Abstract
Eucalyptus, a therapeutic plant mentioned in the ancient Algerian pharmacopeia, specifically two species belonging to the Myrtaceae family, E. radiata and E. cinerea, were investigated in this study for their antibacterial, antioxidant, and anti-inflammatory properties. The study used aqueous extracts (AE) obtained from these plants, and the extraction yields were found to be different. The in vitro antibacterial activity was evaluated using a disc diffusion assay against three typical bacterial strains. The results showed that the two extracts were effective against all three strains. Both extracts displayed significant antioxidant activity compared to BHT. The anti-inflammatory impact was evaluated using a protein (BSA) inhibition denaturation test. The E. radiata extract was found to inhibit inflammation by 85% at a concentration of 250 µg/mL, significantly higher than the Aspirin. All phytoconstituents present good pharmacokinetic characteristics without toxicity except very slight toxicity of terpineol and cineol and a maximum binding energy of −7.53 kcal/mol for its anti-TyrRS activity in silico. The study suggests that the extracts and their primary phytochemicals could enhance the efficacy of antibiotics, antioxidants, and non-steroidal anti-inflammatory drugs (NSAIDs). As pharmaceutical engineering experts, we believe this research contributes to developing natural-based drugs with potential therapeutic benefits.
1. Introduction
Eucalyptus is a member of the Myrtaceae family [1,2] and is native to Australia [3,4]. It is known for its adaptability to different environmental conditions, high productivity, and effortless harvesting, making it a sustainable feedstock supply for phytoconstituents [5,6]. The plant contains a variety of volatile and non-volatile chemicals with diverse biological functions [7]. Extracts from the leaves have been reported to contain phenolics and flavonoids with antioxidant and antimicrobial properties [8], while constituents such as alkaloids, polyphenols, and propanoids exhibit anti-inflammatory, antibacterial, and antiseptic properties [9].
Although most research on the phytochemistry of Eucalyptus has focused on the plant’s essential oils, many non-volatile compounds, including triterpenoids, flavonoids, and tannins, have been isolated from this genus [7]. However, little information is available regarding the potential bioactivities of crude extracts from Eucalyptus, particularly traditional preparations [7].
To address this gap in knowledge, researchers used decoctions as it is the most usual way people take it [7]. Given current ethical concerns about animal testing, the researchers opted to use in vitro and in silico methods to investigate the antibacterial, antioxidant, and anti-inflammatory properties of aqueous extracts of two species of Eucalyptus [10,11]. In silico methods such as virtual screening can be used to examine plant compounds’ receptor interactions quickly and cost-effectively [11]. The study focused on two species of Eucalyptus cultivated in Algeria, for which no reports on the bioactivities of the aqueous extract of the leaves have been found [7].
2. Results
2.1. Extraction Yield
In this context, “yield” refers to the ratio of the amount of extract collected to the total mass of the plants used. The percentages of E. radiata and E. cinerea recovered during the extraction were 27.83 and 4.84%, respectively.
Antibacterial Activity
It was shown that both extracts had substantial antibacterial activity against the tested microorganisms (Table 1). Even so, E. cinerea demonstrated a zone of inhibition of 22.6 mm in diameter against S. aureus, which is a remarkable result. P. aeruginosa, on the other hand, was resistant from the 100 mg/mL concentration. Therefore, only its minimal diameters at 200 and 150 mg/mL were displayed.

Table 1.
Inhibition diameters (mm) of the extracts against the strains tested.
2.2. Antioxidant Activity
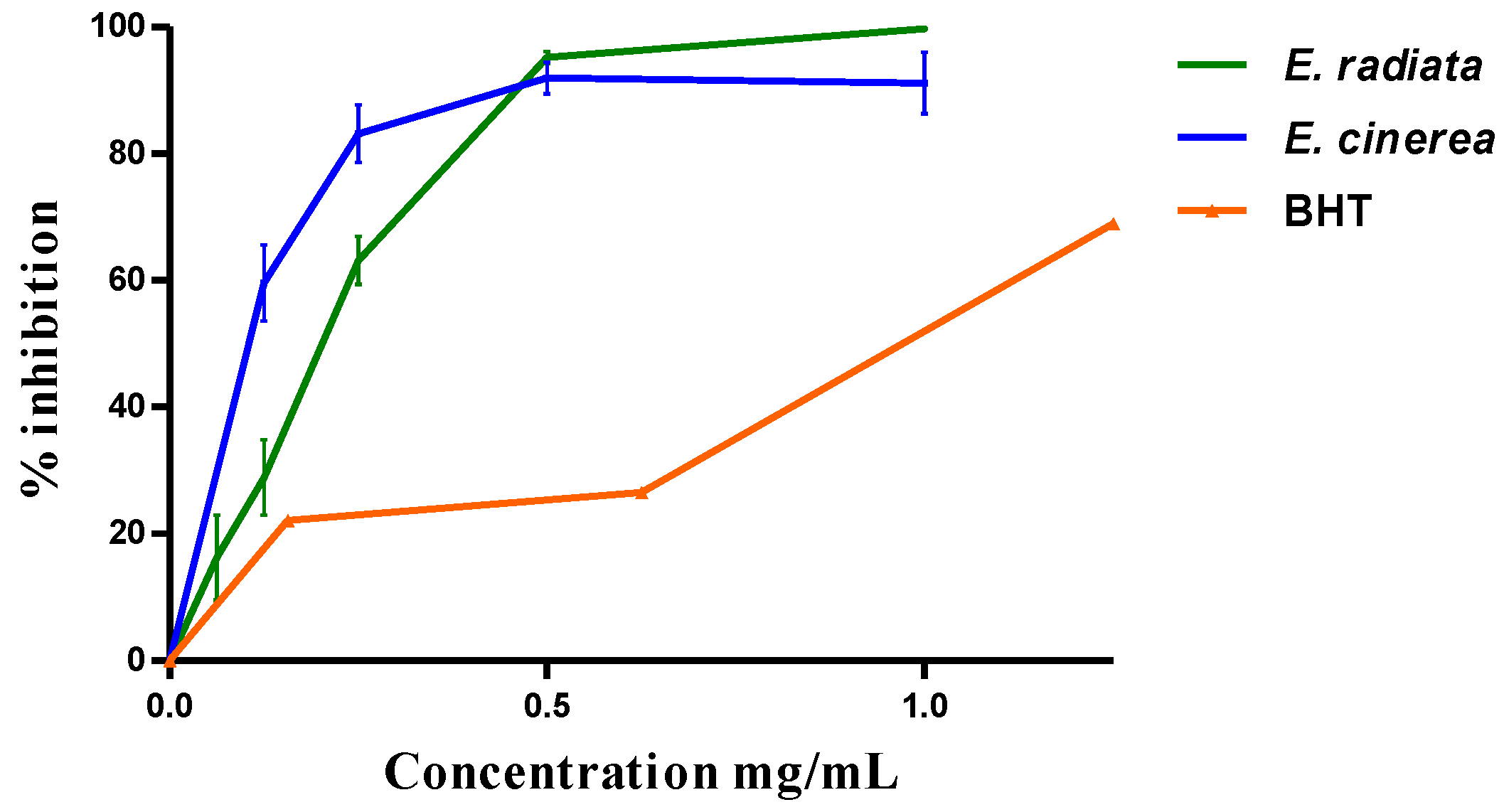
Globally, the two AEs have antioxidant activity. The reduction in DPPH was dose-dependent (Figure 1) with an IC50 of 0.19 + 0.03 mg/mL by E. radiata and 0.15 ± 0.08 mg/mL by E. cinerea compared to the BHT (0.94 ± 0.37 mg/mL).
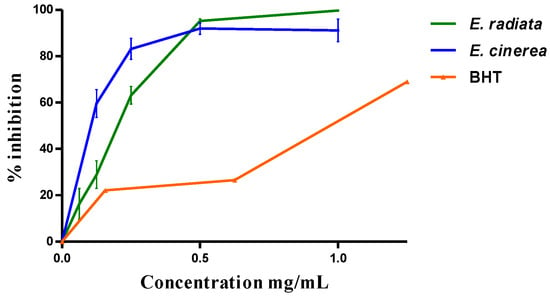
Figure 1.
DPPH scavenging effect of the AEs and this of BHT.
