Development and Characterization of High-Absorption Microencapsulated Organic Propolis EPP-AF® Extract (i-CAPs)
Abstract
:1. Introduction
2. Results
2.1. Development and Characterization
2.1.1. Chemical Characterization
2.1.2. Microencapsulation Efficiency (%ME)
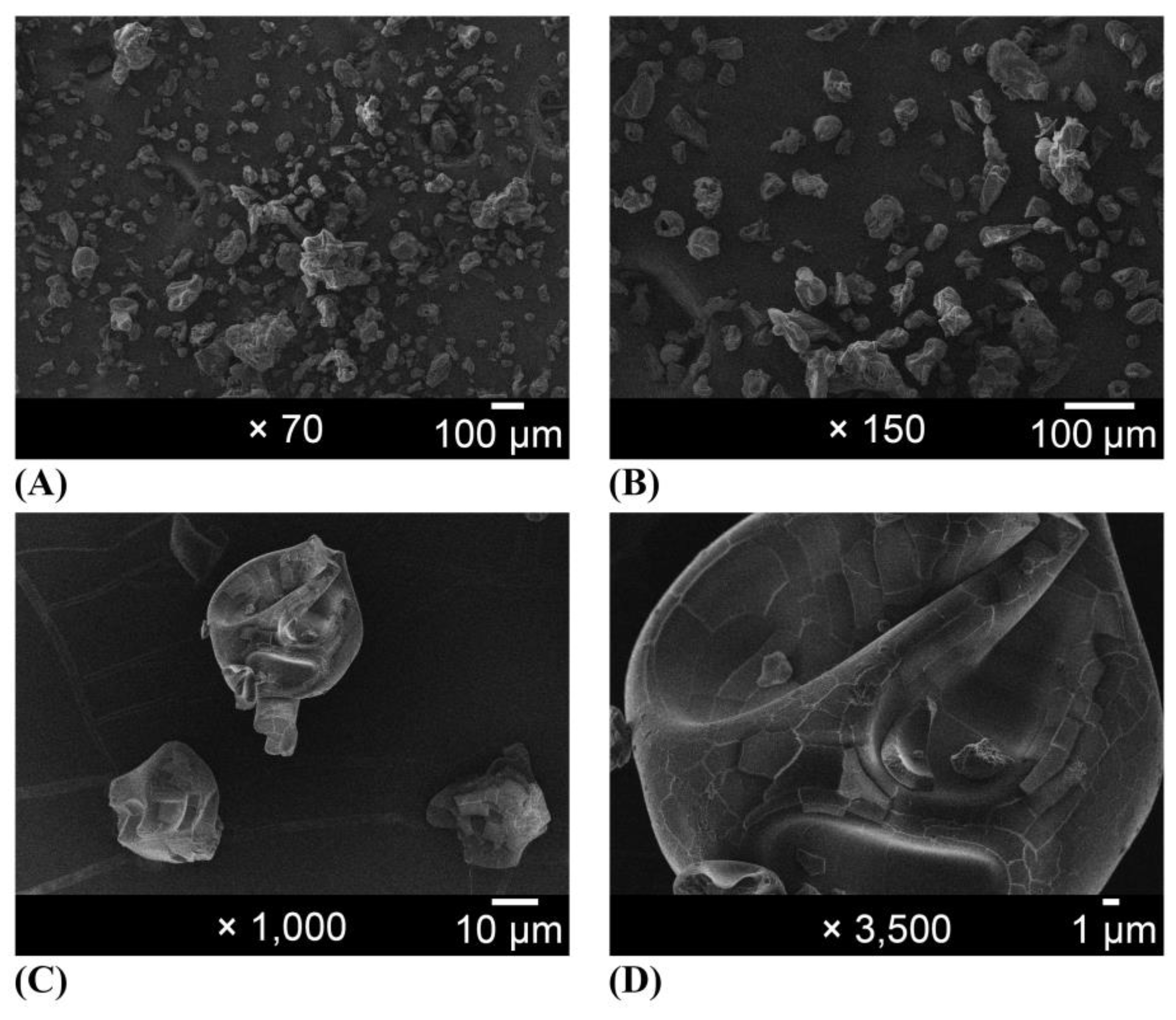
2.1.3. Morphology—Microscopic and Macroscopic Aspects
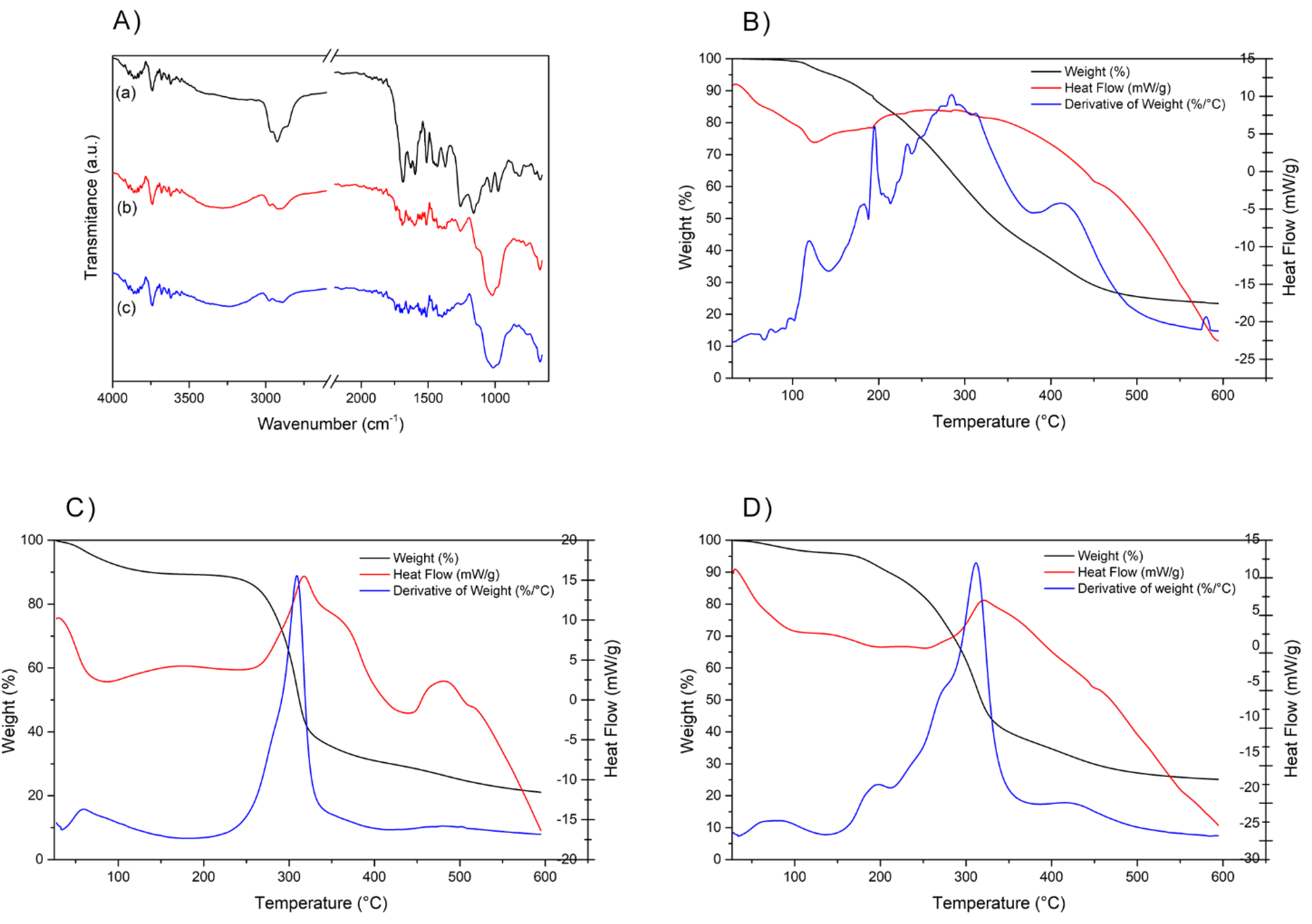
2.1.4. FT-IR and TG/DTG/DSC Characterization
2.1.5. Release Study
2.2. Biological Properties
2.2.1. Antioxidant Activity
2.2.2. Antimicrobial Activity
2.2.3. Cytotoxicity, Anti-Inflammatory, and Antihypocholesterolemia Analyses
2.2.4. Permeability Assays on Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. LC/DAD/ESI-MSn Phenolic Compounds Analysis
4.3. Propolis EPP-AF® and EPP-AF® Microencapsulated Extracts
4.4. Chemical Fingerprint and Characterization
4.5. Total Flavonoid Determination
4.6. Total Phenolic Compounds Determination
4.7. Scanning Electron Microscopy (SEM)
4.8. X-ray Diffraction
4.9. Fourier Transform Infrared Spectroscopy (FT-IR)
4.10. Thermal Analysis
4.11. Microencapsulation Efficiency (ME)
4.12. Total Antioxidant Activity by the Method of Iron Reduction (FRAP)
4.13. Total Antioxidant Activity by DPPH Free Radical Scavenging
4.14. Antimicrobial Activity by Microdilution in Broth
4.15. Release Test
4.16. Cytotoxicity Activity
4.17. Anti-Inflammatory Activity
4.18. Hypocholesterolemic Activity
Cholesterol Quantification
4.19. Permeability Assay on Caco-2 Cells
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grand View Research. Organic Food and Beverages Market Size, Share & Trends Analysis Report by Product (Organic Food, Organic Beverages), by Distribution Channel (Convenience Stores, Specialty Stores), by Region, and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/organic-foods-beverages-market (accessed on 20 August 2023).
