In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic
Abstract
:1. Introduction
- Synthesize the Schiff bases bearing pyrazole scaffold (22a, b and 23a, b).
- Evaluation of in vitro antioxidant, anti-diabetic, anti-Alzheimer’s, and anti-arthritic properties.
- Study the in silico bioinformatics analysis (physicochemical properties, the bioavailability radar, drug-likeness, and medicinal chemistry) of Schiff bases bearing pyrazole scaffold (22a, b and 23a, b).
- Study the molecular lipophilicity potential (MLP) and the molecular polar surface area (PSA) of the derivatives 22a, b and 23a, b.
2. Results and Discussion
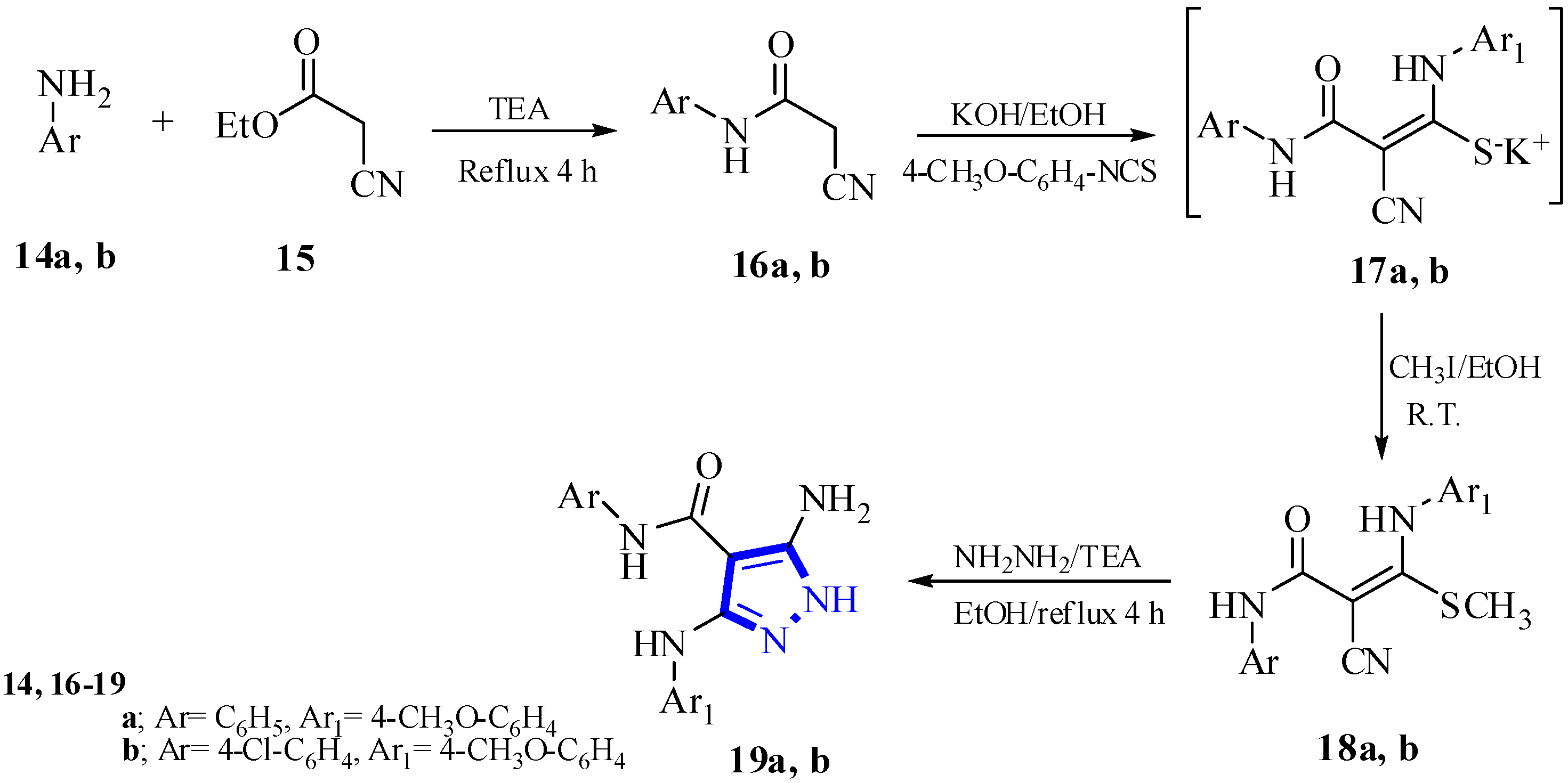
2.1. Chemistry
2.2. In Vitro Biological Activities
2.2.1. Antioxidant Activities
- 5-(2, 5-Dimethoxybenzylideneamino)-3-(4-methoxyphenylamino)-1H-pyrazole derivative 22b exhibited the highest antioxidant activities among all the compounds, which had total antioxidant capacity (TAC) = 42.47 ± 0.09 mg gallic acid/gm, iron-reducing power (IRP) = 24.02 ± 0.05 µg/mL, DPPH radical-scavenging activity (IC50 = 13.20 ± 0.03 µg/mL), and ABTS radical-scavenging activity (%) = 35.11 ± 0.08. After compound 22b in the activity, there was compound 23a, 5-(4-chloro-3-nitrobenzylideneamino)-3-(4-methoxyphenylamino)-N-phenyl-1H-pyrazole derivative, which has total antioxidant capacity (TAC) = 36.85 ± 0.08 mg gallic acid/gm, iron-reducing power (IRP) = 20.84 ± 0.05 µg/mL, DPPH radical-scavenging activity (IC50 = 15.21 ± 0.03 µg/mL), and ABTS radical-scavenging activity (%) = 30.46 ± 0.07.
- The two compounds 22a and 23b have almost the same antioxidant activities (TAC = 34.75 ± 0.08 and 34.56 ± 0.08 mg gallic acid/gm, IRP = 19.65 ± 0.04 and 19.54 ± 0.04 µg/mL, DPPH (IC50) = 16.22 ± 0.04 and 16.13 ± 0.04 µg/mL, and ABTS (%) = 28.73 ± 0.06 and 28.56 ± 0.06, respectively).
2.2.2. Anti-Diabetic and Anti-Alzheimer’s Activities
