Ruthenium-Catalyzed Dehydrogenative Intermolecular O-H/Si-H/C-H Silylation: Synthesis of (E)-Alkenyl Silyl-Ether and Silyl-Ether Heterocycle
Abstract
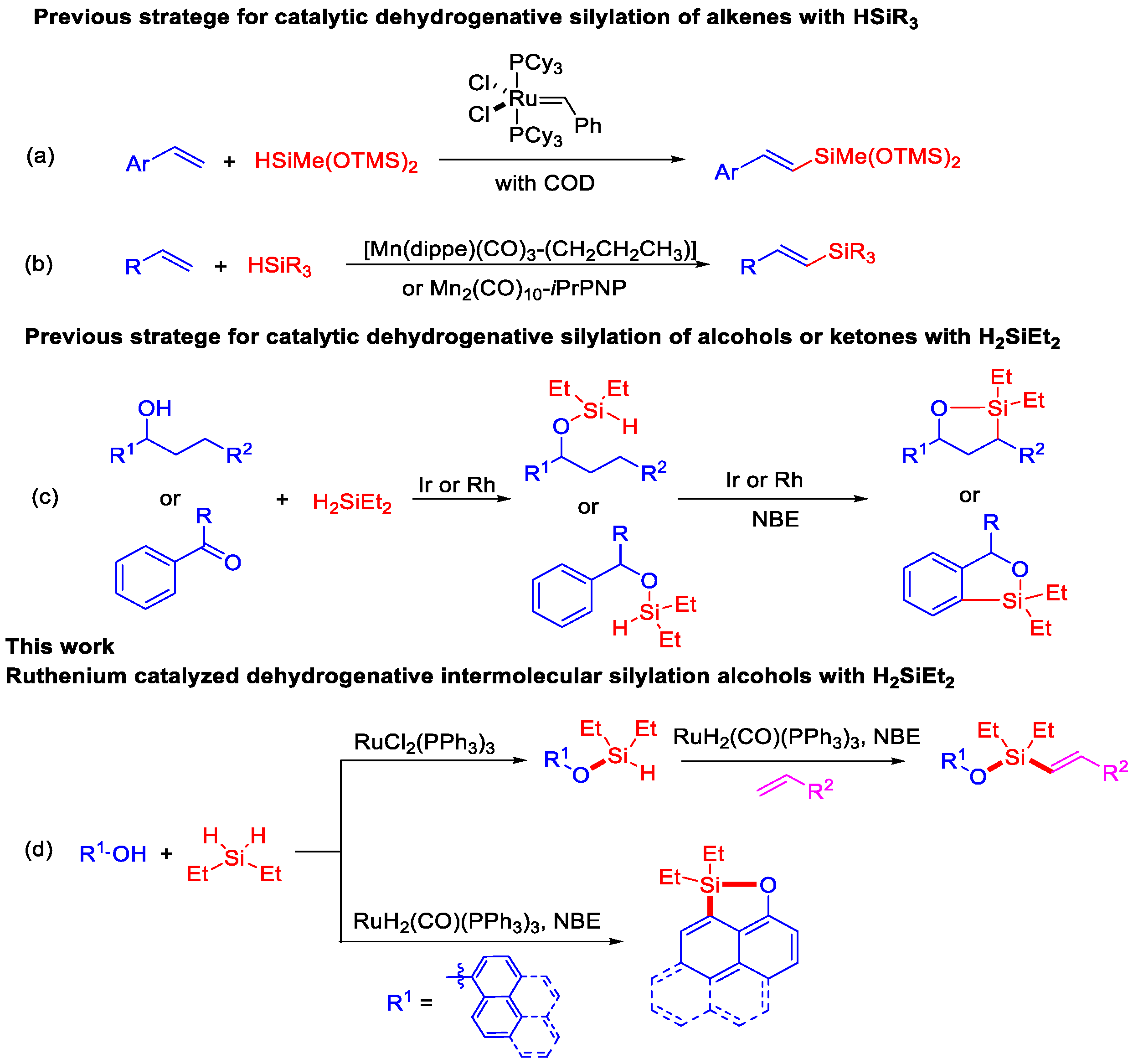
:1. Introduction
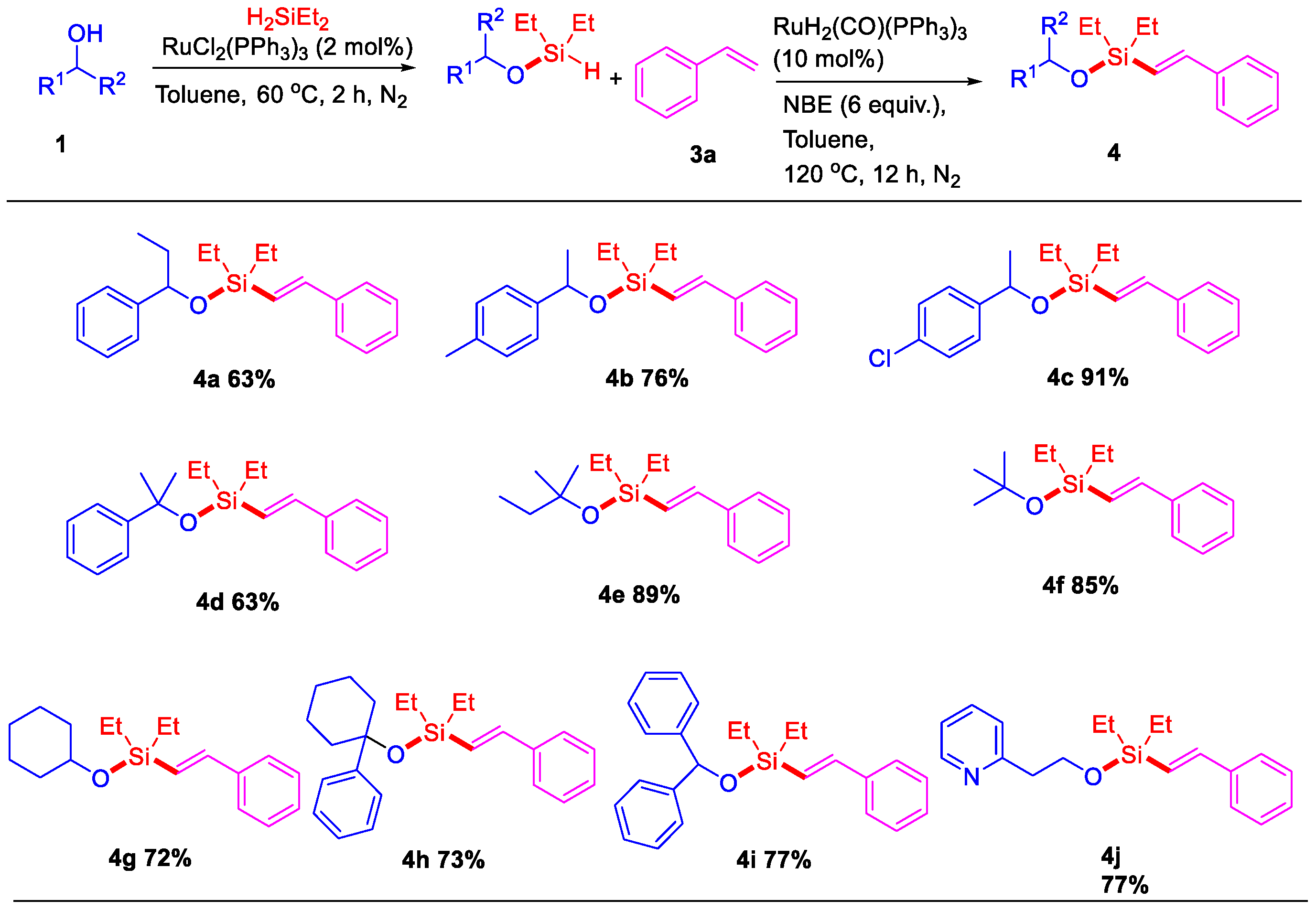
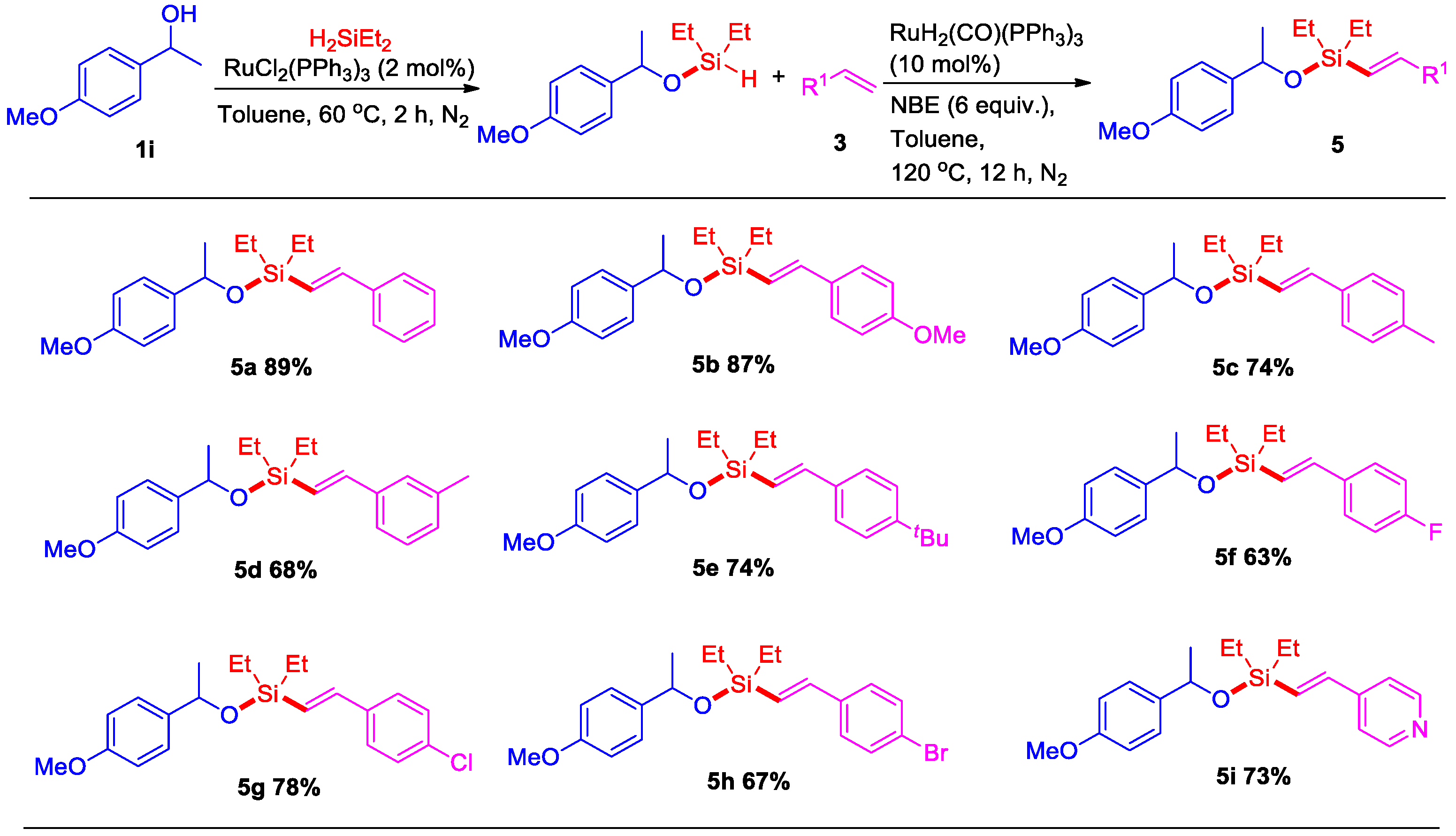
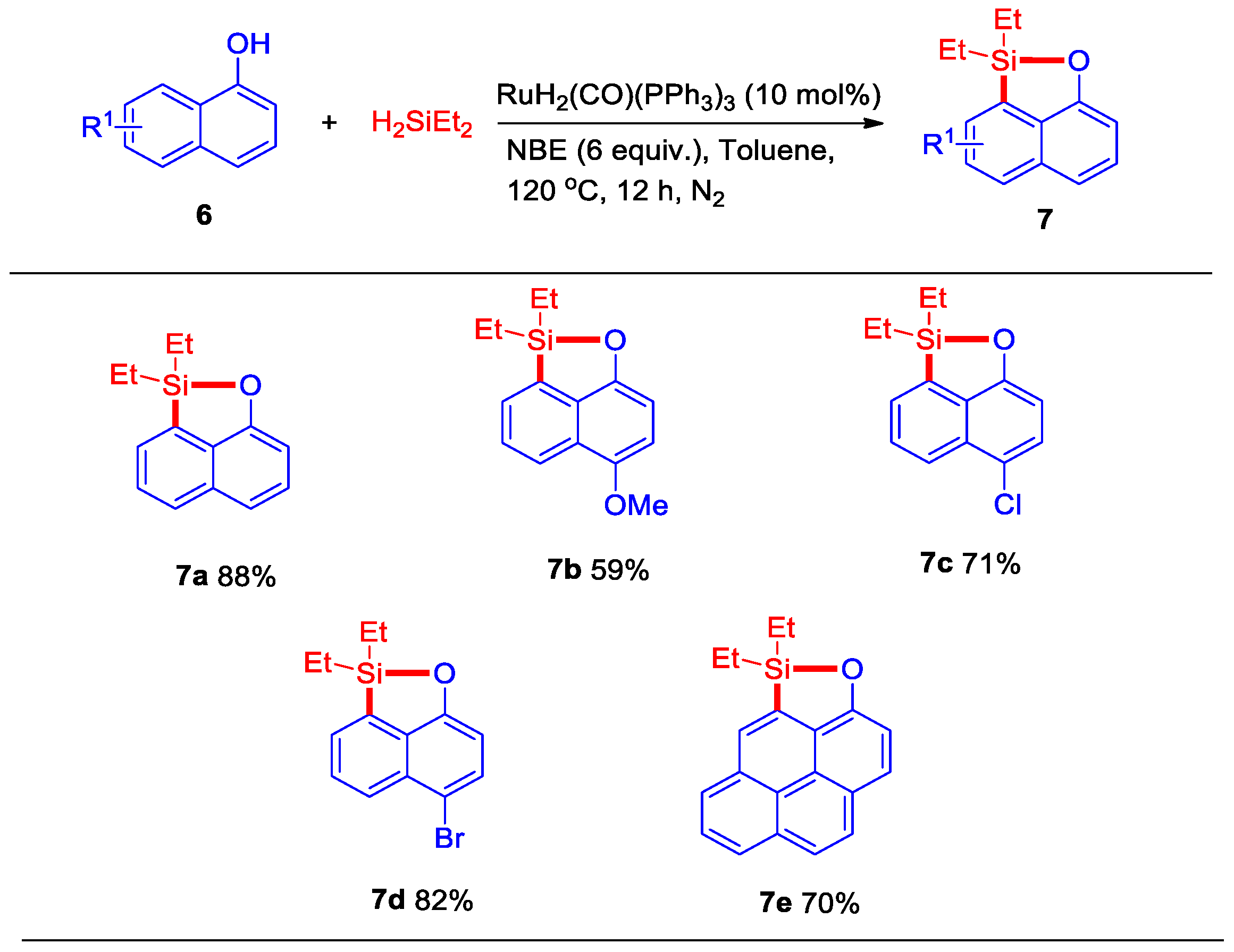
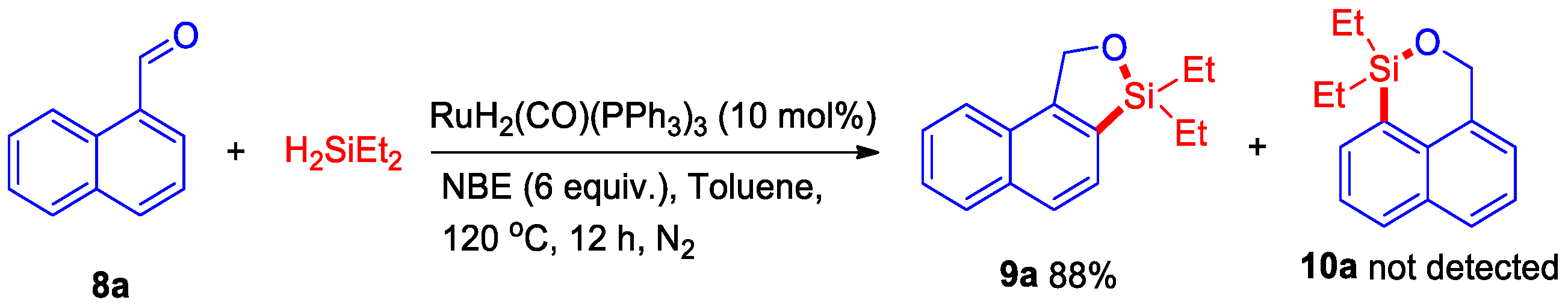
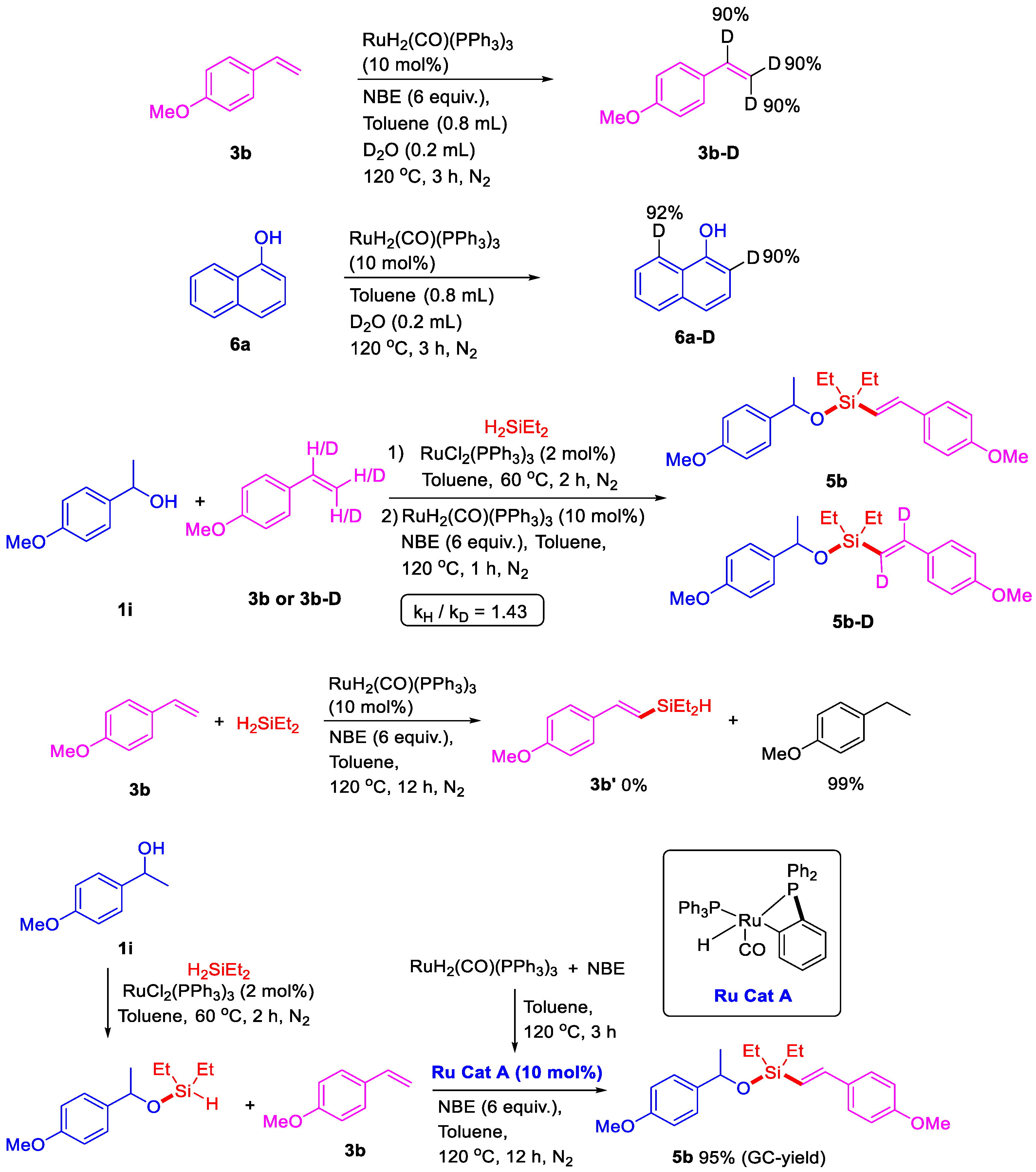
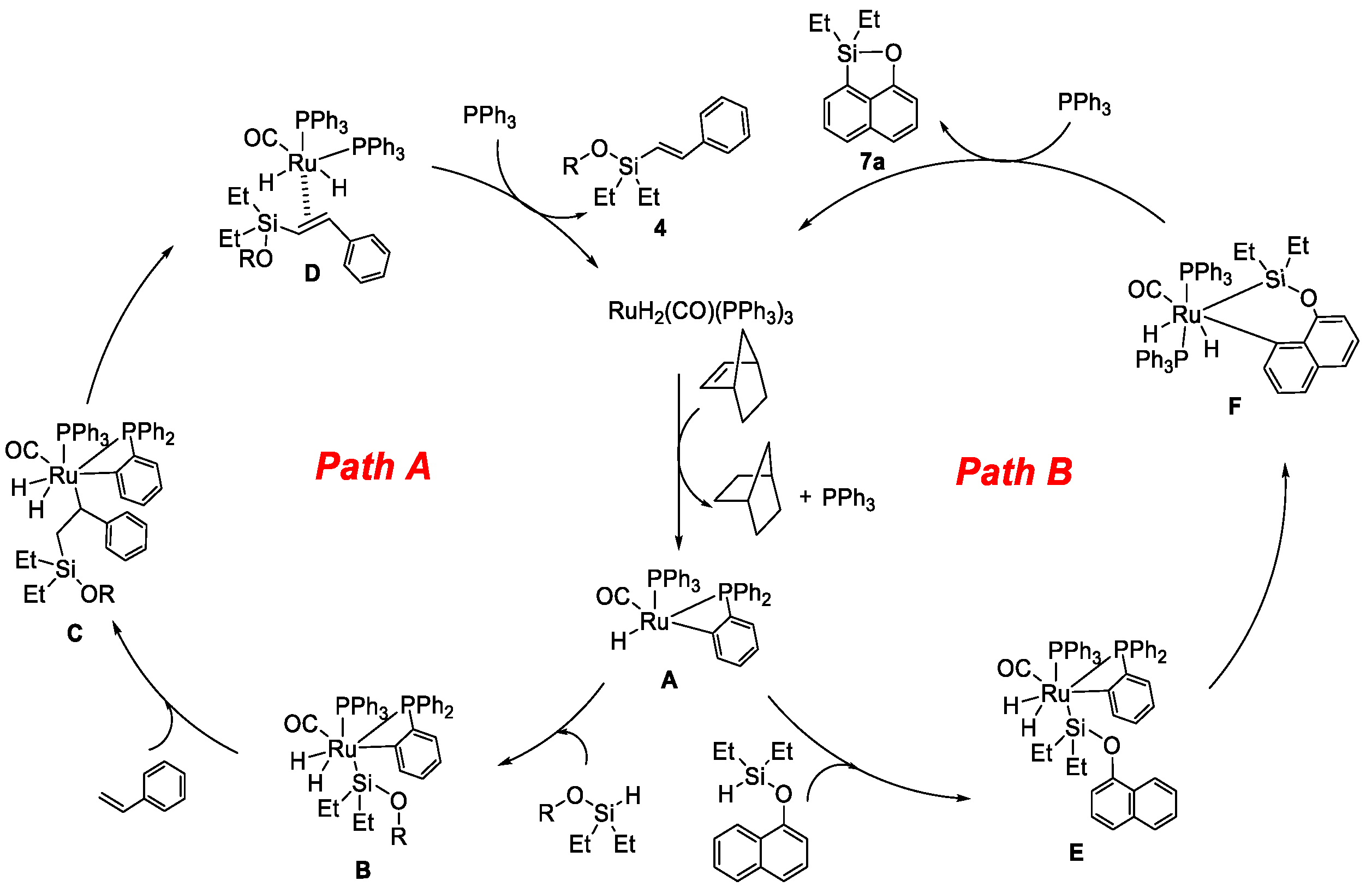
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Ruthenium-Catalyzed Dehydrogenative Intermolecular O–H/Si–H/C–H Silylation of Alcohols with Alkenes
3.3. General Procedure for Ruthenium-Catalyzed Dehydrogenative Intermolecular O–H/Si–H/C–H silylation of naphthalen-1-ol Derivatives
3.4. Characterization Data of Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Min, G.K.; Hernandez, D.; Skrydstrup, T. Efficient Routes to Carbon-Silicon Bond Formation for the Synthesis of SiliconContaining Peptides and Azasilaheterocycles. Acc. Chem. Res. 2013, 46, 457–470. [Google Scholar] [CrossRef]
- Hofmann, R.J.; Vlatkovic, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, NonThermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534–571. [Google Scholar] [PubMed]
- Cheng, C.; Hartwig, J.F. Catalytic Silylation of Unactivated C-H Bonds. Chem. Rev. 2015, 115, 8946–8975. [Google Scholar] [PubMed]