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; Vaz, M.M.O.L.L.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.S.; Damaso, B.; Ramos, C.; et al. Functional properties of Brazilian propolis: From chemical composition until the market. In Superfood and Functional Food-An Overview of Their Processing and Utilization, 1st ed.; Waisundara, V., Shiomi, N., Eds.; Intech Open Limited: London, UK, 2017; pp. 55–98. ISBN 978-953-51-2920-2. [Google Scholar]
- Berretta, A.A.; Zamarrenho, L.G.; Correa, J.A.; de Lima, J.A.; Borini, G.B.; Ambrósio, S.R.; da Silva Barud, H.; Bastos, J.K.; De Jong, D. Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract. Molecules 2023, 28, 3510. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.I.S.; Oki, Y.; Resende, F.M.; Angrisano, P.; Rosa, D.C.P.; Arantes-Garcia, L.; Fernandes, G.H. From Innovation to Market: An Analysis of the Propolis Production Chain. In Baccharis, 1st ed.; Fernandes, G.W., Oki, Y., Barbosa, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 547–564. ISBN 978-3-030-83511-8. [Google Scholar]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Pereira, A.S.; Seixas, F.R.M.S.; Neto, F.R.A. Própolis: 100 anos de pesquisa e suas perspectivas futuras. Quim. Nova 2002, 25, 321–326. [Google Scholar] [CrossRef]
- De Oliveira, R.T.; Nguyen, T.T.K.; Liesch, P.; Verreynne, M.L.; Indulska, M. Exporting to escape and learn: Vietnamese manufacturers in global value chains. J. World Bus. 2021, 56, 101227. [Google Scholar]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, A.; Tan, W.; Yang, W. Development, Physicochemical Properties, and Antibacterial Activity of Propolis Microcapsules. Foods 2023, 12, 3191. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Ramos-Pacheco, B.S.; de la Cruz, G.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Muñoz-Melgarejo, M.; Quispe-Quezada, U.R.; et al. Microencapsulation of Propolis and Honey Using Mixtures of Maltodextrin/Tara Gum and Modified Native Potato Starch/Tara Gum. Foods 2023, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [PubMed]
- Da Silva, T.M.; Barin, J.S.; Lopes, E.J.; Cichoski, A.J.; Flores, E.M.M.; da Silva, C.B. Development, characterization and viability study of probiotic microcapsules produced by complex coacervation followed by freeze-drying. Ciência Rural 2019, 49, 7. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.; Falcao, S.I.; Vale, N.; Almeida-Muradian, L.B.; Vilas-Boas, M. Phenolic composition and antioxidant activity assessment of southeastern and south Brazilian propolis. J. Apic. Res. 2017, 56, 21–31. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; de Souza, M.C.; Arruda, C.; Pereira, R.A.S.; Bastos, J.K. The role of Baccharis dracunculifolia and its chemical profile on green propolis production by Apis mellifera. J. Chem. Ecol. 2020, 46, 150–162. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Nanda, V. Encapsulated bee propolis powder: Drying process optimization and physicochemical characterization. LWT 2022, 155, 112956. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; Andrade, G.R.S.; da Cunha Nascimento, C.; Barbosa, P.F.; Jesus, M.S.; Narain, N. Development and characterization of microencapsules containing spray dried powder obtained from Brazilian brown, green and red propolis. Food Res. Int. 2018, 109, 278–287. [Google Scholar] [CrossRef]
- De Alencar, S.M.; Sartori, A.G.O.; Dag, D.; Batista, P.S.; Rosalen, P.L.; Ikegaki, M.; Kong, F. Dynamic gastrointestinal digestion/intestinal permeability of encapsulated and nonencapsulated Brazilian red propolis: Active compounds stability and bioactivity. Food Chem. 2023, 411, 135469. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert. Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.S.M.C.; Sartori, A.G.O.; Batista, P.S.; do Amaral, J.E.P.G.; da Silva, N.O.; Ikegaki, M.; Rosalen, P.L.; de Alencar, S.M. Simulated gastrointestinal digestion/Caco-2 cell transport: Effects on biological activities and toxicity of a Brazilian propolis. Food Chem. 2023, 403, 134330. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Ministry of Agriculture. Brazilian Ministry of Agriculture: Instruction Normative to Propolis Extracts; n. 3/2001; Ministério da Agricultura e do Abastecimento: São Paulo, Brazil, 2001.
- Arruda, C.; Ribeiro, V.P.; Almeida, M.O.; Mejía, J.A.A.; Casoti, R.; Bastos, J.K. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green própolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Intern. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Fonseca, C.R.; Alencar, S.M.; Thomazini, M.; Balieiro, J.C.C.; Pittia, P. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using arabic gum and OSA starch-based carrier systems. Food Bioprod. 2013, 91, 28–36. [Google Scholar]
- Dos Reis, A.S.; Diedrich, C.; de Moura, C.; Pereira, D.; de Flório Almeida, J.; da Silva, L.D.; Plata-Oviedo, M.S.V.; Tavares, R.A.W.; Carpes, S.T. Physico-chemical characteristics of microencapsulated propolis co-product extract and its effect on storage stability of burger meat during storage at −15 °C. Food Sci. Technol. 2017, 76, 306–313. [Google Scholar]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Poklar Ulrih, N.; Buera, M.P. Propolis encapsulation by spray drying: Characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Ferreira, I.; Melo, D.S.; Santos, M.S.; Dias, D.R.; de Souza, C.O.; Favaro-Trindade, C.S.; Pinho, L.S.; Almeida, R.C.C.; Magalhães-Guedes, K.T.; Schwan, R.F. Non-Lactic Probiotic Beverage Enriched with Microencapsulated Red Propolis: Microorganism Viability, Physicochemical Characteristics, and Sensory Perception. Fermentation 2023, 9, 234. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Lamsen, M.R.; Wang, T.; D’Souza, D.; Dia, V.; Chen, G.; Zhong, Q. Encapsulation of vitamin D3 in gum arabic to enhance bioavailability and stability for beverage applications. J. Food Sci. 2020, 85, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Araújo, A.M., Jr.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid. Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef]
- Tiwari, S.; Mishra, B. Multilayered membrane-controlled microcapsules for controlled delivery of isoniazid. Daru 2011, 19, 41–46. [Google Scholar] [PubMed]
- Kang, Y.R.; Lee, Y.K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Barbosa, M.M. Processo de Purificação de Goma de Cajueiro: Aspectos Técnicos e Ambientais. Ph.D. Thesis, Universidade Federal do Ceará, Ceará, CE, Brazil, 2015. [Google Scholar]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras Castillo, C.J. Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci. Technol. 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Onbas, R.; Kazan, A.; Nalbantsoy, A.; Yesil-Celiktas, O. Cytotoxic and nitric oxide inhibition activities of propolis extract along with microencapsulation by complex coacervation. Plant Foods Hum. Nutr. 2016, 71, 286–293. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 637542, p-Coumaric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/p-Coumaric-acid (accessed on 20 August 2023).
