Exploring DSSC Efficiency Enhancement: SQI-F and SQI-Cl Dyes with Iodolyte Electrolytes and CDCA Optimization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Halogen-Functionalized SQI-F and SQI-Cl Dyes
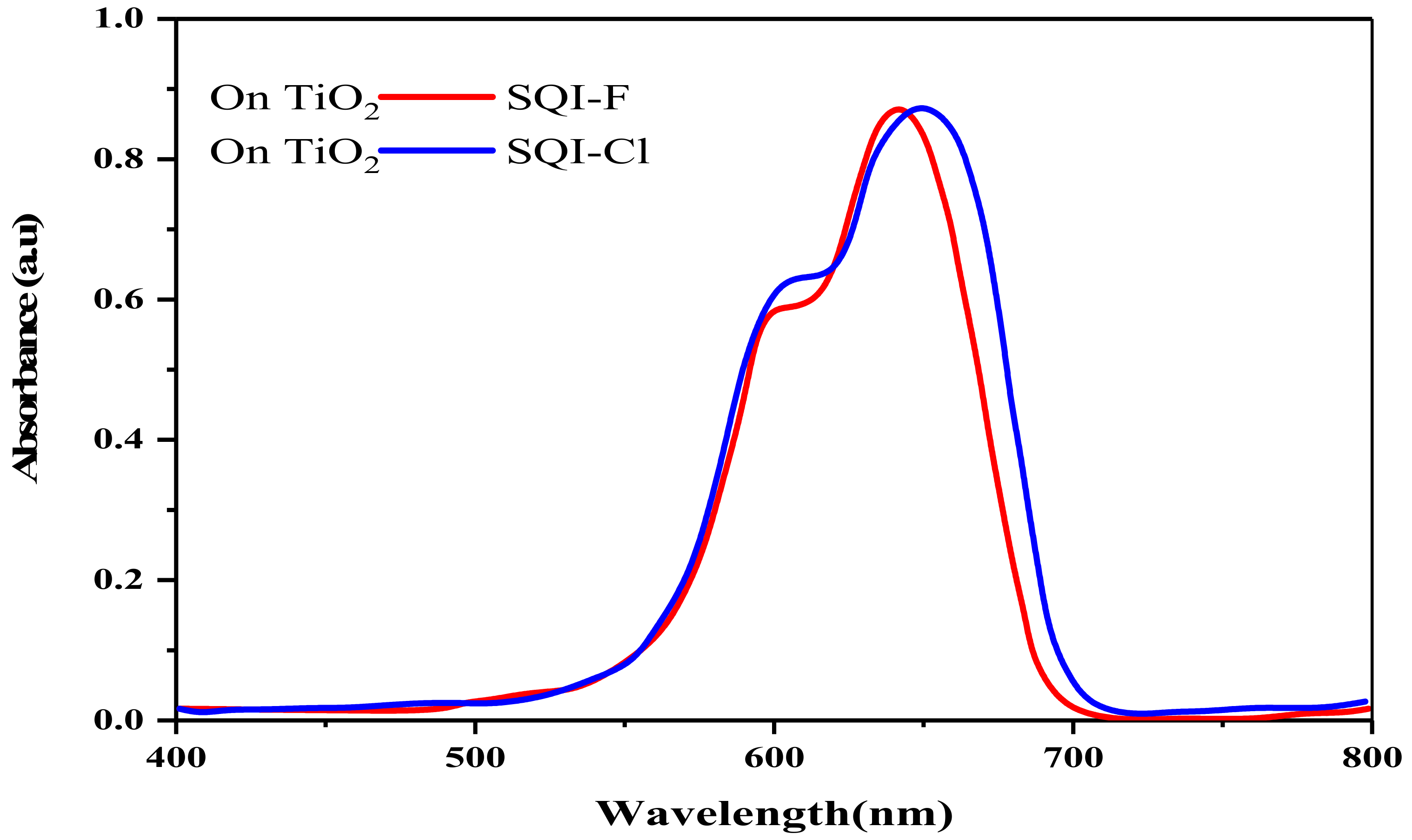
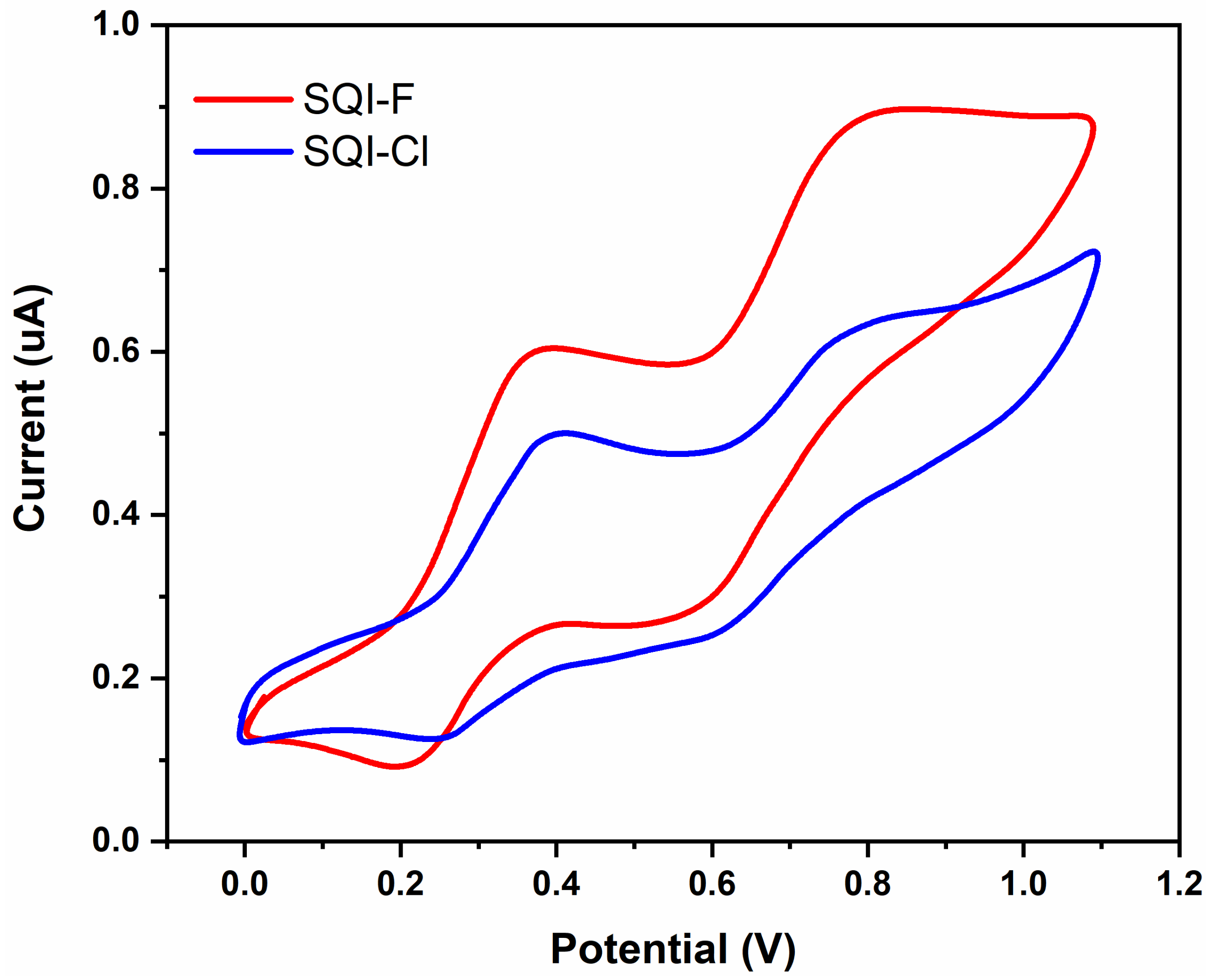
2.2. Evaluation of Photophysical and Electrochemical Attributes
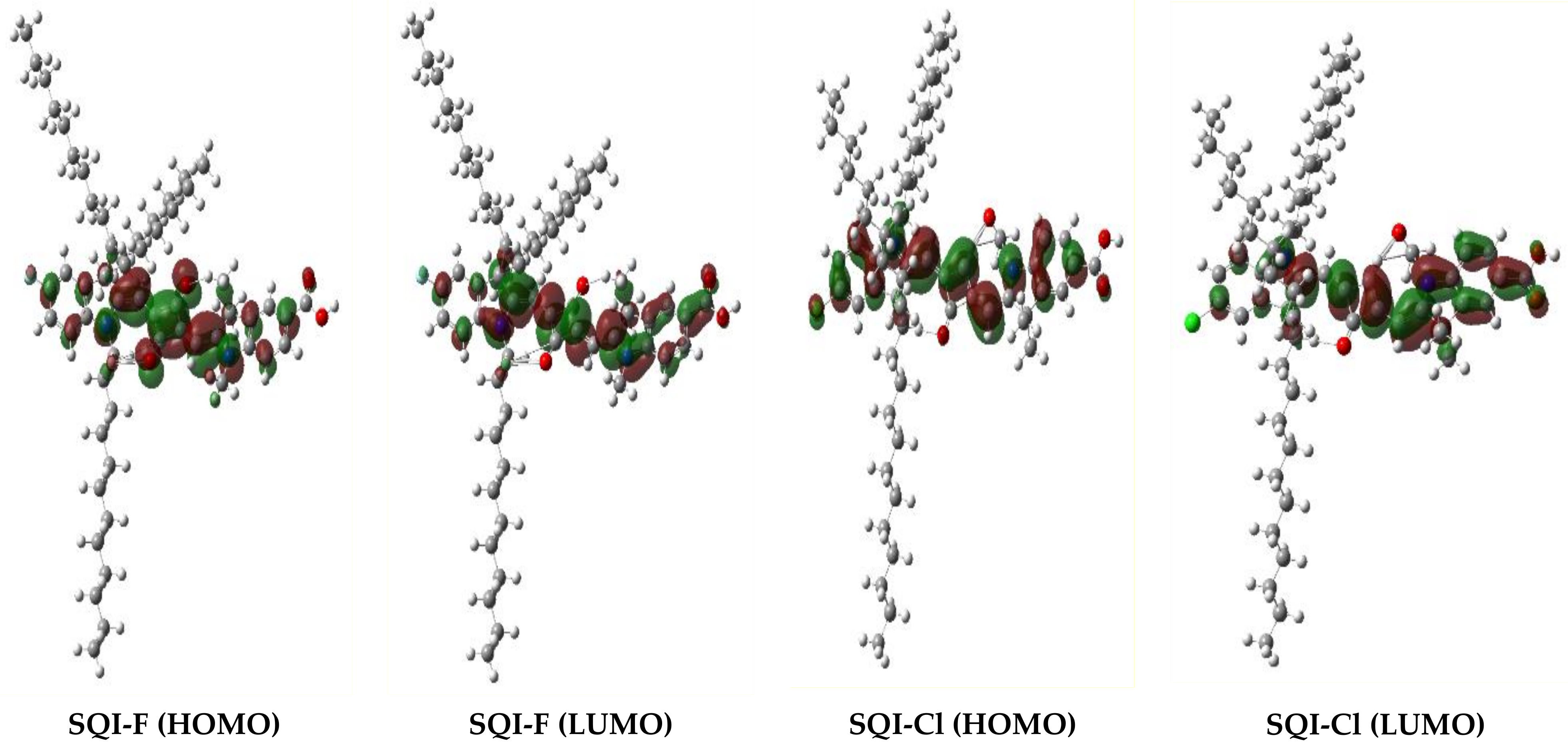
2.3. Calculations Using Density Functional Theory (DFT)
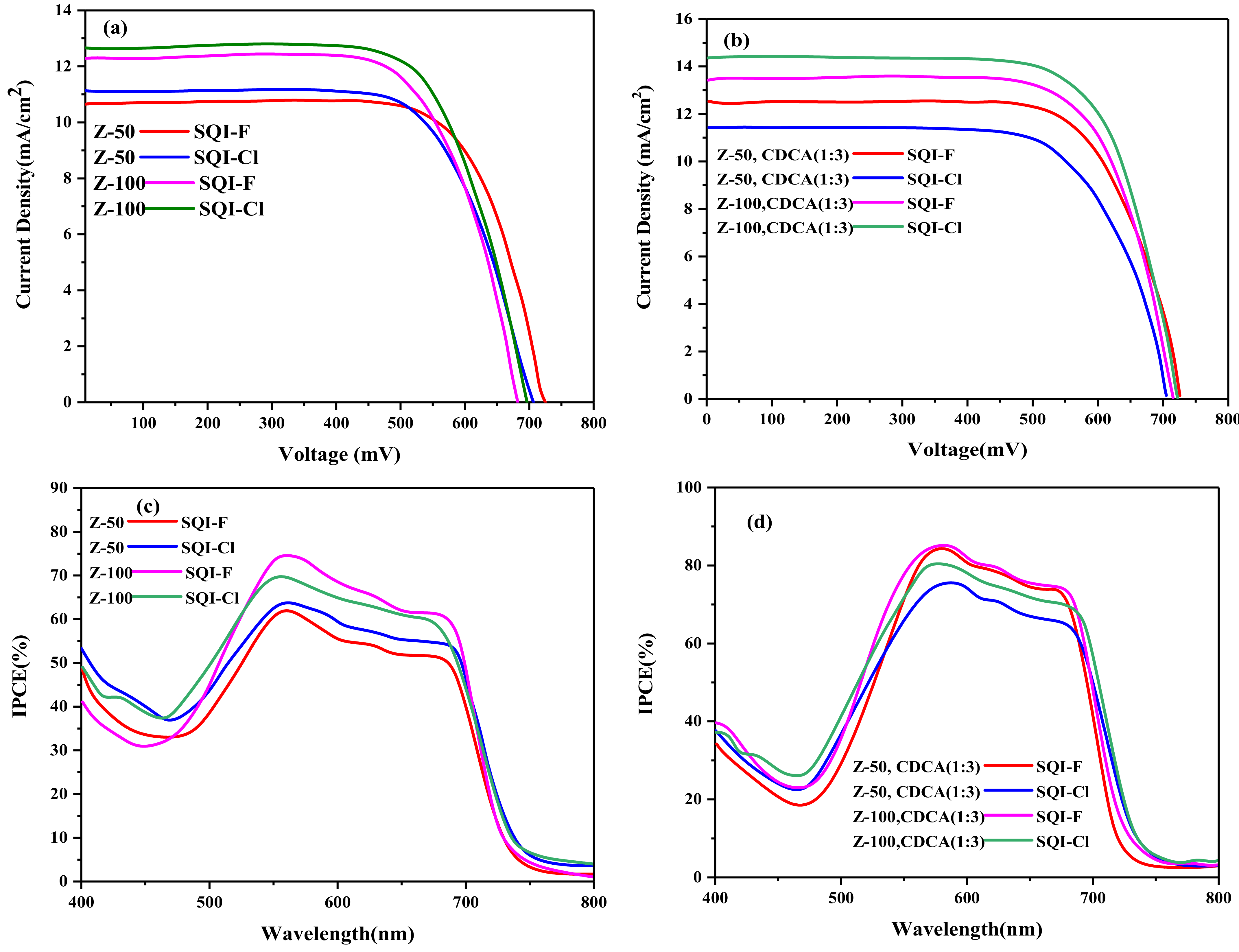
2.4. Characterization of Photovoltaic (SQI-F and SQI-Cl) Properties
2.5. Insights from Electrochemical Impedance Spectroscopy (EIS)
3. Materials and Methods
3.1. Synthesis and Characterization
3.1.1. Synthesis of Compound (2a)
3.1.2. Synthesis of Compound (2b)
3.2. Synthesis of Halogen-Substituted Iodolium Salts (Compounds 3a and 3b)
3.2.1. Synthesis of Compound (3a)
3.2.2. Synthesis of Compound (3b)
3.3. Synthesis of Semi-Squaraine Dye (5)
3.4. Synthesis of Unsymmetrical Squaraine Dyes: SQI-F and SQI-Cl
3.4.1. Synthesis of Unsymmetrical SQI-F Dye
3.4.2. Synthesis of Unsymmetrical SQI-Cl Dye
3.5. DSSC Fabrication Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-horaibi, S.A.; Al-Odayni, A.-B.; Alezzy, A.; ALSaeedy, M.; Al-Adhreai, A.; Saeed, W.S.; Hasan, A. Novel Squaraine Dyes for High-Performance in Dye-Sensitized Solar Cells: Photophysical Properties and Adsorption Behavior on TiO2 with Different Anchoring Groups. J. Mol. Struct. 2023, 1290, 135943. [Google Scholar] [CrossRef]
- Al-horaibi, S.A.; Al-Odayni, A.-B.; Alezzy, A.; ALSaeedy, M.; Saeed, W.; Hasan, A.; El-Shishtawy, R.M. Development of New Co-Sensitizer Based Squaraine Dyes for Enhancing the Performance of DSSC. J. Mol. Struct. 2023, 1292, 136130. