Screening of Multitarget Compounds against Acetaminophen Hepatic Toxicity Using In Silico, In Vitro, and In Vivo Approaches
Abstract
1. Introduction
2. Results
2.1. Percentage Yield
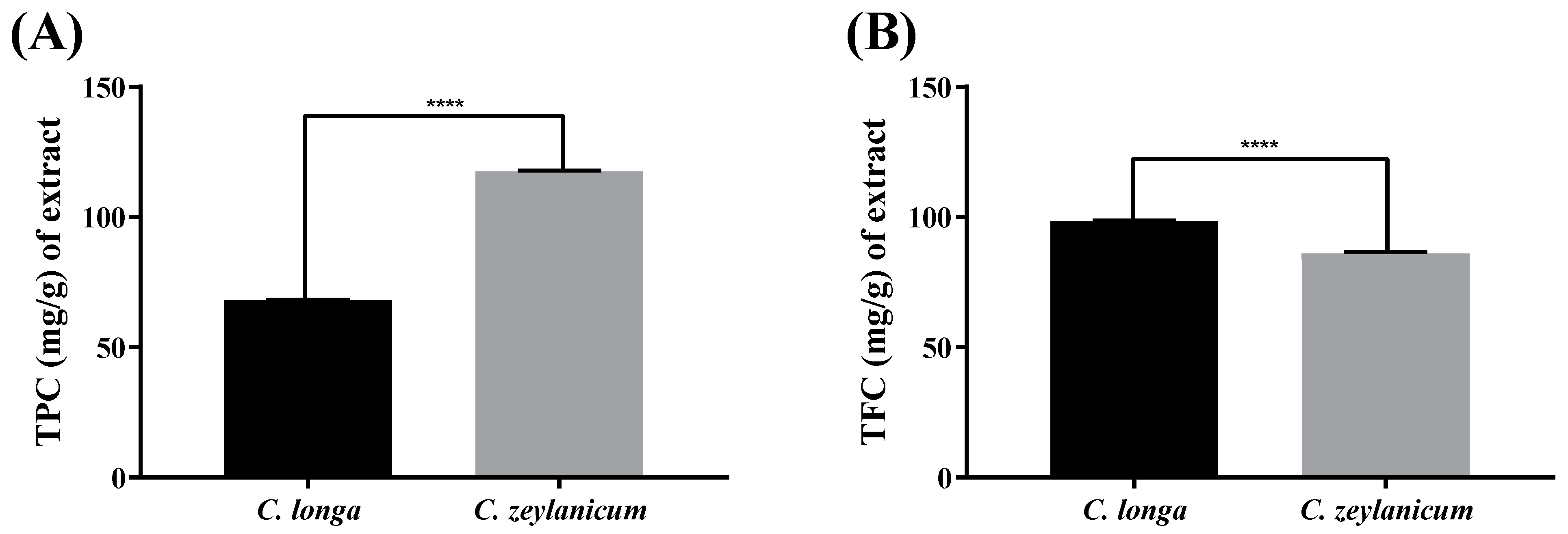
2.2. Total Phenolic Content (TPC)
2.3. Total Flavonoid Content (TFC)
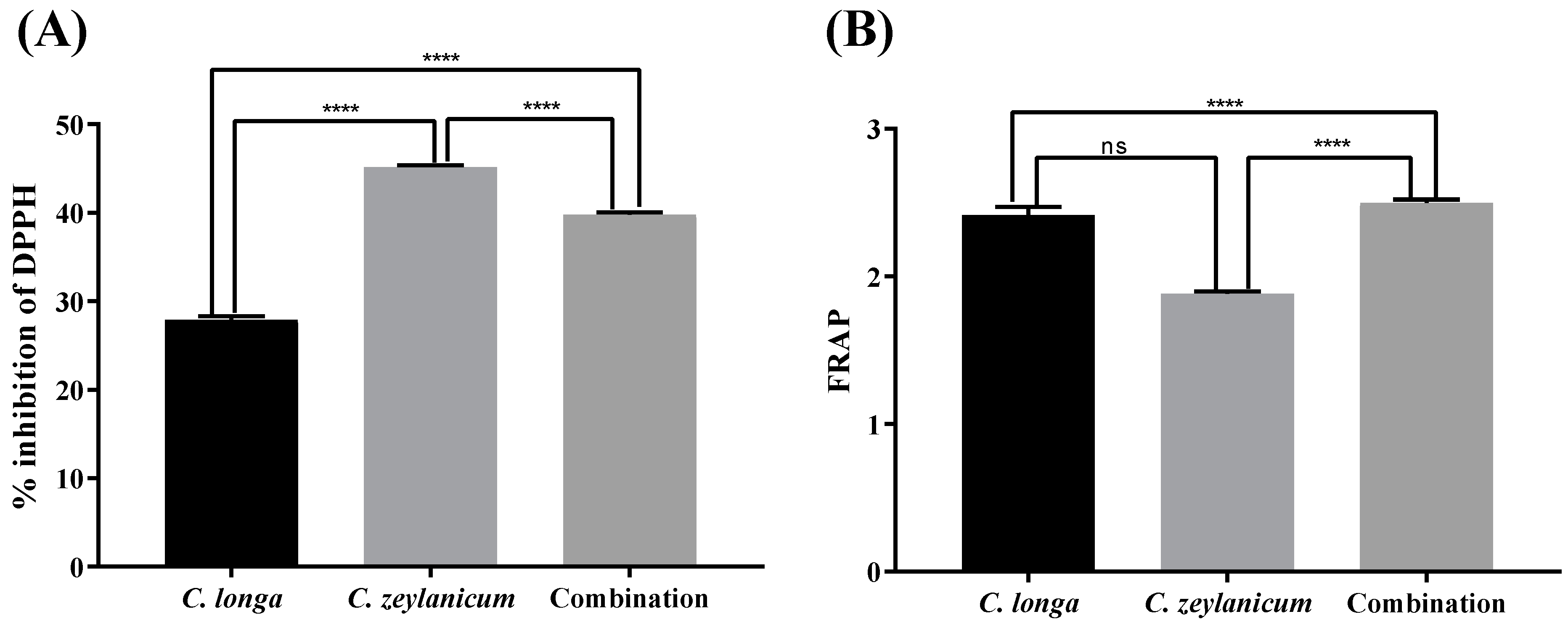
2.4. DPPH Radical Scavenging Activity
2.5. Reducing Power Assay
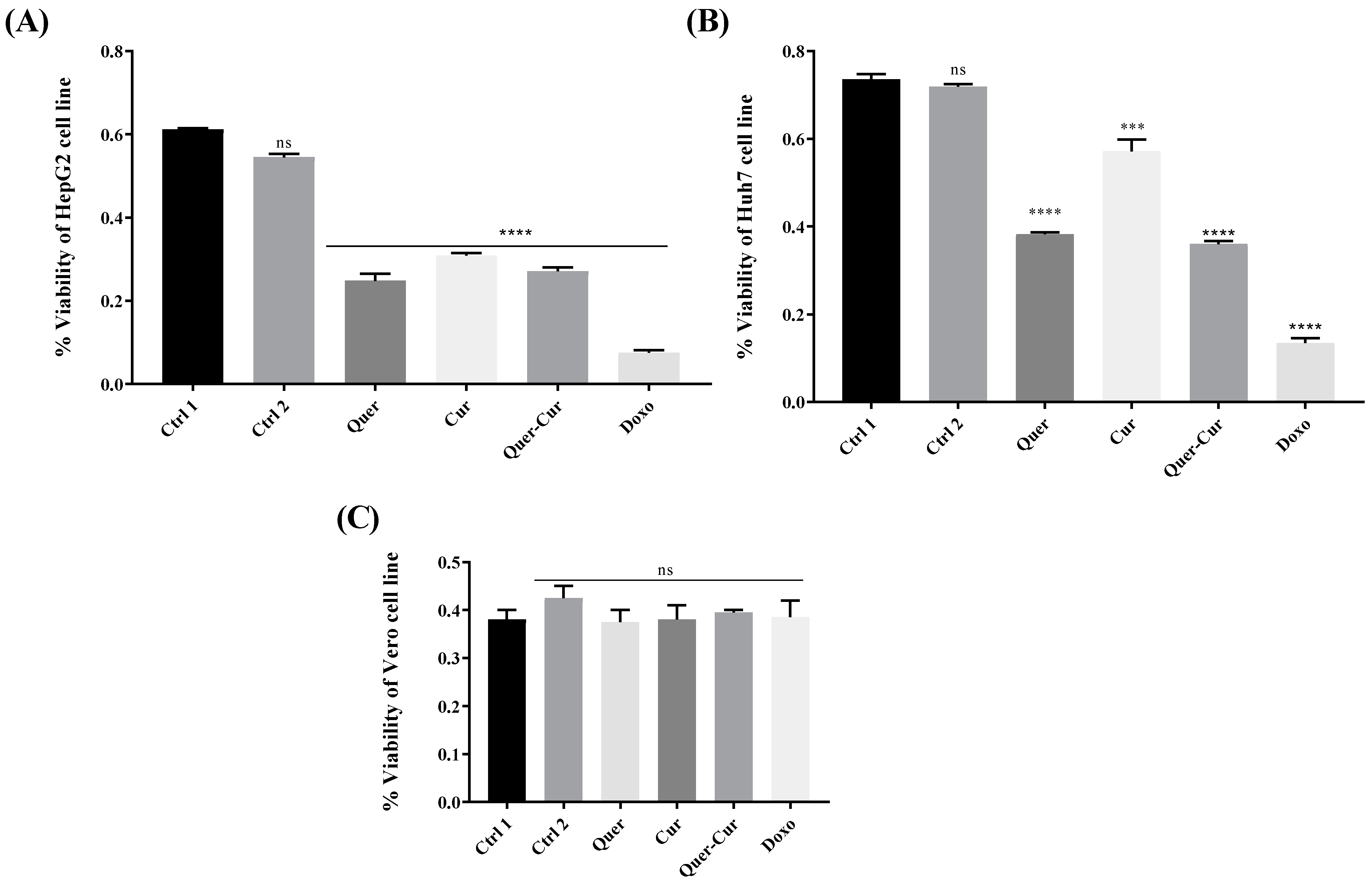
2.6. Cell Viability Assay
2.7. Molecular Docking
2.7.1. Screening
2.7.2. ADME and Drug-Likeness Analysis
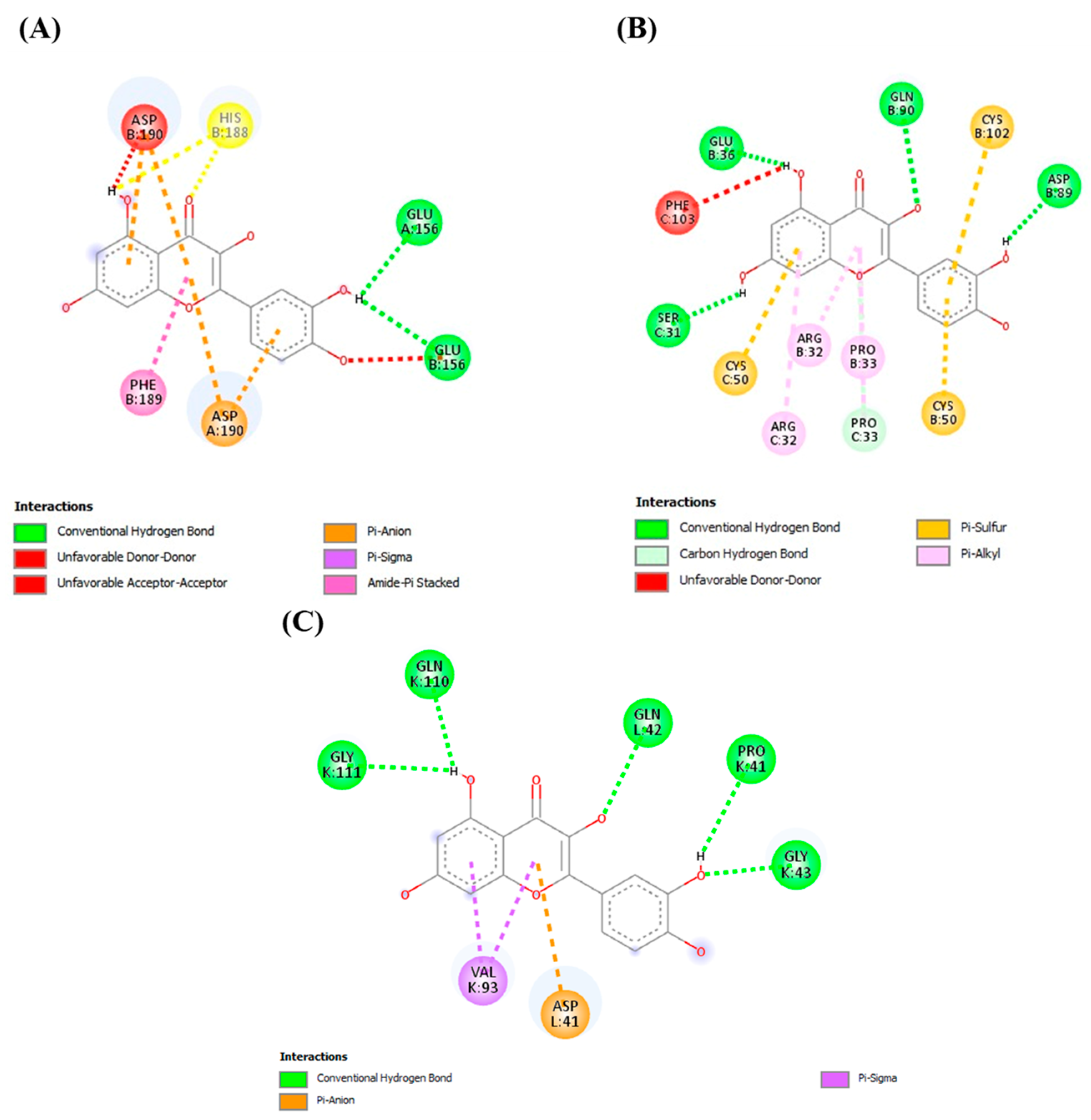
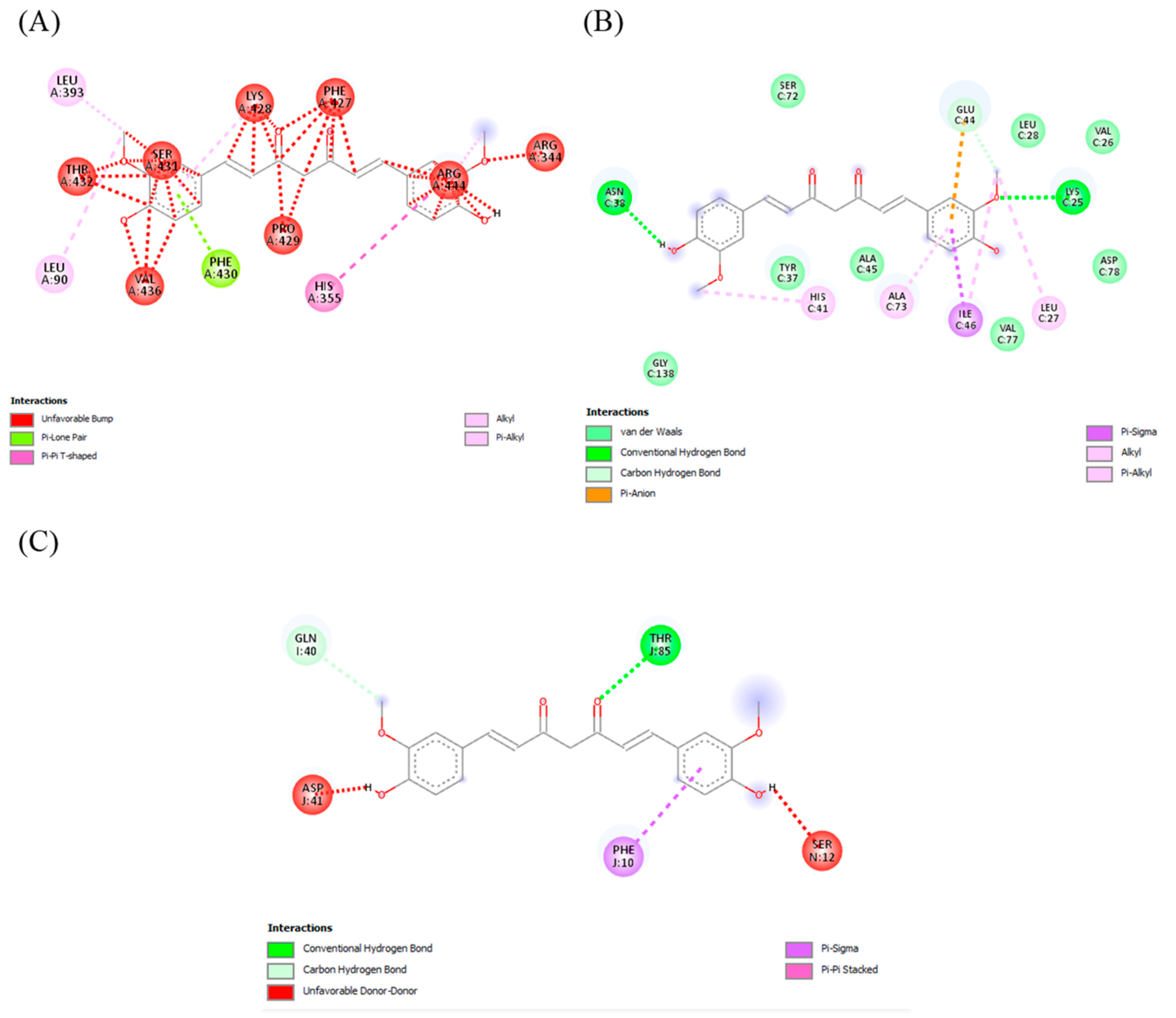
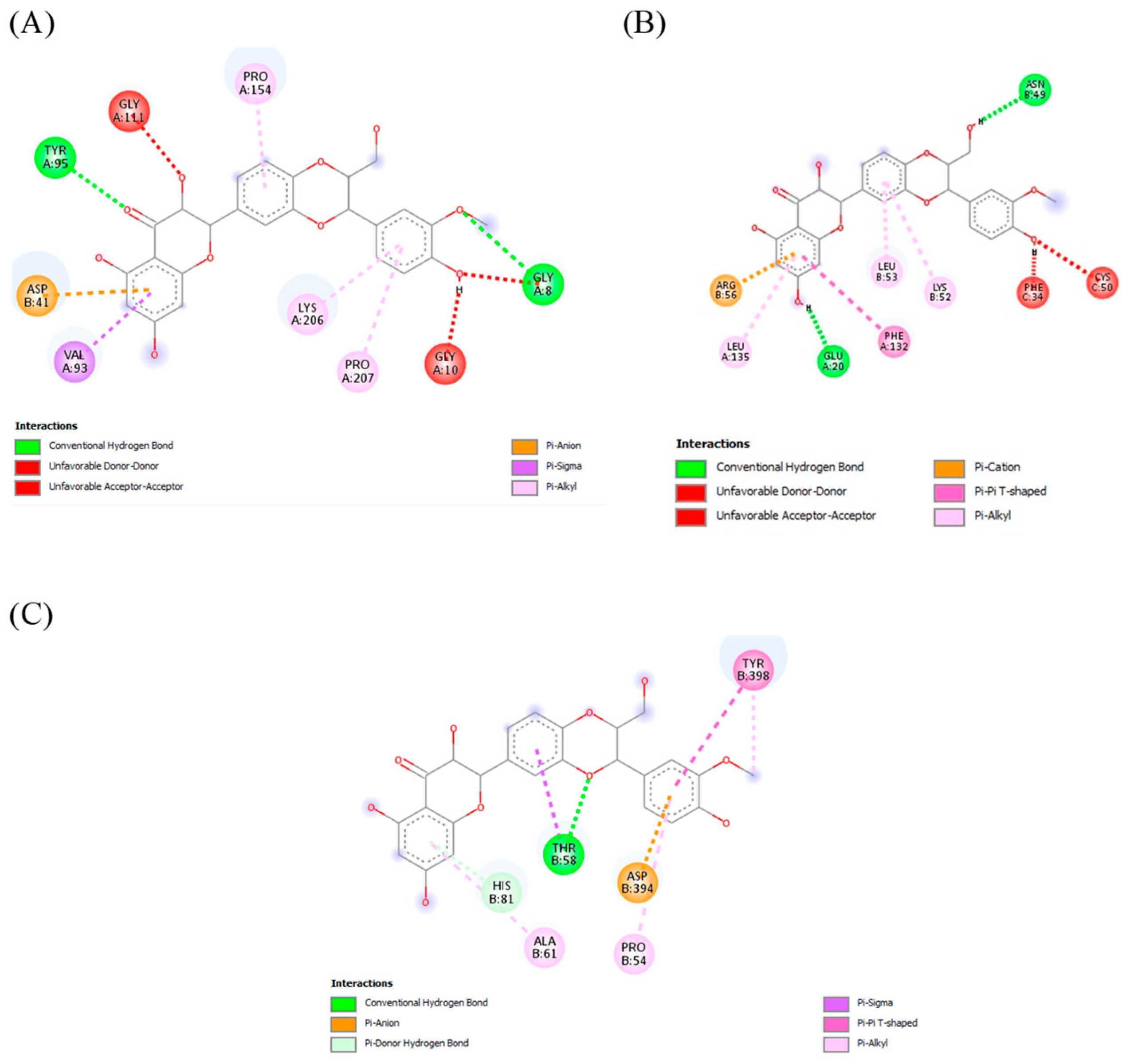
2.7.3. Docking Analysis
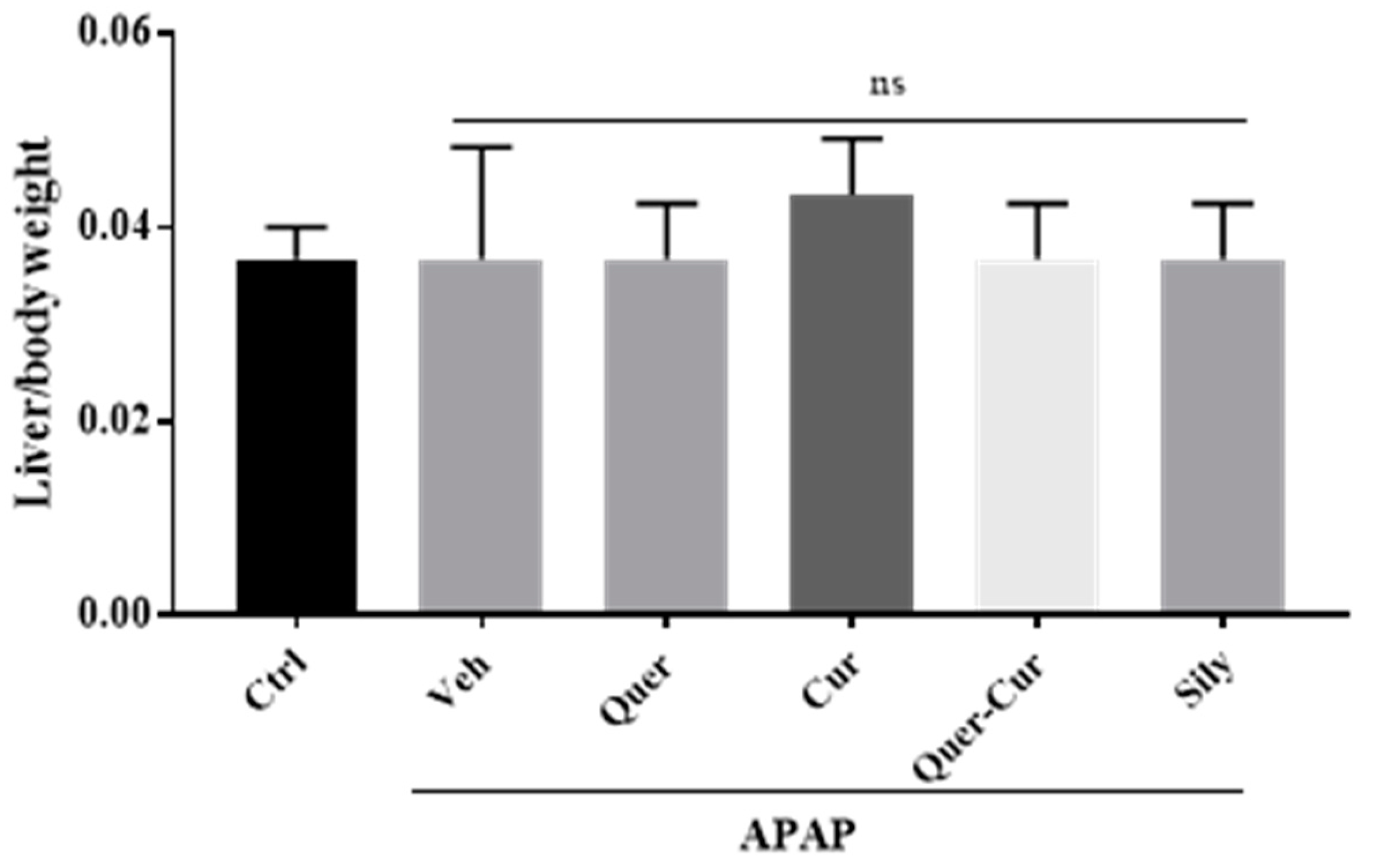
2.8. Liver to Body Weight
2.9. Assessment of Liver Function
