Fabricated Gamma-Alumina-Supported Zinc Ferrite Catalyst for Solvent-Free Aerobic Oxidation of Cyclic Ethers to Lactones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterizations
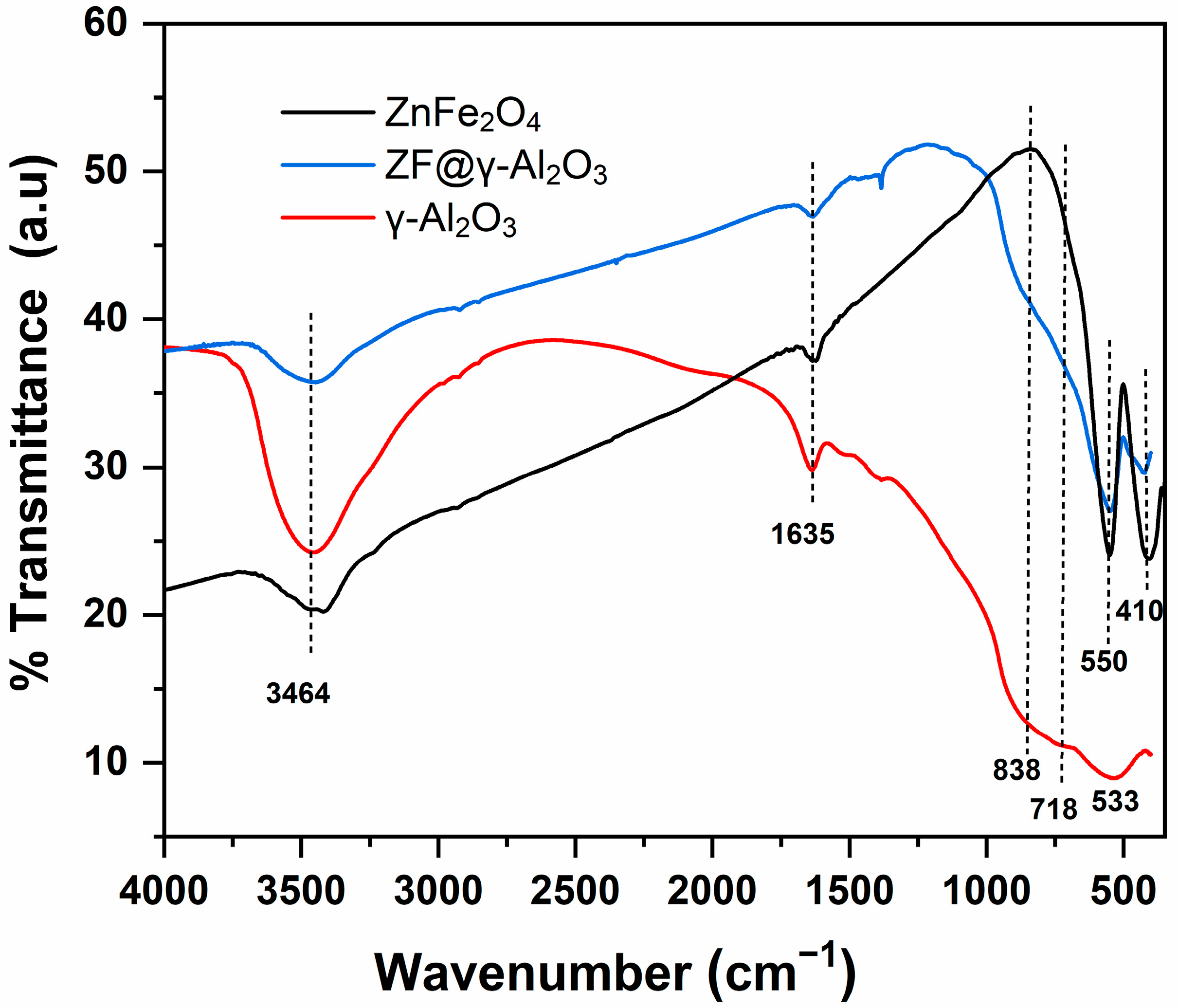
2.1.1. FTIR Analysis
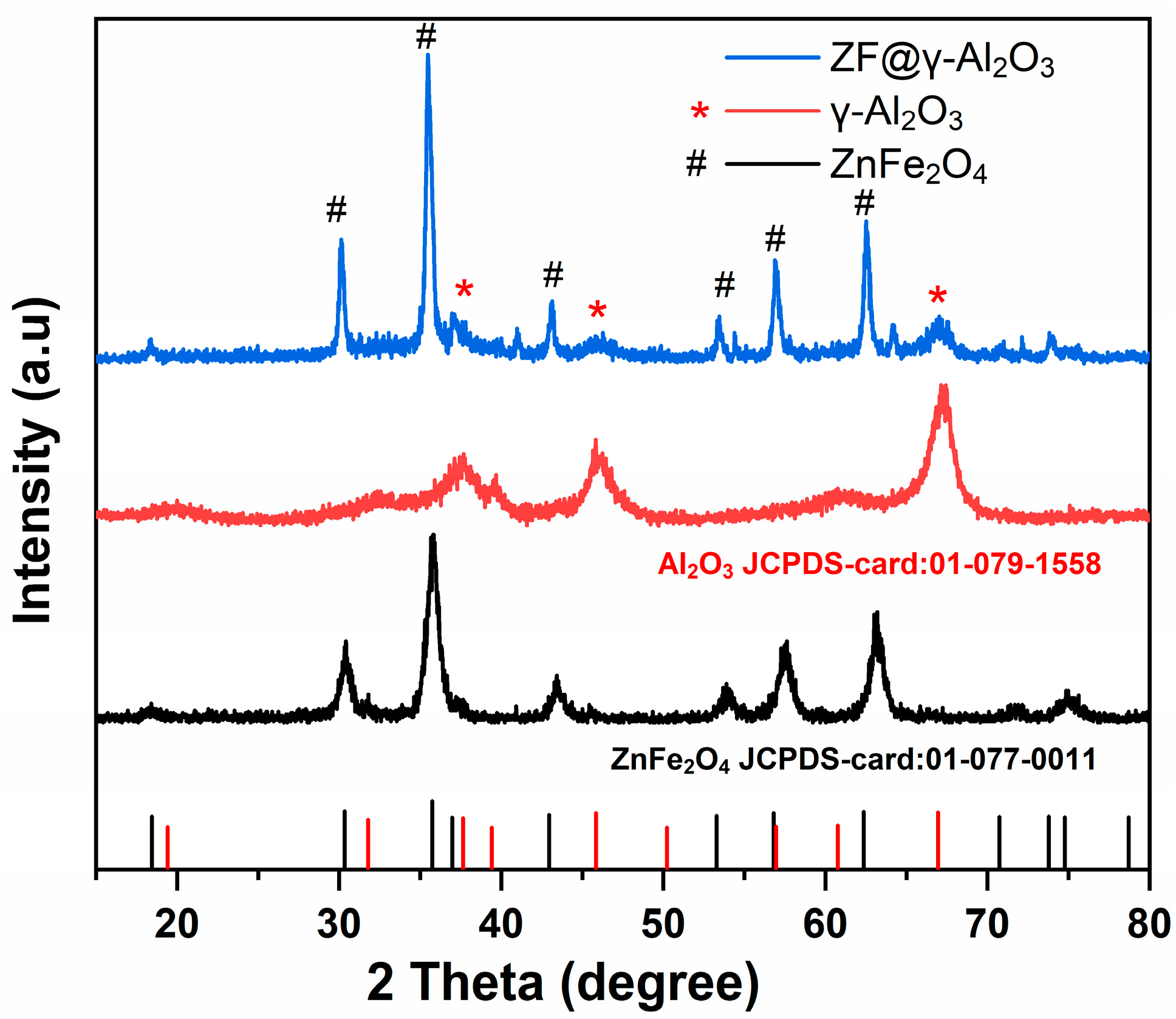
2.1.2. XRD Analysis
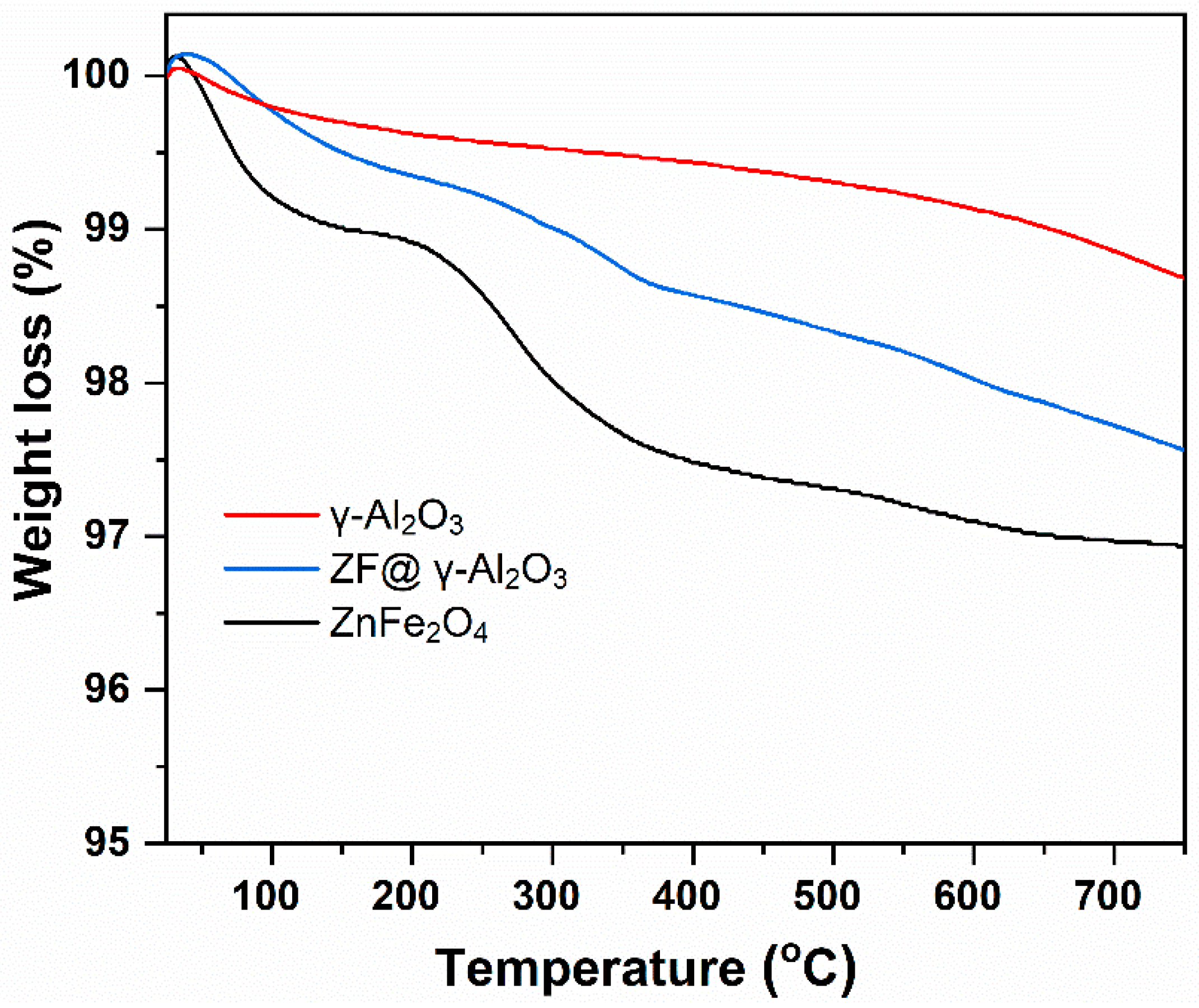
2.1.3. TGA Analysis
2.1.4. BET-Surface Area Analysis
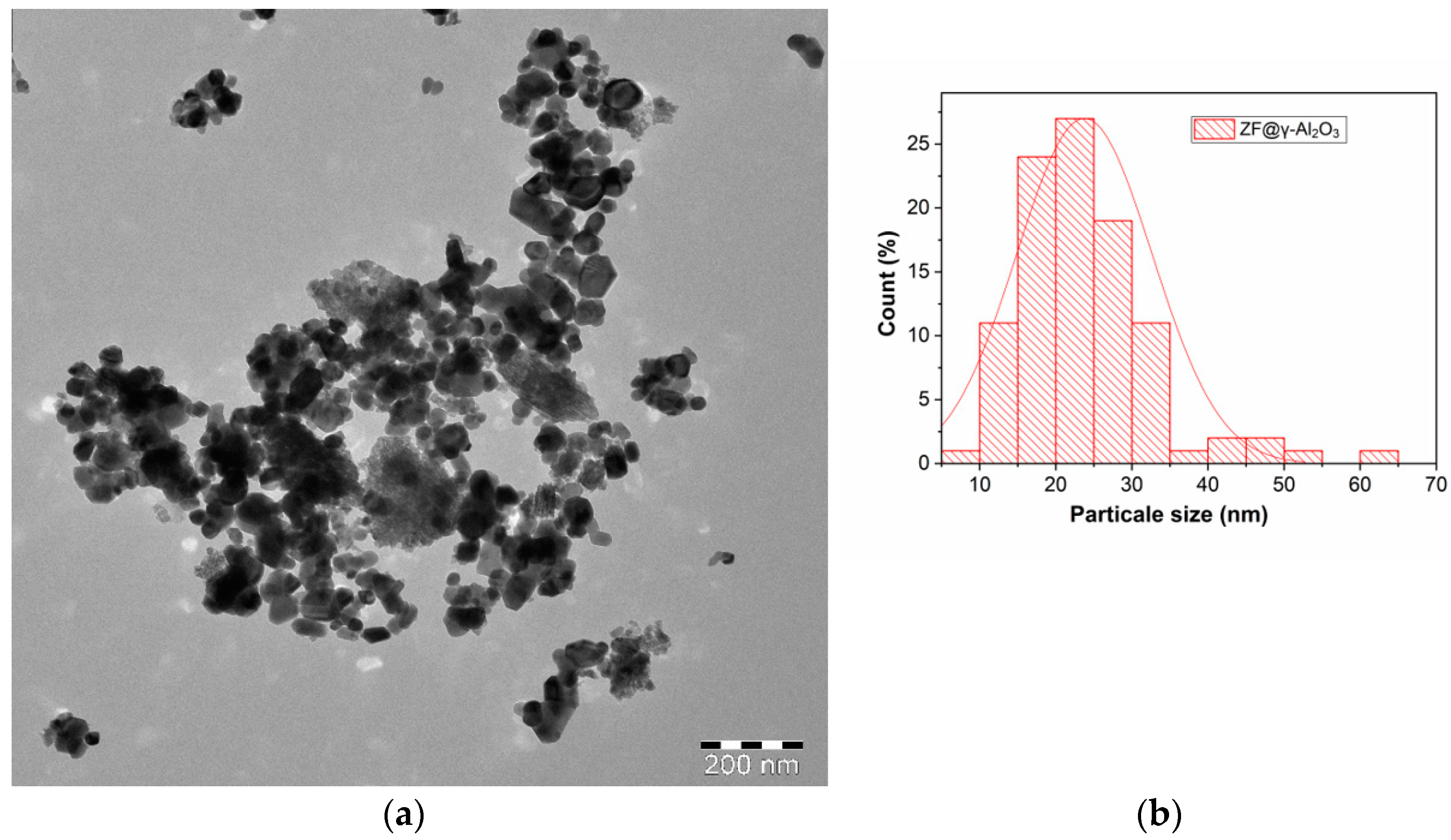
2.1.5. TEM Analysis
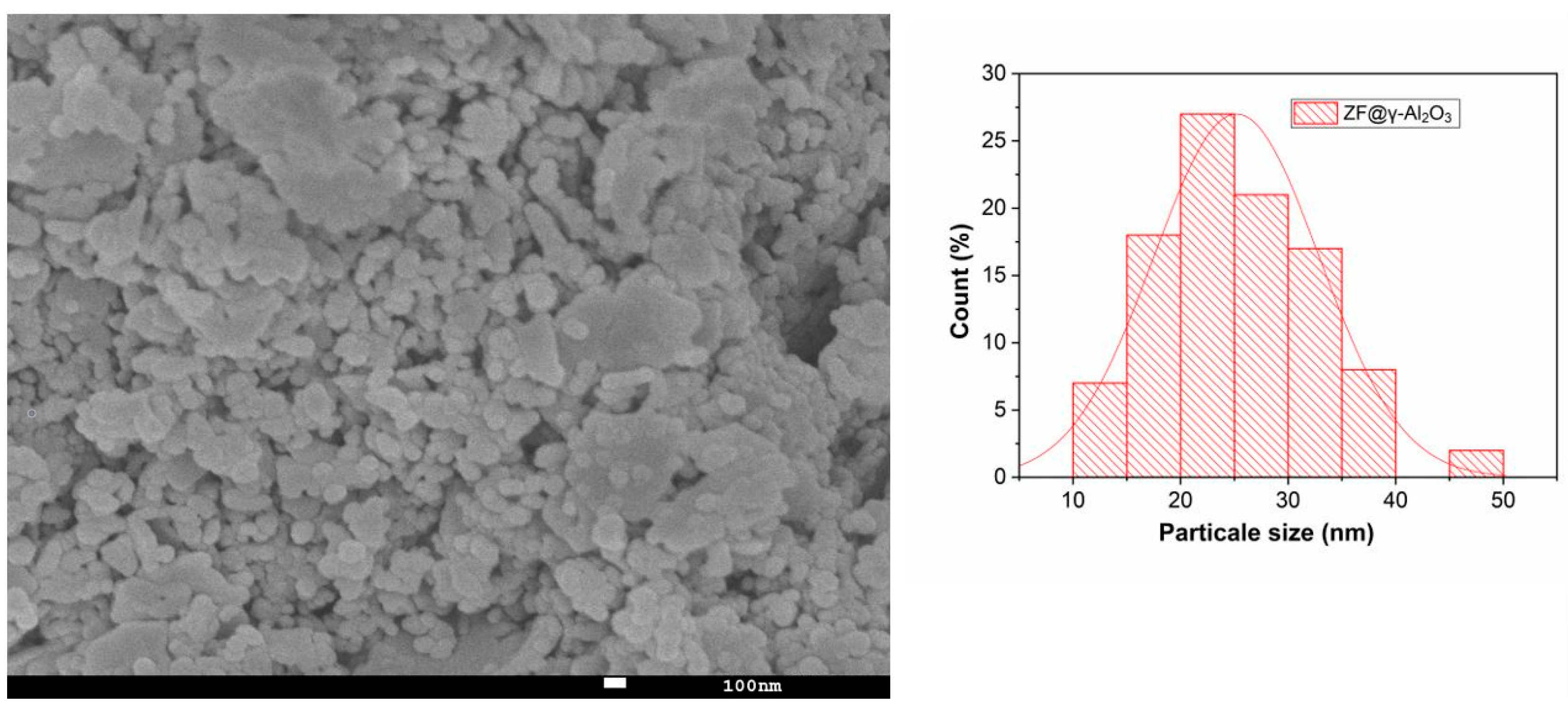
2.1.6. SEM Analysis
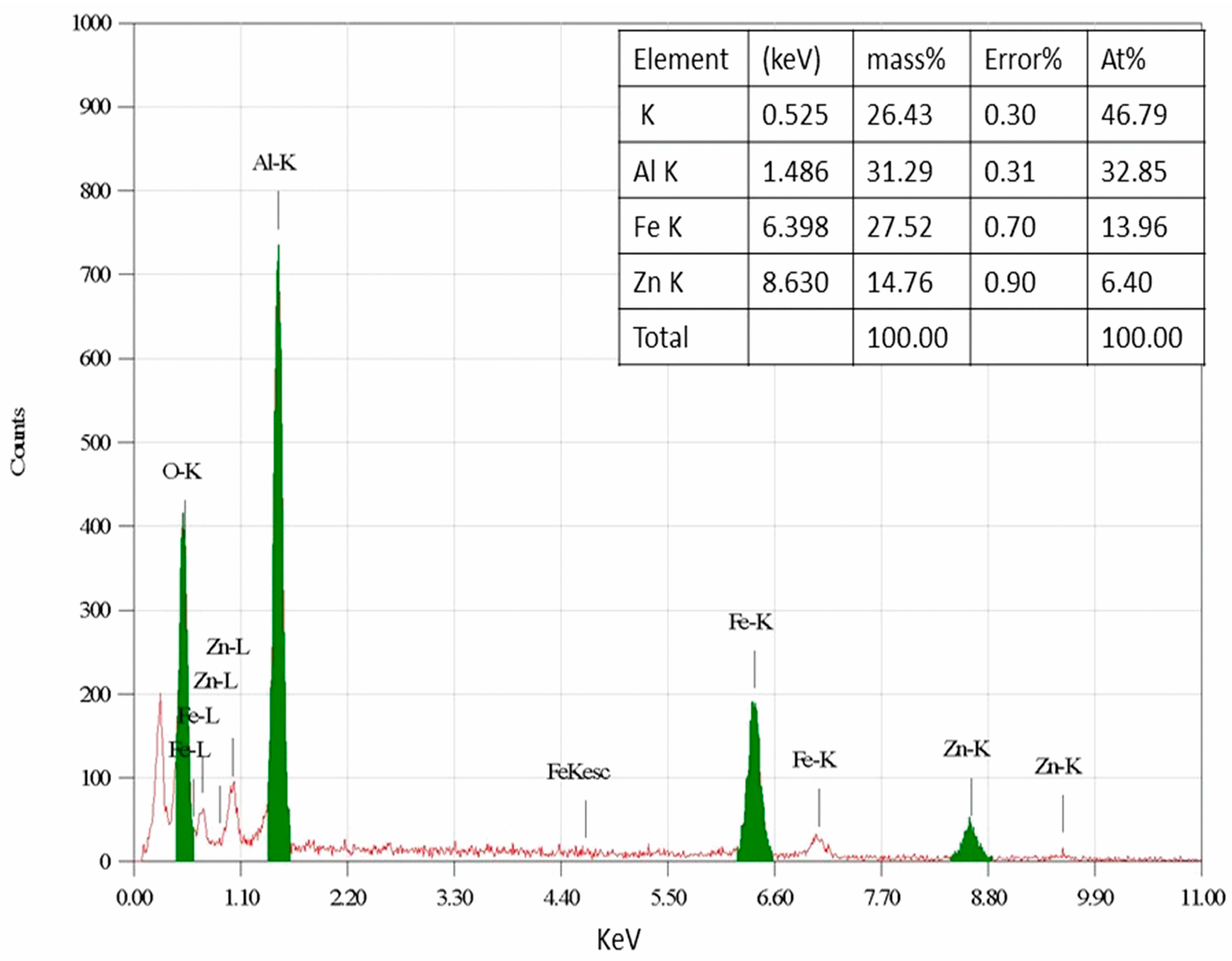
2.1.7. EDS Analysis
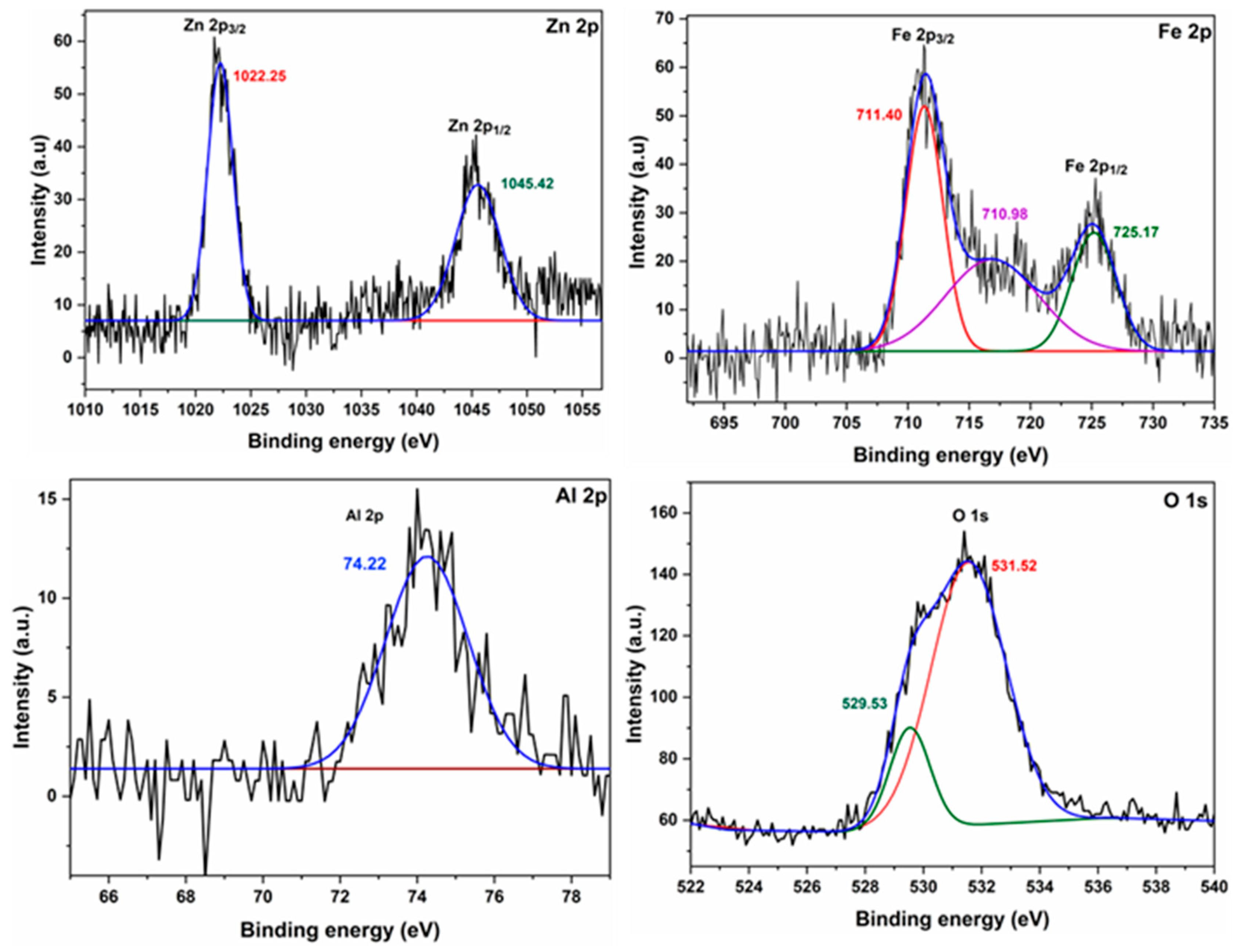
2.1.8. XPS Analysis
2.1.9. ICP Analysis
2.2. Catalytic Activity
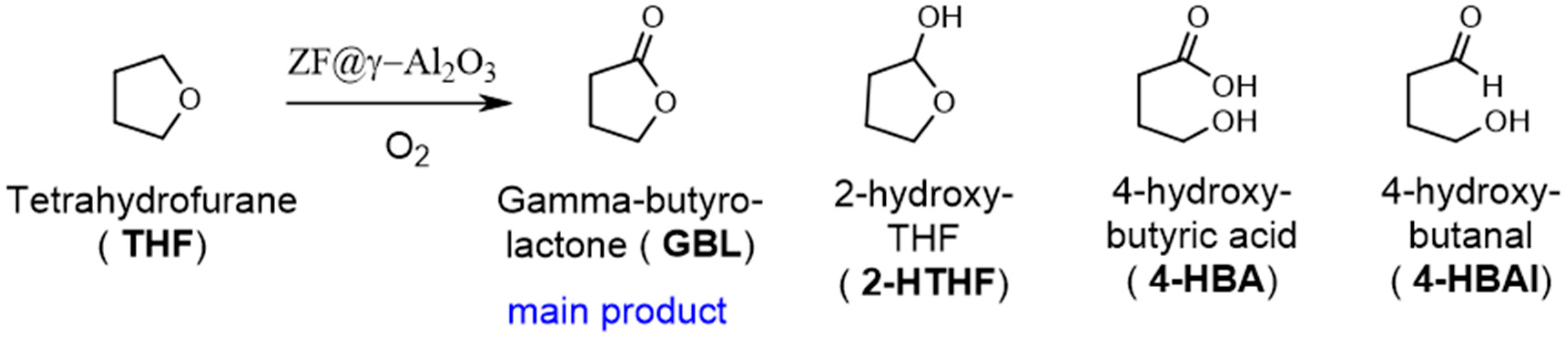
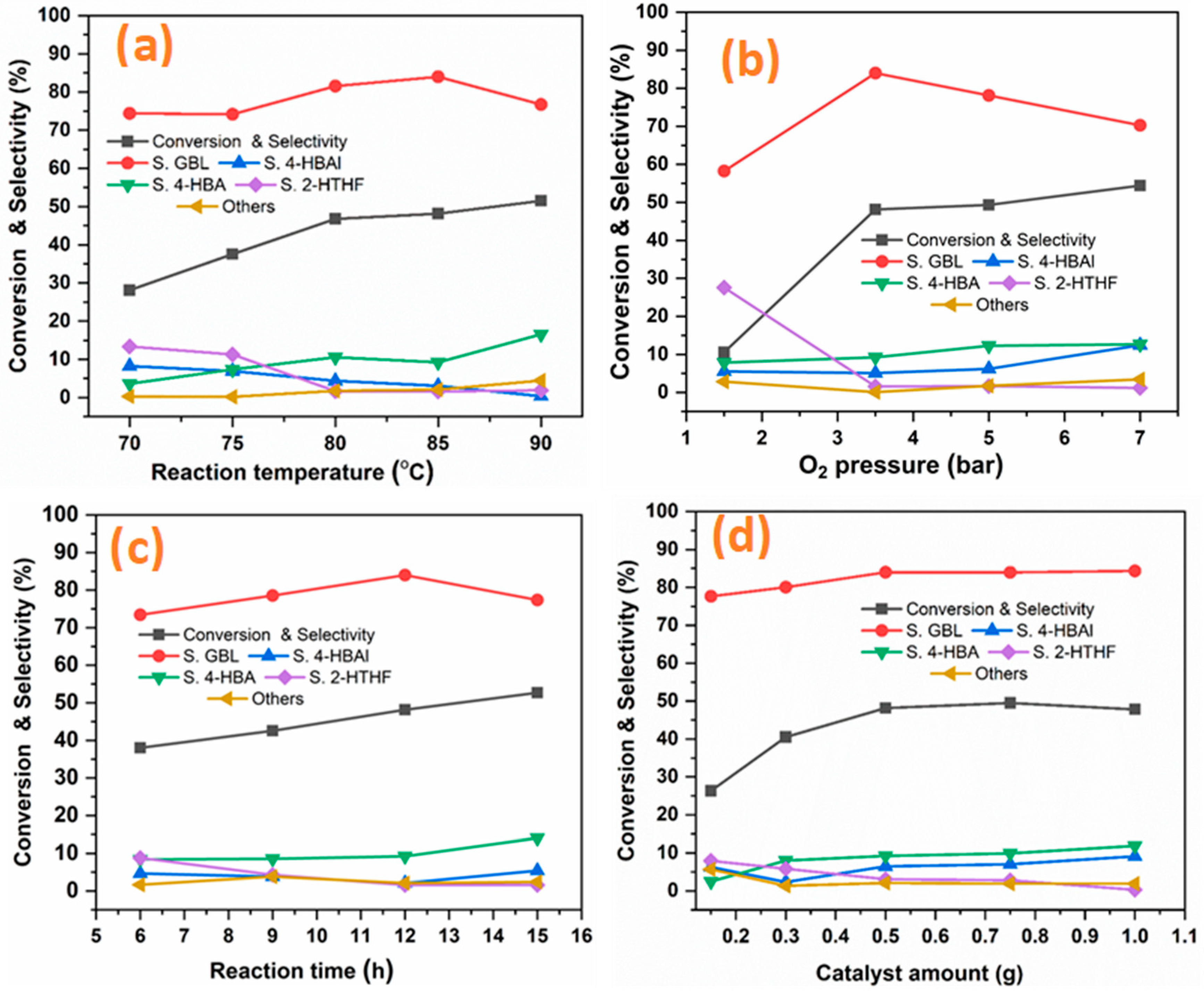
2.2.1. THF Oxidation
2.2.2. Cyclic Ethers Oxidation
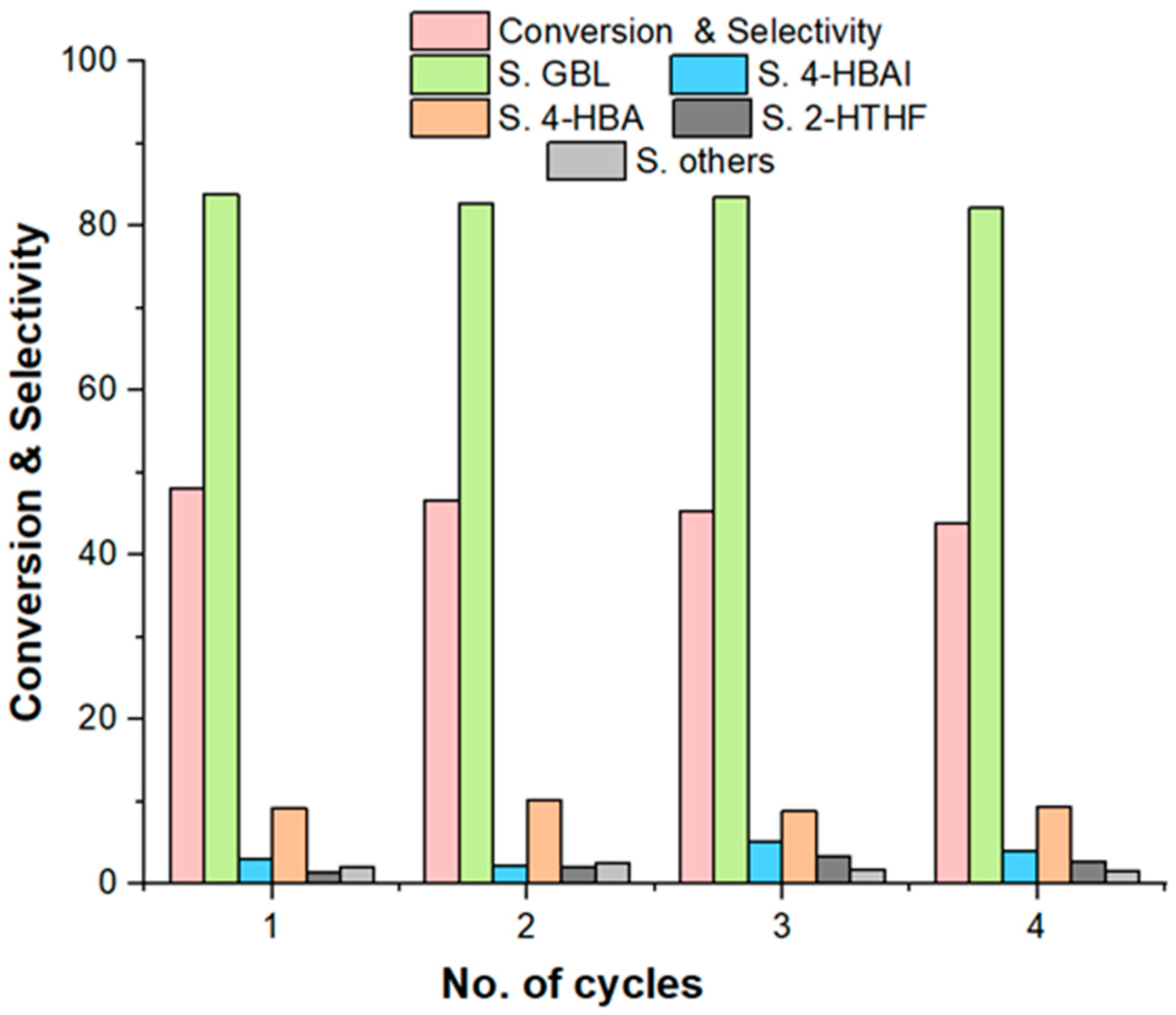
2.3. Reusability
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization of the Catalysts
3.4. Experimental Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cholakov, G.S. Control of pollution in the petroleum industry. Pollut. Control Technol. 2009, 3, 86–107. [Google Scholar]
- Rasul, M.G.; Faisal, I.; Khan, M.M.K. Environmental pollution generated from process industries in Bangladesh. Int. J. Environ. Pollut. 2006, 28, 144–161. [Google Scholar] [CrossRef]
- Makarova, A.S.; Jia, X.; Kruchina, E.B.; Kudryavtseva, E.I.; Kukushkin, I.G. Environmental performance assessment of the chemical industries involved in the Responsible Care® Program: Case study of the Russian Federation. J. Clean. Prod. 2019, 222, 971–985. [Google Scholar] [CrossRef]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chaudhary, A.; Singh, R. Gray chemistry verses green chemistry: Challenges and opportunities. Rasayan J. Chem. 2008, 1, 68–92. [Google Scholar]
- Mulvihill, M.J.; Beach, E.S.; Zimmerman, J.B.; Anastas, P.T. Green chemistry and green engineering: A framework for sustainable technology development. Annu. Rev. Environ. Resour. 2011, 36, 271–293. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]