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic Sensitizers from D–π–A to D–A–π–A: Effect of the Internal Electron-Withdrawing Units on Molecular Absorption, Energy Levels and Photovoltaic Performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Mughal, E.U.; Obaid, R.J.; Sadiq, A.; Alsharif, M.A.; Naeem, N.; Kausar, S.; Altaf, A.A.; Jassas, R.S.; Ahmed, S.; Alsantali, R.I. Chalcone-and Flavone-Based Novel Terpyridine Metal Complexes: Synthesis, Electrochemical, Photophysical, Photovoltaic and Computational Studies. Dyes Pigments 2022, 201, 110248. [Google Scholar] [CrossRef]
- Qin, C.; Numata, Y.; Zhang, S.; Yang, X.; Islam, A.; Zhang, K.; Chen, H.; Han, L. Novel Near-Infrared Squaraine Sensitizers for Stable and Efficient Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 3059–3066. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, Z.; Li, Y.; Wu, W.; Tian, H.; Wang, Z. N-Annulated Perylene-Based Metal-Free Organic Sensitizers for Dye-Sensitized Solar Cells. Chem. Commun. 2015, 51, 4842–4845. [Google Scholar] [CrossRef]
- Qin, C.; Islam, A.; Han, L. Incorporating a Stable Fluorenone Unit into D–A–π–A Organic Dyes for Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 19236–19243. [Google Scholar] [CrossRef]
- Zhong, C.; Gao, J.; Cui, Y.; Li, T.; Han, L. Coumarin-Bearing Triarylamine Sensitizers with High Molar Extinction Coefficient for Dye-Sensitized Solar Cells. J. Power Sources 2015, 273, 831–838. [Google Scholar] [CrossRef]
- Abusaif, M.S.; Fathy, M.; Abu-Saied, M.A.; Elhenawy, A.A.; Kashyout, A.B.; Selim, M.R.; Ammar, Y.A. New Carbazole-Based Organic Dyes with Different Acceptors for Dye-Sensitized Solar Cells: Synthesis, Characterization, Dssc Fabrications and Density Functional Theory Studies. J. Mol. Struct. 2021, 1225, 129297. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef]
- Singh, A.K.; Nithyanandhan, J. Amphiphilic Indoline-Based Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Modulating the Dye-TiO2/Electrolyte Interface for Nonaqueous and Aqueous Electrolytes. ACS Appl. Energy Mater. 2021, 4, 13932–13942. [Google Scholar] [CrossRef]