- In the case of α-amylase inhibition (anti-diabetic activity) and using acarbose as the standard reference (% = 69.11 ± 0.15), we observe that compound 22b showed inhibitor activity of α-amylase (%) = 36.06 ± 0.08, and the next in the activity series is compound 23a with α-amylase inhibition (%) equal to 31.28 ± 0.07. The two compounds 22a and 23b showed almost matching α-amylase inhibition activities equivalent to 29.50 ± 0.06 and 29.34 ± 0.06, respectively.
- In the case of anti-Alzheimer’s activity and using acetylcholinesterase (ACE) inhibition as an indicator for the activity, we observe that compound 22b showed inhibitor activity of acetylcholinesterase (ACE, %) = 20.71 ± 0.05, and the next is 23a with percentage inhibition of acetylcholinesterase equal to 17.97 ± 0.04. The inhibitor activities of the two derivatives 22a and 23b are almost the same and equal to 16.95 ± 0.04 and 16.85 ± 0.04, respectively.
2.2.3. Anti-Arthritic Activity
- -
- In the case of the protein denaturation inhibition, the more potent compound is 23a with an inhibitor percentage equal to 22.56 ± 0.05, and then compound 22b which shows activity equal to 19.57 ± 0.04.
- -
- In the case of the inhibition of proteinase, we also find that compound 23a (inhibition of proteinase% = 20.71 ± 0.05) is the most active among the Schiff bases bearing pyrazole scaffold, then compound 22b (inhibition of proteinase% = 17.97 ± 0.04), then the compound 18a (inhibition of proteinase% = 16.95 ± 0.04), and finally the compound 23b (inhibition of proteinase% = 16.85 ± 0.04), compared to the standard drug, diclofenac sodium (inhibition of proteinase% = 41.88 ± 0.09). Therefore, the order of activities is diclofenac sodium > 23a > 22b > 22a > 23b.
2.3. In Silico Bioinformatics Analysis
2.3.1. Physicochemical Properties
- -
- Molecular weight (MW): sum of the atomic weight values of the atoms in a molecule (optimal: 100~600).