2.3. Anti-Inflammatory Test
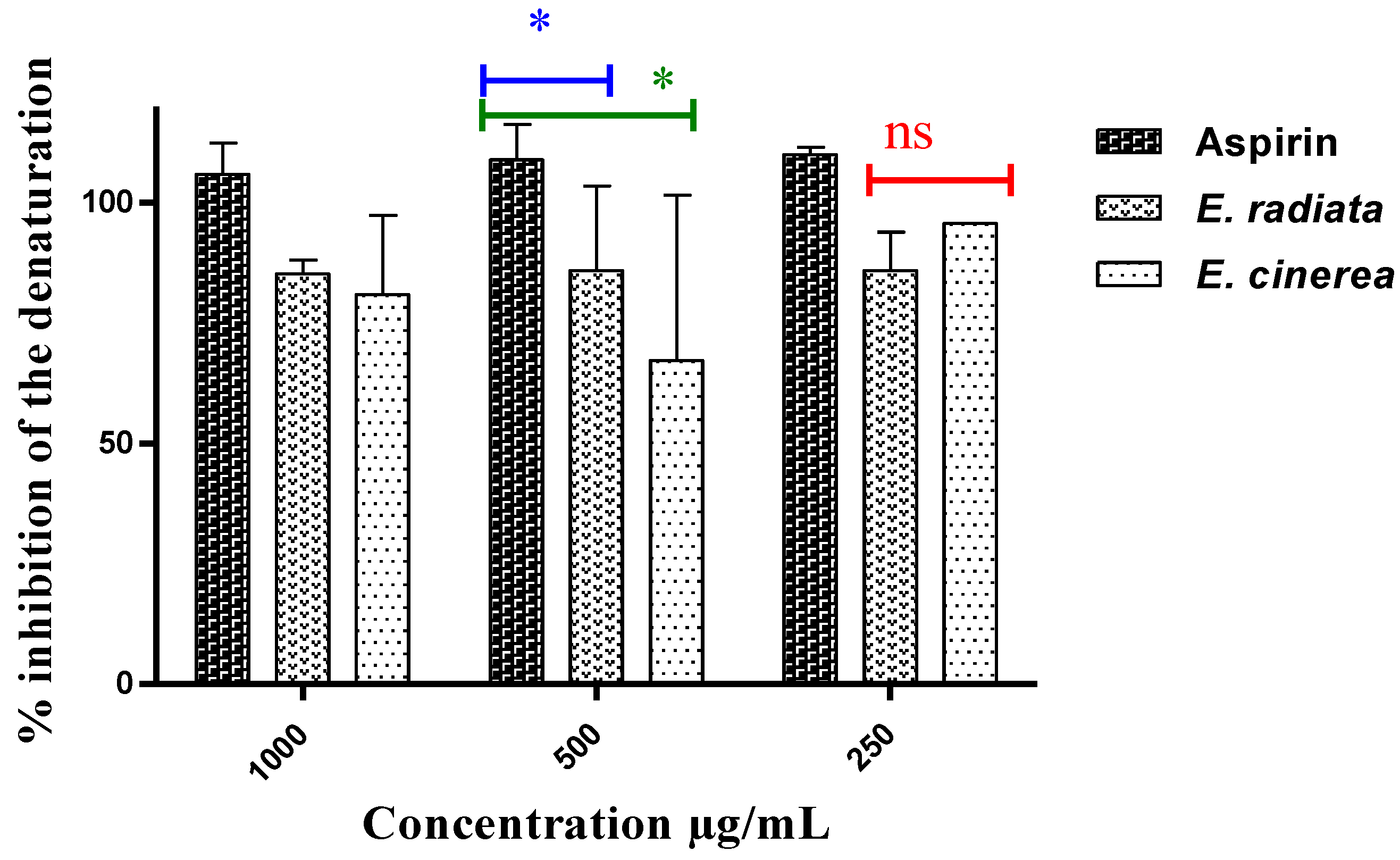
An excellent inhibitory effect on protein denaturation was observed (Figure 2), but no dose-response relationship could be established. The extract from E. radiata was more effective than that from E. cinerea AE in preventing protein denaturation (85.21–85.91% inhibition). At a concentration of 250 µg/mL, the most common NSAID, aspirin, had an inhibition of 110.09%. Statistically, the Tukey’s multiple comparison test revealed that aspirin was significantly more active than the two extracts at a p-value < 0.05.
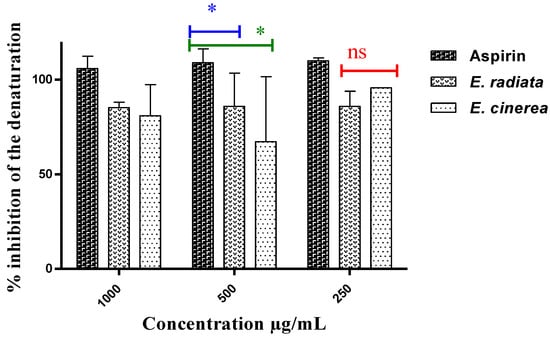
Figure 2.
Inhibition of protein denaturation by the extracts and aspirin (p < 0.05), ns: non-significant, *: Slightly significant difference.
2.4. Docking Results
The scoring phytocompounds and gentamicin are summarized in Table 2. The ellagic acid recorded the best binding energy (−7.53 kcal/mol), but it was still less than gentamicin scoring (−10.04 kcal/mol) and that of the native ligand.

Table 2.
Phytocompound scoring results.
The interactive amino acids of the protein target with phytocompounds are listed as follows (Table 3):

Table 3.
The amino acids involved in the active site of the TyrRS.
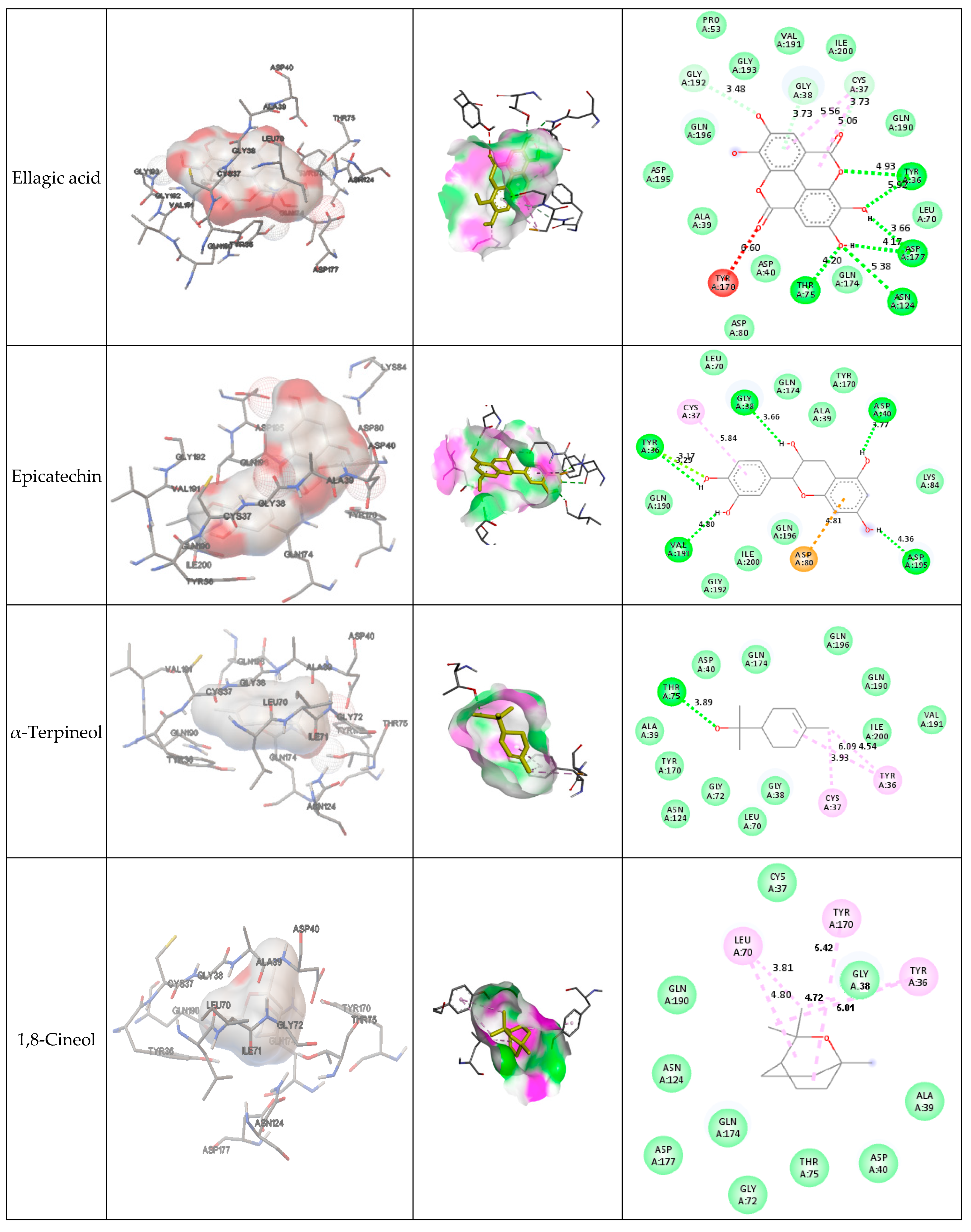
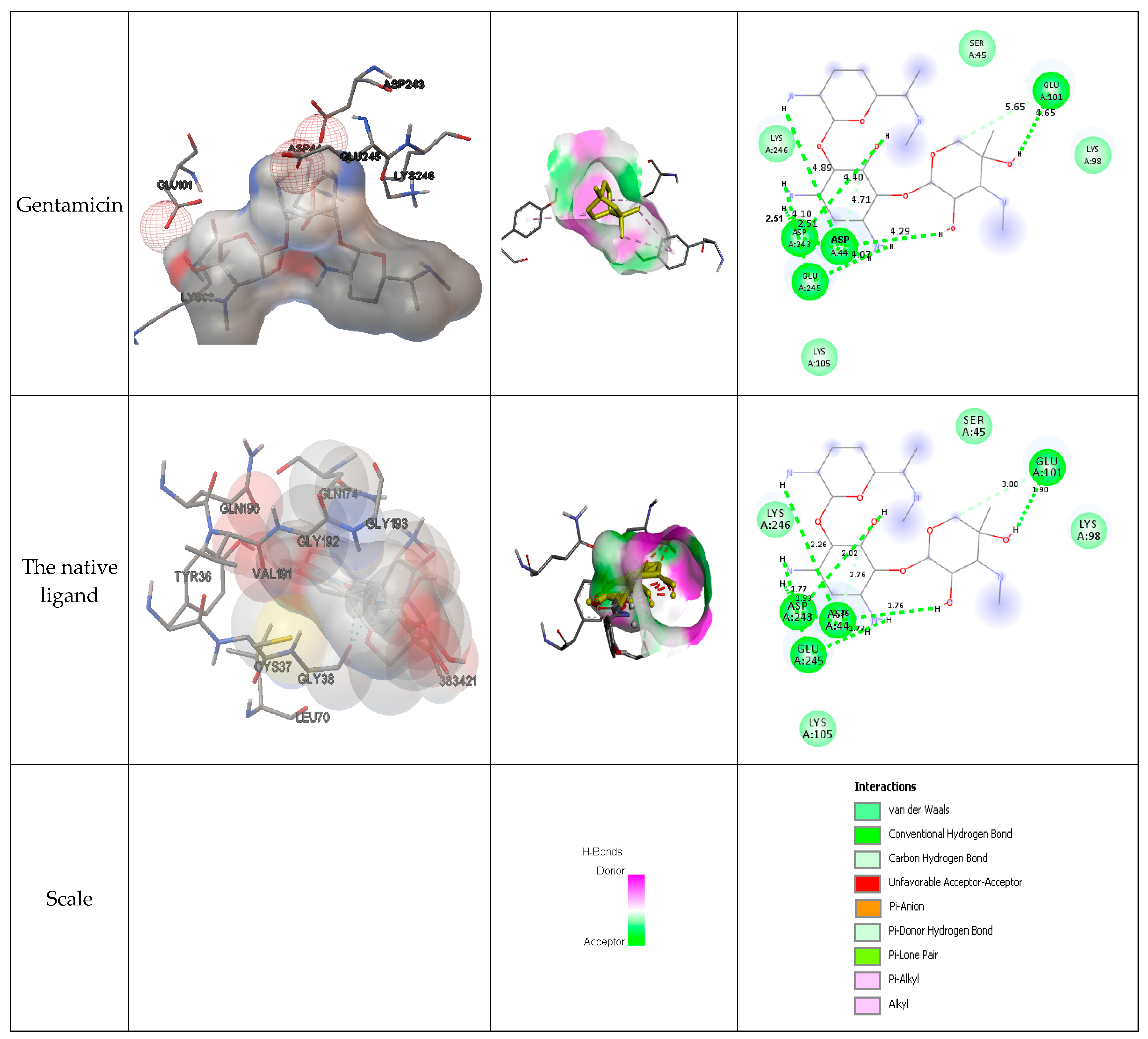