- Singh, A.K.; Mele Kavungathodi, M.F.; Nithyanandhan, J. Alkyl-Group-Wrapped Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Branched Alkyl Chains Modulate the Aggregation of Dyes and Charge Recombination Processes. ACS Appl. Mater. Interfaces 2019, 12, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Alagumalai, A.; Vellimalai, P.; Sil, M.C.; Nithyanandhan, J. Effect of Out-of-Plane Alkyl Group’s Position in Dye-Sensitized Solar Cell Efficiency: A Structure--Property Relationship Utilizing Indoline-Based Unsymmetrical Squaraine Dyes. ACS Appl. Mater. Interfaces 2016, 8, 35353–35367. [Google Scholar] [CrossRef]
- Anderson, A.Y.; Barnes, P.R.F.; Durrant, J.R.; O’Regan, B.C. Quantifying Regeneration in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 2439–2447. [Google Scholar] [CrossRef]
- Otsuka, A.; Funabiki, K.; Sugiyama, N.; Yoshida, T.; Minoura, H.; Matsui, M. Dye Sensitization of ZnO by Unsymmetrical Squaraine Dyes Suppressing Aggregation. Chem. Lett. 2006, 35, 666–667. [Google Scholar] [CrossRef]
- Parlane, F.G.L.; Mustoe, C.; Kellett, C.W.; Simon, S.J.; Swords, W.B.; Meyer, G.J.; Kennepohl, P.; Berlinguette, C.P. Spectroscopic Detection of Halogen Bonding Resolves Dye Regeneration in the Dye-Sensitized Solar Cell. Nat. Commun. 2017, 8, 1761. [Google Scholar] [CrossRef]
- Simon, S.J.C.; Parlane, F.G.L.; Swords, W.B.; Kellett, C.W.; Du, C.; Lam, B.; Dean, R.K.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Halogen Bonding Promotes Higher Dye-Sensitized Solar Cell Photovoltages. J. Am. Chem. Soc. 2016, 138, 10406–10409. [Google Scholar] [CrossRef]
- Curiac, C.; Hunt, L.A.; Sabuj, M.A.; Li, Q.; Baumann, A.; Cheema, H.; Zhang, Y.; Rai, N.; Hammer, N.I.; Delcamp, J.H. Probing Interfacial Halogen-Bonding Effects with Halogenated Organic Dyes and a Lewis Base-Decorated Transition Metal-Based Redox Shuttle at a Metal Oxide Interface in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2021, 125, 17647–17659. [Google Scholar] [CrossRef]