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, characterization and pre-clinical trials of an innovative wound healing dressing based on propolis (EPP-AF®)-containing self-microemulsifying formulation incorporated in biocellulose membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; da Silva, P.F.; Azevedo, L.F.; da Rocha, L.G.; de Moraes Porto, I.C.C.; Lima e Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Alves, C.; Maia, D.S.V.; Krumreich, F.D.; Crizel-Cardoso, M.M.; Fioravante, J.B.; da Silva, W.P.; Borges, C.D.; Zambiazi, R.C. Propolis microparticles produced with pea protein: Characterization and evaluation of antioxidant and antimicrobial activities. Food Hydrocoll. 2019, 87, 703–711. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Lara, E.H.G.; Martins, C.H.G.; Vinholis, A.H.C.; Casemiro, L.A.; Panzeri, H.; Gremião, M.P.D. Preparation and antimicrobial activity of gelatin microparticles containing propolis against oral pathogens. Drug Dev. Ind. Pharm. 2006, 32, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, E.; Figoli, A.; Musatti, A.; Limbo, S.; Drioli, E.; Suevo, R.; Talarico, S.; Rollini, M. An alternative encapsulation approach for production of active chitosan-propolis beads. Int. J. Food Sci. Technol. 2014, 49, 1401–1407. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef]
- Oršoli’c, N.; Jembrek, M.J. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef]
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin C, a cinnamic acid derivative in Brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 497, 437–443. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother. Res. 2012, 26, 1308–1313. [Google Scholar] [CrossRef]