- Li, B.; Dixneuf, P.H. Metal-Catalyzed Silylation of sp3C-H Bonds. Chem. Soc. Rev. 2021, 50, 5062–5085. [Google Scholar] [PubMed]
- Ge, Y.; Huang, X.; Ke, J.; He, C. Transition-Metal-Catalyzed Enantioselective C-H Silylation. Chem. Catal. 2022, 2, 2898–2928. [Google Scholar]
- Ren, L.-Q.; Li, N.; Ke, J.; He, C. Recent Advances in Photo- and Electro-Enabled Radical Silylation. Org. Chem. Front. 2022, 9, 6400–6415. [Google Scholar]
- Hou, J.; Han, X.; Zhang, Y.; Huang, J.; Wang, J.; Yuan, K. Triflic Acid/Silane Promoted Deoxygenative Transformation of Ketones via Carbocations. Org. Lett. 2023, 25, 5709–5713. [Google Scholar] [CrossRef]
- McAtee, J.R.; Martin, S.E.S.; Ahneman, D.T.; Johnson, K.A.; Watson, D.A. Preparation of Allyl and Vinyl Silanes by the Palladium-Catalyzed Silylation of Terminal Olefins: A Silyl-Heck Reaction. Angew. Chem. Int. Ed. 2012, 51, 3663–3667. [Google Scholar] [CrossRef]
- Martin, S.E.S.; Watson, D.A. Preparation of Vinyl Silyl Ethers and Disiloxanes via the Silyl-Heck Reaction of Silyl Ditriflates. J. Am. Chem. Soc. 2013, 135, 13330–13333. [Google Scholar] [CrossRef]
- Matsumoto, K.; Huang, J.; Naganawa, Y.; Guo, H.; Beppu, T.; Sato, K.; Shimada, S.; Nakajima, Y. Direct Silyl-Heck Reaction of Chlorosilanes. Org. Lett. 2018, 20, 2481–2484. [Google Scholar] [CrossRef]
