Green Synthesis of Low-Glycemic Amylose–Lipid Nanocomposites by High-Speed Homogenization and Formulation into Hydrogel
Abstract
:1. Introduction
2. Results
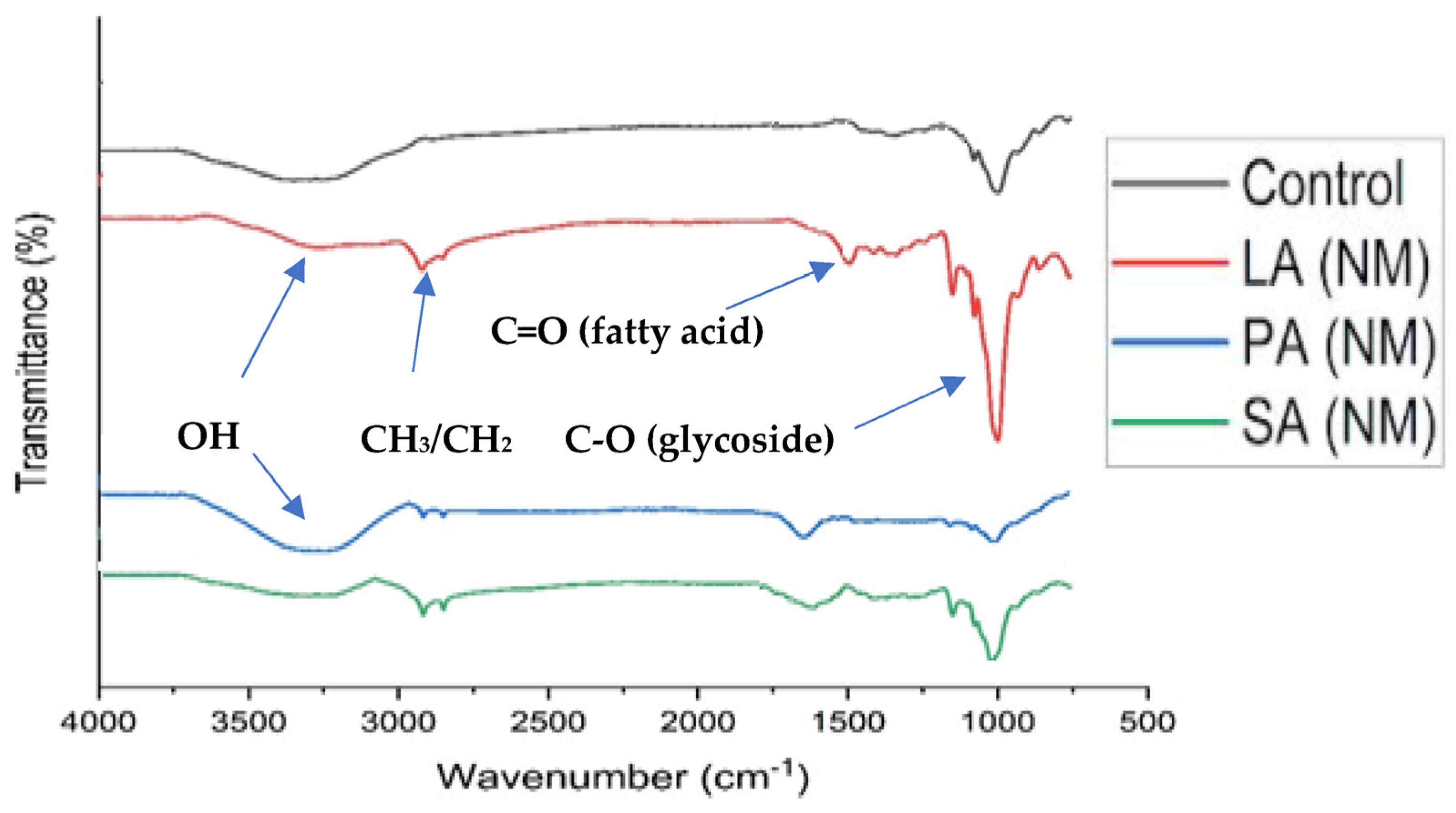
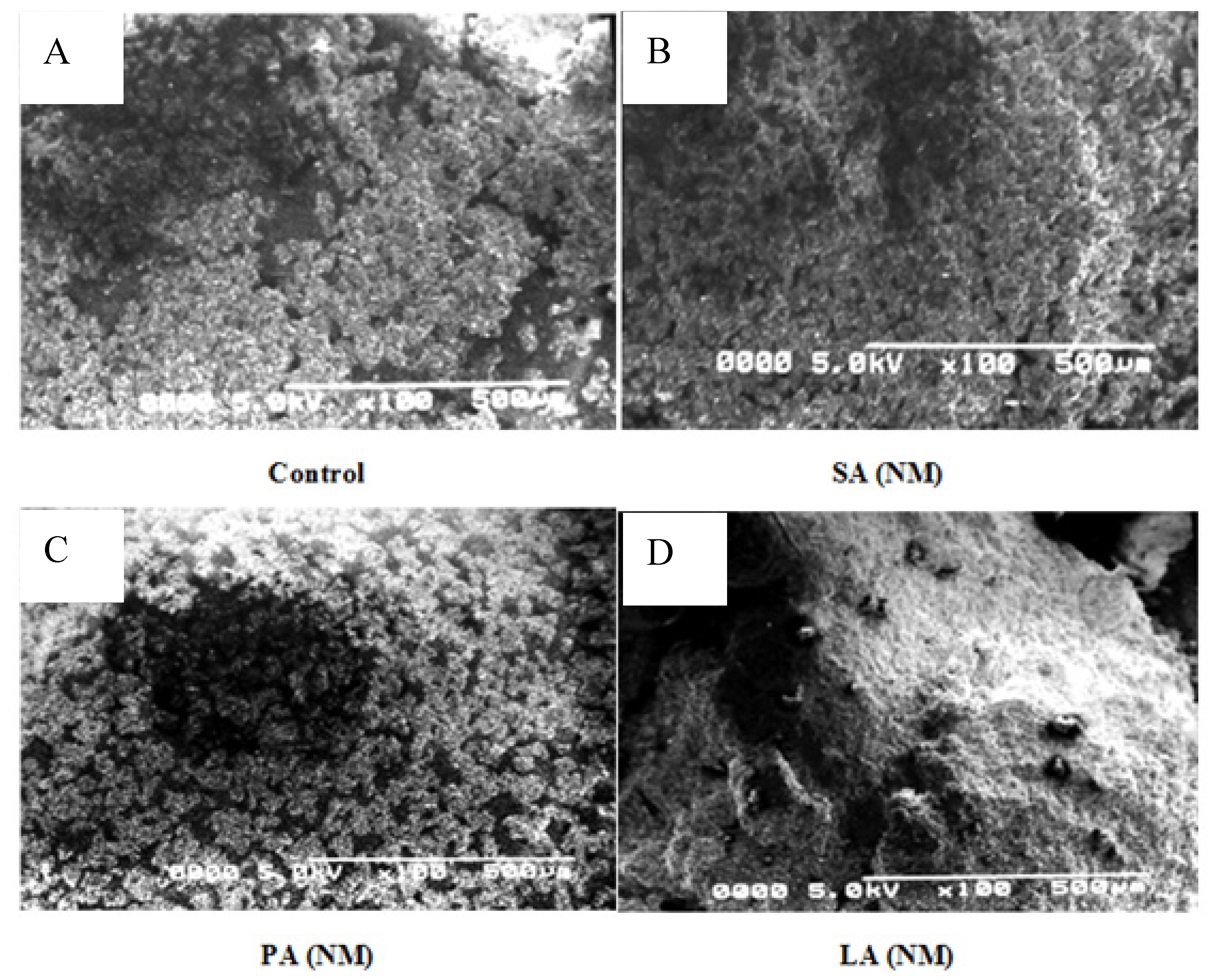
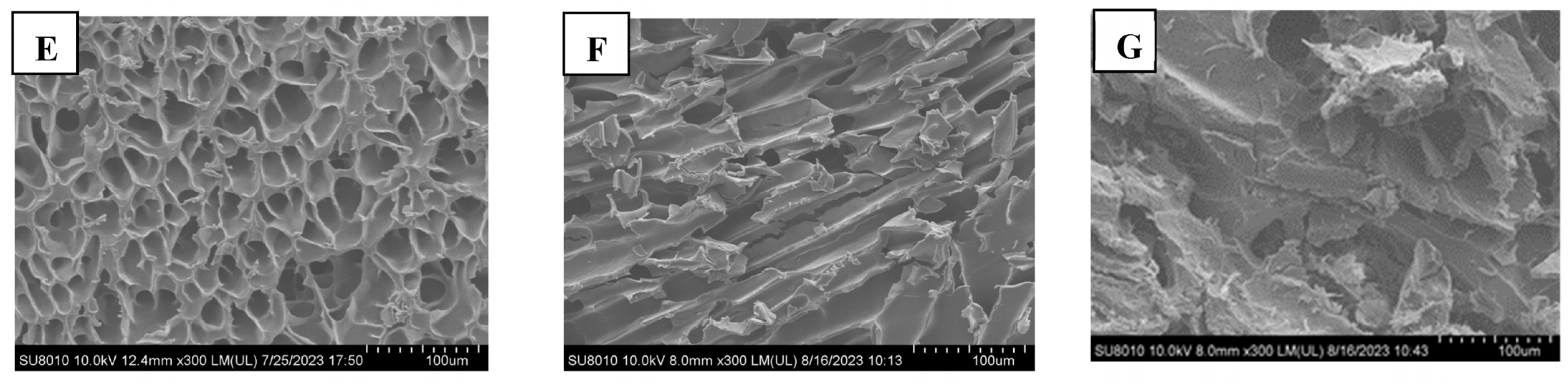
2.1. Structural Characteristics of Isolated ALN
2.2. Complexing Index, Solubility, and Swelling Power of ALN
2.3. In Vitro Digestibility and GI Values
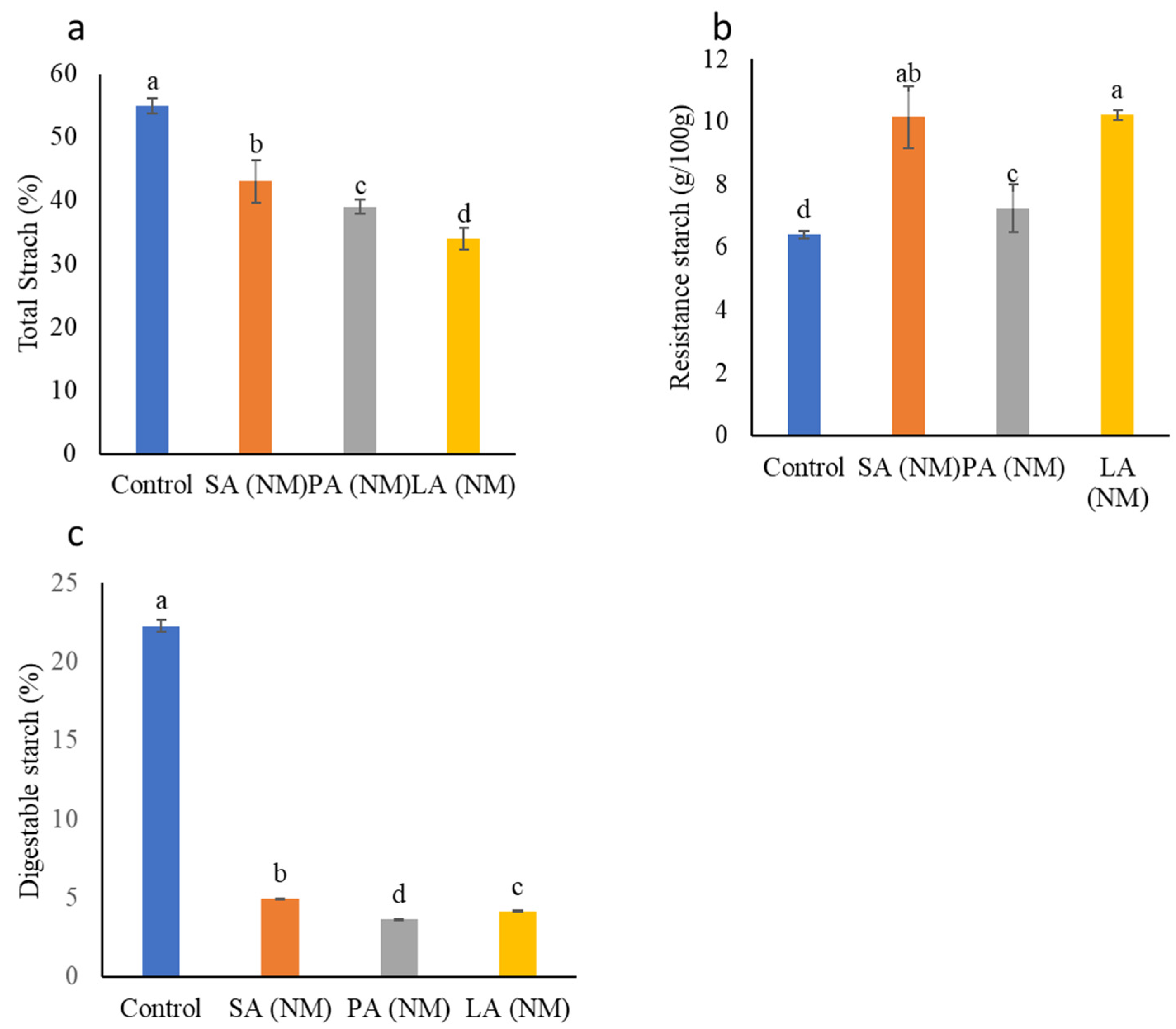
2.4. Total Starch (TS), Resistant Starch (RS) and Digestible Starch (DS)
2.5. Rheology
2.6. Retention of Anthocyanin
3. Materials and Methods
3.1. Preparation of Starch–Fatty Acid Complexes
3.2. Isolation of Amylose–Lipid Nanocomposite Material (ALN) from Starch Fatty Acid Mixtures
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Scanning Electron Microscopy (SEM)
3.5. X-ray Diffraction (XRD)
3.6. Thermal Gravimetric Analysis (TGA)
3.7. Complexing Index (CI)
3.8. Swelling Power (SP) and Solubility (S)
3.9. In Vitro Digestibility and Glycemic Index (GI) of Isolated ALN
3.10. Total Starch
3.11. Resistant Starch and Digestible Starch
3.12. Preparation of Hydrogel
3.13. Rheological Measurements
3.14. Encapsulation and Uptake of Anthocyanin
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shannon, J.C.; Garwood, D.L.; Boyer, C.D. Chapter 3—Genetics and Physiology of Starch Development. In Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 23–82. [Google Scholar]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.R.; Kim, J.Y.; Lim, S.T. Preparation and characterization of crystalline complexes between amylose and C18 fatty acids. LWT-Food Sci. Technol. 2015, 64, 889–897. [Google Scholar] [CrossRef]
