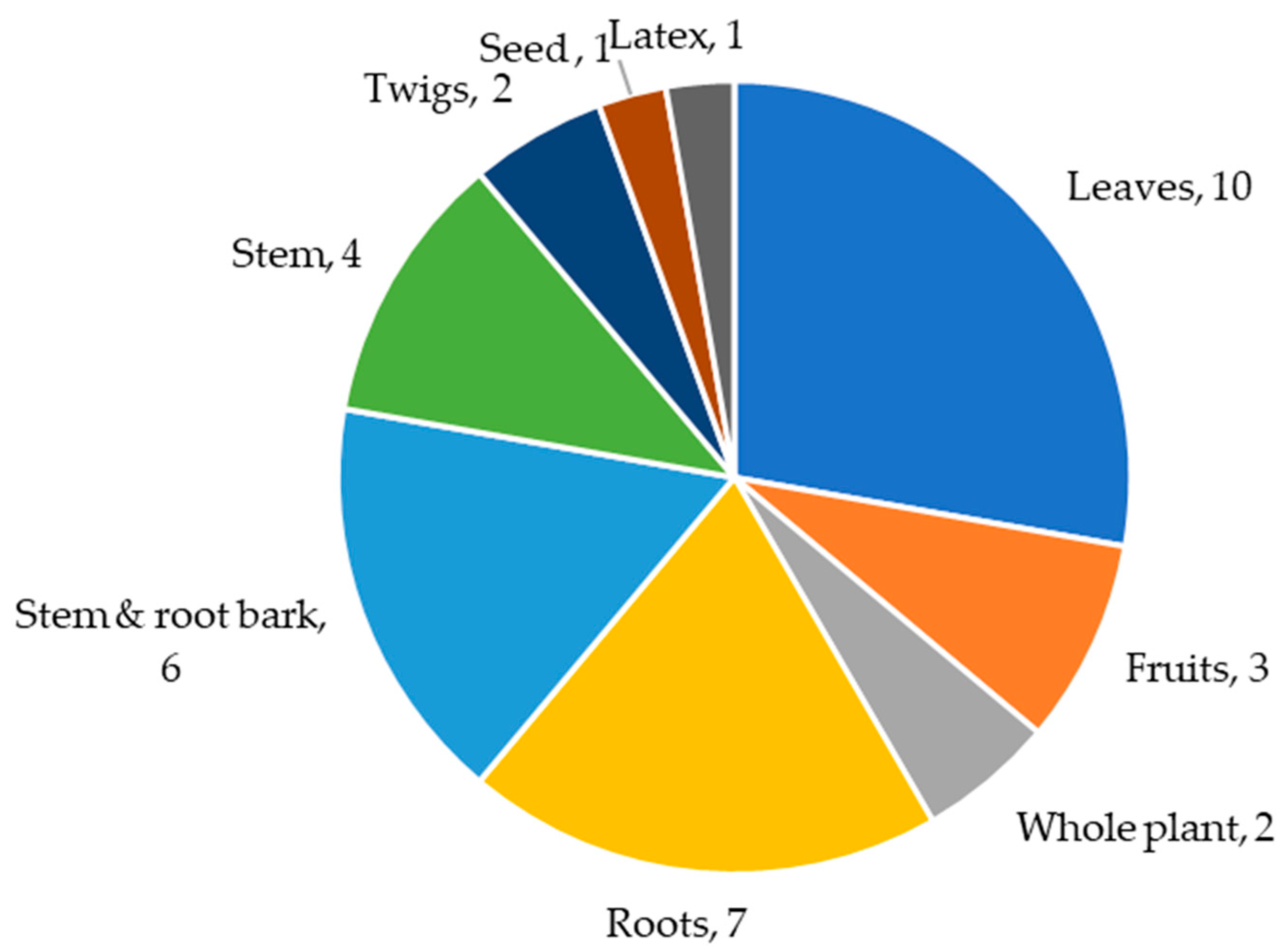
3. Ethnobotany
| Species and Family | Ethnomedicinal Uses |
|---|---|
| Acacia nilotica (L.) Willd. ex Delile (Mimosoideae) | The trunk bark is used to treat diarrhea, colds, bleeding piles, bronchitis and leucoderma [37]. The roots are used to treat cancers and tumors, diabetes, and tuberculosis [38]. Pods are used as antihypertensive and antispasmodic, anti-fertility, and astringent [38]. |
| Acalypha wilkesiana Müll.Arg. (Euphorbiaceae) | The leaves are used to treat diabetes mellitus, malaria, hypertension, skin infections, and gastrointestinal disorders [33,34]. |
| Allium cepa L. (Amaryllidaceae) | The whole plant is used in healing wounds, treating diabetes, pneumonia, headaches, fever, cough, flu, sore throat, high blood pressure, and rheumatism [39]. |
| Aloe secundiflora Engl. (Asphodelaceae) | An infusion from the leaves is used to treat bacterial diseases, ectoparasites, diabetes, fowl typhoid, nose bleeding, malaria, and wounds [40,41]. |
| Carissa edulis (Forssk.) Vahl (Apocynaceae) | A decoction prepared from the leaves is used for indigestion, malaria, and abdominal pain in pregnant women. The root is used to treat chest pains, gonorrhea, swollen glands, back pains, diabetes, syphilis, toothache, epilepsy, and sickle cell anemia [42,43]. |
| Dovyalis abyssinica (A.Rich.) Warb. (Salicaceae) | The leaves and roots are used to treat and manage ulcers, wounds, throat inflammation, pneumonia, malaria, diabetes, and indigestion [41,44]. |
| Dracaena steudneri Schweinf. Ex. Engl. (Asparagaceae) | The leaves are used to treat hernias, asthma, splenomegaly, chest problems, and liver diseases. The stem bark is used to treat liver diseases and measles, and to reduce pain during childbirth [45]. |
| Euphorbia hirta L. (Euphorbiaceae) | A decoction of the whole plant is used to treat respiratory system disorders, diabetes, ulcers, amebic dysentery, gonorrhea, and several types of cancers [46]. |
| Euphorbia tirucalli L. (Euphorbiaceae) | The latex treats cancers, toothaches, skin diseases, intestinal parasites, snake bikes, coughs, scorpion stings, asthma and ear problems [29], and syphilis [47]. The leaves are used to treat skin problems, diabetes, diarrhea, nose ulcers, and hemorrhoids. The stems are used for thorn extraction, and treating swelling, leprosy, paralysis, colic, and gastric problems. The roots are used to treat rheumatism [29]. |
| Faurea saligna Harv. (Proteaceae) | Used to treat sores and wounds, diabetes, fungal infections, candidiasis, and stomach problems [41]. |
| Lactuca inermis Forssk (Asteraceae) | A decoction of the leaves is used to treat joint pain, amebiasis, throat and nose diseases, and diabetes [48]. |
| Manihot esculenta Crantz (Euphorbiaceae) | The leaves are used in treating wounds, diabetes, headache, pain, and hypertension [49]. |
| Myrsine africana L. (Primulaceae) | Used to treat diarrhea, toothache, rheumatism, diabetes, and pulmonary tuberculosis [48,50,51]. |
| Persea americana Mill. (Lauraceae) | Traditionally used to treat rheumatism, bronchitis, urinary infections [52], hypertension, diabetes, stomach aches, and bronchitis [53]. |
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | Used to treat diabetes, high blood pressure, stomach problems, chest pains, fever, and malaria [54,55,56]. |
| Rhamnus prinoides L’Hér. (Rhamnaceae) | A decoction of the leaves is used to treat pneumonia, common colds, chest pain, tonsils, diabetes, back pain, and malaria [57]. |
| Rhamnus staddo A.Rich. (Rhamnaceae) | In East Africa, the stems, roots, fruits and leaves are used to treat malaria, diabetes, and endometritis [41,58]. |
| Rotheca myricoides (Hochst.) Steane and Mabb. (Lamiaceae) | A decoction prepared from the leaves is used to treat and manage diabetes, arthritis, rheumatism, gonorrhea, typhoid, malaria, epilepsy, and cancer [59]. |
| Trimeria grandifolia (Hochst.) Warb. (Salicaceae) | The roots are used to treat back pain, and a decoction of the stem is used to manage postpartum weakness, malaria, and diabetes [41]. |
| Urtica massaica Mildbr (Urticaceae) | A decoction of the leaves is used to treat cancer, diabetes, and malaria [41,60]. |
| Warburgia ugandensis Sprague (Canellaceae) | The leaves and stem bark are used to treat pains, coughs, malaria, colds, toothache, constipation, stomachache, and diabetes [61,62,63]. |
| Zanthoxylum usambarense (Engl.) (Rutaceae) | Decoctions of the leaves and roots are taken to treat stomachache, colds, toothache, and diabetes [64]. |
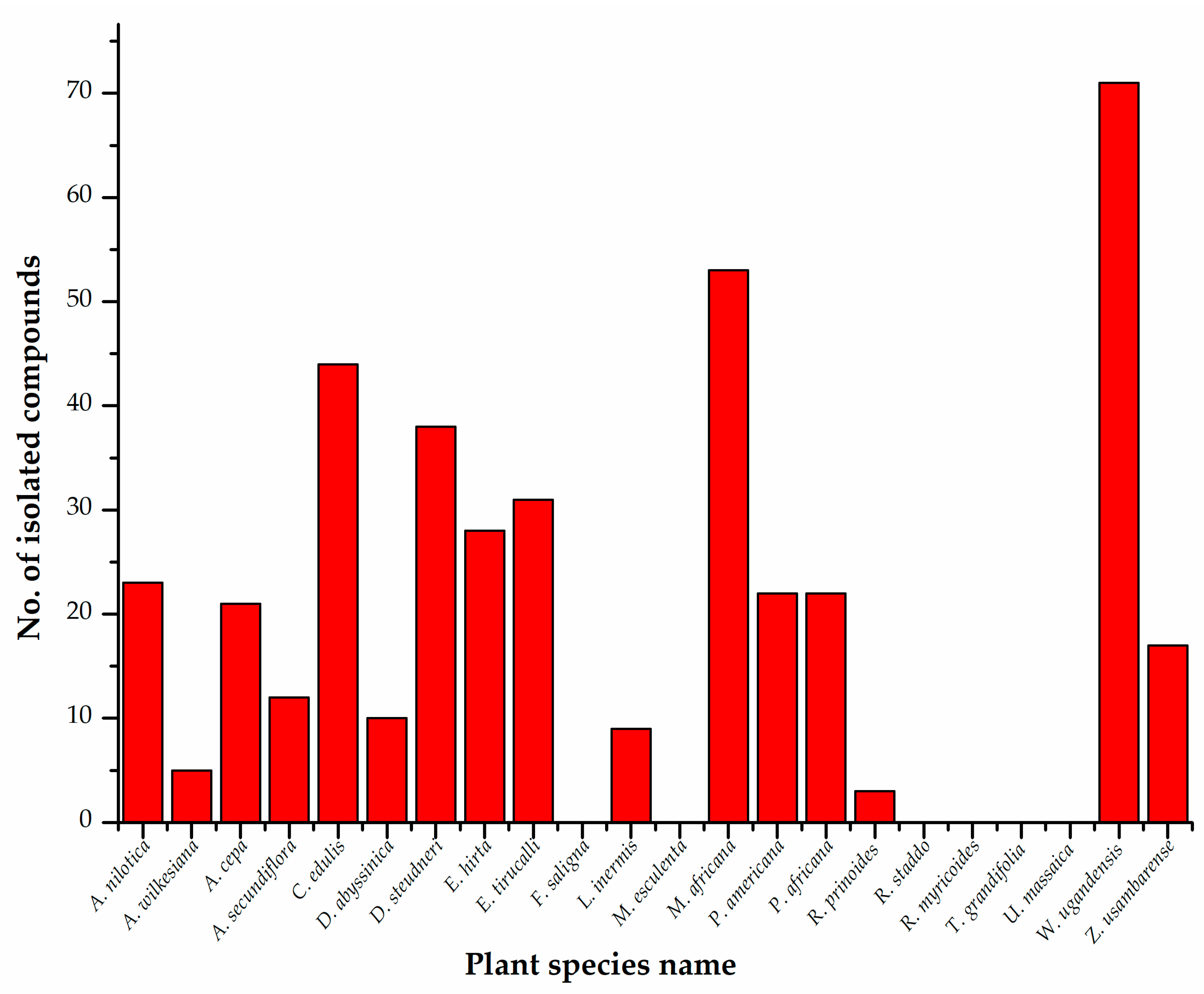
4. Phytochemistry
4.1. Terpenoids
4.2. Flavonoids
4.3. Sterols
4.4. Lignans
4.5. Alkaloids
4.6. Others
| Plant Species and Family | Countries in Which Samples Were Collected for Phytoconstituent Isolation | Plant Parts Used for Extraction | Isolated Chemical Compounds |
|---|---|---|---|
| Acacia nilotica (L.) Willd. ex Delile (Mimosoideae) | India | Stem bark | Kaempferol [37], methyl gallate [85]; catechin, gallocatechin 5-O-gallate, catechin 5-O-gallate, gallic acid, 1-O-galloyl-β-D-glucose, digallic acid, 1,6-di-O-galloyl-β-D-glucose [86]; elagic acid, (-)-Epigallocatechin-7-gallate, and (-)-epigallocatechin-5,7-digallate [87]; catechin, catechin-7-O-gallate, quercetin, quercetin-3-O-β- glucopyranoside, naringenin, naringenin-7-O-β-gluco-pyranoside, chalconaringenin-4′-O-β-glucopyronoside [86]; niloticane [88]; acanilols A and B, lupenone [89,90]. |
| Acalypha wilkesiana Müll.Arg. (Euphorbiaceae) | Nigeria | Leaves, stems, root bark | Rutin, gallic acid [34,91]; corilagin, graraniin, kaempferol 3-O-rutinoside [92]. |
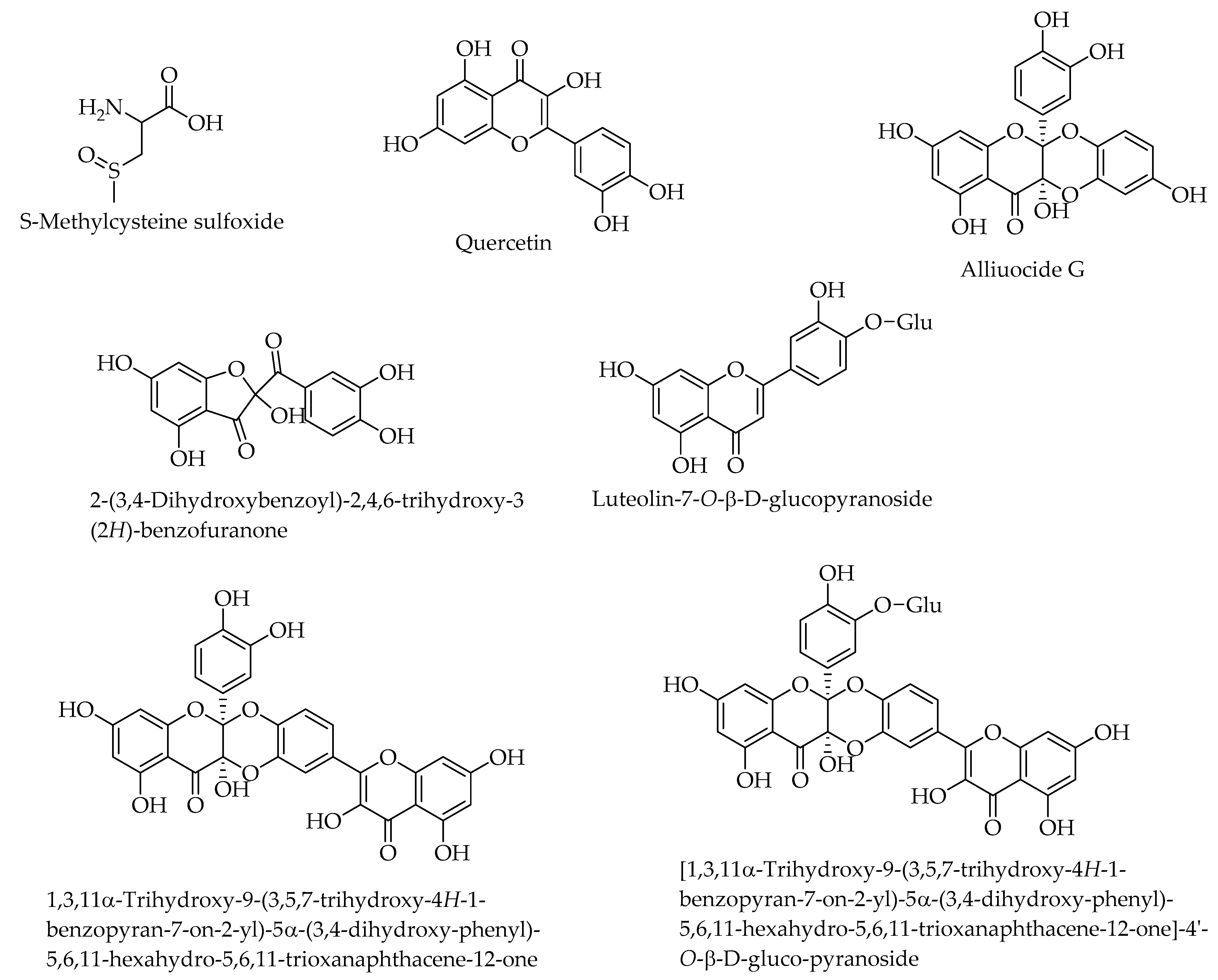
| Allium cepa L. (Amaryllidaceae) | India, Egypt | Whole plant | S-Methylcysteine sulfoxide [93]; alliuocide G, 2-(3,4-Dihydroxybenzoyl)-2,4,6-trihydroxy-3 (2H)-benzofuranone, luteolin-7-O-D-glucopyranoside, quercetin, [1,3,11α-Trihydroxy-9-(3,5,7-trihydroxy-4H-1-benzopyran-7-on-2-yl)-5α-(3,4-dihydroxy-phenyl)-5,6,11-hexahydro-5,6,11-trioxanaphthacene-12-one]-4′-O-D-gluco-pyranoside, 1,3,11α-Trihydroxy-9-(3,5,7-trihydroxy-4H-1-benzopyran-7-on-2-yl)-5α-(3,4-dihydroxy-phenyl)-5,6,11-hexahydro-5,6,11-trioxanaphthacene-12-one [94]; peonidin 3′-glucoside, petunidin 3′-glucoside acetate, petunidin 3′-glucoside acetate, quercetin 3,4-diglucoside, cyanidin 3,40-di-O-β-glucopyranoside, isorhamnetin 3,40 diglucoside, quercetin 7-glucoside, cyanidin 40-O- beta-glucoside, malvidin 3′-glucoside, quercetin-3- monoglucoside, isoalliin, methiin, alliin, N-(gamma-glutamyl)-S-methyl-L-cysteine [95]. |
| Aloe secundiflora Engl. (Asphodelaceae) | Kenya | Roots | 5-Hydroxy-3,6-dimethoxy-2-methylnaphthalene-1,4-dione, laccaic acid D, 3-methoxy-2-methylnaphthalene-1,4-dione, aloesaponols I and II, chrysophanol, ancistroquinone C, helminthosporin, aloesaponarins I and II, soxanthorin, asphodelin [96]. |