- Rémond, E.; Martin, C.; Martinez, J.; Cavelier, F. Silicon-Containing Amino Acids: Synthetic Aspects, Conformational Studies, and Applications to Bioactive Peptides. Chem. Rev. 2016, 116, 11654–11684. [Google Scholar]
- Pérez-Torrente, J.J.; Nguyen, D.H.; Jiménez, M.V.; Modrego, F.J.; Puerta-Oteo, R.; Gómez-Bautista, D.; Iglesias, M.; Oro, L.A. Hydrosilylation of Terminal Alkynes Catalyzed by a ONOPincer Iridium(III) Hydride Compound: Mechanistic Insights into the Hydrosilylation and Dehydrogenative Silylation Catalysis. Organometallics 2016, 35, 2410–2422. [Google Scholar]
- Marciniec, B. Catalysis by Transition Metal Complexes of Alkene Silylation-Recent Progress and Mechanistic Implications. Coordin. Chem. Rev. 2005, 249, 2374–2390. [Google Scholar]
- Zhang, L.; Hang, Z.; Liu, Z.-Q. A Free-Radical-Promoted Stereospecific Decarboxylative Silylation of α,β-Unsaturated Acids with Silanes. Angew. Chem. Int. Ed. 2016, 55, 236–239. [Google Scholar]
- Lu, C.; Lin, Y.; Wang, M.; Zhou, J.; Wang, S.; Jiang, H.; Kang, K.; Huang, L. Nickel-Catalyzed Ring-Opening of Benzofurans for the Divergent Synthesis of ortho-Functionalized Phenol Derivatives. ACS Catal. 2023, 13, 2432. [Google Scholar] [CrossRef]
