Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PFPE-C
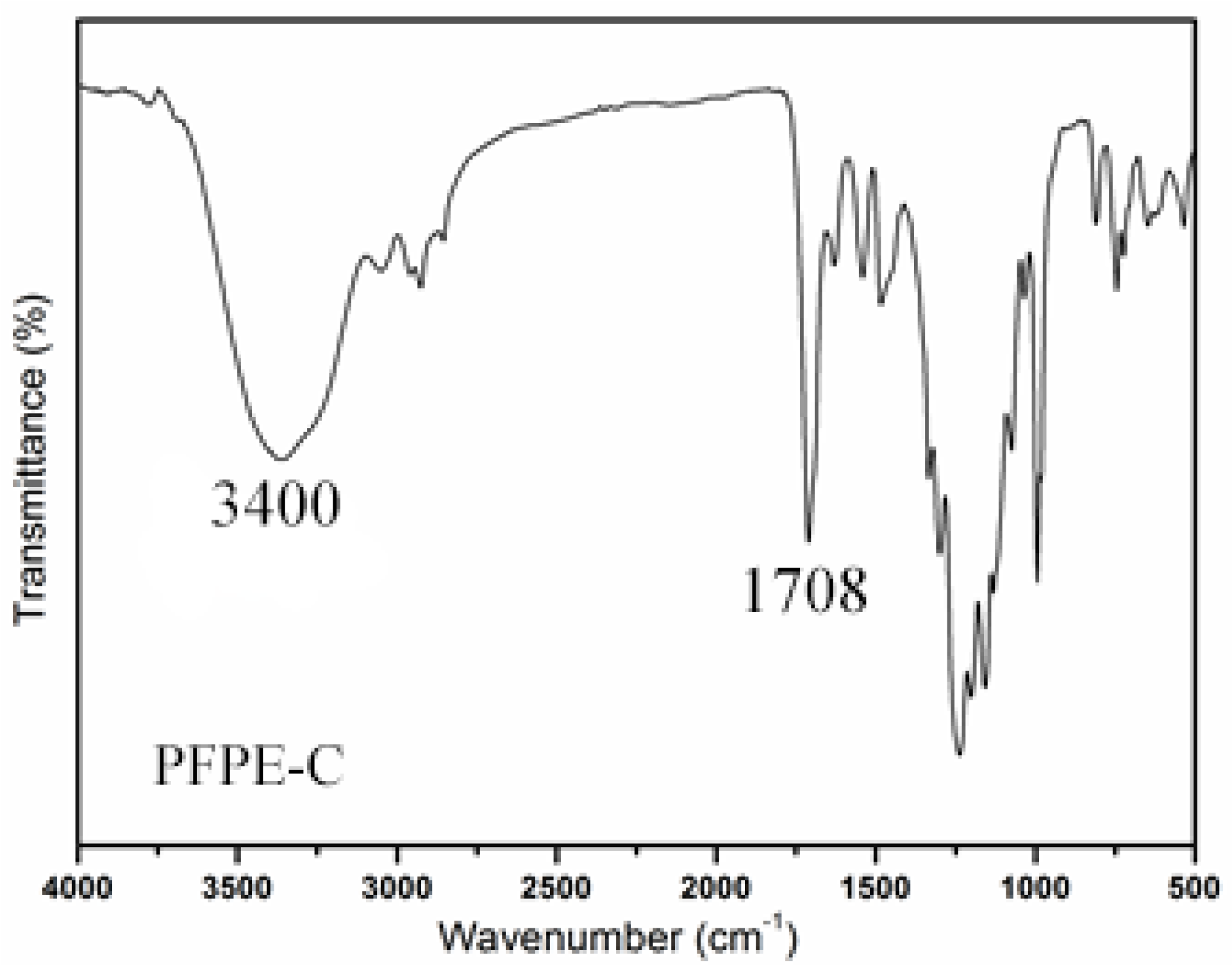
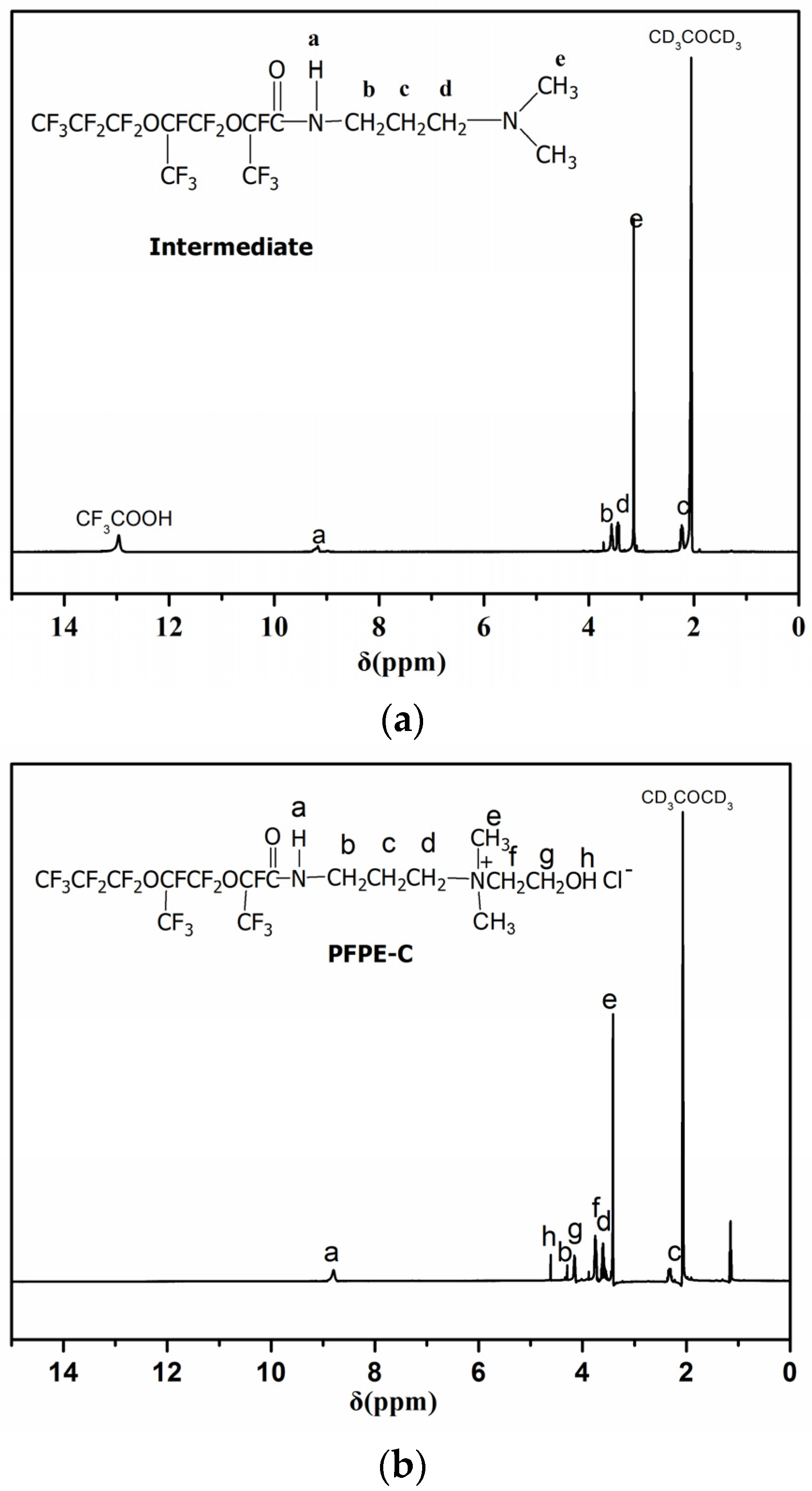
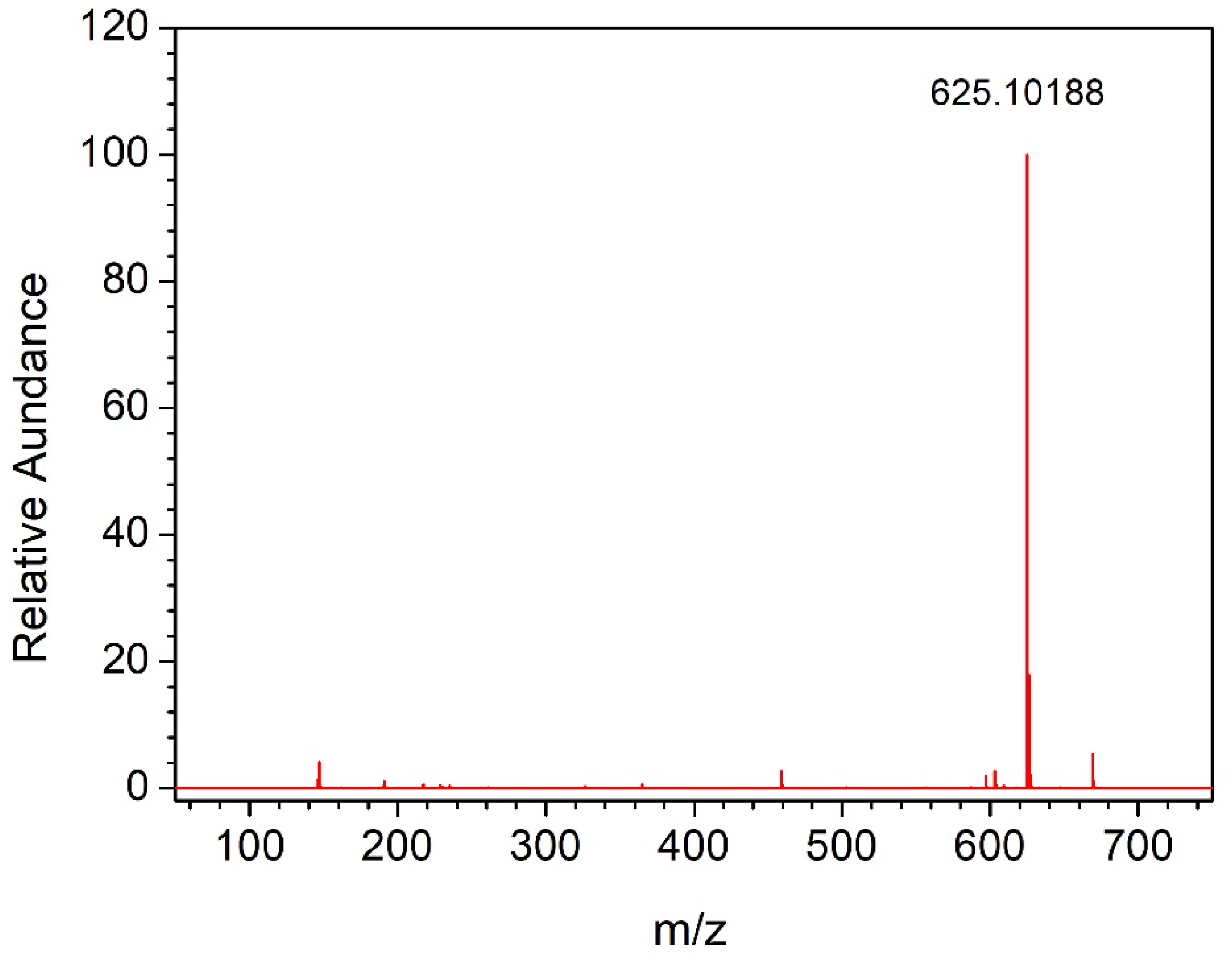
2.3. Characterization of PFPE-C
2.4. Experimental Methods
3. Results and Discussion
3.1. Preparation and Characterization of PFPE-C
3.2. Surface Activity
3.3. Thermodynamic Parameters of PFPE-C Systems
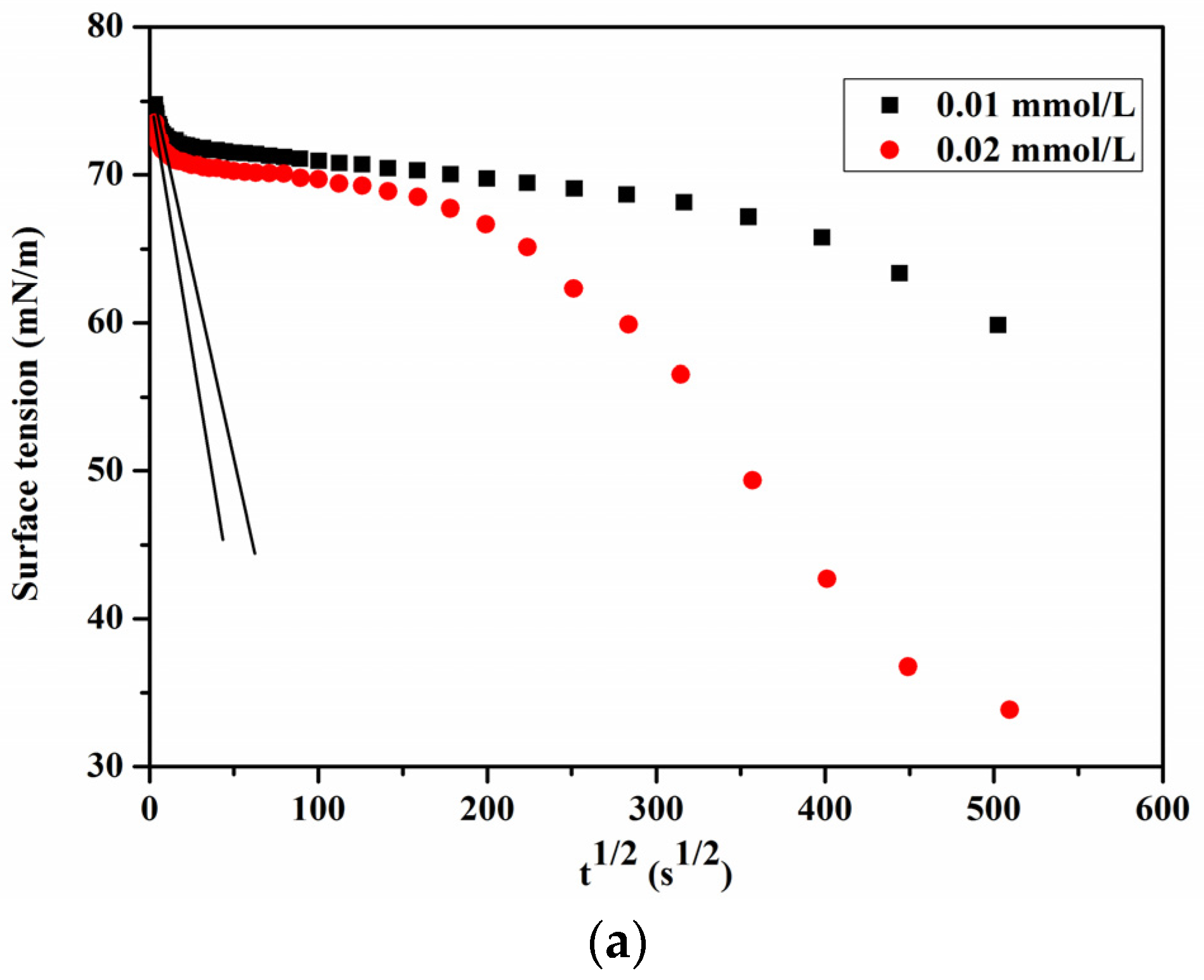
3.4. Adsorption and Diffusion Kinetics of PFPE-C
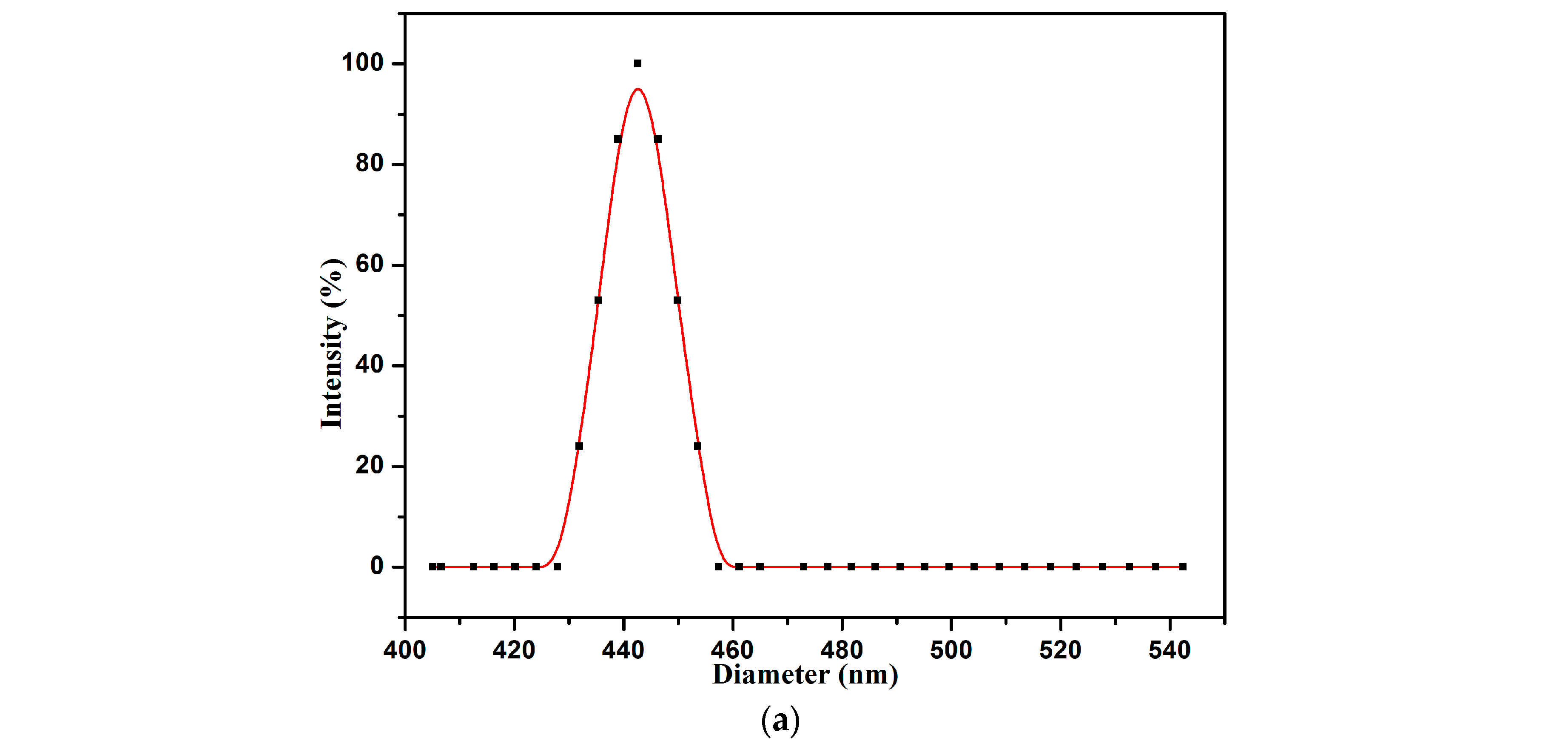
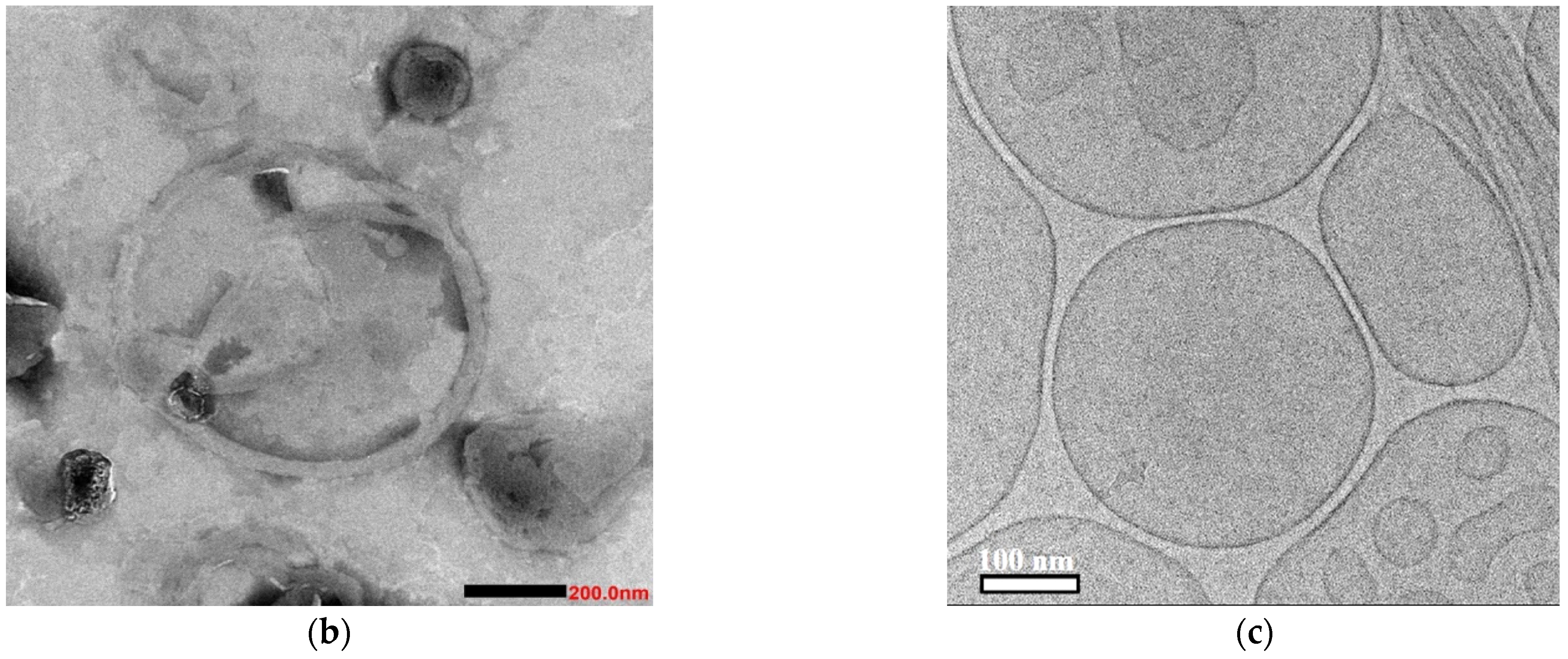
3.5. Aggregation Behavior
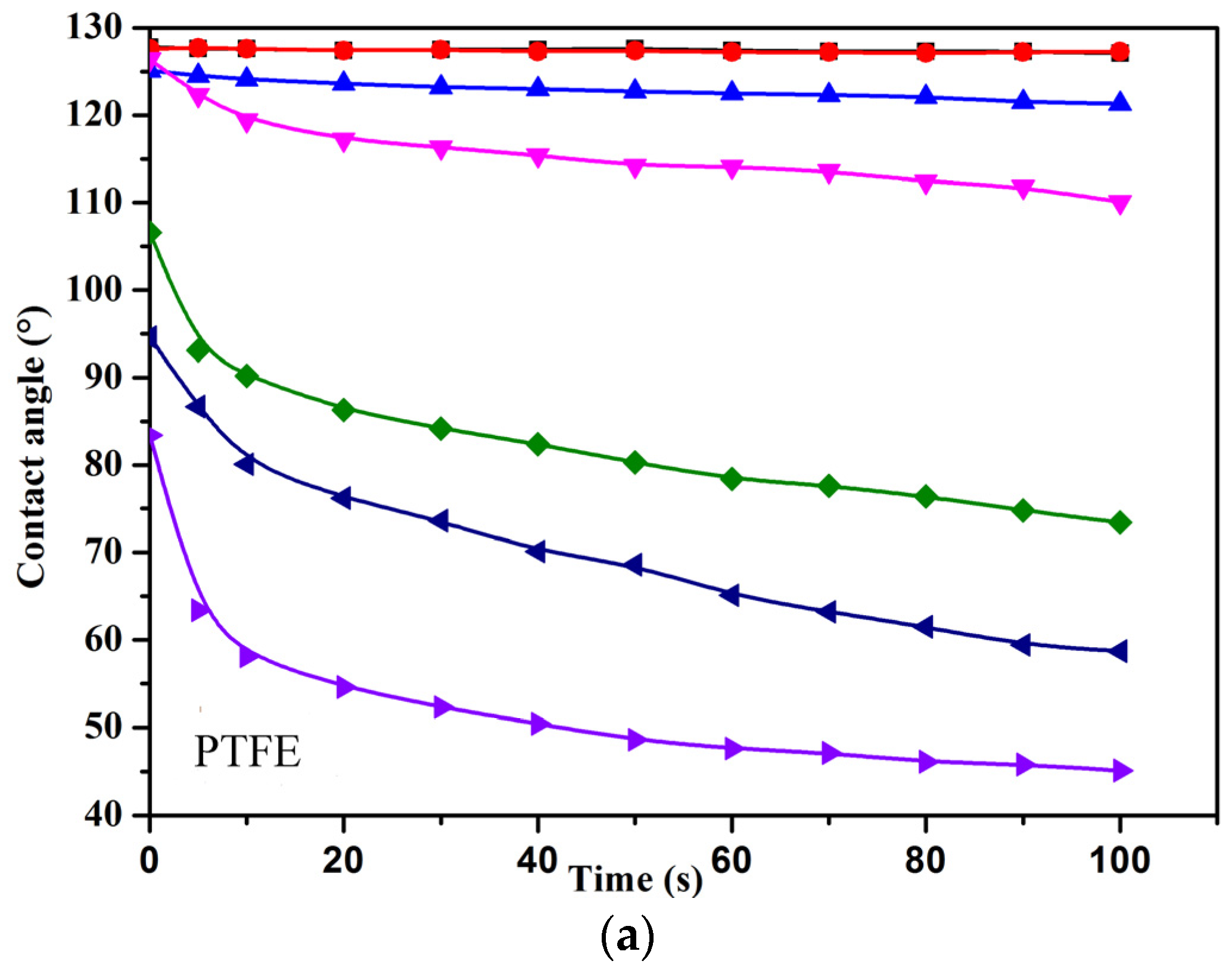
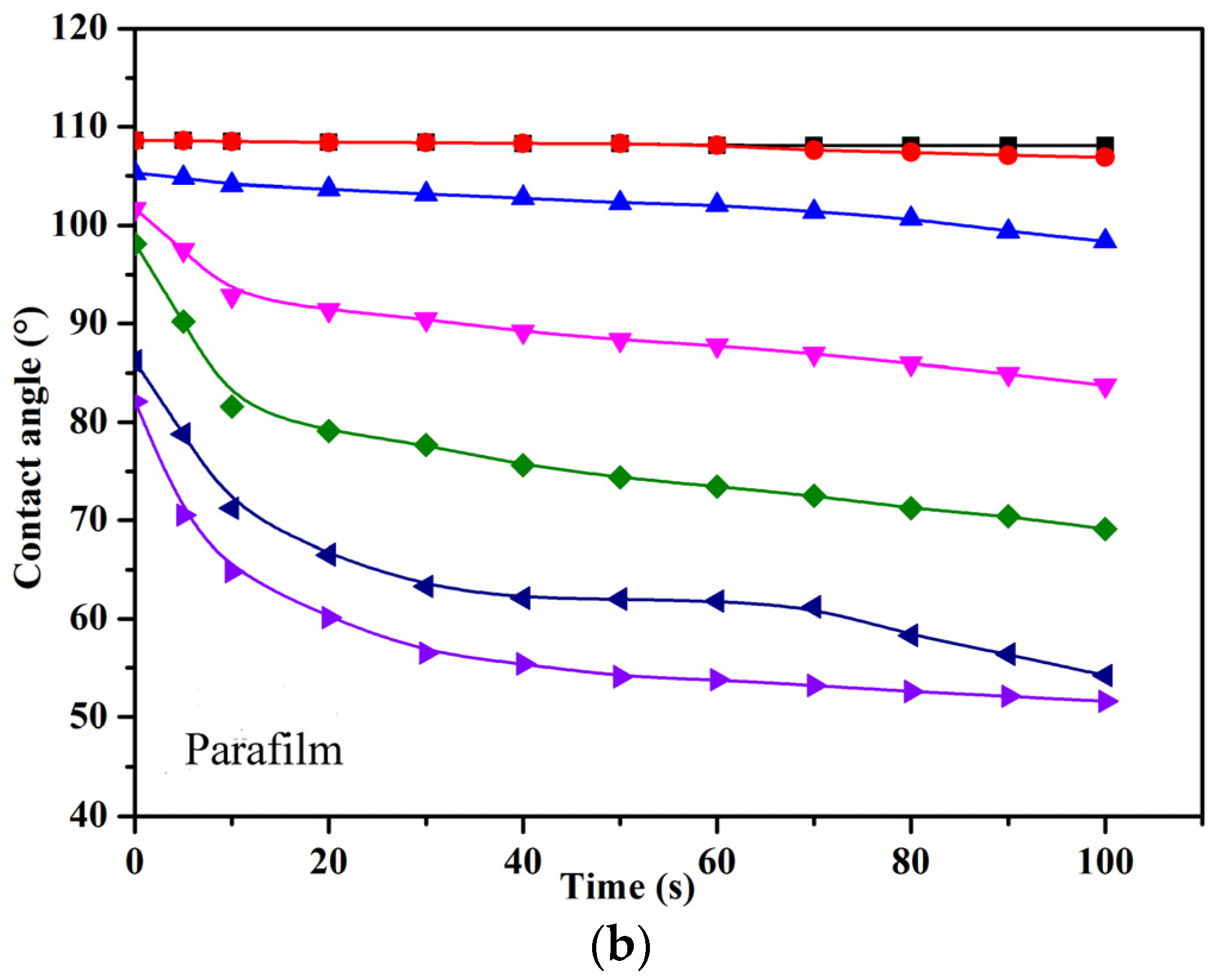
3.6. Wettability of PFPE-C-Based Solutions on Low-Energy Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Améduri, B. The Promising Future of Fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—Part one. Chemosphere 2015, 129, 4–19. [Google Scholar] [CrossRef]