- Syed, N.; Singh, S.; Chaturvedi, S.; Nannaware, A.D.; Khare, S.K.; Rout, P.K. Production of lactones for flavoring and pharmacological purposes from unsaturated lipids: An industrial perspective. Crit. Rev. Food Sci. Nutr. 2022, 1–32. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, H.U.; Kim, T.Y.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Production of 4-hydroxybutyric acid by metabolically engineered Mannheimia succiniciproducens and its conversion to γ-butyrolactone by acid treatment. Metab. Eng. 2013, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bertone, M.E.; Meyer, C.I.; Regenhardt, S.A.; Sebastian, V.; Garetto, T.F.; Marchi, A.J. Highly selective conversion of maleic anhydride to γ-butyrolactone over Ni-supported catalysts prepared by precipitation–deposition method. Appl. Catal. A Gen. 2015, 503, 135–146. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.; Borzecka, W.; Lavandera, I.; Gotor, V. Stereodivergent preparation of valuable γ-or δ-hydroxy esters and lactones through one-pot cascade or tandem chemoenzymatic protocols. ACS Catal. 2014, 4, 386–393. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Bińczak, J.; Dziuba, K.; Chrobok, A. Recent developments in lactone monomers and polymer synthesis and application. Materials 2021, 14, 2881. [Google Scholar] [CrossRef]
- Wang, H.; Ding, G.; Li, X.; She, H.; Zhu, Y.; Li, Y. Sustainable production of γ-valerolactone and δ-valerolactone through the coupling of hydrogenation and dehydrogenation. Sustain. Energy Fuels 2021, 5, 930–934. [Google Scholar] [CrossRef]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Munoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Janecki, T. Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pazos, D.; Giannasi, P.; Rossy, Q.; Esseiva, P. Combining Internet monitoring processes, packaging and isotopic analyses to determine the market structure: Example of Gamma Butyrolactone. Forensic Sci. Int. 2013, 230, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kondawar, S.; Rode, C. Ionic liquids for the sustainable transformation of levulinic acid to gamma-valerolactone (GVL). Curr. Opin. Green Sustain. Chem. 2022, 35, 100607. [Google Scholar] [CrossRef]