- Liu, W.; Lu, W.; Yang, L.; Wu, X.; Zhang, Z. Rhodium-Catalyzed anti-Markovnikov Hydrosilylation of Alkenes. Tetrahedron 2022, 109, 132632. [Google Scholar]
- Greenhalgh, M.D.; Frank, D.J.; Thomas, S.P. Iron-Catalysed Chemo-, Regio-, and Stereoselective Hydrosilylation of Alkenes and Alkynes using a Bench-Stable Iron(II) Pre-Catalyst. Adv. Synth. Catal. 2014, 356, 584–590. [Google Scholar]
- Lu, W.; Li, C.; Wu, X.; Xie, X.; Zhang, Z. [Rh(COD)Cl]2/PPh3-Catalyzed Dehydrogenative Silylation of Styrene Derivatives with NBE as a Hydrogen Acceptor. Organometallics 2020, 39, 3780–3788. [Google Scholar] [CrossRef]
- Zhu, F.; Spannenberg, A.; Wu, X. Rhodium-Catalyzed Carbonylative Synthesis of Silyl-substituted Indenones. Chem. Commun. 2017, 53, 13149–13152. [Google Scholar]
- Chen, B.; Wu, X. Rhodium-Catalyzed Carbonylative Synthesis of Benzosilinones. Org. Lett. 2019, 21, 2899–2902. [Google Scholar] [CrossRef]
- Cheng, C.; Simmons, E.M.; Hartwig, J.F. Iridium-Catalyzed, Diastereoselective Dehydrogenative Silylation of Terminal Alkenes with (TMSO)2MeSiH. Angew. Chem. Int. Ed. 2013, 52, 8984–8989. [Google Scholar]
- Bokka, A.; Jeon, J. Regio- and Stereoselective Dehydrogenative Silylation and Hydrosilylation of Vinylarenes Catalyzed by Ruthenium Alkylidenes. Org. Lett. 2016, 18, 5324–5327. [Google Scholar] [PubMed]