- Lantz, A.; Bertocchio, R. Fluorinated surfactants, application in fire fighting foams. J. Fluor. Chem. 1987, 35, 17. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Adewunmi, A.A.; Mahboob, A.; Murtaza, M.; Zhou, X.; Kamal, M.S. Fluorinated surfactants: A review on recent progress on synthesis and oilfield applications. Adv. Colloid Interface Sci. 2022, 303, 102634. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; Hewat, T. Fluorosurfactants in Enhanced Oil Recovery. Open Pet. Eng. J. 2008, 1, 58–61. [Google Scholar] [CrossRef]
- Sharma, R.; Shelke, S.; Kashani, M.B.; Morose, G.; Christuk, C.; Nagarajan, R. Safer and effective alternatives to perfluoroalkyl-based surfactants in etching solutions for the semiconductor industry. J. Clean. Prod. 2023, 415, 137879. [Google Scholar] [CrossRef]
- Zhao, F.; Jing, Z.; Guo, X.; Li, J.; Dong, H.; Tan, Y.; Liu, L.; Zhou, Y.; Owen, R.; Shearing, P.R.; et al. Trace amounts of fluorinated surfactant additives enable high performance zinc-ion batteries. Energy Storage Mater. 2022, 53, 638–645. [Google Scholar] [CrossRef]
- Li, W.; Liu, H. Novel organic–inorganic hybrid polymer based on fluorinated polyhedral oligomeric silsesquioxanes for stable superamphiphobic fabrics and aluminum corrosion protection. Mater. Today Chem. 2023, 29, 101390. [Google Scholar] [CrossRef]