| Carissa edulis (Forssk.) Vahl (Apocynaceae) | Ghana, Nigeria, Kenya | Roots, fruits, leaves | Hydroxyacetophenone, catalponol, carisson, vanillin, coniferaldehyde, (-)-Nertrachelogenin, scopoletin, isofraxidin, (+)-Lariciresinol, carissanol, carinol [97]; lupeol, oleuropein, carissol [98]; 3-O-acetyl chlorogenic acid, kaempferol 3-O-β-D-glucopyranoside, quercetin-3-O-β-D glucopyranoside, rhamnetin-3-O-β-D glucopyranoside, isorhamnetin-3-O-β-D-glucopyranoside, (+) butyl-O-a-L-rhamnoside [99]; peonidin-3-rutinoside, malvidin-3-O-β-D-(6″-acetylglucoside) [100]; carissaedulosides A-J, [(1S,2S,3S)-1,2,3,4-tetrahydro-3,7-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-6-methoxy-2-naphthalen-yl] methyl β-D-glucopyranoside, sarhamnoloside, (-)-lyoniresinol 9-O-β-D-glucopyranoside, khaephuoside A, (-)-lyoniresinol 9′-O-D-glucopyranoside, scopoletin, guaiacylglycerol, (+)-1-acetoxypinoresinol 4′-β-D-glucoside, acetoxypinoresinol-4′-β-D-glucoside 4″-O-methyl ether, 1-(1-hydroxyethyl)-2-(6-(1-hydroxyethyl)phenoxy)benzene, markhamioside F, 3,4-dimethoxyphenyl-2-O-β-D-apiofuranosyl-(1→2)-β-D-glucopyranoside [81]. |
| Dovyalis abyssinica (A.Rich.) Warb. (Salicaceae) | Kenya | Leaves, twigs, roots | Dovyalicins A, B and E, N-(4-benzoylaminobutyl)-N-(3-dimethylaminopropyl)-3-phenylpropenamide, methyl 1-hydroxy-6-oxocyclohex-2-enecarboxylate, 4-hydroxy-2-(1-hydroxy-6-oxocyclohex-2-enecarbonyloxymethyl)phenyl 2-O-benzoyl-α-D-glucopyranoside, trans-2-{3-O-Acetyl-4-O-[(E)-4-hydroxycinnamoyl-α-D-glucopyranosyloxy}cyclohexanol [84]; benzoic acid, tremulacin, betulinic acid [101]. |
| Dracaena steudneri Schweinf. Ex. Engl. (Asparagaceae) | Kenya | Leaves, fruits | Dihydrooroxylin A, 7-hydroxy-6-methoxyflavanone, 4′,5,7-trihydroxy-6-methylflavanone, 4′-O-methylquercetin, 3,3′-di-O-methylquercetin, kaempferol-3-methyl ether, jaceidin, 7-hydroxy-6-methoxyflavone, 6,8-dimethylchrysin, strobochrysin, 3,5,7-trihydroxy-6-methylflavanone, 3,5,7-trihydroxy-6-methoxyflavanone, 3,7-dihydroxy-6-methoxyflavanone, 3,5,7-trihydroxy-6-methyl-3′,4′-methylenedioxyflavone, 5,7-dihydroxy-3-methoxy-6-methyl-3′,4′-methylenedioxyflavone, 3,5,7-trihydroxy-6-methoxy-3′,4′-methylenedioxyflavone, (2S,3S)-3,7-dihydroxy-6-methoxy-3′,4′-methylenedioxyflavanone, 4′,5,7-trihydroxy-3,3′,8-trimethoxy-6-methylflavone, (2R) 7-hydroxy-2′,8-dimethoxyflavanone [45]; isorhamnetin 3-O-rungioside, kaempferol 3-O-rungioside, quercetin-3-O-β-D-glucoside, isorhamnetin 3-O-β-D-glucopyranoside, 3,3′ -Di-O-methylquercetin 4′-O-β-D-glucoside, quercetin, 3,3′ -Di-O-methyl quercetin, 4-(2ʹ-Formyl-1ʹ-pyrrolyl)butanoic acid [73]. 20,40-dihydroxy-2,30-dimethoxychalcone, kaempferol, 8-(C)-methylquercetagetin-3,6,30-trimethyl ether, alliospiroside A, methylgalangine, 6,8-dimethylchrysin, oleanolic acid, ombuine-3-O-rutinoside (4′,7-dimethylquercetin-3-O-α-L-rhamnopyranosyl-(1-6)-β-D-glucopyranoside), β-sitosterol 3-O-glucopyranoside, betulinic acid, ishigoside, and lupeol [102]. |
| Euphorbia hirta L. (Euphorbiaceae) | China, India | Leaves, stems, latex | Kaempferol, afzelin, quercitrin, and myricitrin [46]; quercetin, quercetin-rhamoside, rutin [103]; isolintetralin, virgatusin, virgatusin 16, urinaligran, phyllanthin, niranthin, 5-demothoxyniranthin, lintetralin, phyltetralin, 7-hydroxy-hinokinin, 5-methoxyursehernin, hypophyllanthin, neonirtetralin, euphorhirtins A-D, 5-methoxyvirgatusin, 7S-ethoxyisolintetralin, 7R-ethoxyisolintetralin, 7R-ethoxy-3-methoxyisolintetralin [80]. |
| Euphorbia tirucalli L. (Euphorbiaceae) | China | Leaves, stem bark, whole plant, roots | Gallic acid, dihydroxybenzoic acid, 4-O-methylgallic acid, ampelopsin, isoquercetin, rutin, ellagic acid, myricetin, avicularin, quercitrin, tricetin, tricetin, 3,3′-dimethoxy-4-O-α-rhamnopyranoside-ellagic acid, quercetin [72]; tirucadalenone, euphorol L, M, N, euphorol D, euphol, lupanone, ergosterol peroxide, vomifoliol, scopoletin, aloe-emodin [104]; 3-O-(2,4,68-Tetradecatetraenoyl) ingenol, 13-O-acetyl-12-O-(2Z,4E-Octadienoyl)-4β-deoxyphorbol, pedilstatin, 4β-Deoxy-phorbol-13-acetate, 4α-deoxy-phorbol-13-acetate, 12-O-(2E,4E,6E,8E-tetradecatetraenoyl)-13-O-isobutyroyl-4β-deoxyphorbol [67]. |
| Faurea saligna Harv. (Proteaceae) | NA | NA | NA |
| Lactuca inermis Forssk (Asteraceae) | Poland | Roots, aerial parts | Scopolin, isofraxoside, 4-hydroxyphenylacetic acid, syringic acid, 9a-hydroxyzaluzanin C, 11b,13-dihydroderivative, ixerin F, 11b,13-dihydroglucozaluzanin C, α-xylofuranosyluracil [105]. |
| Manihot esculenta Crantz (Euphorbiaceae) | NA | NA | NA |
| Myrsine africana L. (Primulaceae) | Kenya, China, Pakistan, South Africa | Leaves, stems | Nepodin, emodin, 5-methoxy-7-hydroxyphthalide, 2-hydroxychrysophanol [106]; myricetin-3-rhamnoside, Myricetin 3-(3″,4″-diacetylrhamnoside), myricetin 7-rharnnoside, gallic acid, myritin 3-xyloside, myricetin, myricetin 3-arabinoside, 3′-O-methylquercetin 3-glucoside, quercetin 3-galactoside, quercetin, kaempferol [107]; Myrsinone, embelin, 5-O-methylembelin, methylvilangin, methylanhydrovilangin [108]; taraxerone, taraxerol, myricadiol, stigmasterol 3-O-β-D-glucoside, a-spinasterol 3-O-β-D-glucoside [68]; muketanin [109]; myricetin 3- galactoside [110]; mearnsetin 3-(2″,4″-diacetylrhamnoside), quercitrin, myricitrin, mearnsitrin, myricetin-3-O-(4″-O-acetyl)-a-L-rhamnopyranoside, mearnsetin-3-O-(4″-O-acetyl)-α-L-rhamnopyranoside, (-)-epicatechin, (-)-epigallocatechin, (-)-epigallocatechin-3-O-gallate, 3′,5′-di-C-β-glucopyranosyl phloretin [111]; Myrsininones A and B [50]; Myrsigenin [112]; (3b,16a,20a)-3,16,28-trihydroxyolean-12-en-29-oic acid 3-{O-β-D-glucopyranosyl-(1-2)-O-[b-d-glucopyranosyl-(1-4)]-a-l-arabinopyranoside}, isolariciresinol 9′-β-D-xylopyranoside, isolariciresinol 9′-β-D-glucopyranoside, lyoniresinol 9′-β-D-glucopyranoside [113]; myricetin 3-O-(2″,4″-di-O-acetyl)-α-L-rhamnopyranoside, mearnsetin 3-O-(4″-Oacetyl)-α-L-rhamnopyranoside, mearnsitrin, myricetin 3-O-(4″-O-acetyl)-α-L-rhamnopyranoside, quercetin 3-O-(3″,4″-di-O-acetyl)-α-L-rhamnoside, rutin, quercetin 3-O-α-L-rhamnopyranoside, myricetin 3-O-α-L-rhamnopyranoside [114]; myrsinane [51]. |
| Persea americana Mill. (Lauraceae) | Brazil, Taiwan, Nigeria | Leaves, seeds, fruits | Kaempferol 3-O-α-D-arabinopyranoside, quercetin 3-O-α-D-arabinopyranoside, afzelin, quercitrin, quercetin 3-O-β-glucopyranoside, quercetin [52]; 1,2R-diacetoxy-4R-hydroxy-n-heptadeca-16-ene, 2R,4R-Diacetoxy-1-hydroxy-n-heptadeca-16-ene, 1,2R-diacetoxy-4R-hydroxy-n-heptadeca-16-yne, 2R,4R-diacetoxy-1-hydroxy-n-heptadeca-16-yne, 1-acetoxy-2R,4R-dihydroxy-n-heptadec-16-ene, 4-acetoxy-1R,2R-dihydroxy-n-heptadec-16-ene, 1-acetoxy-2R,4R-dihydroxy-n-heptadec-16-yne, 1,2R,4R-trihydroxy-n-heptadec-16-yne, 1,4R-diacetoxy-2R-hydroxy-n-heptadeca-16-ene, 1,4R-diacetoxy-2R-hydroxy-n-heptadec-16-yne [115]; isorhamnetin, luteolin, rutin, quercetin, apigenin [116]. |