- Olas, B. Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases. Nutrients 2022, 14, 2267. [Google Scholar] [CrossRef]
- Suryawanshi, N.; Naik, S.; Eswari, J.S. Exopolysaccharides and their applications in food processing industries. Food Sci. Biotechnol. 2022, 5, 22–44. [Google Scholar] [CrossRef]
- Falcão, S.I.; Tomás, A.; Vale, N.; Gomes, P.; Freire, C.; Vilas-Boas, M. Phenolic quantification and botanical origin of Portuguese propolis. Ind. Crops Prod. 2013, 49, 805–812. [Google Scholar] [CrossRef]
- Brazilian Ministry of Agriculture. Brazilian Ministry of Agriculture: Instruction Normative to Livestock and Supply; n. 11/2000; Ministério da Agricultura e do Abastecimento: São Paulo, Brazil, 2000.
- Funari, C.S.; Ferro, V.O. Análise de própolis. Food Sci. Technol. 2006, 26, 171–178. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites, 1st ed.; Wiley: Hoboken, NJ, USA, 1994; ISBN 0632029692. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a meansure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Hwang, H.J.; Ha, J.S.; Jeong, H.S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Patel, J.B.; Burnham, C.A.; Campeau, S.; Conville, P.S.; Doern, C.; Eliopoulos, G.M.; Galas, M.F.; Humphries, R.M.; Jenkins, S.G.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018; ISBN 1-56238-837-1. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Heleno, S.A.; Rudke, A.R.; Calhelha, R.C.; Carocho, M.; Barros, L.; Gonçalves, O.H.; Barreirob, M.F.; Ferreira, I.C.F.R. Development of dairy beverages functionalized with pure ergosterol and mycosterol extracts: An alternative to phytosterol-based beverages. Food Funct. 2017, 8, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Moro, C.; Manchón, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamón, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J. Funct. Foods 2014, 11, 589–597. [Google Scholar] [CrossRef]