- Malzner, F.J.; Brauchli, S.Y.; Constable, E.C.; Housecroft, C.E.; Neuburger, M. Halos Show the Path to Perfection: Peripheral Iodo-Substituents Improve the Efficiencies of Bis (Diimine) Copper (I) Dyes in DSCs. RSC Adv. 2014, 4, 48712–48723. [Google Scholar] [CrossRef]
- Swords, W.B.; Simon, S.J.C.; Parlane, F.G.L.; Dean, R.K.; Kellett, C.W.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Evidence for Interfacial Halogen Bonding. Angew. Chem. 2016, 128, 6060–6064. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Kellett, C.W.; Kennepohl, P.; Berlinguette, C.P. π Covalency in the Halogen Bond. Nat. Commun. 2020, 11, 3310. [Google Scholar] [CrossRef] [PubMed]
- Raithel, A.L.; Meador, W.E.; Kim, T.-Y.; Staples, R.J.; Delcamp, J.H.; Hamann, T.W. Molecular Switch Cobalt Redox Shuttle with a Tunable Hexadentate Ligand. J. Am. Chem. Soc. 2023, 145, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-M.; Lee, H.J.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. Influence of the π-Bridge-Fused Ring and Acceptor Unit Extension in D− π–A-Structured Organic Dyes for Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 52745–52757. [Google Scholar] [CrossRef]
- Nugegoda, D.; Hunt, L.A.; Devdass, A.; Cheema, H.; Fortenberry, R.C.; Jurss, J.W.; Hammer, N.I.; Delcamp, J.H. Lewis Acid–Lewis Base Interactions Promote Fast Interfacial Electron Transfers with a Pyridine-Based Donor Dye in Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 1516–1527. [Google Scholar] [CrossRef]
- Shivashimpi, G.M.; Pandey, S.S.; Watanabe, R.; Fujikawa, N.; Ogomi, Y.; Yamaguchi, Y.; Hayase, S. Effect of Nature of Anchoring Groups on Photosensitization Behavior in Unsymmetrical Squaraine Dyes. J. Photochem. Photobiol. A Chem. 2014, 273, 1–7. [Google Scholar] [CrossRef]
- Bisht, R.; Sudhakar, V.; Mele Kavungathodi, M.F.; Karjule, N.; Nithyanandhan, J. Fused Fluorenylindolenine-Donor-Based Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 26335–26347. [Google Scholar] [CrossRef]