- -
- Volume: van der Waals volume.
- -
- Density: density = MW/volume.
- -
- The number of hydrogen bond acceptors (nHA): sum of all O and N (optimal: 0~12).
- -
- The number of hydrogen bond donors (nHD): sum of all OHs and NHs (optimal: 0~7).
- -
- The number of rotatable bonds (nRot): (optimal: 0~11).
- -
- Number of rings (nRing): smallest set of smallest rings (optimal: 0~6).
- -
- Number of atoms in the biggest ring (MaxRing): number of atoms involved in the biggest ring (optimal: 0~18).
- -
- Number of heteroatoms (nHet): number of non-carbon atoms (hydrogens included, optimal: 1~15).
- -
- The formal charge (fChar) (optimal: −4~4).
- -
- Number of rigid bonds (nRig): number of non-flexible bonds, as opposed to rotatable bonds (optimal: 0~30).
- -
- Flexibility: flexibility = nRot/nRig.
- -
- Stereocenters: number of stereocenters (optimal: ≤2).
- -
- Topological polar surface area (TPSA): sum of tabulated surface contributions of polar fragments (optimal: 0~140).
- -
- logS: the logarithm of aqueous solubility value (optimal: −4~0.5 log mol/L).
- -
- logP: the logarithm of the n-octanol/water distribution coefficient (optimal: 0~3 log mol/L).
- -
- logD7.4: the logarithm of the n-octanol/water distribution coefficients at pH = 7.4 (optimal: 1~3 log mol/L).
2.3.2. Drug-Likeness
- -
- Lipinski rule: this rule held four parameters and its requirements are MW ≤ 500, logP ≤ 5, nHA ≤ 10, and nHD ≤ 5 [43].
- -
- GSK rule: this rule depends on molecular weight (MW) and logP parameters (optimal: MW ≤ 400; logP ≤ 4) [44].
- -
- Pfizer rule: the rule focuses on high logP > 3 and low topological polar surface area (TPSA) < 75 factors [45].
2.3.3. Medicinal Chemistry
2.3.4. Molecular Lipophilicity Potential (MLP)
2.3.5. Molecular Polar Surface Area (PSA)
3. Materials and Methods
3.1. Chemistry
3.2. In Vitro Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Priyanka, D.N.; Harish Prashanth, K.V.; Tharanathan, R.N. A review on potential anti-diabetic mechanisms of chitosan and its derivatives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100188. [Google Scholar] [CrossRef]
- Lima, E.; Medeiros, J. Marine Organisms as Alkaloid Biosynthesizers of Potential Anti-Alzheimer Agents. Mar. Drugs 2022, 20, 75. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F. Anti-arthritic agents: Progress and potential. Bioorg. Med. Chem. 2015, 23, 3059–3080. [Google Scholar] [CrossRef]
- Marchi, R.C.; Campos, I.A.; Santana, V.T.; Carlos, R.M. Chemical implications and considerations on techniques used to assess the in vitro antioxidant activity of coordination compounds. Coord. Chem. Rev. 2022, 451, 214275. [Google Scholar] [CrossRef]
- Sadiq, I.Z. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Shippy, D.C.; Ulland, T.K. Lipid metabolism transcriptomics of murine microglia in Alzheimer’s disease and neuroinflammation. Sci. Rep. 2023, 13, 14800. [Google Scholar] [CrossRef] [PubMed]