- Hollmann, F.; Kara, S.; Opperman, D.J.; Wang, Y. Biocatalytic synthesis of lactones and lactams. Chem. Asian J. 2018, 13, 3601–3610. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Yang, J.; Dong, G.-Q.; Zheng, H.-Y.; Zhang, H.-H.; Xiang, H.-W.; Li, Y.-W. An environmentally benign route to γ-butyrolactone through the coupling of hydrogenation and dehydrogenation. Appl. Catal. B Environ. 2005, 57, 183–190. [Google Scholar] [CrossRef]
- Yu, F.; Chi, Y.; Gao, C.; Chen, R.; Xie, C.; Yu, S. Baeyer-Villiger oxidation of cyclic ketones catalyzed by amino acid ionic liquids. Chem. Res. Chin. Univ. 2020, 36, 865–869. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Cheng, H.; Zhu, X.; Qi, Z. Atomically dispersed Pd catalysts for the selective hydrogenation of succinic acid to γ-butyrolactone. Catal. Today 2016, 276, 55–61. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Chong, Y.; Dumesic, J.A. Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 2012, 5, 8199–8203. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: Optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Zhou, C.; Xiao, Y.; Xu, S.; Li, J.; Hu, C. γ-valerolactone production from furfural residue with formic acid as the sole hydrogen resource via an integrated strategy on Au-Ni/ZrO2. Ind. Eng. Chem. Res. 2020, 59, 17228–17238. [Google Scholar] [CrossRef]
- Tang, D.; Shen, Z.; Lechler, S.; Lu, G.; Yao, L.; Hu, Y.; Huang, X.; Muhler, M.; Zhao, G.; Peng, B. Aerobic oxidative lactonization of diols at room temperature over defective titanium-based oxides in water. J. Catal. 2023, 418, 237–246. [Google Scholar] [CrossRef]
- Budroni, G.; Corma, A. Gold and gold–platinum as active and selective catalyst for biomass conversion: Synthesis of γ-butyrolactone and one-pot synthesis of pyrrolidone. J. Catal. 2008, 257, 403–408. [Google Scholar] [CrossRef]
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 2016, 7, 115–129. [Google Scholar] [CrossRef]
- Alessio, C.; Marco, N.; Maurizio, S.; Alvise, P. Upgrading of Biobased Lactones with Dialkylcarbonates. ACS Sustain. Chem. Eng. 2014, 2, 2131–2141. [Google Scholar]