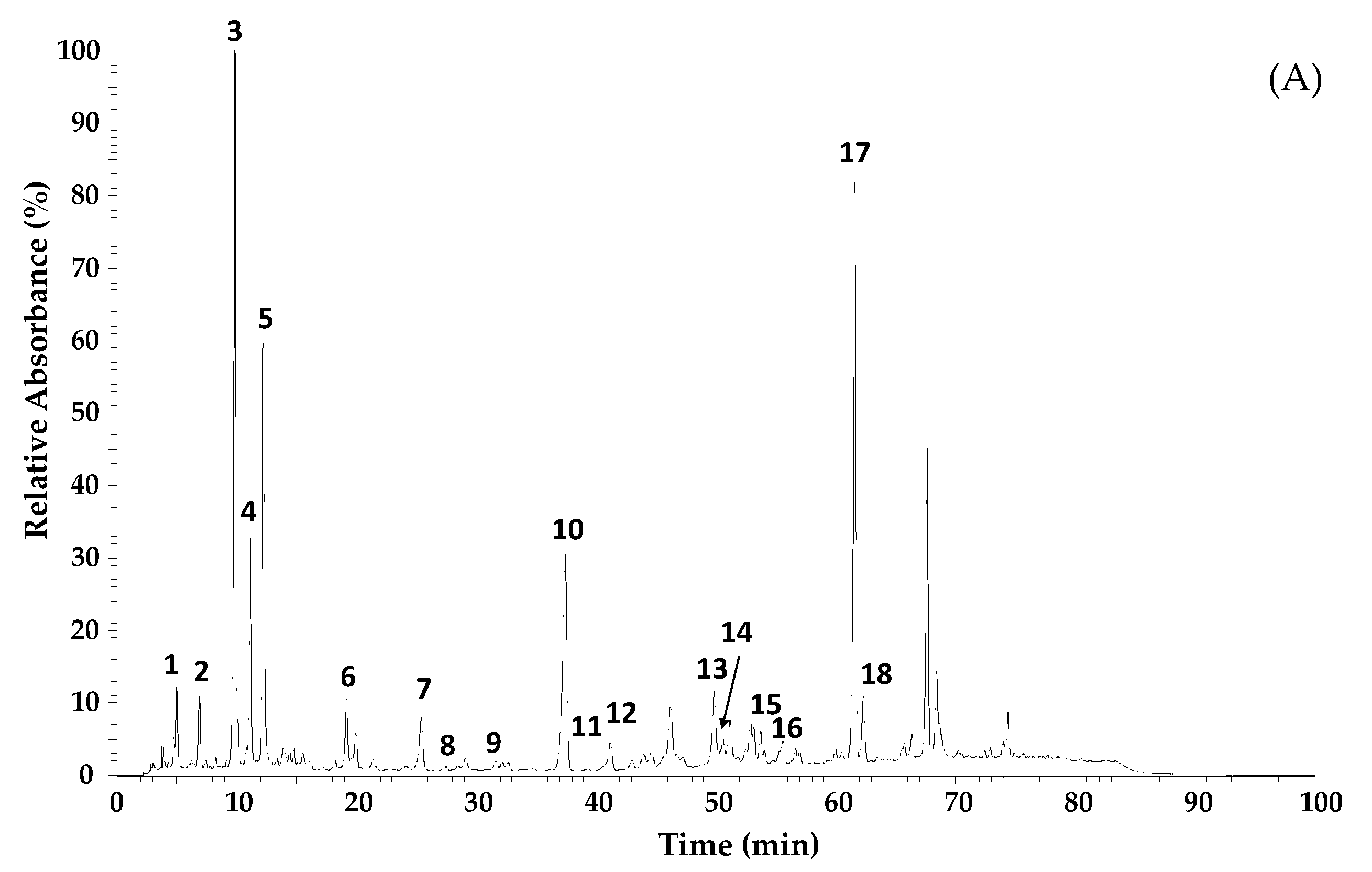
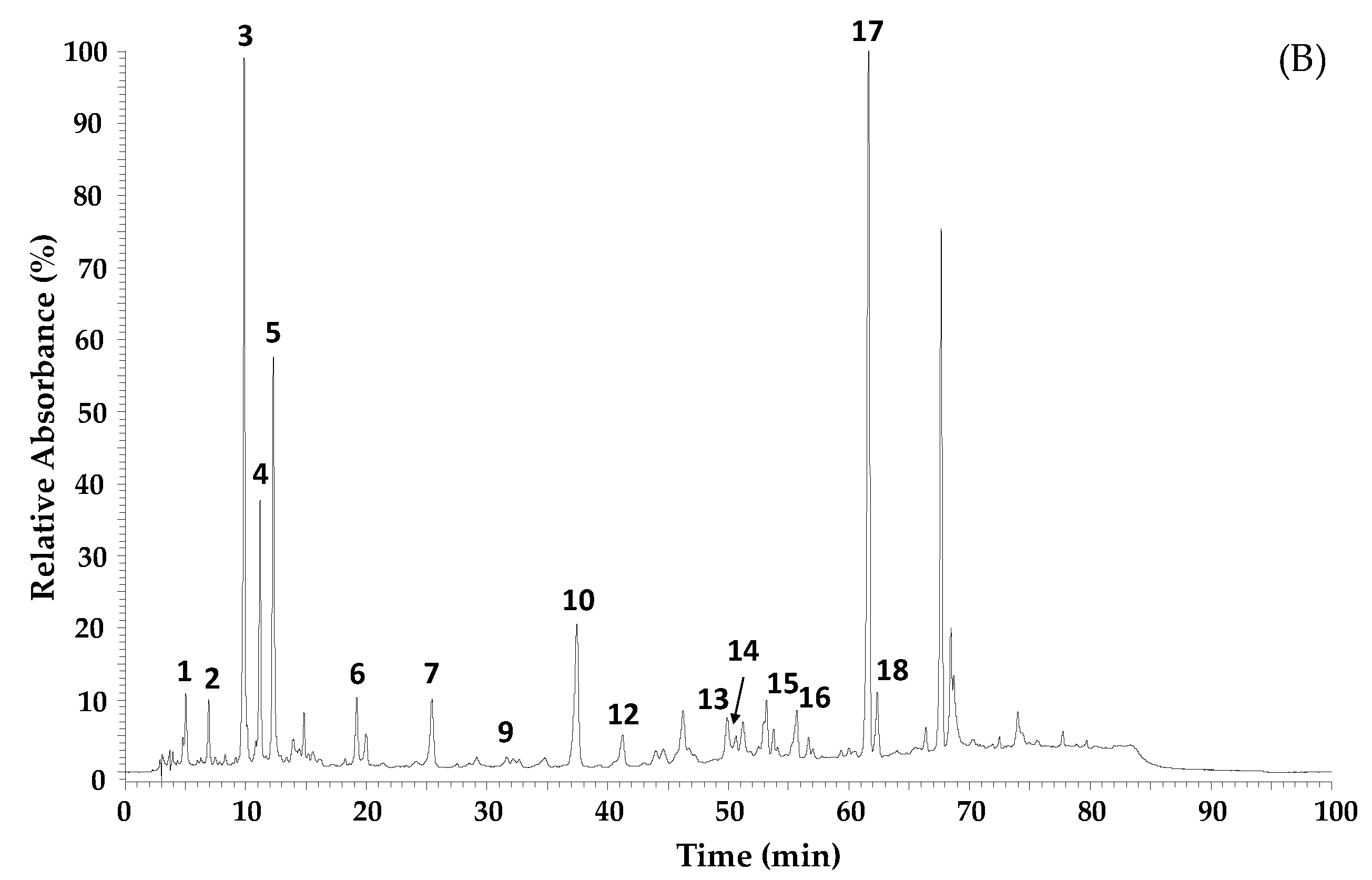


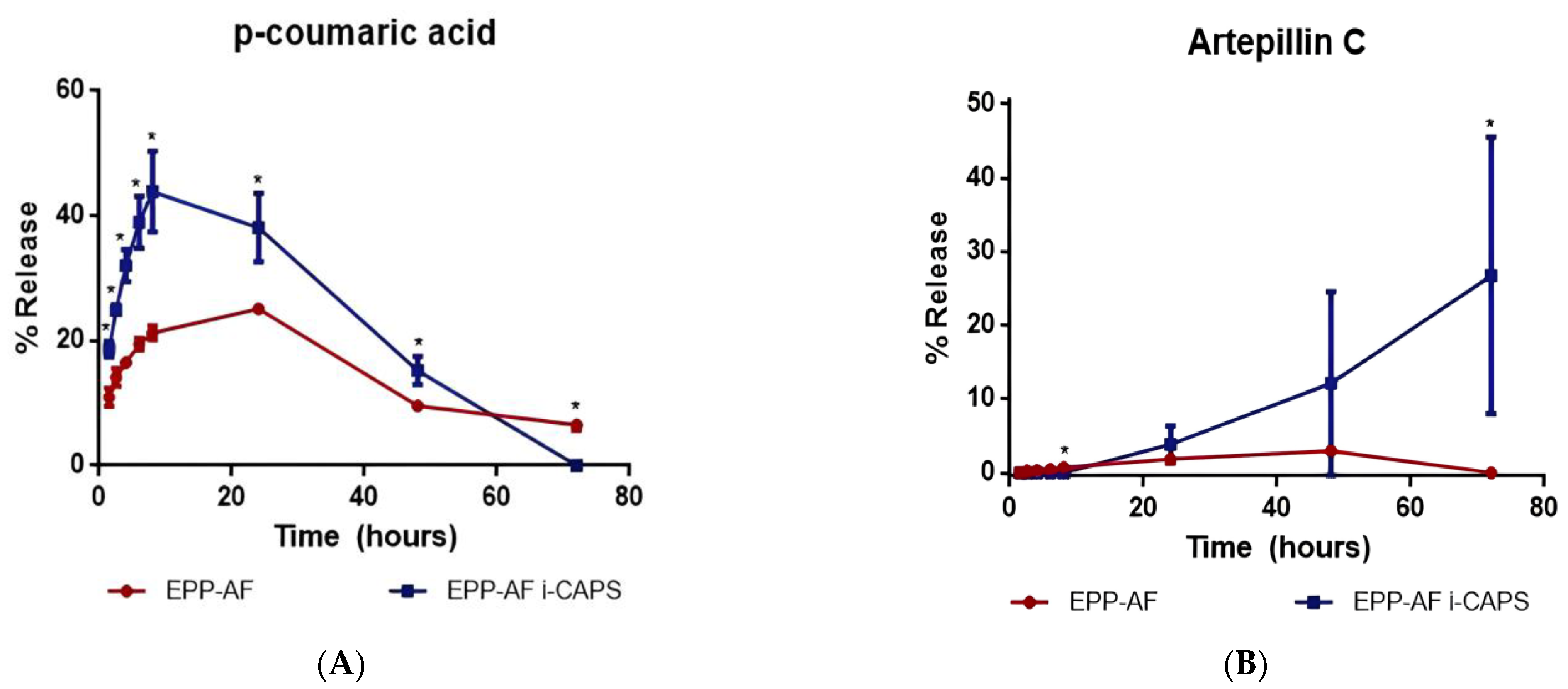
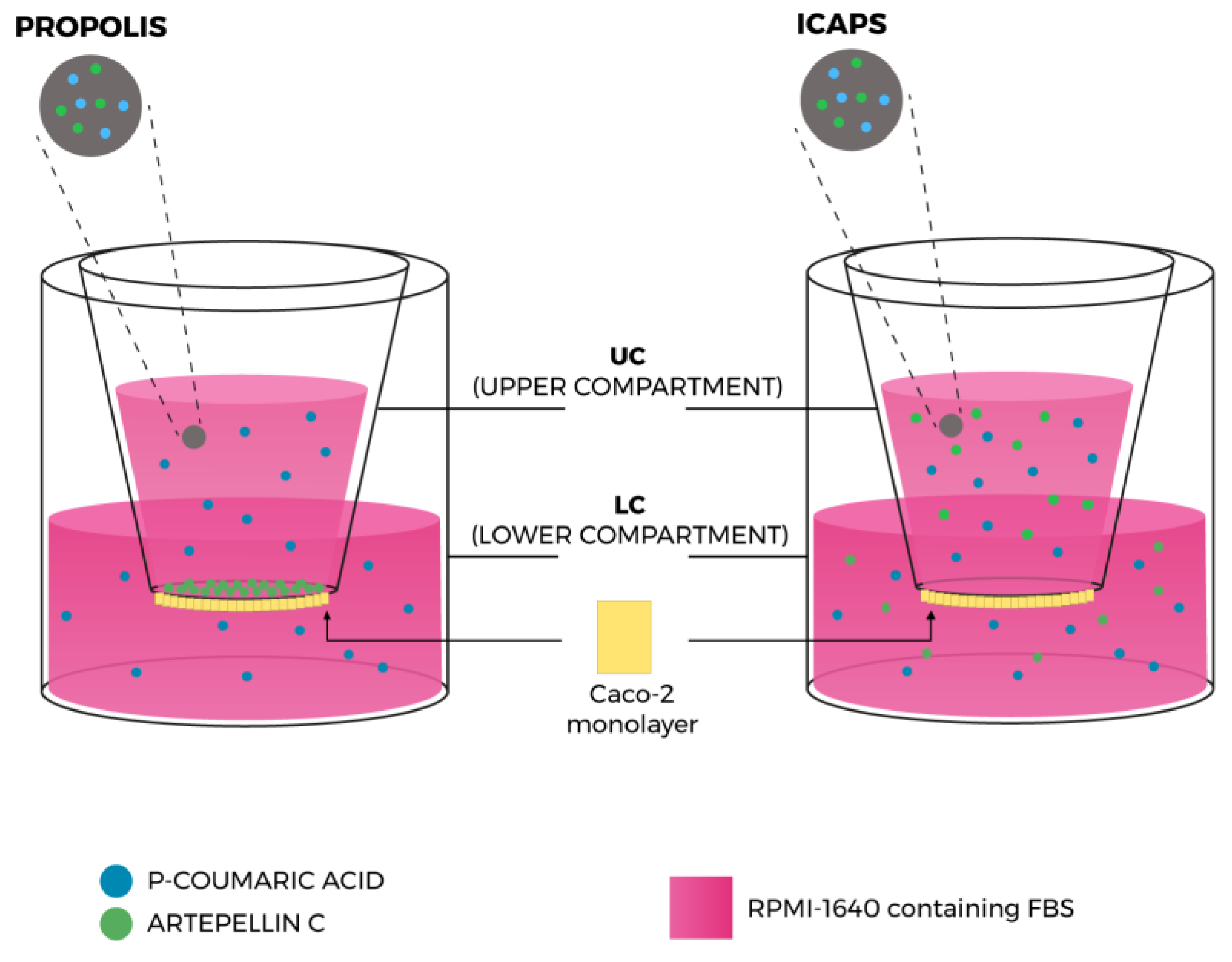
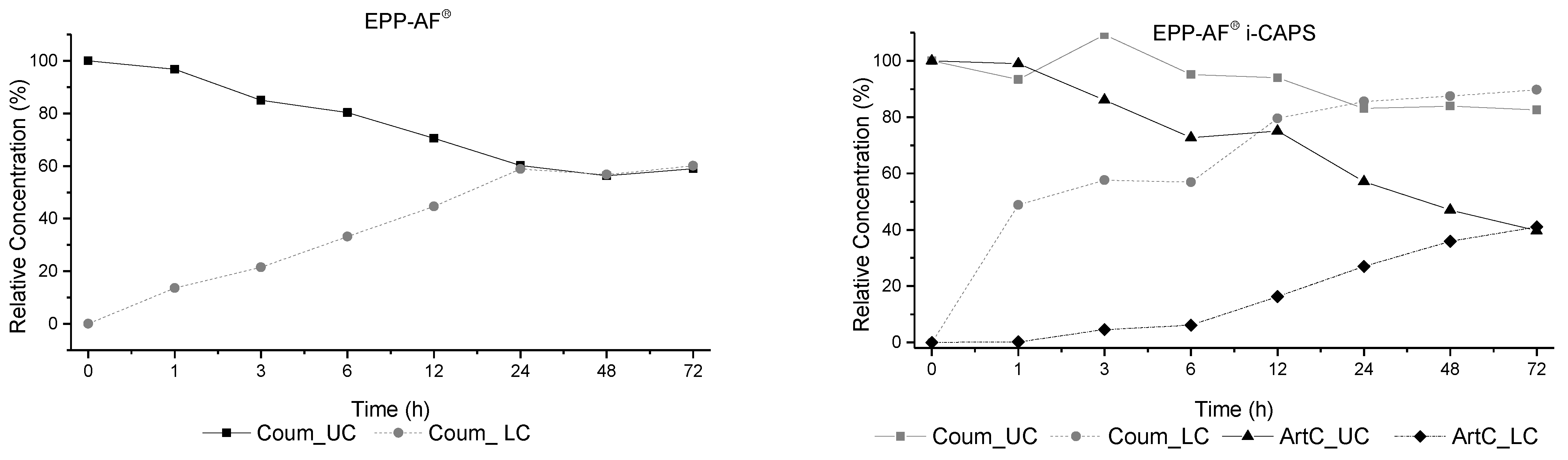
| Nr | RT (min) | λmax (nm) | [M-H]− m/z | MS2 (% Base Peak) | Proposed Compound |
|---|---|---|---|---|---|
| 1 | 5.03 | 299sh, 325 | 353 | 191 (100), 179 (8), 135 (1) | 5-O-Caffeoylquinic acid a,b |
| 2 | 6.93 | 293, 322 | 179 | 135 | Caffeic acid a,b |
| 3 | 9.88 | 310 | 163 | 135 | p-Coumaric acid |
| 4 | 11.19 | 294sh, 325 | 515 | 353 | Dicaffeoylquinic acid b,c |
| 5 | 12.28 | 294sh, 325 | 515 | 353 | Dicaffeoylquinic acid (isomer) b,c |
| 6 | 19.22 | 294sh, 325 | 677 | 515 | Tricaffeoylquinic acid b,c |
| 7 | 25.46 | 293 | 301 | 283 (100), 151 (29) | Dihydrokaempferide b,c |
| 8 | 28.51 | 267, 365 | 285 | 285 (100), 257 (13), 151 (20) | Kaempferol a,b |
| 9 | 31.69 | 321 | 247 | 203 | 5-Isoprenyl caffeic acid b,d |
| 10 | 37.47 | 315 | 231 | 187 | Drupanin b,c |
| 11 | 39.8 | 310 | 327 | 283 | Dihydroconiferyl p-coumarate b,c |
| 12 | 41.26 | 315 | 315 | 271 (100), 241 (70), 285 (59) | Cappilartimisin A b,c,d |
| 13 | 49.90 | 266, 365 | 299 | 284 | Kaempferide b |
| 14 | 50.65 | 266, 365 | 299 | 284 | Kaempferide (isomer) b |
| 15 | 53.78 | 316 | 393 | 349 (100), 163 (91), 199 (52) | 5-Isoprenyl caffeic acid-p-coumaric acid ester b,d |
| 16 | 54.09 | 319 | 315 | 245 (100), 201 (30), 271 (9) | Cappilartimisin A (isomer) b,d |
| 17 | 61.64 | 314 | 299 | 255 | Artepillin C |
| 18 | 62.33 | 284 | 363 | 187 | Baccharin b,e |