Figure 3 presents the interactions with the molecular surface around the studied phytocompounds, as ligands, at the binding site of TyrRS. The pink area shows the electron donor region, and the green represents the electron acceptor region. The 2-D structures show the amino acids involved in the active site of the enzyme as well as the nature of the bonds established.
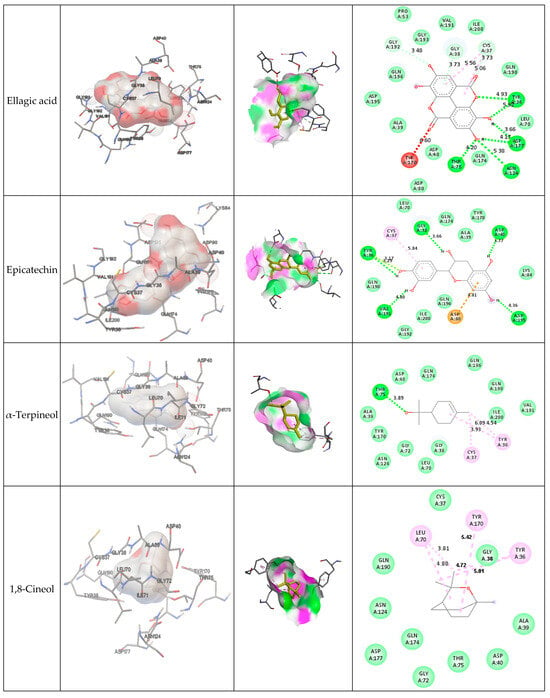
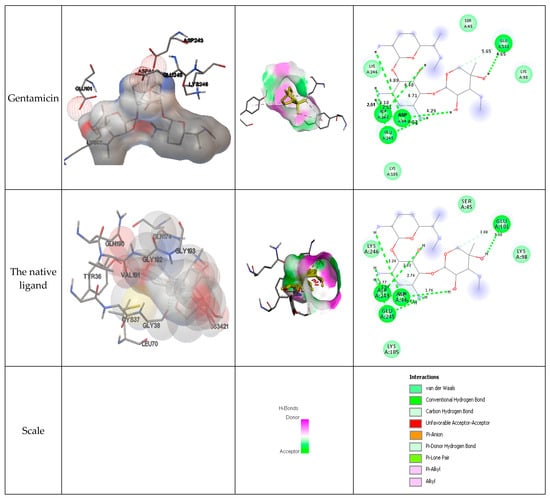
Figure 3.
Interaction and bond distances of ligands inside the active site pocket as shown by molecular surface maps.
2.5. Drug Likeness and ADME Prediction
As a result of the drug-likeness filters, expensive late-stage preclinical and clinical failure can be avoided, allowing for earlier medication development [12], phytocompounds were evaluated for their drug-like characteristics using the SWISS ADME web-based tool, and it was discovered that all compounds followed Lipinski’s rule of five, much like gentamicin (Table 4). The other calculated parameters also show that all compounds were water soluble with high intestinal absorption. Ellagic acid and epicatechin had no toxic risks, while α-terpineol could be moderately irritant. However, 1,8-cineol presented mutagenic and reproductive risks.

Table 4.
Calculated physicochemical and pharmacokinetic parameters of the docked phytocompounds.