- Egu, S.A.; Ali, I.; Khan, K.M.; Chigurupati, S.; Qureshi, U.; Salar, U.; Taha, M.; Felemban, S.G.; Venugopal, V.; Ul-Haq, Z. Syntheses, in vitro, and in silico studies of rhodanine-based schiff bases as potential α-amylase inhibitors and radicals (DPPH and ABTS) scavengers. Mol. Divers. 2022, 27, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, N.; Thomas, R.; Sowrirajan, S.; Manoj, K.P.; Irfan, A. Synthesis, Spectral Characterization, Electronic Structure and Biological Activity Screening of the Schiff Base 4-((4-Hydroxy-3-Methoxy-5-Nitrobenzylidene) Amino)-N-(Pyrimidin-2-yl) Benzene Sulfonamide from 5-Nitrovaniline and Sulphadiazene. Polycycl. Aromat. Compd. 2022, 42, 6818–6835. [Google Scholar] [CrossRef]
- Tokalı, F.S.; Taslimi, P.; Usanmaz, H.; Karaman, M.; Şendil, K. Synthesis, characterization, biological activity and molecular docking studies of novel schiff bases derived from thiosemicarbazide: Biochemical and computational approach. J. Mol. Struct. 2021, 1231, 129666. [Google Scholar] [CrossRef]
- Sayed, F.N.; Mohamed, G.G. Newly synthesized lanthanides complexes of ferrocene-based Schiff base with high biological activities and improved molecular docking data. J. Organomet. Chem. 2022, 977, 122450. [Google Scholar] [CrossRef]
- Surendar, P.; Pooventhiran, T.; Rajam, S.; Bhattacharyya, U.; Bakht, M.A.; Thomas, R. Quasi liquid Schiff bases from trans-2-hexenal and cytosine and l-leucine with potential antieczematic and antiarthritic activities: Synthesis, structure and quantum mechanical studies. J. Mol. Liq. 2021, 334, 116448. [Google Scholar] [CrossRef]
- Said, M.A.; Khan, D.J.; Al-Blewi, F.F.; Al-Kaff, N.S.; Ali, A.A.; Rezki, N.; Aouad, M.R.; Hagar, M. New 1, 2, 3-Triazole scaffold schiff bases as potential anti-COVID-19: Design, synthesis, DFT-molecular docking, and cytotoxicity aspects. Vaccines 2021, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Al-Majid, A.M.; Sholkamy, E.N.; Yousuf, S.; Ayaz, M.; Nawaz, A.; Wadood, A.; Rehman, A.U.; Verma, V.P.; Motiur Rahman, A.F.M.; et al. Synthesis of Spiro-oxindole Analogs Engrafted Pyrazole Scaffold as Potential Alzheimer’s Disease Therapeutics: Anti-oxidant, Enzyme Inhibitory and Molecular Docking Approaches. ChemistrySelect 2022, 7, e202203047. [Google Scholar] [CrossRef]
- Azimi, F.; Azizian, H.; Najafi, M.; Khodarahmi, G.; Saghaei, L.; Hassanzadeh, M.; Ghasemi, J.B.; Faramarzi, M.A.; Larijani, B.; Hassanzadeh, F.; et al. Design, synthesis, biological evaluation, and molecular modeling studies of pyrazole-benzofuran hybrids as new α-glucosidase inhibitor. Sci. Rep. 2021, 11, 20776. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, B.; Hao, Z.; Chen, L.; Cao, L.; Guo, X.; Zhang, N.; Yang, D.; Tang, L.; Fan, Z. Design, synthesis and biological evaluation of pyrazole-aromatic containing carboxamides as potent SDH inhibitors. Eur. J. Med. Chem. 2021, 214, 113230. [Google Scholar] [CrossRef]
- Benazzouz-Touami, A.; Chouh, A.; Halit, S.; Terrachet-Bouaziz, S.; Makhloufi-Chebli, M.; Ighil-Ahriz, K.; Silva, A.M. New Coumarin-Pyrazole hybrids: Synthesis, Docking studies and Biological evaluation as potential cholinesterase inhibitors. J. Mol. Struct. 2022, 1249, 131591. [Google Scholar] [CrossRef]
- Signorello, M.G.; Rapetti, F.; Meta, E.; Sidibè, A.; Bruno, O.; Brullo, C. New series of pyrazoles and imidazo-pyrazoles targeting different cancer and inflammation pathways. Molecules 2021, 26, 5735. [Google Scholar] [CrossRef]