- Punitharasu, V.; Mele Kavungathodi, M.F.; Singh, A.K.; Nithyanandhan, J. $π$-Extended Cis-Configured Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Panchromatic Response. ACS Appl. Energy Mater. 2019, 2, 8464–8472. [Google Scholar] [CrossRef]
- Watson, J.; Rodrigues, R.R.; Delcamp, J.H. Near-Infrared Unsymmetrical Squaraine Core-Based Sensitizers for Co-Sensitized High-Photocurrent Dye-Sensitized Solar Cells. Cell Rep. Phys. Sci. 2022, 3, 100701. [Google Scholar] [CrossRef]
- Islam, N.; Niaz, S.; Manzoor, T.; Pandith, A.H. Theoretical Investigations into Spectral and Non-Linear Optical Properties of Brucine and Strychnine Using Density Functional Theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoudaa, M.; Slimic, H.; Abderrabbaa, M.; ben Salemc, R.; Moussaouic, Y. The Biginelli Reaction in Different Solvents and in Presence of Bismuth Nitrate: Thermodynamical Investigation into the Mechanism by Means of DFT Calculation and Experimental Results. J. Tunis. Chem. Soc. 2017, 19, 105–114. [Google Scholar]
- Singh, G.; Singh, R.; George, N.; Singh, G.; Kaur, G.; Kaur, G.; Singh, H.; Singh, J. ‘Click’-Synthesized PET Based Fluorescent Sensor for Hg (II), Pb (II) and Cr (III) Recognition: DFT and Docking Studies. J. Photochem. Photobiol. A Chem. 2023, 441, 114741. [Google Scholar] [CrossRef]
- Singh, A.K.; Kavungathodi, M.F.M.; Mozer, A.J.; Krishnamoorthy, K.; Nithyanandhan, J. Solvent-Dependent Functional Aggregates of Unsymmetrical Squaraine Dyes on TiO2 Surface for Dye-Sensitized Solar Cells. Langmuir 2022, 38, 14808–14818. [Google Scholar] [CrossRef]
- Pradhan, A.; Morimoto, T.; Saikiran, M.; Kapil, G.; Hayase, S.; Pandey, S.S. Investigation of the Minimum Driving Force for Dye Regeneration Utilizing Model Squaraine Dyes for Dye-Sensitized Solar Cells. J. Mater. Chem. A 2017, 5, 22672–22682. [Google Scholar] [CrossRef]