| Chemical Compounds (n = 3) | EPP-AF® i-CAPS (T0) | EPP-AF® i-CAPS (T24) | EPP-AF® i-CAPS (T36) | % (T24/T0) | % (T36/T0) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| p-Coumaric acid | 7.090 ± 0.037 | 6.888 ± 0.092 | 6.898 ± 0.023 | −2.85 | −2.71 |
| Artepillin C | 33.678 ± 0.993 | 27.693 ± 0.811 * | 28.888 ± 0.350 * | −17.77 | −14.22 |
| Total bioactive compounds | 93.079 ± 1.647 | 84.427 ± 1.812 * | 85.830 ± 0.593 * | −9.30 | −7.79 |
| Samples | FRAP (µmol FeII/mg Propolis Dry Matter) ± SD | DPPH (IC50) (µg Propolis Dry Matter/mL) ± SD |
|---|---|---|
| EPP-AF® | 2.873 ± 0.045 | 6.500 ± 0.062 |
| EPP-AF® i-CAPS | 2.654 ± 0.062 * | 7.342 ± 0.058 * |
| Samples | MBC (Mean ± SD, mg/mL) | |
|---|---|---|
| S. aureus | E. coli | |
| EPP-AF® | 3.44 ± 0.0 | 27.50 ± 0.0 |
| EPP-AF® i-CAPS | 3.44 ± 0.0 | 27.50 ± 0.0 |
| Alcohol 40% v/v | 190.0 ± 0.0 * | 190.0 ± 0.0 * |
| Samples | MBC (Mean ± SD, mg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | S. aureus MRSA | S. epidermidis | E. coli | K. pneumoniae | P. aeruginosa | |
| EPP-AF® (lyophilized) | 55.0 ± 0.0 | 110.0 ± 0.0 | 55.0 ± 0.0 | 110.0 ± 0.0 | 91.67 ± 31.75 | 91.67 ± 31.75 |
| EPP-AF® i-CAPS | 1.72 ± 0.0 * | 5.73 ± 1.98 * | 6.88 ± 0.0 * | 55.0 ± 0.0 * | 27.50 ± 0.0 * | 36.67 ± 15.88 * |
| Cytotoxic Activity (GI50, µg/mL) | Anti-Inflammatory Activity (IC50, µg/mL) | ||||
|---|---|---|---|---|---|
| AGS | Caco-2 | MCF-7 | PLP2 | RAW 264.7 | |
| EPP-AF® | 184 ± 2 a | 241 ± 20 a | 296 ± 23 a | 146 ± 11 a | 86 ± 3 a |
| EPP-AF® i-CAPS | 154 ± 1 b | 117 ± 1 b | 271 ± 25 a | 156 ± 4 a | 59 ± 0.1 b |
| Elipticin | 1.23 ± 0.03 c | 1.21 ± 0.02 c | 1.02 ± 0.02 b | 1.4 ± 0.1 b | - |
| Dexamethasone | - | - | - | - | 6.3 ± 0.4 |
| Cholesterol (µM) | Cholesterol (%) | ||
|---|---|---|---|