- Dreyer, C.; Motoc, D.L.; Koehler, M.; Goldenberg, L. UV LED Curable Perfluoropolyether (PFPE)-Urethane Methacrylate Transparent Coatings for Photonic Applications: Synthesis and Characterization. Polymers 2023, 15, 2983. [Google Scholar] [CrossRef]
- Araz, R.-A.; Mohammad, S.; Kevin, G.; Ning, Y. Nature-inspired surface for simultaneously harvesting water and triboelectric energy from ambient humidity using polymer brush coatings. Nano Energy 2023, 115, 108752. [Google Scholar]
- Shinoda, K.; Hato, M.; Hayashi, T. Physicochemical properties of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 1972, 76, 909–914. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ohno, A.; Esumi, K. The equilibrium and dynamic surface tension properties of partially fluorinated quaternary ammonium salt Gemini surfactants. Langmuir 2006, 22, 4643–4648. [Google Scholar] [CrossRef]
- Dupont, A.; Eastoe, J.; Murray, M.; Martin, L.; Guittard, F.; Taffin de Givenchy, E.; Heenan, R.K. Hybrid Fluorocarbon-Hydrocarbon CO2-philic Surfactants. 1. Synthesis and Properties of Aqueous Solutions. Langmuir 2004, 20, 9953–9959. [Google Scholar] [CrossRef]
- Li, G.-L.; Zheng, L.-Q.; Xiao, J.-X. Synthesis and surface activities of organic solvent-soluble fluorinated surfactants. J. Fluor. Chem. 2009, 130, 674–681. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants: Synthesis, Properties, Applications; M. Marcel Dekker Inc.: New York, NY, USA, 1994. [Google Scholar]
- Alessandro, Z.; Bruno, A. Recent advances on synthesis of potentially non-bioaccumulable fluorinated surfactants. Curr. Opin. Colloid Interface Sci. 2012, 17, 188–195. [Google Scholar]
- Anushka, G.; Nagaraj, P.S.; Soumen, B.; Mallikarjuna, N.N.; Tejraj, M.A. Treatment technologies for removal of per- and polyfluoroalkyl substances (PFAS) in biosolids. Chem. Eng. J. 2023, 453, 139964. [Google Scholar]
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Tammy, C.; Michael, R.H. Sonochemical degradation of perfluorinated surfactants: Power and multiple frequency effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar]