- Jradi, F.M.; Kang, X.; Oneil, D.; Pajares, G.; Getmanenko, Y.A.; Szymanski, P.; Parker, T.C.; El-Sayed, M.A.; Marder, S.R. Near-Infrared Asymmetrical Squaraine Sensitizers for Highly Efficient Dye Sensitized Solar Cells: The Effect of π-Bridges and Anchoring Groups on Solar Cell Performance. Chem. Mater. 2015, 27, 2480–2487. [Google Scholar] [CrossRef]
- Bisht, R.; Singh, A.K.; Nithyanandhan, J. Panchromatic Sensitizer for Dye-Sensitized Solar Cells: Unsymmetrical Squaraine Dyes Incorporating Benzodithiophene $π$-Spacer with Alkyl Chains to Extend Conjugation, Control the Dye Assembly on TiO2, and Retard Charge Recombination. J. Org. Chem. 2017, 82, 1920–1930. [Google Scholar] [CrossRef]
- Matsui, M.; Haishima, Y.; Kubota, Y.; Funabiki, K.; Jin, J.; Kim, T.H.; Manseki, K. Application of Benz [c, d] Indolenine-Based Unsymmetrical Squaraine Dyes to near-Infrared Dye-Sensitized Solar Cells. Dyes Pigments 2017, 141, 457–462. [Google Scholar] [CrossRef]
- Pradhan, S.; Kurokawa, Y.; Pandey, S.S. Design and Synthesis of Novel NIR-Sensitive Unsymmetrical Squaraine Dyes for Molecular Photovoltaics. Phys. Status Solidi 2023, 2300145. [Google Scholar] [CrossRef]
- Singh, A.K.; Maibam, A.; Javaregowda, B.H.; Bisht, R.; Kudlu, A.; Krishnamurty, S.; Krishnamoorthy, K.; Nithyanandhan, J. Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Position of the Anchoring Group Controls the Orientation and Self-Assembly of Sensitizers on the TiO2 Surface and Modulates Its Flat Band Potential. J. Phys. Chem. C 2020, 124, 18436–18451. [Google Scholar] [CrossRef]
- Al-horaibi, S.A.; Asiri, A.M.; El-Shishtawy, R.M.; Gaikwad, S.T.; Rajbhoj, A.S. Synthesis and Characterization of New Squaraine Dyes with Bis-Pendent Carboxylic Groups for Dye-Sensitized Solar Cells. J. Mol. Struct. 2019, 1195, 850–858. [Google Scholar] [CrossRef]
- Al-horaibi, S.A.; Garoon, E.M.; Bhise, N.A.; Gaikwad, S.T.; Rajbhoj, A.S. The Effect of Bis-Carboxylic Groups of Squarylium Dyes on the Efficiency of Dye-Sensitized Solar Cells. Chem. Pap. 2020, 74, 1769–1778. [Google Scholar] [CrossRef]