- Naumov, R.N.; Itazaki, M.; Kamitani, M.; Nakazawa, H. Selective Dehydrogenative Silylation-Hydrogenation Reaction of Divinyldisiloxane with Hydrosilane Catalyzed by an Iron Complex. J. Am. Chem. Soc. 2012, 134, 804–807. [Google Scholar] [CrossRef]
- Gu, J.; Cai, C. Stereoselective Synthesis of Vinylsilanes via Copper-Catalyzed Silylation of Alkenes with Silanes. Chem. Commun. 2016, 52, 10779–10782. [Google Scholar]
- Weber, S.; Glavic, M.; Stöger, B.; Pittenauer, E.; Podewitz, M.; Veiros, L.F.; Kirchner, K. Manganese-Catalyzed Dehydrogenative Silylation of Alkenes Following Two Parallel Inner-Sphere Pathways. J. Am. Chem. Soc. 2021, 143, 17825–17832. [Google Scholar]
- Dong, J.; Yuan, X.-A.; Yan, Z.; Mu, L.; Junyang, M.J.; Zhu, C.; Xie, J. Manganese-Catalysed Divergent Silylation of Alkenes. Nat. Chem. 2021, 13, 182–190. [Google Scholar] [PubMed]
- Li, B.; Driess, M.; Hartwig, J.F. Iridium-Catalyzed Regioselective Silylation of Secondary Alkyl C-H Bonds for the Synthesis of 1,3-Diols. J. Am. Chem. Soc. 2014, 136, 6586–6589. [Google Scholar]
- Lee, T.; Wilson, T.W.; Berg, R.; Ryberg, P.; Hartwig, J.F. Rhodium-Catalyzed Enantioselective Silylation of Arene C-H Bonds: Desymmetrization of Diarylmethanols. J. Am. Chem. Soc. 2015, 137, 6742–6745. [Google Scholar] [PubMed]