3. Materials and Methods
3.1. The Choice of Plants
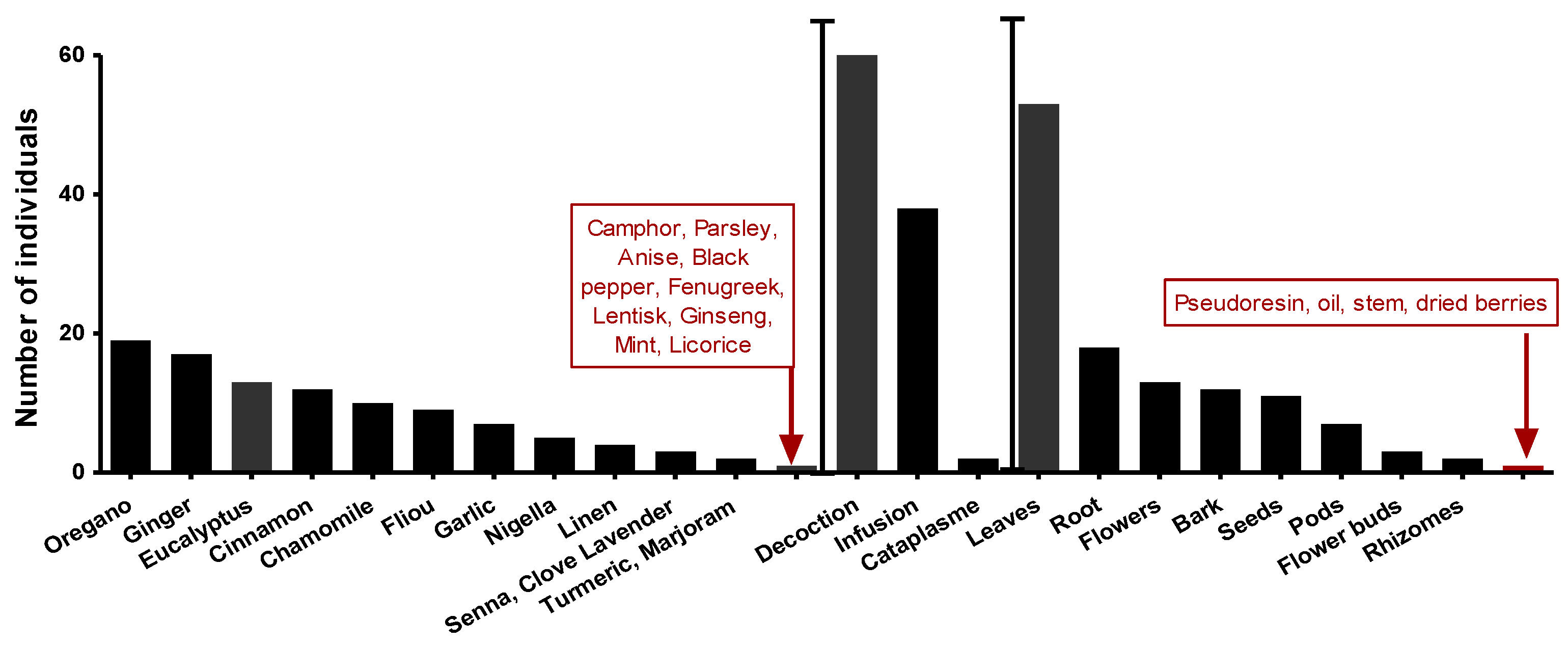
An ethnopharmacological survey (on anti-infectious plants) preceded this work spread over four months and covered all the communes of Setif (northeastern Algeria). A random sample of 75 interviewed people aged between 20 and 67. Eucalyptus (8%) after Origanum 11% and Ginger 10% among the most used plants (Figure 4). Furthermore, leaves represented 48% of plant parts used. A decoction is the most used preparation mode, with the highest rate at 60%.
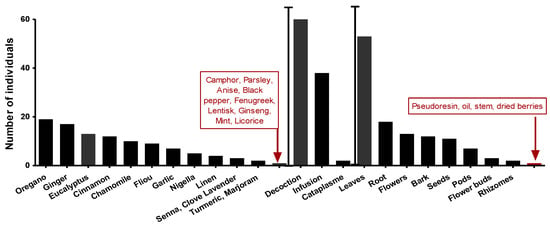
Figure 4.
The most commonly used species, plant parts, and methods of preparation.
In March 2017, young leaves of E. radiata (Figure 5) were harvested from cultivated plants on the Setif 1 university campus. After that, they are dried in the shade at room temperature. A neighborhood herbalist was the source from which we acquired the E. cinerea leaves (Figure 5). Dr. Nouioua provided the botanical confirmation of the species from the Department of Ecology in the FNLS of Sétif 1 University. A temperature of four degrees Celsius is maintained on the plants while they are being stored.

Figure 5.
(a) E. radiata, (b) E. cinerea.
For this investigation, water was selected as the solvent due to its favorable characteristics, including being the least hazardous, least expensive, and most environmentally friendly solvent. The decoction as a conventional extraction method was employed, which involved boiling 20 g of dried leaves with 500 milliliters of cold, distilled water and simmering the mixture for 20 min. After filtration, the aqueous extract (AE) was obtained and air-dried to obtain the final product. The yield percentage was calculated by the ratio between the weight of the obtained dry extract and the initial weight of the plant.
3.2. Antibacterial Activity
Three ATCC bacterial strains, including Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25922), were tested for antibacterial activity using the disc diffusion technique [13]. Filter paper discs impregnated with 50, 100, 150, and 200 mg/mL of the AE are placed on Mueller-Hinton Agar (MHA) inoculated with a 0.5 Mc Farland (108 cell/mL) standard inoculum and then incubated at 37 °C for 24 h. As a reference, we employed gentamicin. As a result, we can calculate the diameter of the inhibition zones for each disc.
3.3. Antioxidant Activity
The antioxidative activity may be tracked by measuring the rate at which the DPPH radical’s absorbance at 517 nm decreases. The method used was that of [14] with slight modifications. First, 50 µL of each AE, at varying concentrations, was mixed with 1250 µL of a methanolic solution of DPPH at 0.004%. After 30 min of incubation at room temperature in the dark, the absorbance was recorded at 517 nm. Butylated hydroxytoluene (BHT) was the standard. Thus, it was treated the same way. As a result, we may calculate the DPPH scavenging activity as follows:
where At represents the absorbance of the test and Ac represents the absorbance of the reference.
3.4. In-Vitro Anti-Inflammatory Test
Protein denaturation inhibition is measured using a modified approach version [15]. When 100 µL of plant extract at varying concentrations (250, 500, and 1000 µg/mL) was mixed with 500 µL of Bovine Serum Albumin (BSA at 1%, the resulting mixture is called a “standard” preparation. For the first 10 min, this combination was left at room temperature and then was heated to 51 °C for 20 min. After bringing the resultant solution to room temperature, its absorbance was measured at 660 nm. As a reference point for success, we used acetylsalicylic acid. Triplicates of the experiment were performed, and the percentage of inhibition of protein denaturation was determined as follows.
In this equation, A1 represents the sample absorbance, A2 denotes the product control absorbance, and A0 represents the absorbance of the positive control (aspirin solution).
GraphPad Prism 5 version 5.03 was used to create the graphs for the ethnopharmacology survey and the in vitro activities.
3.5. Molecular Docking
Phyto-Compounds
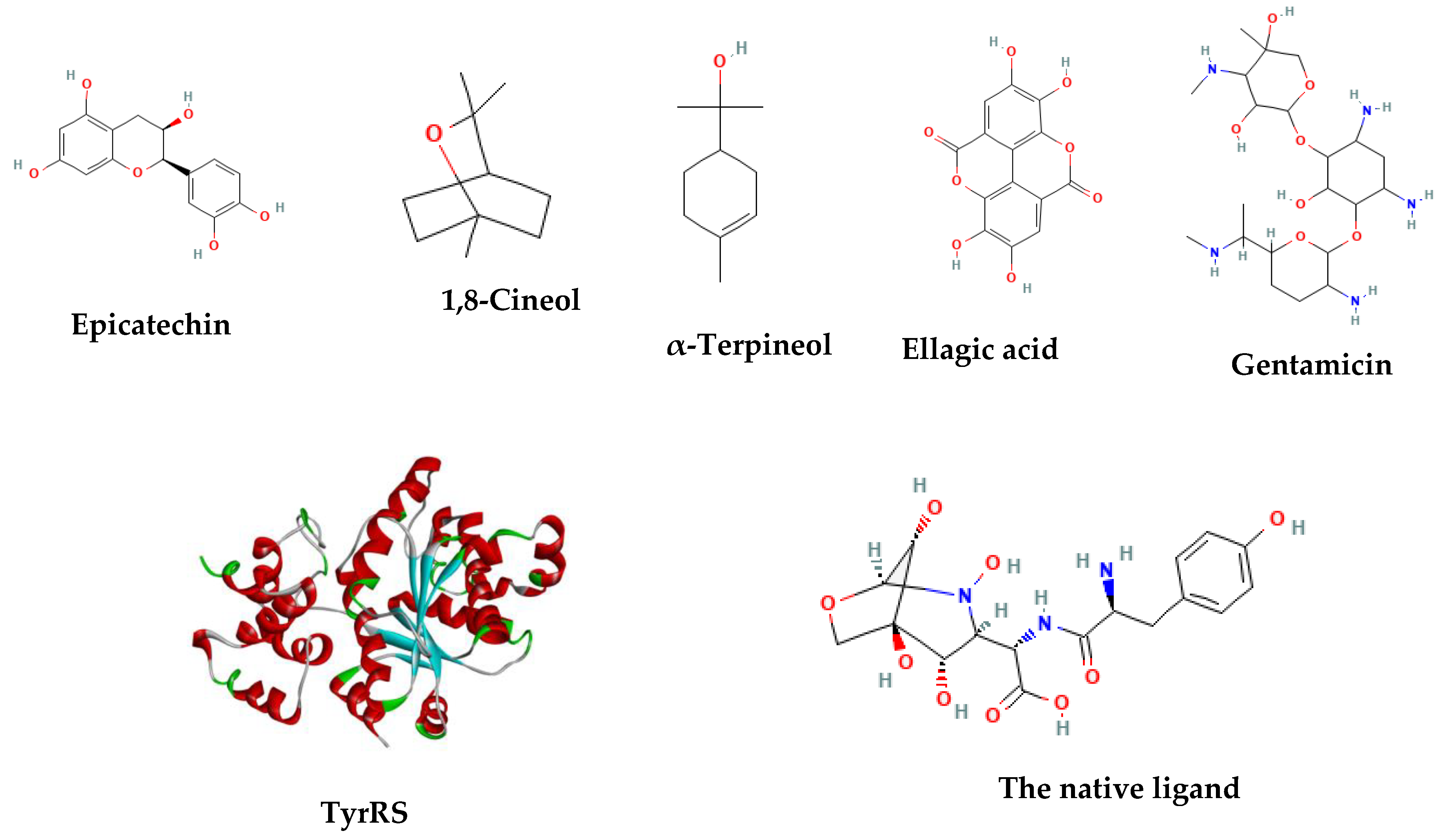
After a thorough literature analysis, the chemicals 1,8-cineol (CID 2758), ellagic acid (CID 5281855), α-terpineol (CID 17100), and epicatechin (CID 72276) were chosen as representative compounds to carry out the molecular docking investigation (Table 5). The standard antibiotic used was gentamicin (CID 3467).