- Silva, R.; Coelho, E.; Aguiar, T.Q.; Domingues, L. Microbial biosynthesis of lactones: Gaps and opportunities towards sustainable production. Appl. Sci. 2021, 11, 8500. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-Valerolactone from Carbohydrates and its Applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Soszka, E.; Jȩdrzejczyk, M.; Keller, N.; Ruppert, A.M. High yield production of 2-methyltetrahydrofuran biofuel with reusable Ni-Co catalysts. Fuel 2023, 332, 126118. [Google Scholar] [CrossRef]
- Dastidar, R.G.; Kim, M.S.; Zhou, P.; Luo, Z.; Shi, C.; Barnett, K.J.; McClelland, D.J.; Chen, E.Y.-X.; Van Lehn, R.C.; Huber, G.W. Catalytic production of tetrahydropyran (THP): A biomass-derived, economically competitive solvent with demonstrated use in plastic dissolution. Green Chem. 2022, 24, 9101–9113. [Google Scholar] [CrossRef]
- Kato, N.; Hamaguchi, Y.; Umezawa, N.; Higuchi, T. Efficient oxidation of ethers with pyridine N-oxide catalyzed by ruthenium porphyrins. J. Porphyr. Phthalocyanines 2015, 19, 411–416. [Google Scholar] [CrossRef]
- Zhao, Y.; Ang, J.Q.L.; Ng, A.W.T.; Yeung, Y.-Y. Oxidative transformation of cyclic ethers/amines to lactones/lactams using a DIB/TBHP protocol. RSC Adv. 2013, 3, 19765–19768. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Shen, X.; Wang, Y.; Du, J.; Chen, B.; Han, B.; Liu, H. Selective aerobic oxidation of cyclic ethers to lactones over Au/CeO2 without any additives. Chem. Commun. 2020, 56, 2638–2641. [Google Scholar] [CrossRef]
- Ali, M.E.; Rahman, M.M.; Sarkar, S.M.; Hamid, S.B.A. Heterogeneous metal catalysts for oxidation reactions. J. Nanomater. 2015, 2014, 209. [Google Scholar] [CrossRef]
- Prati, L.; Rossi, M. Chemoselective catalytic oxidation of polyols with dioxygen on gold supported catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; Volume 110, pp. 509–516. [Google Scholar]
- Sasidharan, M.; Bhaumik, A. Catalytic oxidation of cyclic ethers to lactones over various titanosilicates. J. Mol. Catal. A Chem. 2011, 338, 105–110. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Dou, J.; Tao, F.F. Selective epoxidation of cyclohexene with molecular oxygen on catalyst of nanoporous Au integrated with MoO3 nanoparticles. Appl. Catal. A Gen. 2017, 529, 134–142. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef] [PubMed]