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | Switzerland, Ethiopia, South Africa | Stem bark, leaves | 2α,3α-dihydroxyurs-12-en-28-oic acid, 2a,3fl-dihydroxyurs-12-en-28-oic acid, 2α,3β-dihydroxyolean-12-en-28-oic acid, 3β,24-dihydroxyurs- 12-en-28-oic acid, 2α,3α,23-trihydroxyurs-12-en-28-oic acid, 2α.3α,24-trihydroxyurs-12-en-28-oic acid, 24-O-trans-ferulyl-3flhydroxyurs-12-en-28-oic acid, 24-O-cis-feruly-3β-hydroxy-urs-12-en-28-oic acid, 24-O-trans-ferulyl-2a,3a-dihydroxy-urs-12-en-28-oic acid [117]; friedelin, ursolic acid, maslinic acid, 2 α-hydroxyursolic acid, epimaslinic acid [118]; β-sitosterol, p-hydroxybenzoic acid, oleanoic acid-3-benzoate, oleanoic acid-22-benzoate, benzoic acid [119]; β-sitosterol, β-amyrin, β-sitosterol-3-O-glucoside [120]. |
| Rhamnus prinoides L’Hér. (Rhamnaceae) | Ethiopia | Leaves, stems, roots | Emodin, physcion, emodinanthrone, muszin, rhamnocitrin, rhamnazin, prinoidin, emodinbianthrone, chrysophanol, quercetin, rhamnetin [57]; glucofrangulin A, emodin glucoside B [121]. |
| Rhamnus staddo A.Rich. (Rhamnaceae) | NA | NA | NA |
| Rotheca myricoides (Hochst.) Steane and Mabb. (Lamiaceae) | NA | NA | NA |
| Trimeria grandifolia (Hochst.) Warb. (Salicaceae) | NA | NA | NA |
| Urtica massaica Mildbr (Urticaceae) | NA | NA | NA |
| Warburgia ugandensis Sprague (Canellaceae) | Kenya, Uganda, Ethiopia | Leaves, stem bark | Kaempferide 3-O-bxylosyl (1-2)-b-glucoside, kaempferol 3-O-α-rhamnoside-7,4′-di-O-β-galactoside, kaempferol 3,7,4′-tri-O-β-glucoside, quercetin 3-O-[β-glucosyl (1-2)[α-rhamnosyl (1-6)]-β-glucoside-7-O-a-rhamnoside, quercetin, myricetin, kaempferol, kaempferol 3-rhamnoside, kaempferol 3-arabinoside, quercetin 3-rhamnoside, quercetin 3-glucoside, kaempferol 3-rhamnoside-4′-galactoside, kaempferol 3-rutinoside, myricetin 3-galactoside, kaempferol 3-glucoside [122]; ugandenial A, 11α-hydroxycinnamosmolide, polygodial, mukaadial, dendocarbin A, 9α-hydroxycinnamolide, dendocarbin L, dendocarbin M [69]; 7α-acetylugandensolide, bemadienolide, drimenin, polygodial, warburganal, ugandensidial, 6a-Hydroxymuzigadial, 9-deoxymuzigadial, ugandensolide, deacetoxyugandensolide, cinnamolide, 3β-acetoxycinnamolide [61]; muzigadial, muzigadiolide, cinnamolide-3b-acetate, linoleic acid [123]; polygodial, deacetylugandensolide [124]; nerolidol, warburgin, warburgiadione, pereniporin B, cinnamolide, cinnamolide-3 β-acetate, dendocarbin A, 9 α,11 α-dihydroxy, 6 β-acetyl-cinnamolide, dendocarbin L, 9 α-hydroxycinnamolide, 4(13),7-coloratadien-12,11-olide, 6 α,9 α-dihydroxy-4(13)-7- coloratadien-11,12-dial, 7 β-hydroxy-4(13)-8-coloratadien-11,12-olide, 7 α-hydroxy-8-drimen-11,12-olide, cinnamolide-3 β-ol, deacetylugandensolide, 11 α–hydroxymuzigadiolide [125]; N-cis-grossamide, N-trans-grossamide, 7-hydroxywinterin, 11α-hydroxycinnamosmolide, polygonal acid [126]; 2-[3-[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]-4,5-dihydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one, 2-[3-[2-O-(6-deoxy-α-L-mannopyranosyl)-β-Dglucopyranosyl]-4-hydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one, 4-[(6′-O-β-D-allopyranosyl)-oxy]-hydroxy-benzoicacid cyclic dimeric inner ester, N-trans-caffeoyltyramine, 1-(3,4-dihydroxy-5-methoxyphenyl)-1,2-dihydroxy-7,8-dihydroxy-N-[(3,4-dihydroxyphenyl)ethyl]-N′-[(4-hydroxyphenyl)ethyl]-6-methoxynaphthalene-2,3-dicarboxamide, 1-(3,4-dihydroxy-5-methoxyphenyl)-1,2-dihydroxy-7,8-dihydroxy-N-[(4-hydroxyphenyl)ethyl]-N′-[(4-hydroxyphenyl)ethyl]-6-methoxynaphthalene-2,3-dicarboxamide, 1-(3,4-dihydroxy-5-methoxyphenyl)-1,2-dihydroxy-7,8-dihydroxy-N,N′-bis-[2-(4-hydroxyphenyl)ethyl]-6-methoxynaphthalene-2,3-dicarboxamide, 1-(3,4-dihydroxy-5-methoxyphenyl)-1,2-dihydroxy-6,7-dihydroxy-N,N′-bis-[2-(4-hydroxyphenyl)ethyl]-8-methoxynaphthalene-2,3-dicarboxamide [127]. |
| Zanthoxylum usambarense (Engl.) (Rutaceae) | Kenya | Stems, roots | Usambanoline, (+)-tembetarine, (+)-magnoflorine, (-)-edulinine, (+)-N-methylplatydesmine, (-)-oblongine, (-)-usambarine, jatrorrhizine, (-)-cis-N-methylcanadine, nitidine, chelerythrine [64,128]; canthin-6-one, oxychelerythrine, norchelerythrine, pellitorine, (+)-2,6-bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo[3.3.0]octane ((+)-sesamin, (+)-Piperitol-3,3-dimethylallyl ether [83]. |
5. Antidiabetic Activity of the Selected Medicinal Plants
5.1. In Vitro Antidiabetic Activity of Crude Extracts
5.2. Antidiabetic Activity of Isolated Compounds
| Plant Species | Crude Extracts Tested | Isolated Compounds Tested | Antidiabetic Activities |
|---|---|---|---|
| Acacia nilotica | Ethyl acetate, n-butanol and aqueous extracts of the bark, methanol extract of pods and leaves. | NT | Hypoglycemic and antihyperglycemic effects [139]. |
| Acalypha wilkesiana | Ethyl acetate and ethanol extracts of leaves, stem and root barks. | NT | Inhibition of pancreatic α-amylase [33]. |
| Allium cepa L. | Aqueous and dichloromethane extracts of whole plant. | S-Methylcysteine sulfoxide, quercetin, alliuocide G, 2-(3,4-Dihydroxybenzoyl)-2,4,6-trihydroxy-3 (2H)-benzofuranone, luteolin-7-O-D-glucopyranoside,, [1,3,11α-trihydroxy-9-(3,5,7-trihydroxy-4H-1-benzopyran-7-on-2-yl)-5α-(3,4-dihydroxy-phenyl)-5,6,11-hexahydro-5,6,11-trioxanaphthacene-12-one]-4′-O-D-gluco-pyranoside, 1,3,11α-trihydroxy-9-(3,5,7-trihydroxy-4H-1-benzopyran-7-on-2-yl)-5α-(3,4-dihydroxy-phenyl)-5,6,11-hexahydro-5,6,11-trioxanaphthacene-12-one. | Antihyperglycemic [140], hypoglycemic, and hypolipidemic effects [133,141]. Inhbited clinical hypoglycemic activity in type 1 and type 2 diabetic patients [142]; Quercetin increased insulin levels and reduced blood sugar levels in streptozotocin-induced diabetic rats [141,143]. Alliuocide G, 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3 (2H)-benzofuranone, luteolin-7-O-D-glucopyranoside, quercetin, 1,3,11α-Trihydroxy-9-(3,5,7-trihydroxy-4H-1-benzopyran-7-on-2-yl)-5α-(3,4-dihydroxy-phenyl)-5,6,11-hexahydro-5,6,11-trioxanaphthacene-12-one-4′-O-D-gluco-pyranoside inhibition of α-amylase [94]. Allium cepa extracts inhibited α-glucosidase [144,145]. |
| Aloe secundiflora | Aqueous extract of stem bark. | Aqueous stem bark extracts exhibited in vivo anti-hyperglycemic activity [146]. | |
| Carissa edulis | Ethanolic extract of leaves and methanolic extract of fruits. | Leaf extract exhibited hypoglycemic activity in streptozotocin-induced diabetic rats [147]. α-glucosidase inhibition by the fruit extracts [100]. | |
| Dovyalis abyssinica | NT | NT | NT |
| Dracaena steudneri | NT | NT | NT |
| Euphorbia hirta | Methanolic extract of whole plant. | NT | Methanolic extract inhibited α-glucosidase [2]. |
| Euphorbia tirucalli | Aqueous stem extracts. | NT | α-glucosidase and lipase enzymes inhibitory activity [129]. |
| Faurea saligna | NT | NT | NT |
| Lactuca inermis | NT | NT | NT |