- Lin, Q.; Lin, Z.; Pan, M.; Zheng, Q.; Li, H.; Chen, X.; Darcel, C.; Dixneuf, P.H.; Li, B. Alkene as Hydrogen Trapper to Control the Regio-Selective Ruthenium(II) Catalyzed Ortho C-H Silylation of Amides and Anilides. Org. Chem. Front. 2021, 8, 514–521. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Lin, Q.; Huang, Y.; Li, B. Ruthenium(II) Acetate Catalyzed Synthesis of Silylated Oxazoles via C-H Silylation and Dehalogenation. Org. Lett. 2019, 21, 1134–1138. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Q.; Liao, C.; Chen, J.; Zhang, K.; Liu, Q.; Li, B. Ruthenium(II)/Acetate Catalyzed Intermolecular Dehydrogenative Ortho C-H Silylation of 2-Aryl N-Containing Heterocycles. Org. Biomol. Chem. 2019, 17, 4115–4120. [Google Scholar] [PubMed]
- Chatterjee, B.; Gunanathan, C. Ruthenium Catalyzed Selective Hydrosilylation of Aldehydes. Chem. Commun. 2014, 50, 888–890. [Google Scholar]
- Kakiuchi, F.; Kochi, T.; Mizushima, E.; Murai, S. Room-Temperature Regioselective C-H/Olefifin Coupling of Aromatic Ketones Using an Activated Ruthenium Catalyst with a Carbonyl Ligand and Structural Elucidation of Key Intermediates. J. Am. Chem. Soc. 2010, 132, 17741–17750. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.L.; Sabo-Etienne, S.; Chaudret, B. Highly Selective Dehydrogenative Silylation of Ethylene Using the Bis(dihydrogen) Complex RuH2(H2)2(PCy3)2 as Catalyst Precursor. Organometallics 1995, 14, 1082–1084. [Google Scholar] [CrossRef]