- Sahle-Demessie, E.; Gonzalez, M.A.; Enriquez, J.; Zhao, Q. Selective oxidation in supercritical carbon dioxide using clean oxidants. Ind. Eng. Chem. Res. 2000, 39, 4858–4864. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, H. Palladium-catalyzed oxidation of unsaturated hydrocarbons using molecular oxygen. Acc. Chem. Res. 2012, 45, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, H.; Zhou, Y.; Zhang, L.; Wu, Z.; Yang, S.; Shi, H.; Zhu, Q.; Chen, Y.; Dai, S. Mesoporous MnCeO x solid solutions for low temperature and selective oxidation of hydrocarbons. Nat. Commun. 2015, 6, 8446. [Google Scholar] [CrossRef]
- Abduh, N.A.; Al-Kahtani, A.; Algarni, T.S.; Al-Odayni, A.-B. Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst. Catalysts 2023, 13, 692. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Z.; Wang, Y.; Yang, Q.; Xu, L.; Ding, W. An overview of recent development in composite catalysts from porous materials for various reactions and processes. Int. J. Mol. Sci. 2010, 11, 2152–2187. [Google Scholar] [CrossRef]
- Xue, Z.; Zhong, Z.; Zhang, B.; Xu, C. Performance of catalytic fast pyrolysis using a γ-Al2O3 catalyst with compound modification of ZrO2 and CeO2. Catalysts 2019, 9, 849. [Google Scholar] [CrossRef]
- Firdous, N.; Janjua, N.K. CoPtx/γ-Al2O3 bimetallic nanoalloys as promising catalysts for hydrazine electrooxidation. Heliyon 2019, 5, e01380. [Google Scholar] [CrossRef]
- Atrak, K.; Ramazani, A.; Taghavi Fardood, S. Green synthesis of amorphous and gamma aluminum oxide nanoparticles by tragacanth gel and comparison of their photocatalytic activity for the degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2018, 29, 8347–8353. [Google Scholar] [CrossRef]
- Saafan, S.A.; El-Nimr, M.K.; Hussein, M.M.; Omar, M.K. FTIR, DC, and AC electrical measurements of Mg Zn Nano-ferrites and their composites with Polybenzoxazine. Appl. Phys. A 2021, 127, 800. [Google Scholar] [CrossRef]