| Manihot esculenta | Ethanol and acetone leaves extracts. | NT | Inhibition of α-glucosidase and α-amylase [49,130]. |
| Myrsine africana L. | Methanolic leaves extract. | NT | Leaf extract reduced the levels of blood sugar in diabetes-induced albino rats [148], decreased levels of blood glucose, total cholesterol, glucose-6-phosphatase, glycated hemoglobin, fructose-1-6-bisphosphatase, and triglyceride, and increased levels of HDL cholesterol, insulin, and hexokinase [148]. |
| Persea americana Mill. | Ethanolic and aqueous extracts of leaves, seeds, fruits. | NT | Leafs extract lowered blood sugar levels and improved the metabolism of diabetic rats through the regulation of glucose uptake in the liver and muscles by activating PKB/Akt and reestablishing the equilibrium of intracellular energy [134,135]. Aqueous extracts of leaves and seeds exhibited hypoglycemic effects [53,149,150]. Inhibition of α-amylase and α-glucosidase, hence lowering of post-prandial hyperglycemia [151]. |
| Prunus africana | Aqueous and ethanolic stem bark extracts. | NT | Hypoglycemic effect against diabetic rats [152]. The extracts reduced the dipeptidyl peptidase-4 (DPP-4) enzyme which activates glucagon-like peptides (GLP-1) leading to insulin production in the body, hence controlling body glucose levels [153]. |
| Rhamnus prinoides | NT | NT | NT |
| Rhamnus staddo (Rhamnaceae) | Aqueous extracts of leaves. | NT | Aqueous extract exhibited a hypolipidemic effect [58]. |
| Rotheca myricoides | Aqueous extract of whole plant. | NT | Extracts of R. myricoides exhibited antihyperglycemic and antidyslipidemic effects in diabetic rats [59]. |
| Trimeria grandifolia | NT | NT | NT |
| Urtica massaica | NT | NT | NT |
| Warburgia ugandensis | NT | NT | NT |
| Zanthoxylum usambarense | NT | NT | NT |
5.3. In Silico Antidiabetic Activity of Compounds
5.4. Toxicology Study of Crude Extracts
5.5. Clinical Studies
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mathivanan, K.; Rengasamy, D.; Rajesh, V.; Palani, R.; Jayaraman, P. Phytochemical potential of Euphorbia hirta Linn. And strychnos nux-vomica Linn. With reference to antidiabetic and antioxidant properties. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 1024–1031. [Google Scholar]
- Shilpa, V.; Lekshmi, S.; Swapna, T. In vitro antidiabetic potential of Euphorbia hirta Linn.: A nutritionally significant plant. J. Pharmacogn. Phytochem. 2020, 9, 01–04. [Google Scholar]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, L.; Tang, Y.; Zhao, Z.; Yi, T.; Chen, H. Preparation-related structural diversity and medical potential in the treatment of diabetes mellitus with ginseng pectins. Ann. N. Y. Acad. Sci. 2017, 1401, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, A.K.; Maurya, C.K.; Rai, A.K. PTP1B inhibitors for type 2 diabetes treatment: A patent review (2011–2014). Expert. Opin. Ther. Pat. 2014, 24, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Mohler, M.L.; He, Y.; Wu, Z.; Hwang, D.J.; Miller, D.D. Recent and emerging anti-diabetes targets. Med. Res. Rev. 2009, 29, 125–195. [Google Scholar] [CrossRef]
- Adeshara, K.A.; Diwan, A.G.; Tupe, R.S. Diabetes and complications: Cellular signaling pathways, current understanding and targeted therapies. Curr. Drug Targets 2016, 17, 1309–1328. [Google Scholar] [CrossRef]
- Atlas, D. International diabetes federation. In IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Volume 33, p. 2. [Google Scholar]
- Romero-Daza, N. Traditional Medicine in Africa. Ann. Am. Acad. Political Soc. Sci. 2002, 583, 173–176. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Heo, C.U.; Choi, C.-I. Current progress in pharmacogenetics of second-line antidiabetic medications: Towards precision medicine for type 2 diabetes. J. Clin. Med. 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Olefsky, J.M. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat. Rev. Drug Discov. 2016, 15, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, L.; Huang, X.; Liang, Y.; Wang, X.; Wu, H. Lupenone is a good anti-inflammatory compound based on the network pharmacology. Mol. Divers. 2020, 24, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.W.; Zeng, W.; Niu, Y.; Coghi, P.; Wu, Y.; Sin, W.M.; Ng, S.I.; Gordillo-Martínez, F.; Gao, J.Y.; Law, B.Y. A method for rapid screening of anilide-containing AMPK modulators based on computational docking and biological validation. Front. Pharmacol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Moharram, F.A.-e.; Marzouk, M.S.; El-Shenawy, S.M.; Gaara, A.H.; El Kady, W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012, 64, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W.; Ling, N.; Issa, S.M.; Dite, T.A.; O’Brien, M.T.; Chen, Z.-P.; Galic, S.; Langendorf, C.G.; Steinberg, G.R.; Kemp, B.E. Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem. Biol. 2014, 21, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Li, J.-Y.; Luo, C.; Li, J.; Chen, K.-X.; Li, X.-W.; Guo, Y.-W. Function-oriented synthesis of marine phidianidine derivatives as potential PTP1B inhibitors with specific selectivity. Mar. Drugs 2018, 16, 97. [Google Scholar] [CrossRef]
- Krishnan, N.; Konidaris, K.F.; Gasser, G.; Tonks, N.K. A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem. 2018, 293, 1517–1525. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, M.; He, Y.; Wang, W.; Liu, S. Recent advances in the development of protein tyrosine phosphatase 1B inhibitors for Type 2 diabetes. Future Med. Chem. 2016, 8, 1239–1258. [Google Scholar] [CrossRef]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
- Wang, Q.; Umar Imam, M.; Yida, Z.; Wang, F. Peroxisome proliferator-activated receptor gamma (PPARγ) as a target for concurrent management of diabetes and obesity-related cancer. Curr. Pharm. 2017, 23, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, I.; Candeias, E.; Santos, M.S.; de Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Insulin as a bridge between type 2 diabetes and Alzheimer disease—How anti-diabetics could be a solution for dementia. Front. Endocrinol. 2014, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. Blood Glucose Levels 2018, 1, 1–18. [Google Scholar]
- Ren, Y.; Li, L.; Wan, L.; Huang, Y.; Cao, S. Glucokinase as an emerging anti-diabetes target and recent progress in the development of its agonists. J. Enzym. Inhib. Med. Chem. 2022, 37, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, B.; Singh, K.; Kumar, A. Ethno-medicinal, phytochemical and antimicrobial studies of Euphorbia tirucalli L. J. Phytol. 2010, 2, 65–77. [Google Scholar]
- Zaghlol, A.; Kandil, Z.; Yousif, M.; Salah El Dine, R.; Elkady, W. Phytochemical Analysis of Euphorbia greenwayi Aerial Parts: Antioxidant and Anti-inflammatory Potential. Egypt. J. Chem. 2023. [Google Scholar] [CrossRef]