| Control | UC | 26.86 a | 53.7 a |
| LC | 21.82 C | 43.6 C | |
| EPP-AF® | UC | 35.96 b | 71.9 b |
| LC | 13.29 B | 26.6 B | |
| EPP-AF® i-CAPS | UC | 39.06 c | 78.1 c |
| LC | 10.10 A | 20.2 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berretta, A.A.; De Lima, J.A.; Falcão, S.I.; Calhelha, R.; Amorim, N.A.; Gonçalves, I.S.; Zamarrenho, L.G.; Barud, H.d.S.; Bastos, J.K.; De Jong, D.; et al. Development and Characterization of High-Absorption Microencapsulated Organic Propolis EPP-AF® Extract (i-CAPs). Molecules 2023, 28, 7128. https://doi.org/10.3390/molecules28207128
Berretta AA, De Lima JA, Falcão SI, Calhelha R, Amorim NA, Gonçalves IS, Zamarrenho LG, Barud HdS, Bastos JK, De Jong D, et al. Development and Characterization of High-Absorption Microencapsulated Organic Propolis EPP-AF® Extract (i-CAPs). Molecules. 2023; 28(20):7128. https://doi.org/10.3390/molecules28207128
Chicago/Turabian StyleBerretta, Andresa A., Jéssica A. De Lima, Soraia I. Falcão, Ricardo Calhelha, Nathaly Alcazar Amorim, Isabella Salgado Gonçalves, Luana Gonçalves Zamarrenho, Hernane da Silva Barud, Jairo Kenupp Bastos, David De Jong, and et al. 2023. "Development and Characterization of High-Absorption Microencapsulated Organic Propolis EPP-AF® Extract (i-CAPs)" Molecules 28, no. 20: 7128. https://doi.org/10.3390/molecules28207128
APA StyleBerretta, A. A., De Lima, J. A., Falcão, S. I., Calhelha, R., Amorim, N. A., Gonçalves, I. S., Zamarrenho, L. G., Barud, H. d. S., Bastos, J. K., De Jong, D., & Vilas-Boas, M. (2023). Development and Characterization of High-Absorption Microencapsulated Organic Propolis EPP-AF® Extract (i-CAPs). Molecules, 28(20), 7128. https://doi.org/10.3390/molecules28207128