- Sarala, E.; Madhukara Naik, M.; Vinuth, M.; Rami Reddy, Y.; Sujatha, H. Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J. Mater. Sci. Mater. Electron. 2020, 31, 8589–8596. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Jahin, H.S.; Dessouki, H.A.; Nassar, M.Y. Synthesis and characterization of γ-Al2O3 and α-Al2O3 nanoparticles using a facile, inexpensive auto-combustion approach. Egypt. J. Chem. 2021, 64, 2509–2515. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, X.; Zhang, R.; Zhang, Y.; Chen, J.; Yang, F. Nano ferrites (AFe2O4, A= Zn, Co, Mn, Cu) as efficient catalysts for catalytic ozonation of toluene. RSC Adv. 2020, 10, 5116–5128. [Google Scholar] [CrossRef]
- Varpe, A.S.; Deshpande, M.D. Study of structural, optical, and dielectric properties of sol–gel derived ZnFe2O4–Al2O3 composite nanoparticles. J. Sol-Gel Sci. Technol. 2020, 96, 718–727. [Google Scholar] [CrossRef]
- Ali, S.Y.; Eid, O.I.; Siddig, M.A. The Influence of Cu on the Dielectric Properties of NiZnFe2O4 Synthesized by Solid State Reaction Method. J. Mater. Sci. Chem. Eng. 2020, 8, 14–23. [Google Scholar]
- Etemadinia, T.; Allahrasani, A.; Barikbin, B. ZnFe2O4@ SiO2@ Tragacanth gum nanocomposite: Synthesis and its application for the removal of methylene blue dye from aqueous solution. Polym. Bull. 2019, 76, 6089–6109. [Google Scholar] [CrossRef]
- Shafiee, M.; Hafez Ghoran, S.; Bordbar, S.; Gholami, M.; Naderian, M.; Dehghani, F.S.; Amani, A.M. Rutin: A Flavonoid Precursor for Synthesis of ZnFe2O4 Nanoparticles; Electrochemical Study of Zinc Ferrite-chitosan Nanogel for Doxorubicin Delivery. J. Nanostructures 2021, 11, 114–124. [Google Scholar]
- Urbonavicius, M.; Varnagiris, S.; Pranevicius, L.; Milcius, D. Production of gamma alumina using plasma-treated aluminum and water reaction byproducts. Materials 2020, 13, 1300. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Afshar Ebrahimi, A.; Rahimi, A. Comparison of γ and δ-Al2O3 supported CoMo catalysts in the ydrodesulfurization of straight-run gas oil. Sci. Iran. 2019, 26, 1555–1565. [Google Scholar]