- Priya, C.L.; Rao, K.V.B. A Review o phytochemical ad pharmacological profile of Euphorbia tirucalli. Pharmacologyonline 2011, 2, 384–390. [Google Scholar]
- Lolok, N.; Mashar, H.M.; Annah, I.; Saleh, A.; Yuliastri, W.O.; Isrul, M. Antidiabetic effect of the combination of garlic peel extract (Allium sativum) and onion peel (Allium cepa) in rats with oral-glucose tolerance method. Res. J. Pharm. Technol. 2019, 12, 2153–2156. [Google Scholar] [CrossRef]
- Oyebode, O.A.; Erukainure, O.L.; Koorbanally, N.A.; Islam, M.S. Acalypha wilkesiana ‘Java White’: Identification of Some Bioactive Compounds by Gc-Ms and Their Effects on Key Enzymes Linked to Type 2 Diabete. Acta Pharm. 2018, 68, 425–439. [Google Scholar] [CrossRef] [PubMed]
- El-Khateeb, A.Y.; Azzaz, N.A.-K.E.; Mahmoud, H.I. Phytochemical constituents, hypoglycemic and haematological effects of methanolic Acalypha wilkesiana leaves extract on streptozotocin-induced diabetic rats. Eur. J. Chem. 2014, 5, 430–438. [Google Scholar] [CrossRef]
- Ajayi, E.; Modo, E.; Adebamowo, A.; Banerjee, U.; Tewe, O.; Olorunsogo, O. Inhibitory activity of ethanol extract of Manihot esculenta on mitochondrial membrane permeability transition pore and caspase 3 in type 2 diabetes mellitus. Int. J. Biochem. Res. Rev. 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, E.I.O.; Modo, E.U.; Kiakubu, O.T.; Molehin, O.R. Chapter 35—Diabetes Care and Wound Healing Using Nauclea latifolia, Manihot esculenta, and Other Natural Products. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 545–558. [Google Scholar]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex. Del. Toxicol. Vitr. 2008, 22, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Zaman, S.; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plant Res. 2012, 6, 1492–1496. [Google Scholar]
- Galavi, A.; Hosseinzadeh, H.; Razavi, B.M. The effects of Allium cepa L.(onion) and its active constituents on metabolic syndrome: A review. Iran. J. Basic. Med. Sci. 2021, 24, 3. [Google Scholar]
- Rebecca, W.; Kayser, O.; Hagels, H.; Zessin, K.H.; Madundo, M.; Gamba, N. The phytochemical profile and identification of main phenolic compounds from the leaf exudate of Aloe secundiflora by high-performance liquid chromatography–mass spectroscopy. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2003, 14, 83–86. [Google Scholar] [CrossRef]
- Kamau, L.N.; Mbaabu, P.M.; Karuri, P.G.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in the management of diabetes by traditional healers of Narok County, Kenya. Cellmed 2017, 7, 10. [Google Scholar] [CrossRef]
- Omino, E.; Kokwaro, J. Ethnobotany of Apocynaceae species in Kenya. J. Ethnopharmacol. 1993, 40, 167–180. [Google Scholar] [CrossRef]
- Ngulde, S.I.; Sandabe, U.K.; Tijjani, M.B.; Barkindo, A.A.; Hussaini, I.M. Phytochemical constituents, antimicrobial screening and acute toxicity studies of the ethanol extract of Carissa edulis Vahl. root bark in rats and mice. Am. J. Res. Commun. 2013, 1, 99–110. [Google Scholar]
- Legesse, B.A.; Tamir, A.; Bezabeh, B. Phytochemical screening and antibacterial activity of leaf extracts of Dovyalis abyssinica. J. Emerg. Technol. Innov. Res. 2019, 6, 453–465. [Google Scholar]
- Nchiozem-Ngnitedem, V.-A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Spiteller, M. Inhibition of Proinflammatory Cytokine Release by Flavones and Flavanones from the Leaves of Dracaena steudneri Engl. Planta Med. 2021, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.N.; Sati, S.C.; Kumar, P. Euphorbia hirta Linn-an invasive plant: A review of its traditional uses, phytochemistry and pharmacological properties. System 2021, 17, 22. [Google Scholar]
- Gupta, N.; Vishnoi, G.; Wal, A.; Wal, P. Medicinal Value of Euphorbia tirucalli. Syst. Rev. Pharm. 2013, 4, 40–46. [Google Scholar] [CrossRef]
- Kamau, L.N.; Mbaabu, M.P.; Mbaria, J.M.; Karuri, G.P.; Kiama, S.G. Knowledge and demand for medicinal plants used in the treatment and management of diabetes in Nyeri County, Kenya. J. Ethnopharmacol. 2016, 189, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Laya, A.; Koubala, B.B.; Negi, P.S. Antidiabetic (α-amylase and α-glucosidase) and anti-obesity (lipase) inhibitory activities of edible cassava (Manihot esculenta Crantz) as measured by in vitro gastrointestinal digestion: Effects of phenolics and harvested time. Int. J. Food Prop. 2022, 25, 492–508. [Google Scholar] [CrossRef]
- Kang, L.; Zhou, J.X.; Shen, Z.W. Two novel antibacterial flavonoids from Myrsine africana L. Chin. J. Chem. 2007, 25, 1323–1325. [Google Scholar] [CrossRef]
- Ahmad, B.; Azam, S.; Bashir, S.; Adhikari, A.; Hussain, F. Anti-inflammatory activity and a new compound isolated from aerial parts of Myrsine africana. Afr. J. Biotechnol. 2011, 10, 8465–8470. [Google Scholar]
- De Almeida, A.; Miranda, M.; Simoni, I.; Wigg, M.; Lagrota, M.; Costa, S. Flavonol monoglycosides isolated from the antiviral fractions of Persea americana (Lauraceae) leaf infusion. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1998, 12, 562–567. [Google Scholar]
- Yasir, M.; Das, S.; Kharya, M. The phytochemical and pharmacological profile of Persea americana Mill. Pharmacogn. Rev. 2010, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Ngule, M.C.; Ndiku, M.H.; Ramesh, F. Chemical constituents screening and in vitro antibacterial assessment of Prunus africana bark hydromethanolic extract. Extraction 2014, 4, 85–90. [Google Scholar]
- Mutuma, G.G.; Joseph, N.; King’ori, M.A.; Silas, K. Phytochemical and anti-inflammatory analysis of Prunus africana bark extract. Res. J. Pharmacogn. 2020, 7, 31–38. [Google Scholar]
- Nyamai, D.; Mawia, A.; Wambua, F.; Njoroge, A.; Matheri, F.; Lagat, R.; Burugu, M. Phytochemical profile of Prunus africana stem bark from Kenya. J. Pharmacogn. Nat. Prod. 2015, 1, 8. [Google Scholar]
- Nigussie, G.; Alemu, M.; Ibrahim, F.; Werede, Y.; Tegegn, M.; Neway, S.; ANNİSA, M.E. Phytochemistry, ethnomedicinal uses and pharmacological properties of Rhamnus prinoides: A review. Int. J. Second. Metab. 2021, 8, 136–151. [Google Scholar] [CrossRef]
- Kaidama, W.M.A.; Aqlan, E.M.; El-Sayed, A.I.M. The Hypolipidemic Effect of Aqueous Extract of Crepis rueppellii and Rhamnus staddo on Acetaminophen-Induced Hepatotoxicity of Guinea Pigs. Ann. Agric. Sci. 2023, 61, 41–48. [Google Scholar]
- Chege, B.M.; Waweru, M.P.; Frederick, B.; Nyaga, N.M. The freeze-dried extracts of Rotheca myricoides (Hochst.) Steane & Mabb possess hypoglycemic, hypolipidemic and hypoinsulinemic on type 2 diabetes rat model. J. Ethnopharmacol. 2019, 244, 112077. [Google Scholar] [PubMed]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Opiyo, S.A.; Manguro, L.O.; Okinda-Owuor, P.; Ateka, E.M.; Lemmen, P. 7α-Acetylugandensolide and antimicrobial properties of Warburgia ugandensis extracts and isolates against sweet potato pathogens. Phytochem. Lett. 2011, 4, 161–165. [Google Scholar] [CrossRef]