- Shivashimpi, G.M.; Pandey, S.S.; Watanabe, R.; Fujikawa, N.; Ogomi, Y.; Yamaguchi, Y.; Hayase, S. Novel Unsymmetrical Squaraine Dye Bearing Cyanoacrylic Acid Anchoring Group and Its Photosensitization Behavior. Tetrahedron Lett. 2012, 53, 5437–5440. [Google Scholar] [CrossRef]
- Al-Horaibi, S.A.; Alghamdi, M.T.; Gaikwad, S.T.; Rajbhoj, A.S. Comparison and Determine Characteristics Potentials of HOMO/LUMO and Relationship between Ea and Ip for Squaraine Dyes (SQ1, SQ2) by Using Cyclic Voltammetry and DFT/TD-DFT. Moroc. J. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Irfan, A.; Al-Melfi, M.A.M.; Al-Ghamdi, A.A.; Shalaan, E. The Enhanced Efficiency to 3.6% Based on Organic Dye as Donor and Si/TiO2 Acceptor Bulk Hetero-Junction Solar Cells. J. Photochem. Photobiol. A Chem. 2014, 292, 1–9. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Irfan, A.; Asiri, A.M.; Ammar, Y.A. Molecular Design of New Hydrazone Dyes for Dye-Sensitized Solar Cells: Synthesis, Characterization and DFT Study. J. Mol. Struct. 2012, 1019, 130–134. [Google Scholar] [CrossRef]


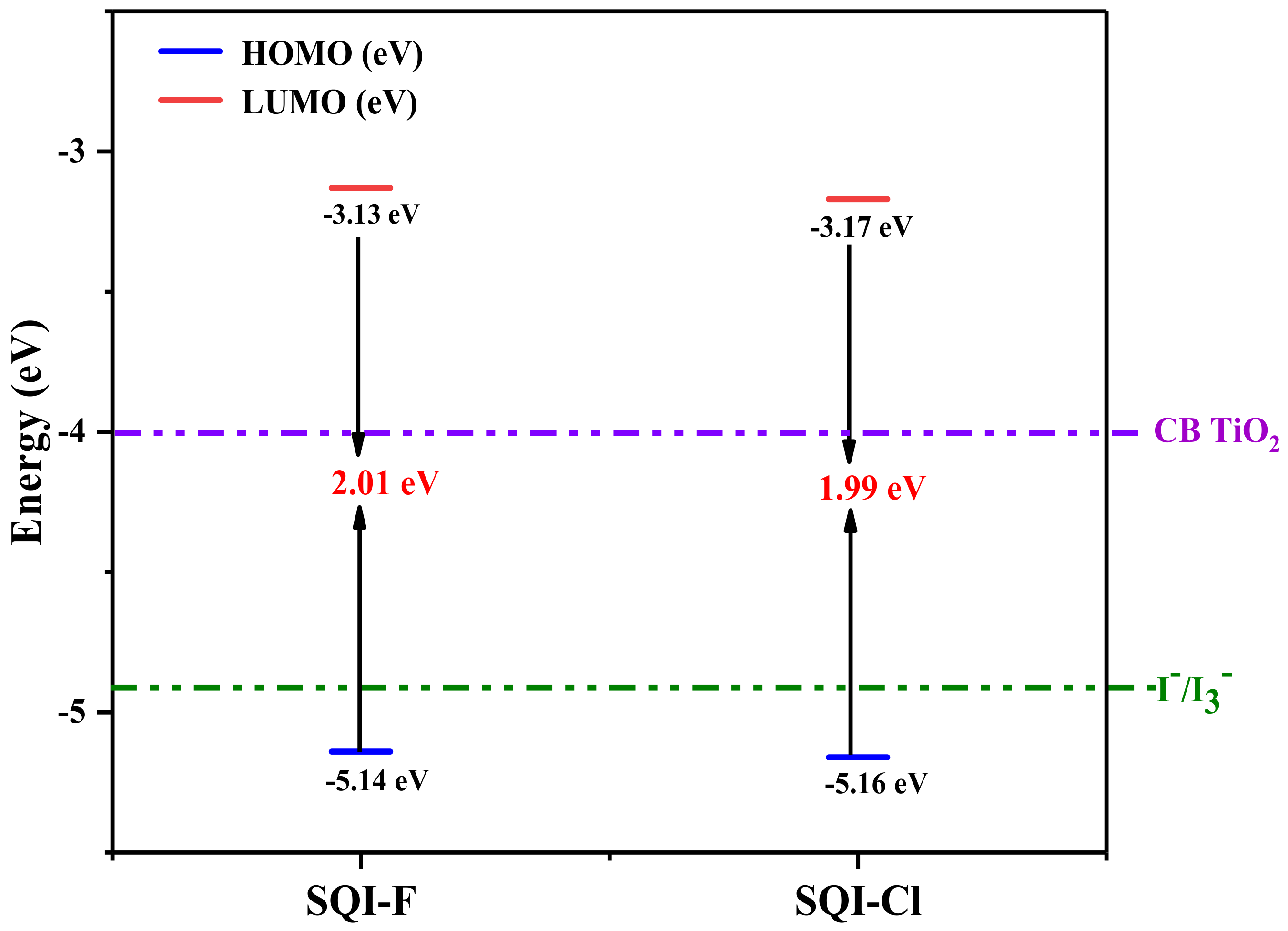

| Dye | λmaxa (nm) a | Ɛmax (M−1 cm−1) a | λem (nm) a | λmax TiO2 (nm) | LHE Δλ (60%) (nm) | Eoxd c (V vs. NHE) | Eg (eV) b | HOMO (eV) c | LUMO (eV) c | HOMO (eV) d | LUMO (eV) d | Eg (eV) d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SQI-F | 641 | 2.1 × 105 | 643 | 642 | 195 | 0.81 | 1.92 | −6.01 | −4.09 | −5.14 | −3.13 | 2.01 |
| SQI-Cl | 643 | 2.5 × 105 | 653 | 651 | 213 | 0.83 | 1.93 | −6.03 | −4.1 | −5.16 | −3.17 | 1.99 |
| Dyes | CDCA (mM) | Jsc (mA/cm2) Z-50/Z-100 | Voc (V) Z-50/Z-100 | FF Z-50/Z-100 | η (%) Z-50/Z-100 |
|---|---|---|---|---|---|
| SQI-F | 0 | 10.70/12.4 | 0.720/0.693 | 0.75/0.73 | 5.62/6.01 |
| SQI-Cl | 0 | 11.12/12.22 | 0.704/0.683 | 0.72/0.72 | 5.47/5.84 |
| SQI-F | 0.3 | 12.22/13.67 | 0.723/0.694 | 0.75/0.73 | 6.46/6.74 |