- Dobrosielska, M.; Zieliński, M.; Frydrych, M.; Pietrowski, M.; Marciniak, P.; Martyła, A.; Sztorch, B.; Przekop, R.E. Sol–Gel Approach for Design of Pt/Al2O3-TiO2 System—Synthesis and Catalytic Tests. Ceramics 2021, 4, 667–680. [Google Scholar] [CrossRef]
- Das, K.K.; Patnaik, S.; Nanda, B.; Pradhan, A.C.; Parida, K. ZnFe2O4-decorated mesoporous Al2O3 modified MCM-41: A solar-light-active photocatalyst for the effective removal of phenol and Cr (VI) from water. ChemistrySelect 2019, 4, 1806–1819. [Google Scholar] [CrossRef]
- Khan, S.; Shah, S.S.; Janjua, N.K.; Yurtcan, A.B.; Nazir, M.T.; Katubi, K.M.; Alsaiari, N.S. Alumina supported copper oxide nanoparticles (CuO/Al2O3) as high-performance electrocatalysts for hydrazine oxidation reaction. Chemosphere 2023, 315, 137659. [Google Scholar] [CrossRef]
- Winiarska, K.; Klimkiewicz, R.; Tylus, W.; Sobianowska-Turek, A.; Winiarski, J.; Szczygieł, B.; Szczygieł, I. Study of the catalytic activity and surface properties of manganese-zinc ferrite prepared from used batteries. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y. Metal-support synergy of supported gold nanoclusters in selective oxidation of alcohols. Catalysts 2020, 10, 107. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, C.; Sun, P.; Gao, G.; Liu, Q.; Huang, Z.; Li, F. Synergistic Catalysis for Promoting Ring-Opening Hydrogenation of Biomass-Derived Cyclic Oxygenates. ACS Catal. 2023, 13, 5170–5193. [Google Scholar] [CrossRef]
- Miceli, M.; Frontera, P.; Macario, A.; Malara, A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591. [Google Scholar] [CrossRef]
- Al-Iessa, M.S.; Al-Zaidi, B.Y.; Almukhtar, R.S.; Shakor, Z.M.; Hamawand, I. Optimization of Polypropylene Waste Recycling Products as Alternative Fuels through Non-Catalytic Thermal and Catalytic Hydrocracking Using Fresh and Spent Pt/Al2O3 and NiMo/Al2O3 Catalysts. Energies 2023, 16, 4871. [Google Scholar] [CrossRef]
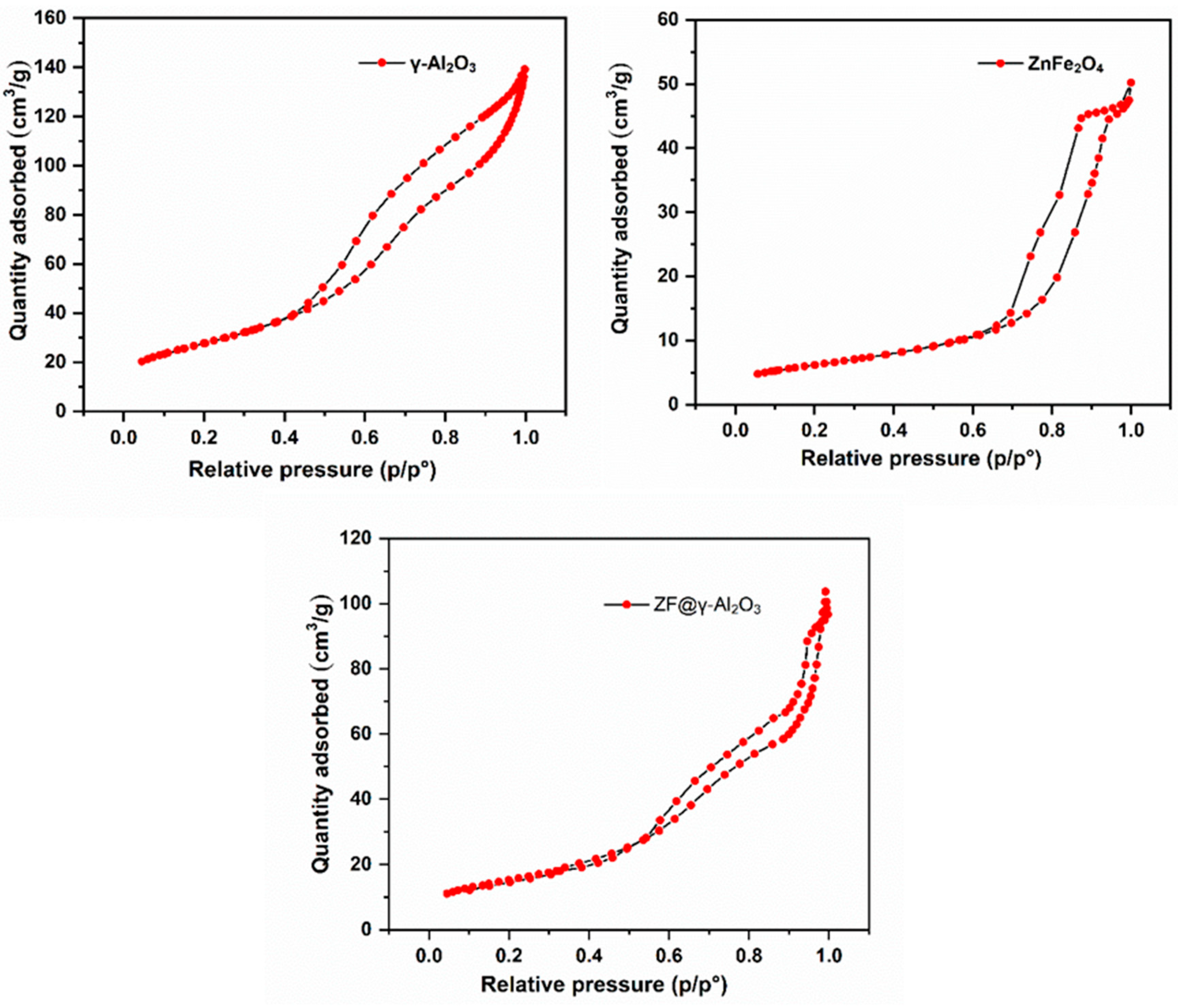

| Catalyst | Total Surface Area (m2/g) | External Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Width (nm) |
|---|---|---|---|---|
| ZnFe2O4 | 22.05 | 14.65 | 0.08 | 13.4 |
| γ-Al2O3 | 100.55 | 67.35 | 0.22 | 78.0 |
| ZF@γ-Al2O3 | 52.04 | 36.33 | 0.16 | 105.2 |