- Kitte, R.; Tretbar, M.; Dluczek, S.; Beckmann, L.; Marquardt, P.; Duenkel, A.; Schubert, A.; Fricke, S.; Tretbar, U.S. Chemical and Cytotoxic Activity of three main Sesquiterpenoids from Warburgia ugandensis. Results Chem. 2021, 3, 100242. [Google Scholar] [CrossRef]
- Maobe, M.A.; Gatebe, E.; Gitu, L.; Rotich, H. Preliminary phytochemical screening of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii region, southwest Kenya. Eur. J. Appl. Sci. 2013, 5, 1–6. [Google Scholar]
- Kato, A.; Moriyasu, M.; Ichimaru, M.; Nishiyama, Y.; Juma, F.D.; Nganga, J.N.; Mathenge, S.G.; Ogeto, J.O. Examination of alkaloidal constituents of Zanthoxylum usambarense by a combination of ion-pair extraction and ion-pair chromatography using sodium perchlorate. Phytochem. Anal. 1995, 6, 89–95. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Cho, D.-Y.; Ezazul Haque, M.; Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxidative Med. Cell. 2018, 2018, 1209801. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.-Z.; Tian, Y.; Zhang, J.-S.; Huang, J.-L.; Tang, G.-H.; Yin, S. A new tigliane-type diterpenoid from Euphorbia tirucalli. Nat. Prod. Res. 2022, 36, 5380–5386. [Google Scholar] [CrossRef] [PubMed]
- Manguro, L.O.A.; Midiwo, J.O.; Kraus, W. Triterpenoids and steroids of Myrsine africana leaves. Planta Med. 1997, 63, 290. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Litaudon, M.; Krief, S.; Martin, M.-T.; Kasenene, J.; Kiremire, B.; Dumontet, V.; Guéritte, F. Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis. Molecules 2009, 14, 3844–3850. [Google Scholar] [CrossRef]
- Nanjala, C.; Odago, W.O.; Rono, P.C.; Waswa, E.N.; Mutinda, E.S.; Oulo, M.A.; Muema, F.W.; Wanga, V.O.; Mkala, E.M.; Kuja, J.; et al. A review on ethnobotany, phytochemistry, and pharmacology of the genus Didymocarpus wall. (Gesneriaceae). J. Ethnopharmacol. 2022, 295, 115404. [Google Scholar] [CrossRef]
- Muema, F.W.; Liu, Y.; Zhang, Y.; Chen, G.; Guo, M. Flavonoids from Selaginella doederleinii Hieron and Their Antioxidant and Antiproliferative Activities. Antioxidants 2022, 11, 1189. [Google Scholar] [CrossRef]
- de Lima, M.d.F.R.; Cavalcante, L.A.; de Araújo Costa, E.C.T.; de Veras, B.O.; da Silva, M.V.; Cavalcanti, L.N.; Araújo, R.M. Bioactivity flavonoids from roots of Euphorbia tirucalli L. Phytochem. Lett. 2021, 41, 186–192. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.-A.; Omosa, L.K.; Derese, S.; Efferth, T.; Spiteller, M. Cytotoxic flavonoids from the seeds of Dracaena steudneri Engl against leukemia cancer cell lines. Phytomed. Plus 2022, 2, 100234. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar] [PubMed]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, S.; Zhou, M.; Yu, W.; Zhang, Y.; He, X. Phytoestrogens and risk of prostate cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2015, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Worawalai, W.; Doungwichitrkul, T.; Rangubpit, W.; Taweechat, P.; Sompornpisut, P.; Phuwapraisirisan, P. Furofuran lignans as a new series of antidiabetic agents exerting α-glucosidase inhibition and radical scarvenging: Semisynthesis, kinetic study and molecular modeling. Bioorg. Chem. 2019, 87, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Sampson, L.; Wang, M.; Manson, J.E.; Rimm, E.; Sun, Q. Lignan Intake and Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2021, 78, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.-L.; Wang, B.; Zhang, L.-T.; Gao, H.-M.; Shen, T.; Lou, H.-X.; Ren, D.-M.; Wang, X.-N. Lignans from Euphorbia hirta L. Nat. Prod. Res. 2020, 36, 26–36. [Google Scholar] [CrossRef]
- Kaunda, J.S.; Qin, X.-J.; Yang, X.-Z.; Mwitari, P.G.; Zhu, H.-T.; Wang, D.; Zhang, Y.-J. Ten new glycosides, carissaedulosides A–J from the root barks of Carissa edulis and their cytotoxicities. Bioorg. Chem. 2020, 102, 104097. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- He, W.; Puyvelde, L.V.; Kimpe, N.D.; Verbruggen, L.; Anthonissen, K.; Flaas, M.V.d.; Bosselaers, J.; Mathenge, S.G.; Mudida, F.P. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.; Nkurunziza, A.-J.; Witt, M.; Oketch-Rabah, H.A.; Jaroszewski, J.W.; Stærk, D. Dovyalicin-type spermidine alkaloids from Dovyalis species. J. Nat. Prod. 2006, 69, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gupta, A.; Mukherji, A. Invasive Acacia nilotica a problematic weed is a source of potent methyl gallate. IJSR 2014, 10, 1193–1195. [Google Scholar]
- Salem, M.M.; Davidorf, F.H.; Abdel-Rahman, M.H. In vitro anti-uveal melanoma activity of phenolic compounds from the Egyptian medicinal plant Acacia nilotica. Fitoterapia 2011, 82, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Rather, L.J.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Eldeen, I.; Van Heerden, F.; Van Staden, J. In vitro biological activities of niloticane, a new bioactive cassane diterpene from the bark of Acacia nilotica subsp. kraussiana. J. Ethnopharmacol. 2010, 128, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Ahmadu, A.; Abdulkarim, A.; Grougnet, R.; Myrianthopoulos, V.; Tillequin, F.; Magiatis, P.; Skaltsounis, A.-L. Two new peltogynoids from Acacia nilotica Delile with kinase inhibitory activity. Planta Med. 2010, 76, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Ahmadu, A.; Agunu, A.; Grougnet, R.; Magiatis, P.; Skaltsounis, A.; Tillequin, F. New peltogynoids from Acacia nilotica. Planta Med. 2008, 74, PB157. [Google Scholar] [CrossRef]
- Ikewuchi, J.C.; Onyeike, E.N.; Uwakwe, A.A.; Ikewuchi, C.C. Effect of aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’ Muell Arg (Euphorbiaceae) on the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan induced diabetic rats. J. Ethnopharmacol. 2011, 137, 1415–1424. [Google Scholar] [CrossRef]
- Adesina, S.; Idowu, O.; Ogundaini, A.; Oladimeji, H.; Olugbade, T.; Onawunmi, G.; Pais, M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Acalypha hispida. Phytother. Res. 2000, 14, 371–374. [Google Scholar] [CrossRef]
- Kumari, K.; Augusti, K. Antidiabetic and antioxidant effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) as compared to standard drugs in alloxan diabetic rats. Indian J. Exp. Biol. 2002, 40, 1005–1009. [Google Scholar]
- Mohamed, G.A. Alliuocide G, a new flavonoid with potent α-amylase inhibitory activity from Allium cepa L. Arkivoc 2008, 11, 202–209. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Uddin, T.M.; Zidan, B.R.M.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.J.; Khusro, A. Allium cepa: A treasure of bioactive phytochemicals with prospective health benefits. Evid. Based Complement. Altern. Med. 2022, 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Induli, M.; Cheloti, M.; Wasuna, A.; Wekesa, I.; Wanjohi, J.M.; Byamukama, R.; Heydenrich, M.; Makayoto, M.; Yenesew, A. Naphthoquinones from the roots of Aloe secundiflora. Phytochem. Lett. 2012, 5, 506–509. [Google Scholar] [CrossRef]