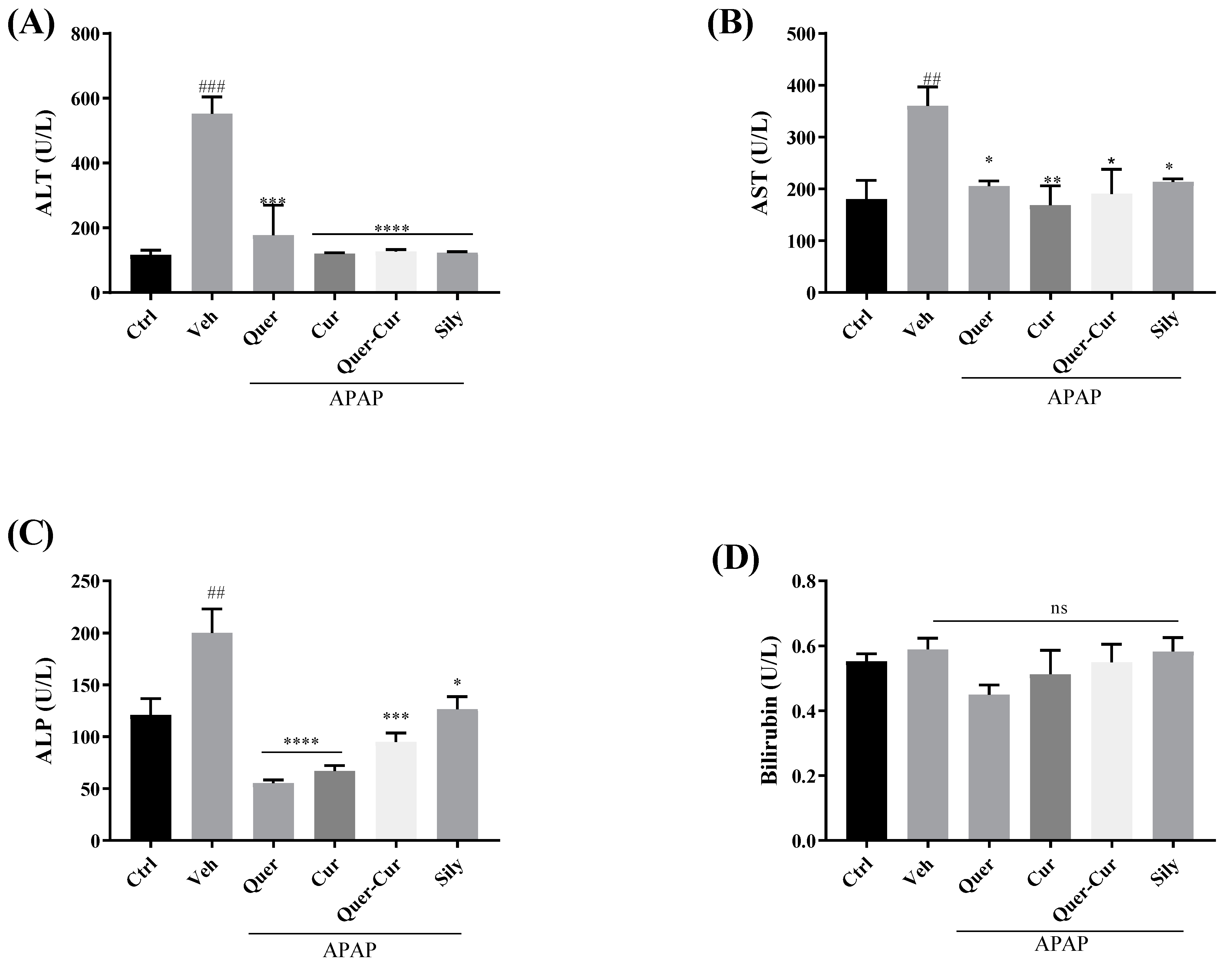
2.9.1. ALT, AST, ALP, and Bilirubin
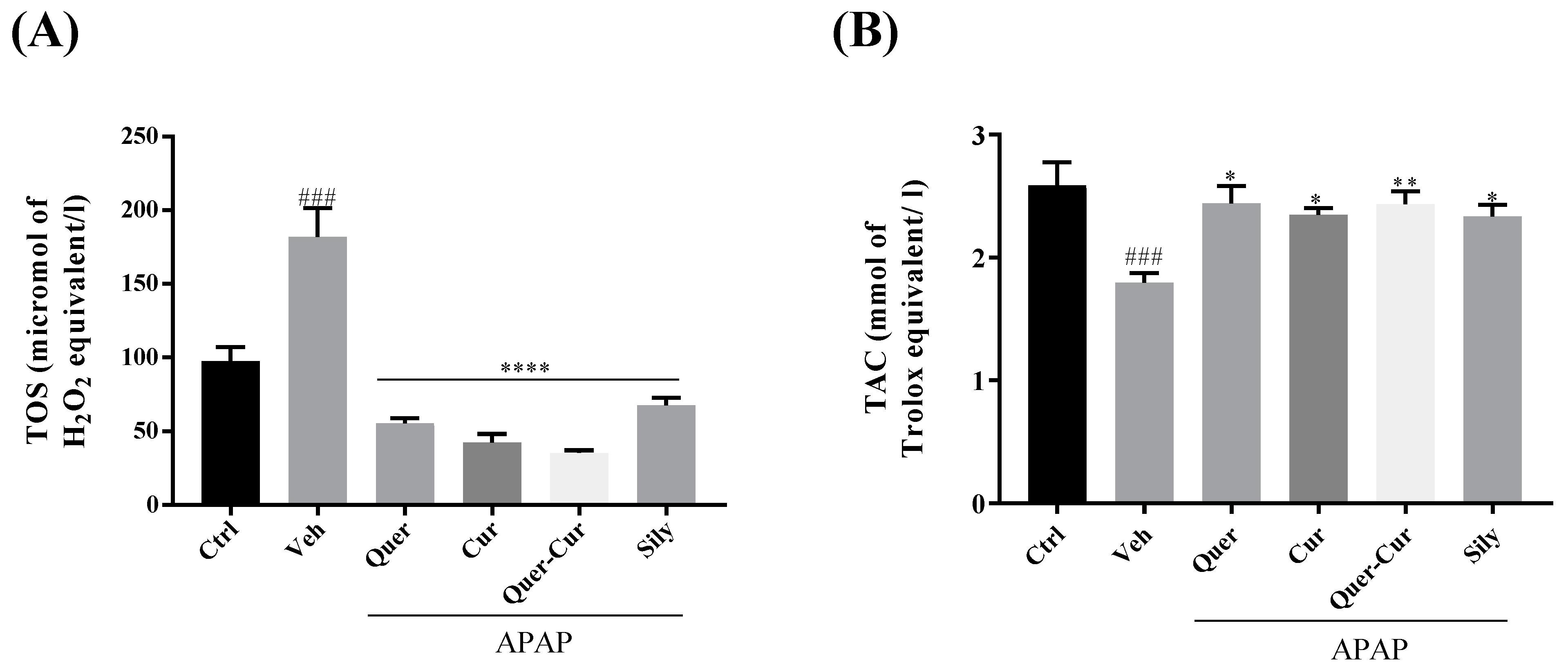
2.9.2. Analysis of TAC and TOS
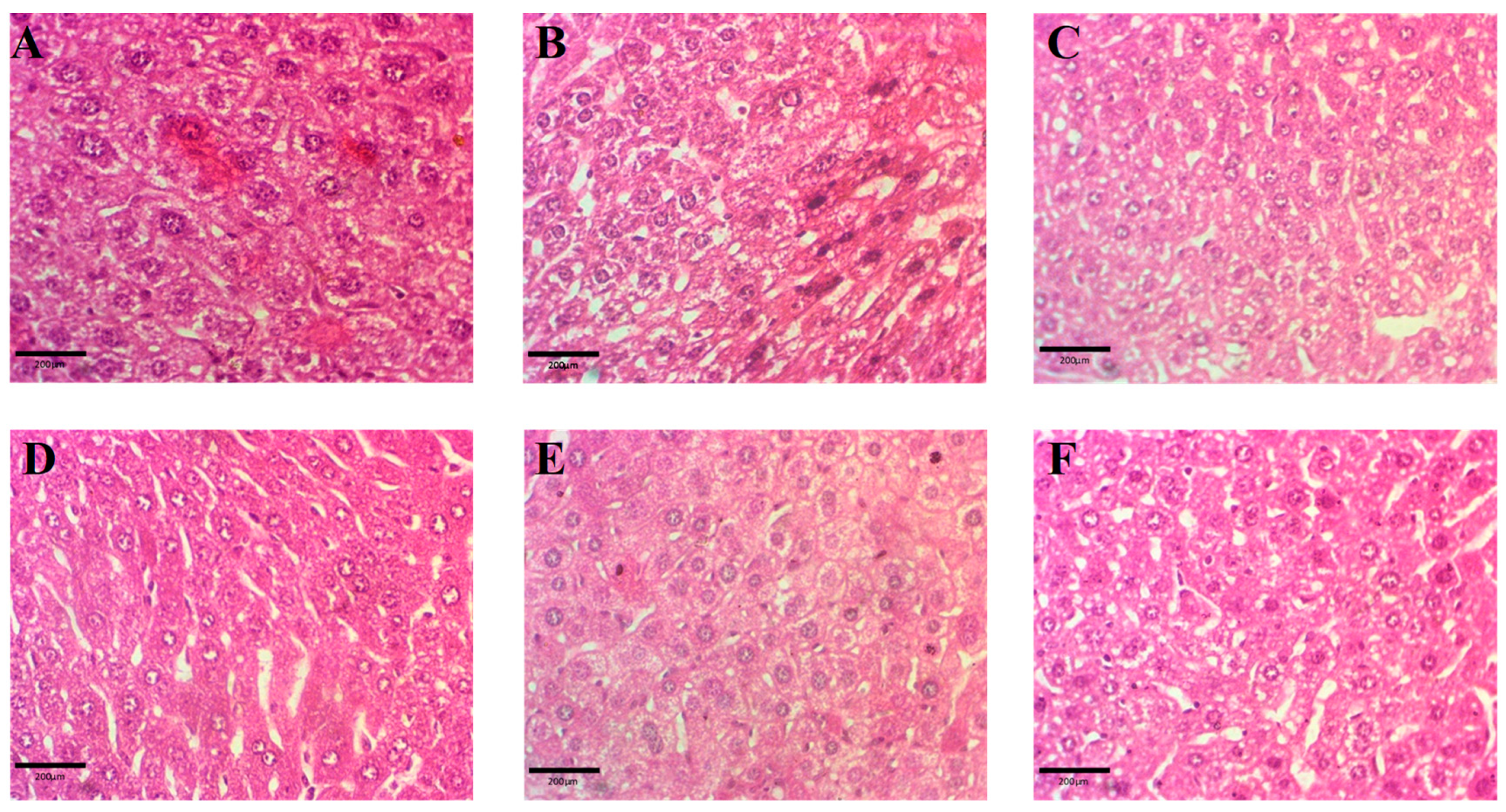
2.9.3. Histopathology
3. Discussion
4. Materials and Methods
4.1. Chemicals and Cell Lines
4.2. Collection of the Sample Plant and Its Preparation
4.3. In Vitro Study
4.3.1. Total Phenolic and Flavonoid Contents
4.3.2. Antioxidative Activity
4.4. Cell Viability Assay
4.5. In Silico Analysis
4.5.1. Screening
4.5.2. Protein and Ligand Preparation
4.5.3. Docking Analysis
4.6. In Vivo Studies
4.6.1. Mouse Model
4.6.2. Levels of ALT, AST, ALP, and Bilirubin
4.6.3. Total Antioxidative Activity (TAC)
4.6.4. Total Oxidative Stress (TOS)