| SQI-Cl | 0.3 | 11.20/12.09 | 0.707/0.680 | 0.72/0.71 | 5.55/5.64 |
| b SQIND-1 | 0.3 | 5.88 | 0.48 | 0.74 | 2.1 |
| c SQ-A | 0 | 6.71 | 0.58 | 0.72 | 2.82 |
| Dye | Rct (Ω cm2) Z-50/Z-100 | Cμ (mF) Z-50/Z-100 | τ (ms) Z-50/Z-100 |
|---|---|---|---|
| SQI-F | 4.42/4.45 | 0.394/0.43 | 1.74/1.83 |
| SQI-F (CDCA 1:3) | 4.87/4.91 | 0.415/0.42 | 2.02/1.96 |
| SQI-Cl | 4.67/6.03 | 0.434/0.439 | 2.02/2.64 |
| SQI-Cl (CDCA 1:3) | 4.64/5.52 | 0.430/0.403 | 2.64/2.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-horaibi, S.A.; Al-Odayni, A.-B.; ALSaeedy, M.; Al-Ostoot, F.H.; Al-Salihy, A.; Alezzy, A.; Al-Adhreai, A.; Saif, F.A.; Yaseen, S.A.; Saeed, W.S. Exploring DSSC Efficiency Enhancement: SQI-F and SQI-Cl Dyes with Iodolyte Electrolytes and CDCA Optimization. Molecules 2023, 28, 7129. https://doi.org/10.3390/molecules28207129
Al-horaibi SA, Al-Odayni A-B, ALSaeedy M, Al-Ostoot FH, Al-Salihy A, Alezzy A, Al-Adhreai A, Saif FA, Yaseen SA, Saeed WS. Exploring DSSC Efficiency Enhancement: SQI-F and SQI-Cl Dyes with Iodolyte Electrolytes and CDCA Optimization. Molecules. 2023; 28(20):7129. https://doi.org/10.3390/molecules28207129
Chicago/Turabian StyleAl-horaibi, Sultan A., Abdel-Basit Al-Odayni, Mohammed ALSaeedy, Fares Hezam Al-Ostoot, Adel Al-Salihy, Abdulmajeed Alezzy, Arwa Al-Adhreai, Faizaa A. Saif, Salama A. Yaseen, and Waseem Sharaf Saeed. 2023. "Exploring DSSC Efficiency Enhancement: SQI-F and SQI-Cl Dyes with Iodolyte Electrolytes and CDCA Optimization" Molecules 28, no. 20: 7129. https://doi.org/10.3390/molecules28207129
APA StyleAl-horaibi, S. A., Al-Odayni, A.-B., ALSaeedy, M., Al-Ostoot, F. H., Al-Salihy, A., Alezzy, A., Al-Adhreai, A., Saif, F. A., Yaseen, S. A., & Saeed, W. S. (2023). Exploring DSSC Efficiency Enhancement: SQI-F and SQI-Cl Dyes with Iodolyte Electrolytes and CDCA Optimization. Molecules, 28(20), 7129. https://doi.org/10.3390/molecules28207129