Table 5.
The main phytocompounds found in aqueous extracts of Eucalyptus species.
In Figure 6, the 2-D structures and functional groups of the four main compounds present in the extracts are displayed, along with gentamicin and the native ligand of the enzyme. These compounds were subjected to in silico testing to assess their ability to impede the aminoacylation process facilitated by TyrRS (PDB code 1JII). To determine their binding affinities, components of the highly effective in vitro extracts were evaluated and compared to the standard antibiotic, gentamicin. The 3-D structures of the phytocompounds and gentamicin were obtained in CID format from the PubChem database.
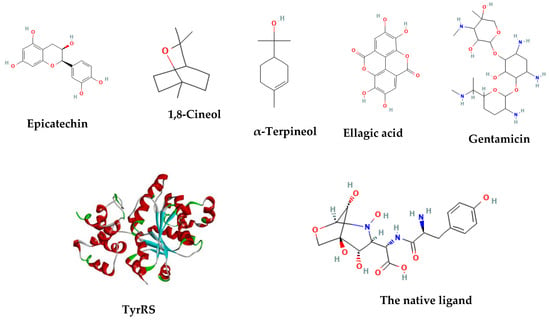
Figure 6.
The TyrRS (1JII) crystal structure and the 2D structures of the tested compounds.
3.6. Docking Analysis and Protein Preparation
AutoDock Tools 1.5.7 was utilized to determine the binding affinity of the selected phytoconstituents to the binding site of the bacterial tyrosyl-tRNA synthetase. The program implements the gradient optimization method in its local optimization process and ranks the ligands based on the empirical binding free energy (ΔG in kcal/mol) function [12]. The 3D structures of the target enzymes were obtained in PDB format from the Protein Data Bank. PDBQT format files were generated by removing water molecules, polar hydrogen, and Kollman charges were added to protein residues, and the protein’s native ligand was eliminated (2-Amino-3-(4-Hydroxyphenyl)Propionylamino)-(2,4,5,8-Tetrahydroxy-7-Oxa-2-Aza-Bi cyclo[3.2.1]Oct-3-Yl) Acetic Acid). The Lamarckian Genetic docking algorithm was employed and executed ten times. The open-source program Babel 2.4.1 was used to convert the SDF files of the substances under investigation into PDB files. The interactions were visualized in two dimensions using Discovery Studio v.16.1.0.15350 software. H-bonds between the ligands and interacting residues are depicted as lines and balls with a distance range of Å.
3.7. Drug Likeness, ADME/Toxicity Prediction
Lipinski’s method was employed to assess the drug-like properties of phytocompounds, which sets limits on four specific physicochemical parameters [27]. Typically, these are the characteristics of an orally active drug: the octanol-water partition coefficient (milogP) and the number of hydrogen bond donors (n-OH and n-NH) should not exceed 5, and the number of hydrogen bond acceptors (n-ONs) should be less than 10. The molecular weight (MW) should be below 500 D, and no more than one violation should occur [28]. Molinspiration Cheminformatics online tools and SwissADME online tools were used to predict physicochemical and pharmacokinetic parameters, while OSIRIS Property Explorer online tools were used to predict toxicity risks.
4. Discussion
According to research studies [29,30,31], decoction is Algeria’s most commonly used method for obtaining active plant chemicals. Water is preferred over organic solvents due to its safety, affordability, environmental friendliness, and accessibility for extracting phenolic compounds [32]. Traditional healers and practitioners mostly use it as a solvent [33]. However, the boiling process involved in decoction may lead to the degradation of the medicinal components of plants [34]. It has been reported that conventional extraction at 100 °C yields the highest amount of flavonoids and phenols (active compounds). However, it is impossible to obtain the best yield of all compounds simultaneously [35], as the chemical content of the extract varies depending on the preparation method [36]. A study on the aqueous leaf extracts of E. camaldulensis revealed high levels of polyphenols, saponins, and flavonoids in both qualitative and quantitative analyses [37]. The main component of Eucalyptus essential oils is 1,8-cineol, a monoterpene that dissolves in water at 19.85 °C [19] and is commonly found in Eucalyptus species [38,39,40,41,42,43].
4.1. Efficacy against Bacteria
According to [44], antibiotic-resistant bacteria significantly threaten public health globally. These synthetic antibiotics, especially in developing nations, are expensive and deficient for the treatment of diseases caused by pathogenic microorganisms and have incidental effects and adulterations [45]. However, medicinal plants have yielded antimicrobial compounds that offer a new tool for combatting bacterial illnesses [1]. The presence of phytochemicals in plant fractions, such as flavonoids and polyphenols, may be responsible for the disruptive bacterial membrane effect [8]. For bacterial illnesses such as sinusitis, sore throat, angina, cough, bronchitis, and urinary tract infections, [46] recommends a decoction of Eucalyptus leaves in water at 10–20 g/L as a daily beverage.
Similarly, the phenolic and saponin contents of the E. microcorys aqueous extract have been credited with its antibacterial capabilities [7]. Eucalyptus species have revealed strong antibacterial abilities against various bacteria [47], such as S. aureus, L. monocytogenes, Bacillus, K. pneumoniae, E. faecalis, P. aeruginosa, S. enteritidis, and E. coli. Leaf extract from E. camaldulensis showed anti-virulence and membrane disruption actions against Gram-positive L. monocytogenes [8]. However, P. aeruginosa is frequently resistant to multiple antibiotics [48] due to its outer membrane’s low permeability [49], leading to therapeutic failures [50]. It should be noted that antibacterials also target DNA topoisomerase [51] and other bacterial proteases [52].
Antioxidants in Eucalyptus extracts indicate the likely presence of compounds that can interact with free radicals by acting as an electron donor or hydrogen atom donor and producing a scavenging activity [53]. Eucalyptus leaf extracts effectively ameliorate hydrogen peroxide-induced oxidative stress by increasing cell viability, glutathione levels, and antioxidant enzyme activity and by decreasing the production of free radicals and lipid peroxidation levels [54]. The rich phenolic content of the aqueous fraction is responsible for superior antioxidant activity [8] by capturing free radicals through hydroxyl groups to reduce oxidative stress [55]. Polyphenolic substances, such as ellagic acid and epicatechin, are particularly plentiful in the Eucalyptus genus and responsible for the antioxidant effects of its extracts [32]. Ellagic acid, in particular, found in E. cinerea, has been demonstrated to possess potent antioxidant activity [39] that surpasses tocopherol [18]. Additionally, flavonoids increase the likelihood of antioxidant activity in Eucalyptus extracts [47]. Notably, conventional extraction at 90 °C for phenols is crucial for optimizing antioxidant efficiency [35].
4.2. Results in Reducing Inflammation
The determination of protein denaturation is a valuable tool for detecting anti-inflammatory compounds without the need for animal testing [56]. Protein denaturation is the primary cause of inflammation [15]. It is associated with the denaturation of tissue proteins (in vivo) and the onset of inflammatory and arthritic complications due to auto-antigen production [57]. Herbal extracts, which serve as safe and novel sources, can be evaluated for their anti-inflammatory efficacy [58], as an alternative to non-steroidal anti-inflammatory drugs, which have undesirable side effects such as gastrointestinal toxicity, renal injury, hepatotoxicity, hypertension, and allergic skin reactions [59,60]. Additionally, 1,8-cineol can be used for chronic inflammation management as a potent cytokine suppressant [39]. Inhalation of 1,8-cineol prior to the ovalbumin challenge was found to reduce the levels of pro-inflammatory cytokines TNF-α and IL-1β and prevent the decrease of the anti-inflammatory cytokine IL-10 in the bronchoalveolar fluid [41]. It is speculated that the activity may be due to the polyphenolic content, including flavonoids [61], such as ellagic acid and epicatechin. The extracts in the present study exhibited higher activity at lower concentrations. In contrast, the standard drug Aspirin showed the best activity, indicating that the anti-inflammatory activity of these extracts is not related to protection against protein denaturation.