- Achenbach, H.; Waibel, R.; Addae-Mensah, I. Lignans and other constituents from Carissa edulis. Phytochemistry 1983, 22, 749–753. [Google Scholar] [CrossRef]
- Tolo, F.; Rukunga, G.; Muli, F.; Ochora, J.; Irungu, B.; Muthaura, C.; Wanjiku, C.; Mungai, G.; Ngoc, Q.; Hashimoto, K. The antiviral activity of compounds isolated from Kenyan Carissa edulis (Forssk.) Vahl. JMPR 2010, 4, 1517–1522. [Google Scholar]
- Al-Youssef, H.M.; Hassan, W.H. Chemical constituents of Carissa edulis Vahl. Arab. J. Chem. 2017, 10, 109–113. [Google Scholar] [CrossRef]
- Ojerinde, O.S.; Gwatau, D.D.; Falang, K.D.; Odumosu, P.O.; Kolawole, J.A. Nutritional composition, antioxidant assay and α-glucosidase inhibitory flavonoids from the fruits of Carissa edulis Vahl (Apocynaceae). J. Pharm. Bioresour. 2021, 18, 122–132. [Google Scholar] [CrossRef]
- Chirchir, K.D.; Cheplogoi, K.P.; Omolo, O.J.; Langat, K.M. Chemical constituents of Solanum mauense (Solanaceae) and Dovyalis abyssinica (Salicaceae). Int. J. Biol. Chem. Sci. 2018, 12, 999–1007. [Google Scholar] [CrossRef]
- Mouzié, C.M.; Guefack, M.-G.F.; Kianfé, B.Y.; Serondo, H.U.; Ponou, B.K.; Siwe-Noundou, X.; Teponno, R.B.; Krause, R.W.; Kuete, V.; Tapondjou, L.A. A new chalcone and antimicrobial chemical constituents of Dracaena stedneuri. Pharmaceuticals 2022, 15, 725. [Google Scholar] [CrossRef]
- Huang, L.; Chen, S.; Yang, M. Euphorbia hirta (Feiyangcao): A review on its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plant Res. 2012, 6, 5176–5185. [Google Scholar]
- Duong, T.-H.; Beniddir, M.A.; Genta-Jouve, G.; Nguyen, H.-H.; Nguyen, D.-P.; Mac, D.-H.; Boustie, J.; Chavasiri, W.; Le Pogam, P. Further terpenoids from Euphorbia tirucalli. Fitoterapia 2019, 135, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Kisiel, W. Chemical constituents from Lactuca inermis, a wild African species. Biochem. Syst. Ecol. 2014, 55, 104–106. [Google Scholar] [CrossRef]
- Li, X.-H.; McLaughlin, J.L. Bioactive compounds from the root of Myrsine africana. J. Nat. Prod. 1989, 52, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Arot, L.O.M.; Midiwo, J.O.; Kraus, W. A flavonol glycoside from Myrsine africana leaves. Phytochemistry 1996, 43, 1107–1109. [Google Scholar] [CrossRef]
- Manguro, L.O.A.; Midiwo, J.O.; Kraus, W.; Ugi, I. Benzoquinone derivatives of Myrsine africana and Maesa lanceolata. Phytochemistry 2003, 64, 855–862. [Google Scholar] [CrossRef]
- Midiwo, J.O.; Arot, L.M. New Dialkyl Benzoquinones from Fruits of Myrsine africana L. and Maesa lanceolata, Forsk. Nat. Prod. Lett. 1996, 8, 11–14. [Google Scholar] [CrossRef]
- Manguro, L.O.; Midiwo, J.O.; Kraus, W. A new flavonol Tetraglycoside from Myrsine africana leaves. Nat. Prod. Lett. 1996, 9, 121–126. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, C.; Zhu, D. A new acetylated flavonoid glycoside from Myrsine africana L. Notes 2009, 30, 2111. [Google Scholar]
- Azam, S.; Bashir, S.; Ahmad, B. Anti-spasmodic action of crude methanolic extract and a new compound isolated from the aerial parts of Myrsine africana. BMC Complement. Altern. Med. 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Zou, Y.P.; Tan, C.H.; Wang, B.D.; Zhu, D.Y.; Kim, S.K. Chemical constituents from Myrsine africana L. Helv. Chim. 2008, 91, 2168–2173. [Google Scholar]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [PubMed]
- Lee, T.-H.; Tsai, Y.-F.; Huang, T.-T.; Chen, P.-Y.; Liang, W.-L.; Lee, C.-K. Heptadecanols from the leaves of Persea americana var. americana. Food Chem. 2012, 132, 921–924. [Google Scholar]
- Owolabi, M.; Coker, H.; Jaja, S. Bioactivity of the phytoconstituents of the leaves of Persea americana. J. Med. Plants Res. 2010, 4, 1130–1135. [Google Scholar]
- Fourneau, C.; Hocquemiller, R.; Cavé, A. Triterpenes from Prunus africana bark. Phytochemistry 1996, 42, 1387–1389. [Google Scholar]
- Catalano, S.; Ferretti, M.; Marsili, A.; Morelli, I. New constituents of Prunus africana bark extract. J. Nat. Prod. 1984, 47, 910. [Google Scholar] [PubMed]
- Deresa, D.A.; Abdissa, Z.; Gurmessa, G.T.; Abdissa, N. Chemical constituents of the stem bark of Prunus africana and Evaluation of their Antibacterial Activity. J. Turk. Chem. Soc. Sect. Chem. 2022, 9, 395–414. [Google Scholar]
- Maiyoa, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-oglucoside and β-amyrin from Prunus africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar]
- Bezabih, M.; Abegaz, B.M. Glucofrangulin A diacetate from the fruits of Rhamnus prinoids. Bull. Chem. Soc. Ethiop. 1998, 12, 45–48. [Google Scholar]
- Manguro, L.O.A.; Ugi, I.; Lemmen, P.; Hermann, R. Flavonol glycosides of Warburgia ugandensis leaves. Phytochemistry 2003, 64, 891–896. [Google Scholar]
- Wube, A.A.; Bucar, F.; Gibbons, S.; Asres, K. Sesquiterpenes from Warburgia ugandensis and their antimycobacterial activity. Phytochemistry 2005, 66, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Kioy, D.; Gray, A.I.; Waterman, P.G. A comparative study of the stem-bark drimane sesquiterpenes and leaf volatile oils of Warburgia ugandensis and W. stuhlmannii. Phytochemistry 1990, 29, 3535–3538. [Google Scholar] [CrossRef]
- Gonfa, T.; Fisseha, A.; Thangamani, A. Isolation, characterization and drug-likeness analysis of bioactive compounds from stem bark of Warburgia ugandensis Sprague. Chem. Data Collect. 2020, 29, 100535. [Google Scholar] [CrossRef]
- Zhuang, X.-C.; Zhang, Y.-L.; Chen, G.-L.; Liu, Y.; Hu, X.-L.; Li, N.; Wu, J.-L.; Guo, M.-Q. Identification of anti-inflammatory and anti-proliferative neolignanamides from Warburgia ugandensis employing multi-target affinity Ultrafiltration and LC-MS. Pharmaceuticals 2021, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.-C.; Chen, G.-L.; Liu, Y.; Zhang, Y.-L.; Guo, M.-Q. New lignanamides with antioxidant and anti-inflammatory activities screened out and identified from Warburgia ugandensis combining affinity ultrafiltration LC-MS with SOD and XOD enzymes. Antioxidants 2021, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Moriyasu, M.; Ichimaru, M.; Nishiyama, Y.; Juma, F.D.; Nganga, J.N.; Mathenge, S.G.; Ogeto, J.O. Isolation of alkaloidal constituents of Zanthoxylum usambarense and Zanthoxylum chalybeum using ion-pair HPLC. J. Nat. Prod. 1996, 59, 316–318. [Google Scholar] [CrossRef]
- Altamimi, M.; Jaradat, N.; Alham, S.; Al-Masri, M.; Bsharat, A.; Alsaleh, R.; Sabobeh, R. Antioxidant, anti-enzymatic, antimicrobial and cytotoxic properties of Euphorbia tirucalli L. bioRxiv 2019. bioRxiv:17.879692. [Google Scholar]
- Okoro, I.O. Two extracts from Manihot esculenta leaves efficiently inhibit α-glucosidase and α-amylase: A new approach for the management of diabetes. Iran. J. Toxicol. 2020, 14, 131–138. [Google Scholar] [CrossRef]