- Arijaje, E.O.; Wang, Y.J. Effects of chemical and enzymatic modifications on starch-linoleic acid complex formation. Food Chem. 2017, 217, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose structural characteristics, methods of preparation, significance, and potential applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Le Thanh-Blicharz, J.; Lewandowicz, G.; Błaszczak, W.; Prochaska, K. Starch modified by high-pressure homogenisation of the pastes—Some structural and physico-chemical aspects. Food Hydrocoll. 2012, 27, 347–354. [Google Scholar] [CrossRef]
- Tang, J.; Yi, W.; Yan, J.; Chen, Z.; Fan, H.; Zaldivar-Silva, D.; Wang, S. Highly absorbent bio-sponge based on carboxymethyl chitosan/poly-γ-glutamic acid/platelet-rich plasma for hemostasis and wound healing. Int. J. Biol. Macromol. 2023, 247, 125754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Xie, P.; Wang, S. Biomedical Applications of Electrets: Recent Advance and Future Perspectives. J. Func. Biomater. 2023, 14, 320. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- García-Astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Uddin, M.; Khan, N.M.; Ali, F.; Khan, Z.U.; Muhammad, N.; Iqbal, J.; Rehman, N.; Jan, A.K.; Ahmad, S. Green Production and Structural Evaluation of Maize Starch–Fatty Acid Complexes Through High-Speed Homogenization. J. Polym. Environ. 2020, 28, 3110–3115. [Google Scholar] [CrossRef]
- Yan, X.; Diao, M.; Li, C.; Lu, C.; Zhao, P.; Zhang, T. Formation and properties of starch-palmitic acid complex nanoparticles and their influence on Pickering emulsions. Int. J. Biol. Macromol. 2022, 204, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.S.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer degradation and in vitro release of a model protein from poly(d,l-lactide-co-glycolide) nano- and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Narváez-González, E.D.; de Dios Figueroa-Cárdenas, J.; Taba, S.; Tostado, E.C.; Peniche, R.Á.M.; Sánchez, F.R. Relationships Between the Microstructure, Physical Features, and Chemical Composition of Different Maize Accessions from Latin America. Cereal. Chem. 2006, 83, 595–604. [Google Scholar] [CrossRef]