4.6.5. Histopathological Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, L.N.; Chalasani, N. Epidemiology of Idiosyncratic Drug-Induced Liver Injury; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 337–347. [Google Scholar]
- Pandit, A.; Sachdeva, T.; Bafna, P. Drug-induced hepatotoxicity: A review. J. Appl. Pharm. Sci. 2012, 2, 233–243. [Google Scholar] [CrossRef]
- Laatikainen, O.; Sneck, S.; Turpeinen, M. Medication-related adverse events in health care—What have we learned? A narrative overview of the current knowledge. Eur. J. Clin. Pharmacol. 2022, 78, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 523, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Thorgeirsson, S.S.; Potter, W.Z.; Jollow, D.J.; Keiser, H. Acetaminophen-induced hepatic injury: Protective role of glutathione in man and rationale for therapy. Clin. Pharmacol. Ther. 1974, 16, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Eugenio-Pérez, D.; Montes de Oca-Solano, H.A.; Pedraza-Chaverri, J. Role of food-derived antioxidant agents against acetaminophen-induced hepatotoxicity. Pharm. Biol. 2016, 54, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity—Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Setty, S.R.; Quereshi, A.A.; Swamy, A.V.; Patil, T.; Prakash, T.; Prabhu, K.; Gouda, A.V. Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia 2007, 78, 451–454. [Google Scholar] [CrossRef]
- Abdel-Daim, M.; Abushouk, A.I.; Reggi, R.; Yarla, N.S.; Palmery, M.; Peluso, I. Association of antioxidant nutraceuticals and acetaminophen (paracetamol): Friend or foe? J. Food Drug Anal. 2018, 26, S78–S87. [Google Scholar] [CrossRef]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid Based Complement Altern. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Krup, V.; Prakash, L.H.; Harini, A. Pharmacological activities of turmeric (Curcuma longa Linn): A review. J. Homeop. Ayurv. Med. 2013, 2, 4. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive Compounds of Turmeric (Curcuma longa L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Cham, Switzerland, 2021; pp. 297–318. [Google Scholar]
- Błaszczyk, N.; Rosiak, A.; Kałużna-Czaplińska, J. The potential role of cinnamon in human health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, J.A.; Arshad, A.; Asif, P.; Rashid, H.; Arshad, M.I. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed. Pharmacother. 2019, 109, 2285–2292. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, W.; Wang, R.; Wang, Y.; Ouyang, D. Research advances in molecular modeling in cyclodextrins. Curr. Pharm. Des. 2017, 23, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, W. Molecular docking for drug discovery and development: A widely used approach but far from perfect. Future Sci. 2016, 8, 1707–1710. [Google Scholar] [CrossRef]
- Scotti, L.; Mendonca Junior, F.J.B.; Ishiki, H.M.; Ribeiro, F.F.; Singla, R.K.; Barbosa Filho, J.M.; DaSilva, M.S.; T. Scotti, M. Docking studies for multi-target drugs. Curr. Drug. Tar. 2017, 18, 592–604. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar. Drug. 2022, 20, 141. [Google Scholar] [CrossRef]