- Shen, J.; Bai, Y.; Yin, Q.; Wang, W.; Ma, X.; Wang, G. Adsorption, aggregation and wetting behaviors of biodegradable surfactant: Perfluoropolyether quaternary ammonium salt. J. Ind. Eng. Chem. 2017, 56, 82–89. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Li, Q. Physicochemical properties of quaternary ammonium surfactants with hydroxyethyl groups. Tenside Surfactants Deterg. 2014, 51, 54–58. [Google Scholar] [CrossRef]
- GB/T 15818-2006; Test Method for Biodegradability of Surfactants. Standards Press of China: Beijing, China, 2006.
- Mehta, S.K.; Bhasin, K.K.; Kumar, A.; Dham, S. Micellar behavior of dodecyldimethylethyl ammonium bromide and dodecyltrimethylammonium chloride in aqueous media in the presence of diclofenac sodium. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 17–25. [Google Scholar] [CrossRef]
- Vanyúr, R.; Biczók, L.; Miskolczy, Z. Micelle formation of 1-alkyl-3-methylimidazolium bromide ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 256–261. [Google Scholar] [CrossRef]
- Nusselder, J.J.H.; Engberts, J.B. Toward a better understanding of the driving force for micelle formation and micellar growth. J. Colloid Interface Sci. 1992, 148, 353–361. [Google Scholar] [CrossRef]
- Xu, W.; Song, A.; Dong, S.; Chen, J.; Hao, J. A Systematic Investigation and Insight into the Formation Mechanism of Bilayers of Fatty Acid/Soap Mixtures in Aqueous Solutions. Langmuir 2013, 29, 12380–12388. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interfac. Adv. Colloid Interface Sci. 2000, 85, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Shi, X.; Zhang, E.; Pan, Y.; Li, X.; Wang, H.; Liu, W. Synthesis and properties of stellate lactosamide quaternary ammonium surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127317. [Google Scholar] [CrossRef]
- Wen, Y.; Ge, X.; Gao, W.; Wei, W.; Qiao, Y.; Chang, H. Synthesis and aggregation properties of ethylene glycol ester-based cationic Gemini surfactants. Colloid Interface Sci. Commun. 2020, 37, 100274. [Google Scholar] [CrossRef]
- Hoffmann, H.; Ebert, G. Surfactants, Micelles and Fascinating Phenomena. Angew. Chem. Int. Ed. 1988, 27, 902–912. [Google Scholar] [CrossRef]