- Parija, A.; Sunoj, R.B. Mechanism of Catalytic Functionalization of Primary C-H Bonds Using a Silylation Strategy. Org. Lett. 2013, 15, 4066–4069. [Google Scholar] [PubMed]
- Delpech, F.; Mansas, J.; Leuser, H.; Sabo-Etienne, S.; Chaudret, B. Ruthenium-Catalyzed Silylation of Ethylene by Disilanes. Organometallics 2000, 19, 5750–5757. [Google Scholar]
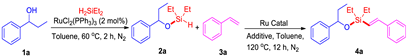
| Entry | Ru Catalyst | Additive | Solvent | Yield (%) |
|---|---|---|---|---|
| 1 | RuHCl(CO)(PPh3)3 | NBE | toluene | 88 |
| 2 | [RuCl2(p-cymene)]2 | NBE | toluene | 50 |
| 3 | [RuCl2(COD)]n | NBE | toluene | 12 |
| 4 | RuH2(CO)(PPh3)3 | NBE | toluene | 98 |
| 5 | RuCl2(PPh3)3 | NBE | toluene | 53 |
| 6 | Ru3(CO)12 | NBE | toluene | 42 |
| 7 | RuH2(CO)(PPh3)3 | NBE | toluene | 74 b |
| 8 | RuH2(CO)(PPh3)3 | cyclohexene | toluene | 7 |
| 9 | RuH2(CO)(PPh3)3 | t-butyl acrylate | toluene | 11 |
| 10 | RuH2(CO)(PPh3)3 | ɑ-methylstyrene | toluene | 44 |
| 11 | RuH2(CO)(PPh3)3 | NBE | toluene | 15 c |
| 12 | RuH2(CO)(PPh3)3 | NBE | MeOH | trace d |
| 13 | RuH2(CO)(PPh3)3 | NBE | THF | trace d |
| 14 | RuH2(CO)(PPh3)3 | NBE | CH3CN | trace c |