- Islam, M.S.; Al-Majid, A.M.; Sholkamy, E.N.; Yousuf, S.; Ayaz, M.; Nawaz, A.; Wadood, A.; Rehman, A.U.; Verma, V.P.; Bari, A.; et al. Synthesis, molecular docking and enzyme inhibitory approaches of some new chalcones engrafted pyrazole as potential antialzheimer, antidiabetic and antioxidant agents. J. Mol. Struct. 2022, 1269, 133843. [Google Scholar] [CrossRef]
- Eweas, A.F.; El-Nezhawy, A.O.H.; Abdel-Rahman, R.F.; Baiuomy, A.R. Design, Synthesis. Vivo Anti-inflammatory, Analgesic Activities and Molecular Docking of Some Novel Pyrazolone Derivatives. Med. Chem. 2015, 5, 458–466. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Alharbi, O.; Al-Odayni, A.B.; Abduh, N.A. Quinoline-and Isoindoline-Integrated Polycyclic Compounds as Antioxidant, and Antidiabetic Agents Targeting the Dual Inhibition of α-Glycosidase and α-Amylase Enzymes. Pharmaceuticals 2023, 16, 1222. [Google Scholar] [CrossRef] [PubMed]
- Şener, N.; Özkinali, S.; Altunoglu, Y.C.; Yerlikaya, S.; Gökçe, H.; Zurnaci, M.; Gür, M.; Baloglu, M.C.; Şener, İ. Antiproliferative properties and structural analysis of newly synthesized Schiff bases bearing pyrazole derivatives and molecular docking studies. J. Mol. Struct. 2021, 1241, 130520. [Google Scholar] [CrossRef]
- Sangani, C.B.; Makwana, J.A.; Duan, Y.T.; Tarpada, U.P.; Patel, Y.S.; Patel, K.B.; Dave, V.N.; Zhu, H.L. Design, synthesis, and antibacterial evaluation of new Schiff’s base derivatives bearing nitroimidazole and pyrazole nuclei as potent E. coli FabH inhibitors. Res. Chem. Intermed. 2015, 41, 10137–10149. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone comprising compounds as promising anti-infective agents: Chemistry and structure-property relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Noce, B.; Di Bello, E.; Fioravanti, R.; Mai, A. LSD1 inhibitors for cancer treatment: Focus on multi-target agents and compounds in clinical trials. Front. Pharmacol. 2023, 14, 1120911. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.A. Bioinformatics and network pharmacology-based study to elucidate the multi-target pharmacological mechanism of the indigenous plants of Medina valley in treating HCV-related hepatocellular carcinoma. Saudi Pharm. J. 2023, 31, 1125–1138. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mohammed, A.F.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents. Pharmaceuticals 2023, 16, 1039. [Google Scholar] [CrossRef]
- Kumari, M.; Waseem, M.; Subbarao, N. Discovery of multi-target mur enzymes inhibitors with anti-mycobacterial activity through a Scaffold approach. J. Biomol. Struct. Dyn. 2023, 41, 2878–2899. [Google Scholar] [CrossRef]
- Ragab, M.A.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Okda, H.E.; Elgendy, B.; Ibrahim, T.S.; Abd-Alhaseeb, M.M.; Gratteri, P.; Supuran, C.T.; et al. 4-(5-Amino-pyrazol-1-yl) benzenesulfonamide derivatives as novel multi-target anti-inflammatory agents endowed with inhibitory activity against COX-2, 5-LOX and carbonic anhydrase: Design, synthesis, and biological assessments. Eur. J. Med. Chem. 2023, 250, 115180. [Google Scholar] [CrossRef]
- Nandi, S.; Chauhan, B.; Tarannum, H.; Khede, M.K. Multi-target polypharmacology of 4-aminoquinoline compounds against malaria, tuberculosis and cancer. Curr. Top. Med. Chem. 2023, 23, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.K.; Mitra, S.; Cordeiro, M.N.D. Designing multi-target drugs for the treatment of major depressive disorder. Expert Opin. Drug Discov. 2023, 18, 643–658. [Google Scholar] [CrossRef]