- Guan, J.; Eskridge, K.M.; Hanna, M.A. Acetylated starch-polylactic acid loose-fill packaging materials. Ind. Crops Prod. 2005, 22, 109–123. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, J.Y.; Lee, E.J.; Chung, H.J.; Lim, S.T. Impact of single and dual modifications on physicochemical properties of japonica and indica rice starches. Carbohyd. Polym. 2015, 122, 77–83. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Papastergiadis, E.; Raphaelides, S.N.; Kontominas, M.G. Morphological characteristics, oxidative stability and enzymic hydrolysis of amylose-fatty acid complexes. Carbohydr. Polym. 2016, 141, 106–115. [Google Scholar] [CrossRef]
- Konstantakos, S.; Marinopoulou, A.; Papaemmanouil, S.; Emmanouilidou, M.; Karamalaki, M.; Kolothas, E.; Saridou, E.; Papastergiadis, E.; Karageorgiou, V. Preparation of model starch complex hydrogels. Food Hydrocoll. 2019, 96, 365–372. [Google Scholar] [CrossRef]
- Chanvrier, H.; Uthayakumaran, S.; Appelqvist, I.A.M.; Gidley, M.J.; Gilbert, E.P.; López-Rubio, A. Influence of Storage Conditions on the Structure, Thermal Behavior, and Formation of Enzyme-Resistant Starch in Extruded Starches. J. Agric. Food Chem. 2007, 55, 9883–9890. [Google Scholar] [CrossRef]
- Chung, H.J.; Jeong, H.Y.; Lim, S.T. Effects of acid hydrolysis and defatting on crystallinity and pasting properties of freeze-thawed high amylose corn starch. Carbohydr. Polym. 2003, 54, 449–455. [Google Scholar] [CrossRef]
- Lebail, P.; Buleon, A.; Shiftan, D.; Marchessault, R.H. Mobility of lipid in complexes of amylose–fatty acids by deuterium and 13C solid state NMR. Carbohydr. Polym. 2000, 43, 317–326. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Beninca, C.; Demiate, I.; Lacerda, L.; Carvalho, M.A.; Ionashiro, M.; Schnitzler, E. Thermal behavior of corn starch granules modified by acid treatment at 30 and 50 °C. Eclét. Quím. 2008, 3, 33. [Google Scholar] [CrossRef]