| 15 | RuH2(CO)(PPh3)3 | NBE | 1,4-dioxane | 13 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Lin, Q.; Li, J.; Xu, S.; Lv, S.; Xie, F.; Wang, J.; Li, B. Ruthenium-Catalyzed Dehydrogenative Intermolecular O-H/Si-H/C-H Silylation: Synthesis of (E)-Alkenyl Silyl-Ether and Silyl-Ether Heterocycle. Molecules 2023, 28, 7186. https://doi.org/10.3390/molecules28207186
Huang Z, Lin Q, Li J, Xu S, Lv S, Xie F, Wang J, Li B. Ruthenium-Catalyzed Dehydrogenative Intermolecular O-H/Si-H/C-H Silylation: Synthesis of (E)-Alkenyl Silyl-Ether and Silyl-Ether Heterocycle. Molecules. 2023; 28(20):7186. https://doi.org/10.3390/molecules28207186
Chicago/Turabian StyleHuang, Ziwei, Qiao Lin, Jiefang Li, Shanshan Xu, Shaohuan Lv, Feng Xie, Jun Wang, and Bin Li. 2023. "Ruthenium-Catalyzed Dehydrogenative Intermolecular O-H/Si-H/C-H Silylation: Synthesis of (E)-Alkenyl Silyl-Ether and Silyl-Ether Heterocycle" Molecules 28, no. 20: 7186. https://doi.org/10.3390/molecules28207186
APA StyleHuang, Z., Lin, Q., Li, J., Xu, S., Lv, S., Xie, F., Wang, J., & Li, B. (2023). Ruthenium-Catalyzed Dehydrogenative Intermolecular O-H/Si-H/C-H Silylation: Synthesis of (E)-Alkenyl Silyl-Ether and Silyl-Ether Heterocycle. Molecules, 28(20), 7186. https://doi.org/10.3390/molecules28207186