4.3. Investigation of Docking
The use of in silico models as a preliminary screening tool for predicting a medication’s effect on cells and aiding in experimental research trial design has potential. The predictions’ results are noteworthy and helpful in designing experiments due to the likelihood of false positives in the selected chemical leads for biological activity [11]. This approach has been successful in the pharmaceutical sector [62]. The binding affinity of phytocompounds to their respective protein targets determines their scores. A higher binding affinity is achieved with lower binding energies [12]. In our study, ellagic acid (EA) exhibited the best free binding energy (−7.53 kcal/mol) and the best specific bond energies (vdW + Hbond + Desolvation) (−8.48 kcal/mol) among the docked compounds. EA has been reported to be effective against various pathogens, including bacteria, fungi, and parasites [63], and has antioxidant and anti-inflammatory properties [64]. Polyphenols such as ellagic acid and epicatechin interact with proteins primarily through hydrophobic interactions and hydrogen bonds via their aromatic and phenolic nuclei [65], consistent with our findings (Table 3). α-Terpineol also exhibited antibacterial properties [66,67], demonstrating in vitro activity against P. slundensis, E. coli, and S. aureus, while 1,8-cineol only demonstrated activity against E. coli [68]. It can be concluded that cineol, widely recognized as an antibacterial agent [69,70,71,72,73], does not act via the same mechanism as the other compounds and therefore does not have the same enzymatic target. The binding energies of the phytocompounds were ranked in the following order: Native ligand > gentamicin > ellagic acid > epicatechin > α-terpineol > 1,8-cineol. Hydrogen bonds are essential for the interaction between inhibitors and receptors [28]. The absence of hydrogen bonds in cineol (No H bonds) resulted in its inactivity and demonstrated that its primary target differs from TyrRS.
4.4. Prediction of ADME/Toxicity and Similarity to Existing Drugs
An in silico analysis was performed to investigate the pharmacokinetic and toxicity properties of the compounds. Lipinski’s drug-likeness rules were met by all compounds without any violations, except gentamicin which had two violations. Furthermore, the compounds showed a high potential for absorption by the human intestine, which is in contrast to gentamicin, which has low gastrointestinal absorption and thus is administered in the injectable form [55]. The LogP and TPSA values indicate that the compounds have the potential for intramuscular, cutaneous, and intravenous administration. Notably, all compounds demonstrated good solubility, which is a critical factor for successful drug development, as poor solubility may affect xenobiotics’ pharmacokinetic and pharmacodynamic properties [74]. Regarding their safety profile on cytochromes P450 (1A2, 2C1, 2C9, 2D6, and 3A4), all phytoconstituents were deemed safe except for ellagic acid, which was found to be an inhibitor of CYP1A2. Despite this, Japan has approved it as an existing food additive [64]. P450 (CYP) enzymes are ubiquitous enzymes that play a crucial role in the metabolism of pharmaceuticals [75] and are involved in the activation and detoxification of endogenous and xenobiotic chemicals [76]. Interestingly, flavonoids have been shown to induce the biosynthesis of several CYPs, and the enzymatic activities of CYPs can be modulated (inhibited or stimulated) by these compounds [77].
5. Conclusions
Several factors, including availability, ease of preparation, safety, and affordability, drive herbal preparations’ consumption. In Algeria, Eucalyptus extracts are widely used in traditional medicine, but their use lacks standardization. The aqueous extract obtained from leaves of E. radiata and E. cinerea was tested for eventual bioactivities. In general, the results of this study indicated that the extracts possess potent antibacterial activity where E. coli and S. aureus were susceptible to the two Eucalyptus probably because of the presence of Ellagic acid and Epicatechin which showed strong TyRS bond energies. The extracts were found to possess radical scavenging on the DPPH radical and anti-inflammatory activities during albumin denaturation assay. In silico, results show a prominent binding of the ligand with the bacterial enzyme target. In addition, phytoconstituent docked were found to be free of cytotoxicity, supporting their future exploitation for oral or topical application. Thus, it can be concluded that aqueous extract of leaves of E. radiate and E. cinerea can be used as antibacterial, antioxidant, and anti-inflammatory agents or for the preparation of nutraceutical products and in food industries.
Author Contributions
Conceptualization, M.E. and H.E.; methodology, Y.B., M.M.S.-B., S.A.Z. and F.S.A.; validation, B.E. and Y.B.; formal analysis, M.E., H.E. and F.S.A.; investigation, Y.B. and B.E.; data curation, M.E. and M.M.S.-B.; writing—original draft preparation, Y.B. and F.S.A.; writing—review and editing, M.M.S.-B., M.W.M.A. and H.E.; supervision, Y.B. and S.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia (IFKSURC-1-0823).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research (IFKSURC-1-0823).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Kahla, Y.; Zouari-Bouassida, K.; Rezgui, F.; Trigui, M.; Tounsi, S. Efficacy of Eucalyptus cinerea as a Source of Bioactive Compounds for Curative Biocontrol of Crown Gall Caused by Agrobacterium Tumefaciens Strain B6. BioMed Res. Int. 2017, 2017, 9308063. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, J.; Zhang, Y.; Xiao, Y.; Zhang, X.; Zhong, L.; Liu, H.; Chen, B. Genome-Wide Identification of MicroRNAs Involved in the Somatic Embryogenesis of Eucalyptus. G3 Genes Genomes Genetics 2021, 11, jkab070. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Saber, F.R. Chemical Composition and Bioactivity of the Volatile Oil from Leaves and Stems of Eucalyptus cinerea. Pharm. Biol. 2014, 52, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Abouzkhar, F.A.; Yangui, I.; Messaoud, C.; Ben Jamâa, M.L. Eucalyptus Leaf Diseases Associated with Neofusicoccum spp. in North Africa. J. Arid Environ. 2022, 197, 104662. [Google Scholar] [CrossRef]
- Boeno, D.; Silva, R.F.; Almeida, H.S.; Rodrigues, A.C.; Vanzan, M.; Andreazza, R. Influence of Eucalyptus Development under Soil Fauna. Braz. J. Biol. 2020, 80, 345–353. [Google Scholar] [CrossRef]
- Penín, L.; López, M.; Santos, V.; Alonso, J.L.; Parajó, J.C. Technologies for Eucalyptus Wood Processing in the Scope of Biorefineries: A Comprehensive Review. Bioresour. Technol. 2020, 311, 123528. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, Antibacterial and Antifungal Properties of an Aqueous Extract of Eucalyptus microcorys Leaves. S. Afr. J. Bot. 2017, 112, 180–185. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Syukri, D.M.; Voravuthikunchai, S.P. Bioactive Fractions of Eucalyptus Camaldulensis Inhibit Important Foodborne Pathogens, Reduce Listeriolysin O-Induced Haemolysis, and Ameliorate Hydrogen Peroxide-Induced Oxidative Stress on Human Embryonic Colon Cells. Food Chem. 2021, 344, 128571. [Google Scholar] [CrossRef]
- Teixeira, A.; DaCunha, D.C.; Barros, L.; Caires, H.R.; Xavier, C.P.R.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Eucalyptus globulus Labill. Decoction Extract Inhibits the Growth of NCI-H460 Cells by Increasing the P53 Levels and Altering the Cell Cycle Profile. Food Funct. 2019, 10, 3188–3197. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N. In vitro Study of Anti-Inflammatory Properties of Methanolic Extract Fruits from Rosa Canina L. (Rosaceae). Nutr. Santé 2020, 9, 117–125. [Google Scholar] [CrossRef]
- Olugbodi, J.O.; Tincho, M.B.; Oguntibeju, O.O.; Olaleye, M.T.; Akinmoladun, A.C. Glyphaea brevis—In vitro Antioxidant and In Silico Biological Activity of Major Constituents and Molecular Docking Analyses. Toxicol. In Vitro 2019, 59, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Salaria, D.; Rolta, R.; Patel, C.N.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in Silico Analysis of Thymus serpyllum Essential Oil as Bioactivity Enhancer of Antibacterial and Antifungal Agents. J. Biomol. Struct. Dyn. 2021, 40, 10383–10402. [Google Scholar] [CrossRef]
- CLSI M02-A11; Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard—Eleventh Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; p. 76.