- Ge, X.; Guo, Y.; Zhao, J.; Zhao, J.; Shen, H.; Yan, W. Dielectric barrier discharge cold plasma combined with cross-linking: An innovative way to modify the multi-scale structure and physicochemical properties of corn starch. Int. J. Biol. Macromol. 2022, 215, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Alfenas, R.D.C.G. Effect of the glycemic index on lipid oxidation and body composition. Nutr. Hosp. 2011, 26, 48–55. [Google Scholar]
- Salmerón, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary Fiber, Glycemic Load, and Risk of NIDDM in Men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef]
- Jimenez-Cruz, A.; Loustaunau-López, V.; Bacardi-Gascon, M. The use of low glycemic and high satiety index food dishes in Mexico: A low-cost approach to prevent and control obesity and diabetes. Nutr. Hosp. 2006, 21, 353–356. [Google Scholar]
- Higgins, J.A. Resistant starch: Metabolic effects and potential health benefits. J. AOAC Int. 2004, 87, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.A.; Fong, J.N.; Maiya, M.; Kung, S.; Sarkissian, A.; Nashef, N.; Wang, W. Chilled Potatoes Decrease Postprandial Glucose, Insulin, and Glucose-dependent Insulinotropic Peptide Compared to Boiled Potatoes in Females with Elevated Fasting Glucose and Insulin. Nutrients 2019, 11, 2066. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Dreher, C.J.; Berry, J.W. Fleming SE. Starch digestibility of foods: A nutritional perspective. Crit. Rev. Food Sci. Nut. 1984, 20, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Robin, F. Slowly digestible starch—Its structure and health implications: A review. Trends. Food Sci. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2021, 13, 622–633. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Ki, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng C 2020, 115, 111096. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Cuthbert, W.O.; Ray, S.S.; Emmambux, N.M. Isolation and characterization of nanoparticles from tef and maize starch modified with stearic acid. Carbohydr. Polym. 2017, 168, 86–93. [Google Scholar] [CrossRef]
- Tang, M.C.; Copeland, L. Analysis of complexes between lipids and wheat starch. Carbohydr. Polym. 2007, 67, 80–85. [Google Scholar] [CrossRef]
- McCleary, B.V.; McNally, M.; Rossiter, P. Collaborators: Measurement of Resistant Starch by Enzymatic Digestion in Starch and Selected Plant Materials: Collaborative Study. J. AOAC Int. 2002, 85, 1103–1111. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F.A. starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Ji, D.; Kim, J. Recent strategies for strengthening and stiffening tough hydrogels. Adv. NanoBiomed. Res. 2021, 1, 2100026. [Google Scholar] [CrossRef]


| Control | SA (NM) | PA (NM) | LA (NM) | |
|---|---|---|---|---|
| Complexing index | ------- | 45.38 ± 0.004 b | 29.94 ± 4.960 c | 40.9 ± 0.000 a |
| Solubility | 84.5 ± 0.04 a | 47.5 ± 0.009 d | 79 ± 0.078 b | 59.51 ± 0.005 c |
| Swelling power | 13.75 ± 0.001 a | 8.25 ± 0.003 c | 5.75 ± 0.007 d | 10.75 ± 0.002 b |
| GOPOD Parameters | Control | SA (NM) | PA (NM) | LA (NM) |
|---|---|---|---|---|
| Slope | 0.00089 ± 0.0007 | 0.00073 ± 0.0002 | −0.00227 ± 0.0002 | −0.00019 ± 0.0002 |
| Intercept | −1.04558 ± 0.0001 | −0.95603 ± 0.000 | −0.57345 ± 0.000 | −0.99518 ± 0.000 |
| Kt (h−1) | −0.00205 ± 0.000 | −0.00168 ± 0.000 | 0.00523 ± 0.0004 | 0.000426 ± 0.000 |
| T1/2 (h) | −337.938 ± 0.000 | −412.489 ± 0.000 | 132.502 ± 0.710 | 1625.087 ± 1.777 |
| Co (µg/mL) | 0.090036 ± 0.005 | 0.110655 ± 0.000 | 0.267021 ± 0.0003 | 0.101117 ± 0.0008 |