- Sadek, K.U.; Mekheimer, R.A.; Abd-Elmonem, M.; Abo-Elsoud, F.A.; Hayallah, A.M.; Mostafa, S.M.; Abdellattif, M.H.; Abourehab, M.A.; Farghaly, T.A.; Elkamhawy, A. Recent Developments in the Synthesis of Hybrid Heterocycles, A Promising Approach to Develop Multi-target Antibacterial Agents. J. Mol. Struct. 2023, 1286, 135616. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.; Wang, Y.; Wang, X.; Gou, S. Discovery of phthalazino [1,2-b]quinazolinone derivatives as multi-target HDAC inhibitors for the treatment of hepatocellular carcinoma via activating the p53 signal pathway. Eur. J. Med. Chem. 2022, 229, 114058. [Google Scholar] [CrossRef]
- Hassan, A.S. Antimicrobial evaluation, in silico ADMET prediction, molecular docking, and molecular electrostatic potential of pyrazole-isatin and pyrazole-indole hybrid molecules. J. Iran. Chem. Soc. 2022, 19, 3577–3589. [Google Scholar] [CrossRef]
- Khatab, T.K.; Hassan, A.S.; Hafez, T.S. V2O5/SiO2 as an efficient catalyst in the synthesis of 5-amino-pyrazole derivatives under solvent free condition. Bull. Chem. Soc. Ethiop. 2019, 33, 135–142. [Google Scholar] [CrossRef]
- Hassan, A.S.; Morsy, N.M.; Awad, H.M.; Ragab, A. Synthesis, molecular docking, and in silico ADME prediction of some fused pyrazolo [1,5-a]pyrimidine and pyrazole derivatives as potential antimicrobial agents. J. Iran. Chem. Soc. 2022, 19, 521–545. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Omar, N.I.; El-Feky, A.M.; Hasan, E.A.; Ibrahim, N.E.S.; Youssef, A.M. In vitro study on effect of zinc oxide nanoparticles on the biological activities of croton Tiglium L. Seeds extracts. Asian Pac. J. Cancer Prev. 2022, 23, 2671–2686. [Google Scholar] [CrossRef]
- Azeem, M.N.A.; Ahmed, O.M.; Shaban, M.; Elsayed, K.N. In vitro antioxidant, anticancer, anti-inflammatory, anti-diabetic and anti-Alzheimer potentials of innovative macroalgae bio-capped silver nanoparticles. Environ. Sci. Pollut. Res. 2022, 29, 59930–59947. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Faisal, S.; Alotaibi, A.; Ullah, R.; Hussain, T.; Rizwan, M.; Zaman, N.; Iqbal, M.; Iqbal, A.; Ali, Z. Zingiber officinale driven bioproduction of ZnO nanoparticles and their anti-inflammatory, anti-diabetic, anti-Alzheimer, anti-oxidant, and anti-microbial applications. Inorg. Chem. Commun. 2022, 140, 109274. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Ertl, P.; Schuffenhauer, A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J. Cheminform. 2009, 1, 8. [Google Scholar] [CrossRef]
- Ertl, P.; Roggo, S.; Schuffenhauer, A. Natural product-likeness score and its application for prioritization of compound libraries. J. Chem. Inf. Model 2008, 48, 68–74. [Google Scholar] [CrossRef]
- Alam, M.S.; Lee, D.U.; Bari, M.L. Antibacterial and cytotoxic activities of Schiff base analogues of 4-aminoantipyrine. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 613–619. [Google Scholar] [CrossRef]








| Derivatives | Antioxidant Activity | Scavenging Activity | ||
|---|---|---|---|---|
| TAC (mg Gallic Acid/gm) | IRP (µg/mL) | DPPH (IC50 µg/mL) | ABTS (%) | |