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A.N. Antioxidant and Biocidal Activities of Carum nigrum (Seed) Essential Oil, Oleoresin, and Their Selected Components. J. Agric. Food Chem. 2006, 54, 174–181. [Google Scholar] [CrossRef]
- Reshma, A.K.; Arun, K.P. In vitro Anti-Inflammatory, Antioxidant and Nephroprotective Studies on Leaves of Aegle marmelos and Ocimum sanctum. Asian J. Pharm. Clin. Res. 2014, 7. [Google Scholar]
- Haddad, M.; Herent, M.-F.; Tilquin, B.; Quetin-Leclercq, J. Effect of Gamma and E-Beam Radiation on the Essential Oils of Thymus vulgaris Thymoliferum, Eucalyptus radiata, and Lavandula angustifolia. J. Agric. Food Chem. 2007, 55, 6082–6086. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-Guided Fractionation Driven by In vitro α-Amylase Inhibition Assays of Essential Oils Bearing Specialized Metabolites with Potential Hypoglycemic Activity. Plants 2020, 9, 1242. [Google Scholar] [CrossRef]
- Coppen, J.J.W. Eucalyptus: The Genus Eucalyptus; Taylor and Francis: London, UK, 2002; ISBN 0-415-27879-1. [Google Scholar]
- Ali, K.; Ahmed, B.; Ansari, S.M.; Saquib, Q.; Al-Khedhairy, A.A.; Dwivedi, S.; Alshaeri, M.; Khan, M.S.; Musarrat, J. Comparative in Situ ROS Mediated Killing of Bacteria with Bulk Analogue, Eucalyptus Leaf Extract (ELE)-Capped and Bare Surface Copper Oxide Nanoparticles. Mater. Sci. Eng. C 2019, 100, 747–758. [Google Scholar] [CrossRef]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Costa, P.C.; Bahia, M.F. Oxygen and Nitrogen Reactive Species Are Effectively Scavenged by Eucalyptus globulus Leaf Water Extract. J. Med. Food 2009, 12, 175–183. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Ku, Y.H. Aqueous Eucalyptus globulus Leaf Extract-Mediated Biosynthesis of MgO Nanorods. Appl. Biol. Chem. 2018, 61, 197–208. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus globulus Leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Puig, C.G.; Reigosa, M.J.; Valentão, P.; Andrade, P.B.; Pedrol, N. Unravelling the Bioherbicide Potential of Eucalyptus globulus Labill: Biochemistry and Effects of Its Aqueous Extract. PLoS ONE 2018, 13, e0192872. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Hirun, S.; Chuen, T.L.K.; Goldsmith, C.D.; Munro, B.; Bowyer, M.C.; Chalmers, A.C.; Sakoff, J.A.; Phillips, P.A.; Scarlett, C.J. Physicochemical, Antioxidant and Anti-Cancer Activity of a Eucalyptus robusta (Sm.) Leaf Aqueous Extract. Ind. Crops Prod. 2015, 64, 167–174. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Bond, D.R.; Chalmers, A.C.; Bowyer, M.C.; Scarlett, C.J. Eucalyptus microcorys Leaf Extract Derived HPLC-Fraction Reduces the Viability of MIA PaCa-2 Cells by Inducing Apoptosis and Arresting Cell Cycle. Biomed. Pharmacother. 2018, 105, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Phytotoxic Activity and Chemical Composition of Aqueous Volatile Fractions from Eucalyptus Species. PLoS ONE 2014, 9, e93189. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Adjissi, L.; Chafai, N.; Benbouguerra, K.; Kirouani, I.; Hellal, A.; Layaida, H.; Elkolli, M.; Bensouici, C.; Chafaa, S. Synthesis, Characterization, DFT, Antioxidant, Antibacterial, Pharmacokinetics and Inhibition of SARS-CoV-2 Main Protease of Some Heterocyclic Hydrazones. J. Mol. Struct. 2022, 1270, 134005. [Google Scholar] [CrossRef]
- Benarba, B. Medicinal Plants Used by Traditional Healers from South-West Algeria: An Ethnobotanical Study. J. Intercult. Ethnopharmacol. 2016, 5, 320. [Google Scholar] [CrossRef]
- Belhouala, K.; Benarba, B. Medicinal Plants Used by Traditional Healers in Algeria: A Multiregional Ethnobotanical Study. Front. Pharmacol. 2021, 12, 760492. [Google Scholar] [CrossRef]
- Bouafia, M.; Amamou, F.; Gherib, M.; Benaissa, M.; Azzi, R.; Nemmiche, S. Ethnobotanical and Ethnomedicinal Analysis of Wild Medicinal Plants Traditionally Used in Naâma, Southwest Algeria. Vegetos 2021, 34, 654–662. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-Assisted Extraction of Eucalyptus robusta Leaf for the Optimal Yield of Total Phenolic Compounds. Ind. Crops Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Kaneria, M.; Kanani, B.; Chanda, S. Assessment of Effect of Hydroalcoholic and Decoction Methods on Extraction of Antioxidants from Selected Indian Medicinal Plants. Asian Pac. J. Trop. Biomed. 2012, 2, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Aït Abderrahim, L.; Boussaid, M.; Taibi, F.; Achir, M.; Souana, K.; Benaissa, T.; Farhi, K.H.; Naamani, F.Z.; Nait Said, K. Unraveling the Ethnopharmacological Potential of Medicinal Plants Used in Algerian Traditional Medicine for Urinary Diseases. Eur. J. Integr. Med. 2021, 44, 101339. [Google Scholar] [CrossRef]
- Periche, A.; Castelló, M.L.; Heredia, A.; Escriche, I. Influence of Extraction Methods on the Yield of Steviol Glycosides and Antioxidants in Stevia rebaudiana Extracts. Plant Foods Hum. Nutr. 2015, 70, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Alghoraibi, I.; Soukkarieh, C.; Zein, R.; Alahmad, A.; Walter, J.-G.; Daghestani, M. Aqueous Extract of Eucalyptus camaldulensis Leaves as Reducing and Capping Agent in Biosynthesis of Silver Nanoparticles. Inorg. Nano-Met. Chem. 2020, 50, 895–902. [Google Scholar] [CrossRef]
- Anigbo, A.A.; Avwioroko, O.J.; Cholu, C.O. Phytochemical Constituents, Antimalarial Efficacy, and Protective Effect of Eucalyptus camaldulensis Aqueous Leaf Extract in Plasmodium Berghei-Infected Mice. Prev. Nutr. Food Sci. 2020, 25, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.; Palmer, A.; Nixon, R. Eucalyptus Oil: Contact Allergy and Safety: Eucalyptus Oil Contact Allergy And Safety. Contact Dermat. 2015, 72, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review: Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Tian, Y.; Dong, F.; Zhou, X.; Yang, X. Repellent, Insecticidal and Antimicrobial Activities of Leaf Essential Oils from Three Eucalyptus Species. Chem. Biodivers. 2020, 17, e1900580. [Google Scholar] [CrossRef]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New Perspectives for Mucolytic, Anti-Inflammatory and Adjunctive Therapy with 1,8-Cineol in COPD and Asthma: Review on the New Therapeutic Approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Usha-Raja-Nanthini, A.; Valli, G.; Subramanian Shivakumar, M. Comparative Efficacy of Eucalyptus globulus (Labill) Hydrodistilled Essential Oil and Temephos as Mosquito Larvicide. Nat. Prod. Res. 2020, 34, 2626–2629. [Google Scholar] [CrossRef]
- Danna, C.; Cornara, L.; Smeriglio, A.; Trombetta, D.; Amato, G.; Aicardi, P.; De Martino, L.; De Feo, V.; Caputo, L. Eucalyptus gunnii and Eucalyptus oulverulenta’ Baby Blue’ Essential Oils as Potential Natural Herbicides. Molecules 2021, 26, 6749. [Google Scholar] [CrossRef] [PubMed]
- Obeizi, Z.; Benbouzid, H.; Ouchenane, S.; Yılmaz, D.; Culha, M.; Bououdina, M. Biosynthesis of Zinc Oxide Nanoparticles from Essential Oil of Eucalyptus globulus with Antimicrobial and Anti-Biofilm Activities. Mater. Today Commun. 2020, 25, 101553. [Google Scholar] [CrossRef]