| Catalyst | Al (mol%) | Zn (mol%) | Fe (mol%) |
|---|---|---|---|
| ZF@γ-Al2O3 | 58.78 | 12.53 | 28.69 |
| Catalyst | Conversion (%) | Selectivity of GBL (%) |
|---|---|---|
| ZF@γ-Al2O3 | 48.1 | 83.9 |
| ZnFe2O4 (ZF) | 18.1 | 63.3 |
| γ-Al2O3 | 11.7 | 32.6 |
| Blank | 3.2 | 0 |
| Entry | Substrate | Conversion | S. Lactone | Product |
|---|---|---|---|---|
| 1 |  | 40 | 86 | 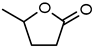 |
| 2 |  | 76 49 a | 34 62 a | 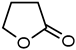 |
| 3 | 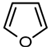 | -- | -- | - |
| 4 | 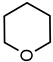 | 11 28 b | 83 89 b | 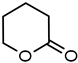 |
| 5 | 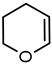 | 38 49 a | 61 72 a | 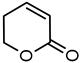 |
| 6 | 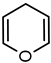 | 68 | 25 |  |
| 7 | 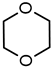 | 11 | 72 | 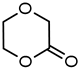 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduh, N.A.Y.; Al-Kahtani, A.A.; Amer, M.S.; Algarni, T.S.; Al-Odayni, A.-B. Fabricated Gamma-Alumina-Supported Zinc Ferrite Catalyst for Solvent-Free Aerobic Oxidation of Cyclic Ethers to Lactones. Molecules 2023, 28, 7192. https://doi.org/10.3390/molecules28207192
Abduh NAY, Al-Kahtani AA, Amer MS, Algarni TS, Al-Odayni A-B. Fabricated Gamma-Alumina-Supported Zinc Ferrite Catalyst for Solvent-Free Aerobic Oxidation of Cyclic Ethers to Lactones. Molecules. 2023; 28(20):7192. https://doi.org/10.3390/molecules28207192
Chicago/Turabian StyleAbduh, Naaser A. Y., Abdullah A. Al-Kahtani, Mabrook S. Amer, Tahani Saad Algarni, and Abdel-Basit Al-Odayni. 2023. "Fabricated Gamma-Alumina-Supported Zinc Ferrite Catalyst for Solvent-Free Aerobic Oxidation of Cyclic Ethers to Lactones" Molecules 28, no. 20: 7192. https://doi.org/10.3390/molecules28207192
APA StyleAbduh, N. A. Y., Al-Kahtani, A. A., Amer, M. S., Algarni, T. S., & Al-Odayni, A.-B. (2023). Fabricated Gamma-Alumina-Supported Zinc Ferrite Catalyst for Solvent-Free Aerobic Oxidation of Cyclic Ethers to Lactones. Molecules, 28(20), 7192. https://doi.org/10.3390/molecules28207192