- Subramanian, S.P.; Bhuvaneshwari, S.; Prasath, G.S. Antidiabetic and antioxidant potentials of Euphorbia hirta leaves extract studied in streptozotocin-induced experimental diabetes in rats. Gen. Physiol. Biophys. 2011, 30, 278–285. [Google Scholar] [CrossRef]
- Maurya, A.K.; Tripathi, S.; Ahmed, Z.; Sahu, R.K. Antidiabetic and antihyperlipidemic effect of Euphorbia hirta in streptozotocin induced diabetic rats. Der Pharm. Lett. 2012, 4, 703–707. [Google Scholar]
- Ozougwu, J.C. Anti-diabetic effects of Allium cepa (onions) aqueous extracts on alloxan-induced diabetic Rattus novergicus. J. Med. Plants Res. 2011, 5, 1134–1139. [Google Scholar]
- Lima, C.; Vasconcelos, C.; Costa-Silva, J.; Maranhão, C.; Costa, J.; Batista, T.; Carneiro, E.; Soares, L.; Ferreira, F.; Wanderley, A. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2012, 141, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Amanze, J.C.; Oni, A.I.; Grant, S.; Iyobhebhe, M.; Elebiyo, T.C.; Rotimi, D.; Asogwa, N.T.; Oyinloye, B.E.; Ajiboye, B.O. Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Sci. Rep. 2022, 12, 2919. [Google Scholar] [CrossRef] [PubMed]
- Natsume, N.; Yonezawa, T.; Saito, Y.; Woo, J.-T.; Teruya, T. Prenylflavonoids from fruit of Macaranga tanarius promote glucose uptake via AMPK activation in L6 myotubes. J. Nat. Med. 2021, 75, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arya, A.; Nisha, P.; Jayamurthy, P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Rawat, S. Phytopharmacological evaluation of Acacia nilotica Deile bark extract and its fractions for its effect on antidiabetic and antioxidant activities of glucose metabolism in alloxan induced diabetic rats. Inven. Imapact Ethnopharmacol. 2012, 3, 169–173. [Google Scholar]
- Habbu, P.; Madagundi, S.; Shastry, R.; Vanakudri, R.; Kulkarni, V. Preparation and evaluation of antidiabetic activity of Allium cepa-phospholipid complex (phytosome) in streptozotocin induced diabetic rats. RGUHS J. Pharm. Sci. 2015, 5, 132–141. [Google Scholar] [CrossRef]
- Khaki, A.; Fathi, A.F.; Ahmadi, A.H.; Rezazadeh, S.; Rastegar, H.; Imani, A. Compartments of quercetin & Allium cepa (onion) on blood glucose in diabetic rats. J. Med. Plants 2010, 9, 107–112. [Google Scholar]
- Eldin, I.M.T.; Ahmed, E.M.; Abd, E.H. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environ. Health Insights 2010, 4, EHI-S5540. [Google Scholar] [CrossRef]
- Shetty, A.; Rashmi, R.; Rajan, M.; Sambaiah, K.; Salimath, P. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr. Res. 2004, 24, 373–381. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jo, S.-H.; Jang, H.-D.; Lee, M.S.; Kwon, Y.-I. Antioxidant activity and α-glucosidase inhibitory potential of onion (Allium cepa L.) extracts. Food Sci. Biotechnol. 2010, 19, 159–164. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Karagecili, H.; Alwasel, S.; Gulcin, İ. Potential Antioxidant, Anticholinergic, Antidiabetic and Antiglaucoma Activities and Molecular Docking of Spiraeoside as A Secondary Metabolite of Onion (Allium cepa). Saudi Pharm. J. 2023, 31, 101760. [Google Scholar] [CrossRef]
- Abdirahman, Y.; Nyamai, D.; Njagi, J.; Agyirifo, D.; Ngugi, M.; Gathumbi, P.; Ngeranwa, J.; Njagi, E. In-Vivo Anti-hyperglycemic Activity and Safety of the Aqueous Stem Bark Extracts of Aloe secundiflora. Med. Aromat Plants 2015, S1, 3. [Google Scholar]
- El-Fiky, F.K.; Abou-Karam, M.A.; Afify, E.A. Effect of Luffa aegyptiaca (seeds) and Carissa edulis (leaves) extracts on blood glucose level of normal and streptozotocin diabetic rats. J. Ethnopharmacol. 1996, 50, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Amare, Y.E. Methanolic extract of Myrsine africana leaf ameliorates hyperglycemia and dyslipidemia in alloxan-induced diabetic albino mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 3987656. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, A.N.; Okorie, A.; Orisakwe, O.E. Hypoglycaemic and tissue-protective effects of the aqueous extract of Persea americana seeds on alloxan-induced albino rats. Malays. J. Med. Sci. 2013, 20, 31. [Google Scholar] [PubMed]
- Umoh, I.; Samuel, O.; Kureh, T.; Davies, K. Antidiabetic and hypolipidaemic potentials of ethanol fruit pulp extract of Persea americana (avocado pear) in rats. J. Afr. Assos. Physiol. Sci. 2019, 7, 59–63. [Google Scholar]
- Alhassan, A.; Sule, M.; Lawal, A. In vitro inhibitory activities of Persea americana seed extracts on α-amylase and α-glucosidas. Bayero J. Pure Appl. Sci. 2017, 10, 546–552. [Google Scholar] [CrossRef]
- Maina, J.; Kareru, P.; Gatebe, E.; Rotich, H.; Githira, P.; Njonge, F.; Kiman, D.; Mutembei, J. Hypoglycemic effects of selected herbal drug formulations from the Kenyan market. J. Nat. Prod. Plant Res. 2014, 4, 10–17. [Google Scholar]
- Komakech, R.; Kang, Y. Ethnopharmacological potential of African cherry [Prunus africana]. J. Herb. Med. 2019, 17, 100283. [Google Scholar] [CrossRef]
- Trinh, Q.; Le, L. An investigation of antidiabetic activities of bioactive compounds in Euphorbia hirta Linn using molecular docking and pharmacophore. Med. Chem. Res. 2014, 23, 2033–2045. [Google Scholar] [CrossRef]
- Al-Nour, M.Y.; Ibrahim, M.M.; Elsaman, T. Ellagic acid, Kaempferol, and Quercetin from Acacia nilotica: Promising combined drug with multiple mechanisms of action. Curr. Pharmacol. Rep. 2019, 5, 255–280. [Google Scholar] [CrossRef]
- York, R.G.; Parker, R.; Haber, L. Test methods for assessing female reproductive and developmental toxicology. Hayes’ Princ. Methods Toxicol. 2014, 6, 1637–1722. [Google Scholar]
- Silva, A.C.P.; de Faria, D.E.P.; Santo Borges, N.B.d.E.; de Souza, I.A.; Peters, V.M.; de Oliveira Guerra, M. Toxicological screening of Euphorbia tirucalli L.: Developmental toxicity studies in rats. J. Ethnopharmacol. 2007, 110, 154–159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muema, F.W.; Nanjala, C.; Oulo, M.A.; Wangchuk, P. Phytochemical Content and Antidiabetic Properties of Most Commonly Used Antidiabetic Medicinal Plants of Kenya. Molecules 2023, 28, 7202. https://doi.org/10.3390/molecules28207202
Muema FW, Nanjala C, Oulo MA, Wangchuk P. Phytochemical Content and Antidiabetic Properties of Most Commonly Used Antidiabetic Medicinal Plants of Kenya. Molecules. 2023; 28(20):7202. https://doi.org/10.3390/molecules28207202
Chicago/Turabian StyleMuema, Felix Wambua, Consolata Nanjala, Millicent Akinyi Oulo, and Phurpa Wangchuk. 2023. "Phytochemical Content and Antidiabetic Properties of Most Commonly Used Antidiabetic Medicinal Plants of Kenya" Molecules 28, no. 20: 7202. https://doi.org/10.3390/molecules28207202
APA StyleMuema, F. W., Nanjala, C., Oulo, M. A., & Wangchuk, P. (2023). Phytochemical Content and Antidiabetic Properties of Most Commonly Used Antidiabetic Medicinal Plants of Kenya. Molecules, 28(20), 7202. https://doi.org/10.3390/molecules28207202