- Shen, J.; Bai, Y.; Tai, X.; Wang, W.; Wang, G. Surface activity, spreading, and aggregation behavior of ecofriendly perfluoropolyether amide propyl betaine in aqueous solution. ACS Sustain. Chem. Eng. 2018, 6, 6183–6191. [Google Scholar] [CrossRef]
- Zhi, L.; Li, X.; Wang, H.; Xue, Y.; Zhang, Q.; Wang, X. Dynamic Surface Properties of Eco-Friendly Cationic Saccharide Surfactants at the Water/Air Interface. Tenside Surfactants Deterg. 2019, 56, 473–483. [Google Scholar] [CrossRef]

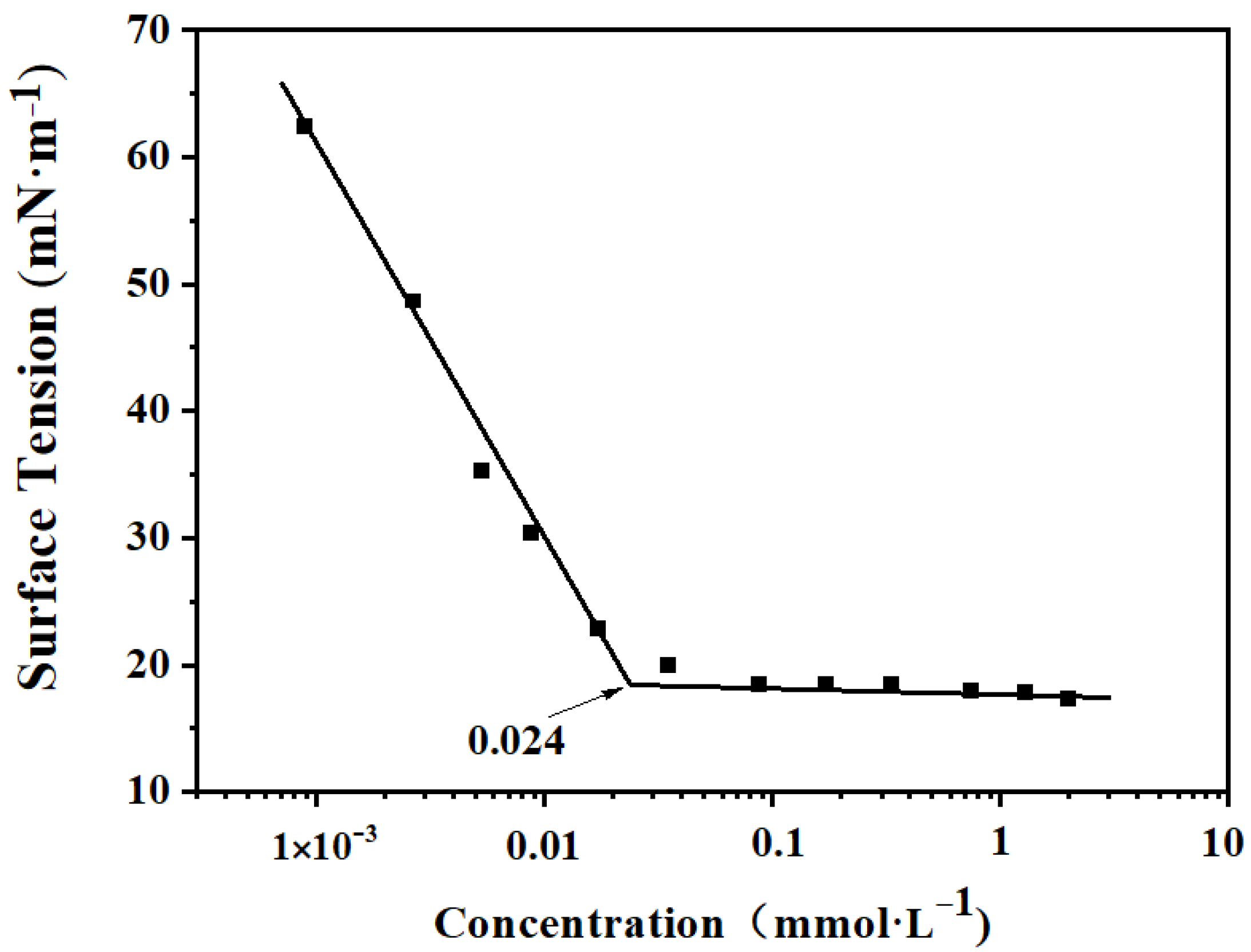
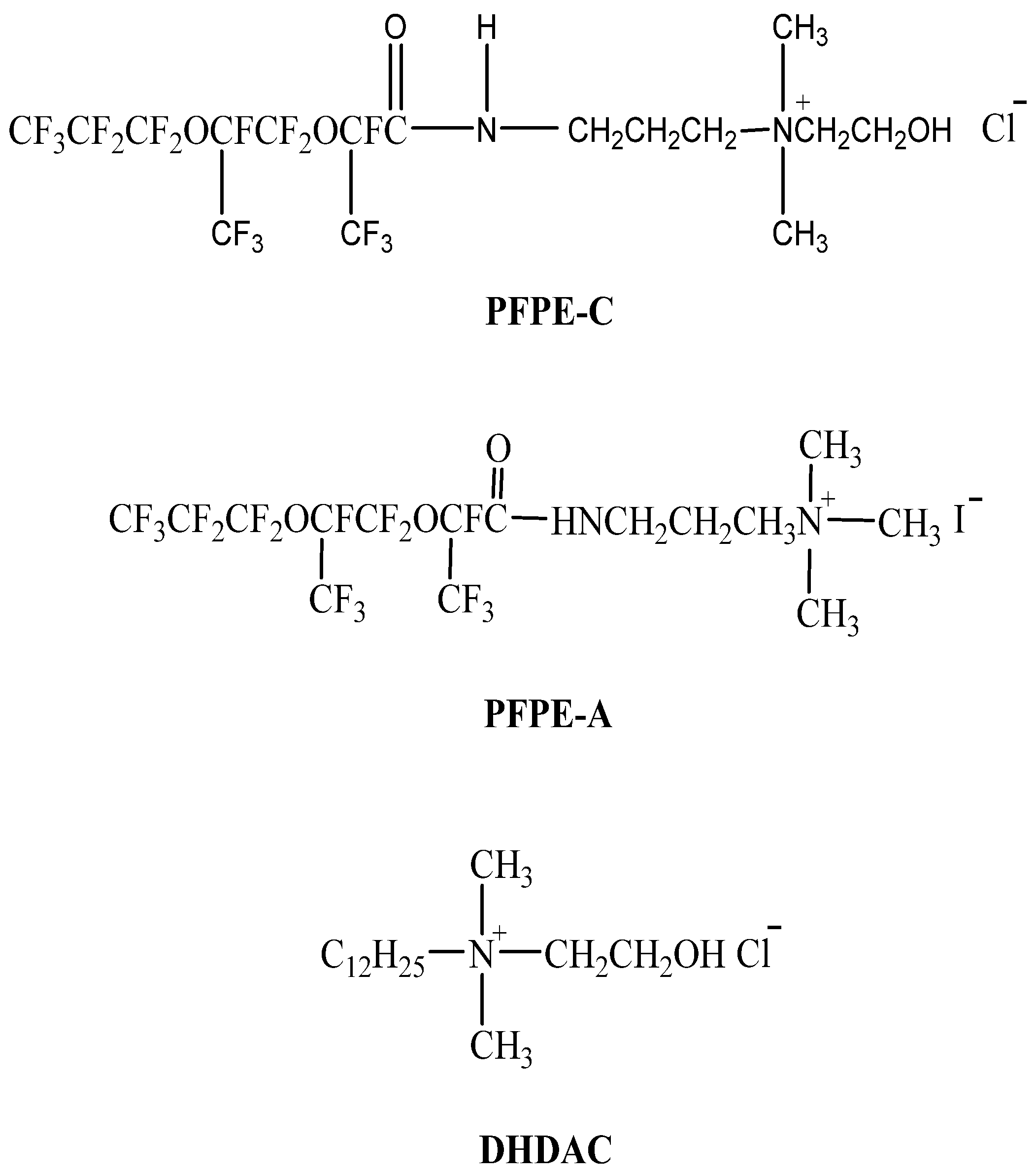

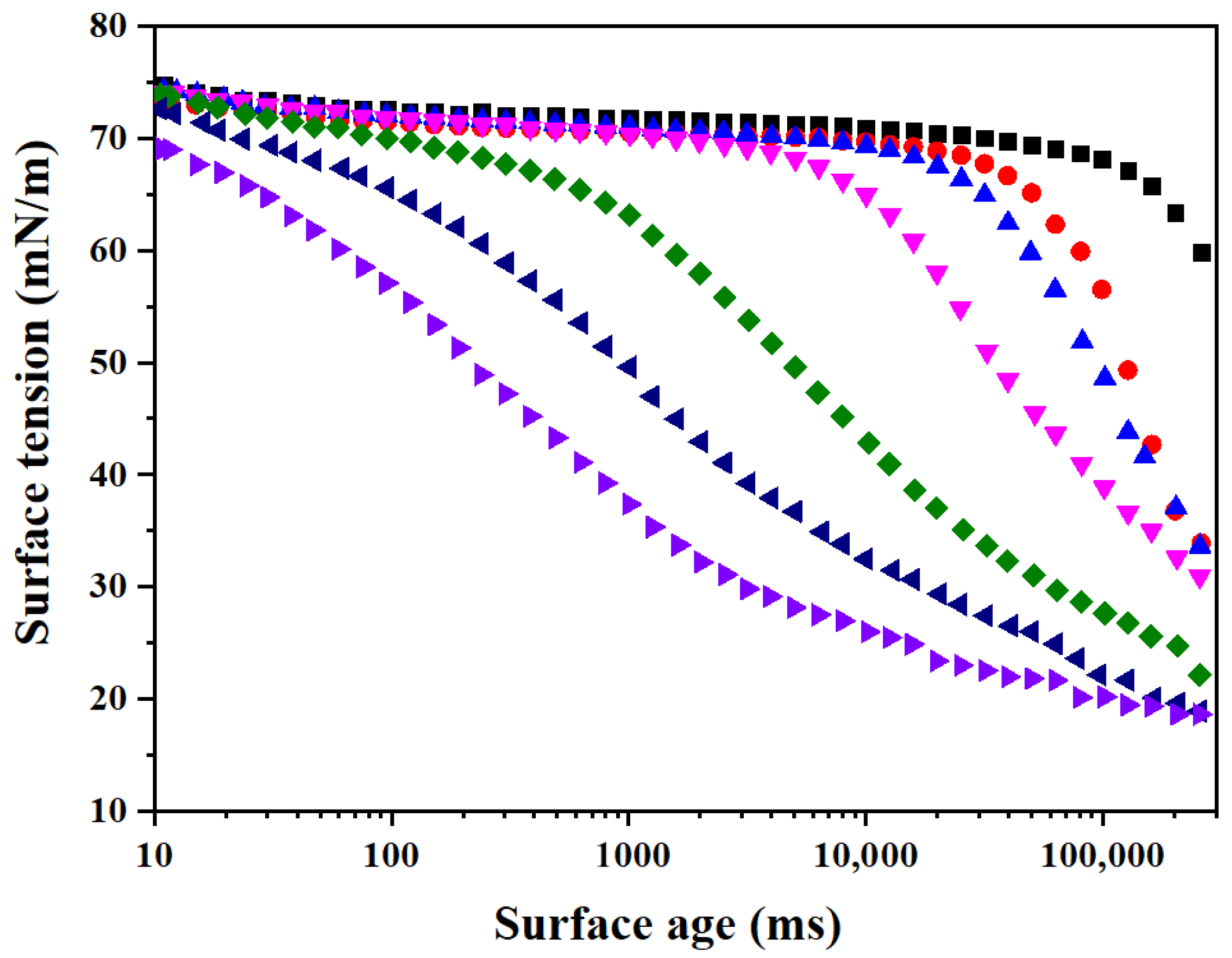

| Surfactant | (mN/m) | CMC (mmol/L) | (mol/cm2) | (Å2) |
|---|---|---|---|---|
| PFPE-C | 17.35 | 2.40 × 10−2 | 2.75 | 60.38 |
| PFPE-A [21] | 16.41 | 1.02 | 1.84 | 90.25 |
| DHDAC [22] | 30.71 | 11.69 | 2.75 | 60.40 |
| Surfactant | T (°C) | CMC (mmol/L) | (kJ/mol) | (kJ/mol) | (kJ/mol) | |
|---|---|---|---|---|---|---|
| PFPE-C | 25 | 2.41 × 10−2 | 0.37 | −36.11 | −9.19 | −26.92 |
| 35 | 2.66 × 10−2 | 0.39 | −37.50 | −9.96 | −26.20 | |