| 22a | 34.75 ± 0.08 | 19.65 ± 0.04 | 16.22 ± 0.04 | 28.73 ± 0.06 |
| 22b | 42.47 ± 0.09 * | 24.02 ± 0.05 * | 13.20 ± 0.03 * | 35.11 ± 0.08 * |
| 23a | 36.85 ± 0.08 | 20.84 ± 0.05 | 15.21 ± 0.03 | 30.46 ± 0.07 |
| 23b | 34.56 ± 0.08 | 19.54 ± 0.04 | 16.13 ± 0.04 | 28.56 ± 0.06 |
| STD | - | - | 4.05 ± 0.01 | 39.09 ± 0.09 |
| Ascorbic Acid | ||||
| Derivatives | Anti-Diabetic Activity | Anti-Alzheimer’s Activity | Anti-Arthritic Activity | |
|---|---|---|---|---|
| α-Amylase Inhibition (%) | Acetylcholinesterase (ACE) Inhibition (%) | Proteinase Denaturation (%) | Inhibition of Proteinase (%) | |
| 22a | 29.50 ± 0.06 | 16.95 ± 0.04 | 18.45 ± 0.04 | 16.95 ± 0.04 |
| 22b | 36.06 ± 0.08 * | 20.71 ± 0.05 * | 19.57 ± 0.04 | 17.97 ± 0.04 |
| 23a | 31.28 ± 0.07 | 17.97 ± 0.04 | 22.56 ± 0.05 * | 20.71 ± 0.05 * |
| 23b | 29.34 ± 0.06 | 16.85 ± 0.04 | 18.35 ± 0.04 | 16.85 ± 0.04 |
| STD | 69.11 ± 0.15 | - | 49.33 ± 0.11 | 41.88 ± 0.09 |
| Acarbose | Diclofenac Sodium | |||
| Derivatives | 22a | 22b | 23a | 23b |
|---|---|---|---|---|
| Molecular Weight (MW) | 471.190 | 505.150 | 490.120 | 524.080 |
| Volume | 479.897 | 495.108 | 468.876 | 484.087 |
| Density | 0.982 | 1.020 | 1.045 | 1.083 |
| nHA | 9 | 9 | 10 | 10 |
| nHD | 3 | 3 | 3 | 3 |
| nRot | 9 | 9 | 8 | 8 |
| nRing | 4 | 4 | 4 | 4 |
| MaxRing | 6 | 6 | 6 | 6 |
| nHet | 9 | 10 | 11 | 12 |
| fChar | 0 | 0 | 0 | 0 |
| nRig | 26 | 26 | 27 | 27 |
| Flexibility | 0.346 | 0.346 | 0.296 | 0.296 |
| Stereocenters | 0 | 0 | 0 | 0 |
| TPSA | 113.090 | 113.090 | 137.770 | 137.770 |
| logS | −6.608 | −7.121 | −6.434 | −6.807 |
| logP | 4.421 | 5.190 | 4.711 | 5.455 |
| logD7.4 | 3.783 | 3.910 | 3.915 | 3.807 |
| Derivatives | 22a | 22b | 23a | 23b |
|---|---|---|---|---|
| Molecular Weight (MW) | 471.190 | 505.150 | 490.120 | 524.080 |
| nHA | 9 | 9 | 10 | 10 |
| nHD | 3 | 3 | 3 | 3 |
| TPSA | 113.090 | 113.090 | 137.770 | 137.770 |
| logP | 4.421 | 5.190 | 4.711 | 5.455 |
| Lipinski Rule | Accepted | Rejected | Accepted | Rejected |
| GSK Rule | Rejected | Rejected | Rejected | Rejected |
| Pfizer Rule | Accepted | Accepted | Accepted | Accepted |
| SA Score | 2.886 | 2.928 | 2.986 | 3.027 |
| NP Score | −0.903 | −1.033 | −1.395 | −1.448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkahtani, H.M.; Almehizia, A.A.; Al-Omar, M.A.; Obaidullah, A.J.; Zen, A.A.; Hassan, A.S.; Aboulthana, W.M. In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules 2023, 28, 7125. https://doi.org/10.3390/molecules28207125
Alkahtani HM, Almehizia AA, Al-Omar MA, Obaidullah AJ, Zen AA, Hassan AS, Aboulthana WM. In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules. 2023; 28(20):7125. https://doi.org/10.3390/molecules28207125
Chicago/Turabian StyleAlkahtani, Hamad M., Abdulrahman A. Almehizia, Mohamed A. Al-Omar, Ahmad J. Obaidullah, Amer A. Zen, Ashraf S. Hassan, and Wael M. Aboulthana. 2023. "In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic" Molecules 28, no. 20: 7125. https://doi.org/10.3390/molecules28207125
APA StyleAlkahtani, H. M., Almehizia, A. A., Al-Omar, M. A., Obaidullah, A. J., Zen, A. A., Hassan, A. S., & Aboulthana, W. M. (2023). In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules, 28(20), 7125. https://doi.org/10.3390/molecules28207125