| Vd (L) | 555.3325 ± 0.0001 | 451.8567 ± 0.001 | 187.251 ± 0.0001 | 494.4761 ± 0.007 |
| Clearness (L/h) | −1.13881 ± 0.0000 | −0.75914 ± 0.000 | 0.979344 ± 0.001 | 0.210864 ± 0.000 |
| AUC 0 to t | 0 | 0 | 0 | 0 |
| AUC 1 to t | 19.61 ± 0.0001 | 23.76 ± 0.0002 | 32.7 ± 0.0000 | 17.02 ± 0.0000 |
| AUC t to Infinity | −1.06469 ± 0.000 | −1.46821 ± 0.000 | 0.455695 ± 0.0002 | 4.103756 ± 0.0000 |
| GI (%) | 76.86 ± 1.271 | 40.29 ± 0.001 | 40.58 ± 0.001 | 40.26 ± 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.M.; Uddin, M.; Falade, E.O.; Khan, F.A.; Wang, J.; Shafique, M.; Alnemari, R.M.; Abduljabbar, M.H.; Ahmad, S. Green Synthesis of Low-Glycemic Amylose–Lipid Nanocomposites by High-Speed Homogenization and Formulation into Hydrogel. Molecules 2023, 28, 7154. https://doi.org/10.3390/molecules28207154
Khan NM, Uddin M, Falade EO, Khan FA, Wang J, Shafique M, Alnemari RM, Abduljabbar MH, Ahmad S. Green Synthesis of Low-Glycemic Amylose–Lipid Nanocomposites by High-Speed Homogenization and Formulation into Hydrogel. Molecules. 2023; 28(20):7154. https://doi.org/10.3390/molecules28207154
Chicago/Turabian StyleKhan, Nasir Mehmood, Misbah Uddin, Ebenezer Ola Falade, Farman Ali Khan, Jian Wang, Muhammad Shafique, Reem M. Alnemari, Maram H. Abduljabbar, and Shujaat Ahmad. 2023. "Green Synthesis of Low-Glycemic Amylose–Lipid Nanocomposites by High-Speed Homogenization and Formulation into Hydrogel" Molecules 28, no. 20: 7154. https://doi.org/10.3390/molecules28207154
APA StyleKhan, N. M., Uddin, M., Falade, E. O., Khan, F. A., Wang, J., Shafique, M., Alnemari, R. M., Abduljabbar, M. H., & Ahmad, S. (2023). Green Synthesis of Low-Glycemic Amylose–Lipid Nanocomposites by High-Speed Homogenization and Formulation into Hydrogel. Molecules, 28(20), 7154. https://doi.org/10.3390/molecules28207154