| 45 | 2.89 × 10−2 | 0.40 | −38.69 | −10.69 | −28.01 |
| Surfactant | C0 (mmol/L) | Deff (m2/s) | Ds (m2/s) | Deff/Ds |
|---|---|---|---|---|
| PFPE-C | 0.01 | 2.34 × 10−8 | 5.33 × 10−10 | 43.90 |
| 0.02 | 9.66 × 10−9 | 1.65 × 10−11 | 585.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, J.; Liu, J.; Wang, Y.; Zhi, L.; Shen, J.; Wang, G. Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group. Molecules 2023, 28, 7151. https://doi.org/10.3390/molecules28207151
Dou J, Liu J, Wang Y, Zhi L, Shen J, Wang G. Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group. Molecules. 2023; 28(20):7151. https://doi.org/10.3390/molecules28207151
Chicago/Turabian StyleDou, Jiangxun, Jiaoyan Liu, Yan Wang, Lifei Zhi, Jixian Shen, and Guoyong Wang. 2023. "Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group" Molecules 28, no. 20: 7151. https://doi.org/10.3390/molecules28207151
APA StyleDou, J., Liu, J., Wang, Y., Zhi, L., Shen, J., & Wang, G. (2023). Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group. Molecules, 28(20), 7151. https://doi.org/10.3390/molecules28207151