- Najda, A.; Bains, A.; Chawla, P.; Kumar, A.; Balant, S.; Walasek-Janusz, M.; Wach, D.; Kaushik, R. Assessment of Anti-Inflammatory and Antimicrobial Potential of Ethanolic Extract of Woodfordia Fruticosa Flowers: GC-MS Analysis. Molecules 2021, 26, 7193. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.A.; Chretien, L.D.; Olive Vivien, N.E.; Djaouda, M.; Aoudou, Y.; Roger, T.; Moïse, N. Use of the Aqueous Extract of Eucalyptus Microcorys for the Treatment in Microcosm, of Water Containing Enterococcus faecalis: Hierarchisation of Cells’ Inhibition Factors. H2Open J. 2018, 1, 47–56. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas Aeruginosa: All Roads Lead to Resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, Function and Regulation of Pseudomonas aeruginosa Porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Lupo, A.; Haenni, M.; Madec, J.-Y. Antimicrobial Resistance in Acinetobacter spp. and Pseudomonas spp. Microbiol. Spectr. 2018, 6, 3. [Google Scholar] [CrossRef]
- Bush, N.G.; Evans-Roberts, K.; Maxwell, A. DNA Topoisomerases. EcoSal Plus 2015, 6, 1–34. [Google Scholar] [CrossRef]
- Culp, E.; Wright, G.D. Bacterial Proteases, Untapped Antimicrobial Drug Targets. J. Antibiot. 2017, 70, 366–377. [Google Scholar] [CrossRef]
- Traversier, M.; Gaslonde, T.; Lecso, M.; Michel, S.; Delannay, E. Comparison of Extraction Methods for Chemical Composition, Antibacterial, Depigmenting and Antioxidant Activities of Eryngium maritimum. Int. J. Cosmet. Sci. 2020, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Trabolsi, C.; Takash Chamoun, W.; Hijazi, A.; Nicoletti, C.; Maresca, M.; Nasser, M. Study of Neuroprotection by a Combination of the Biological Antioxidant (Eucalyptus Extract) and the Antihypertensive Drug Candesartan against Chronic Cerebral Ischemia in Rats. Molecules 2021, 26, 839. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Oliveira, M.S.; Fernandes, D.A.; Ferreira, M.D.L.; Cordeiro, L.V.; Souza, M.F.V.; Fernandes, L.M.D.; Souza, H.D.S.; Oliveira Filho, A.A.; Pessoa, H.L.F.; et al. In Silico, in vitro, and Ex vivo Studies of the Toxicological and Pharmacological Properties of the Flavonoid 5,7-Dihydroxy-3,8,4’-Trimethoxy. Braz. J. Med. Biol. Res. 2021, 54, e11203. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in vitro Anti-Inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-Biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Akhtar, S.; Ismail, T.; Yuan, Y.; Ahmad, N.; Tawab, A.; Ismail, A.; Barnard, R.T.; Cooper, M.A.; Blaskovich, M.A.T.; et al. Syzygium cumini(L.), Skeels Fruit Extracts: In vitro and in Vivo Anti-Inflammatory Properties. J. Ethnopharmacol. 2021, 271, 113805. [Google Scholar] [CrossRef] [PubMed]
- Modak, D.; Paul, S.; Sarkar, S.; Thakur, S.; Bhattacharjee, S. Validating Potent Anti-Inflammatory and Anti-Rheumatoid Properties of Drynaria quercifolia Rhizome Methanolic Extract through in vitro, in Vivo, in Silico and GC-MS-Based Profiling. BMC Complement. Med. Ther. 2021, 21, 89. [Google Scholar] [CrossRef]
- Chaiya, P.; Senarat, S.; Phaechamud, T.; Narakornwit, W. In vitro Anti-Inflammatory Activity Using Thermally Inhibiting Protein Denaturation of Egg Albumin and Antimicrobial Activities of Some Organic Solvents. Mater. Today Proc. 2022, 65, 2290–2295. [Google Scholar] [CrossRef]
- Sava, A.; Buron, F.; Routier, S.; Panainte, A.; Bibire, N.; Constantin, S.M.; Lupașcu, F.G.; Focșa, A.V.; Profire, L. Design, Synthesis, In Silico and In vitro Studies for New Nitric Oxide-Releasing Indomethacin Derivatives with 1,3,4-Oxadiazole-2-Thiol Scaffold. Int. J. Mol. Sci. 2021, 22, 7079. [Google Scholar] [CrossRef]
- Babar, Z.M.; Jaswir, I.; Tareq, A.M.; Ali Reza, A.S.M.; Azizi, W.M.; Hafidz, M.; Ahfter, F.; Hasan, M.; Farhad, S.; Uddin, M.M.R.; et al. In Vivo Anxiolytic and in vitro Anti-Inflammatory Activities of Water-Soluble Extract (WSE) of Nigella sativa (L.) Seeds. Nat. Prod. Res. 2021, 35, 2793–2798. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, H.; Wang, M.; Chow, A.; Fang, M. Metabolomics and In Silico Docking-Directed Discovery of Small-Molecule Enzyme Targets. Anal. Chem. 2021, 93, 3072–3081. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, A.; Azam, M.; Allemailem, K.S.; Alrumaihi, F.; Almatroudi, A.; Alhumaydhi, F.A.; Azam, F.; Khan, S.H.; Zofair, S.F.F.; et al. Liposomal Ellagic Acid Alleviates Cyclophosphamide-Induced Toxicity and Eliminates the Systemic Cryptococcus neoformans Infection in Leukopenic Mice. Pharmaceutics 2021, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, V.; Solaipriya, S.; Sivaramakrishnan, V. Role of Ellagic Acid for the Prevention and Treatment of Liver Diseases. Phytother. Res. 2021, 35, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Cesar, P.H.S.; Trento, M.V.C.; Konig, I.F.M.; Marcussi, S. Catechin, and Epicatechin as an Adjuvant in the Therapy of Hemostasis Disorders Induced by Snake Venoms. J. Biochem. Mol. Toxicol. 2020, 34, e22604. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.-K. Antimicrobial Effect of Linalool and α-Terpineol against Periodontopathic and Cariogenic Bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zhang, L.; An, P.; Qi, J.; Yu, X.; Lu, J.; Ren, X. Antifungal Mechanisms of α-Terpineol and Terpene-4-Alcohol as the Critical Components of Melaleuca alternifolia Oil in the Inhibition of Rot Disease Caused by Aspergillus Ochraceus in Postharvest Grapes. J. Appl. Microbiol. 2019, 126, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.N.; Csehi, B.; József, S.; Ferenc, H.; Kiskó, G.; Dalmadi, I.; Friedrich, L. Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods 2021, 10, 1855. [Google Scholar] [CrossRef]
- Moo, C.-L.; Osman, M.A.; Yang, S.-K.; Yap, W.-S.; Ismail, S.; Lim, S.-H.-E.; Chong, C.-M.; Lai, K.-S. Antimicrobial Activity and Mode of Action of 1,8-Cineol against Carbapenemase-Producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can Eucalyptol Replace Antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef]
- Juergens, U. Anti-Inflammatory Properties of the Monoterpene 1.8-Cineol: Current Evidence for Co-Medication in Inflammatory Airway Diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Ultrasonic Nanoemulsification of Food Grade Trans-Cinnamaldehyde: 1,8-Cineol and investigation of the Mechanism of Antibacterial Activity. Ultrason. Sonochem. 2017, 35, 415–421. [Google Scholar] [CrossRef]
- Ulloa-Benítez, Á.; Medina-Romero, Y.M.; Sánchez-Fernández, R.E.; Lappe-Oliveras, P.; Roque-Flores, G.; Duarte Lisci, G.; Herrera Suárez, T.; Macías-Rubalcava, M.L. Phytotoxic and Antimicrobial Activity of Volatile and Semi-Volatile Organic Compounds from the Endophyte Hypoxylon anthochroum Strain Blaci Isolated from Bursera Lancifolia (Burseraceae). J. Appl. Microbiol. 2016, 121, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Gosling, D.J.; Patissier, C.; Rodde, S.; Zhou, L.; Faller, B. What Is Modulating Solubility in Simulated Intestinal Fluids? Eur. J. Pharm. Sci. 2010, 41, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Morrison, I.J.; Zhang, J.; Lin, J.; Murray, J.E.; Porter, R.; Langat, M.K.; Sadgrove, N.J.; Barker, J.; Zhang, G.; Delgoda, R. Potential Chemopreventive, Anticancer and Anti-Inflammatory Properties of a Refined Artocarpin-Rich Wood Extract of Artocarpus heterophyllus Lam. Sci. Rep. 2021, 11, 6854. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Tanaka, K.; Takenaka, S.; Murayama, N.; Martin, M.V.; Foroozesh, M.K.; Yamazaki, H.; Guengerich, F.P.; Komori, M. Structure-Function Relationships of Inhibition of Human Cytochromes P450 1A1, 1A2, 1B1, 2C9, and 3A4 by 33 Flavonoid Derivatives. Chem. Res. Toxicol. 2010, 23, 1921–1935. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Stiborová, M. Flavonoids-Potent and Versatile Biologically Active Compounds Interacting with Cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).