- Lee, G.-H.; Lee, H.-Y.; Choi, M.-K.; Chung, H.-W.; Kim, S.-W.; Chae, H.-J. Protective effect of Curcuma longa L. Extract on CCl 4-induced acute hepatic stress. BMC Res. Notes 2017, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). ACTA Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Wands, J.R.; Eken, A.; Osna, N.A.; Weinman, S.A.; Machida, K.; Joe Wang, H. Alcohol and hepatitis C virus–interactions in immune dysfunctions and liver damage. Alcohol. Clin. Exp. Res. 2010, 34, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, X.; Ren, Y.; Liu, C.; Yuan, A.; Zheng, L.; Li, B.; Zhang, Y. Identification of a novel farnesoid X receptor agonist, kaempferol-7-O-rhamnoside, a compound ameliorating drug-induced liver injury based on virtual screening and in vitro validation. Toxicol. Appl. Pharmacol. 2022, 454, 116251. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, N.; Chung, H.-J.; Alam, R.; Emon, N.U.; Alam, S.; Kabir, M.F.; Islam, M.M.; Hong, S.-T.; Sarkar, T.; Sarker, M.M.R.; et al. Chemico-pharmacological screening of the methanol extract of Gynura nepalensis DC deciphered promising antioxidant and hepatoprotective potentials: Evidenced from in vitro, in vivo, and computer-aided studies. Molecules 2022, 27, 3474. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P. Role of free radicals in liver diseases. Hepatol. Int. 2009, 3, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e14. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Saxena, J. Screening of total phenolic and flavonoid content in conventional and non-conventional species of curcuma. J. Pharmacogn. Phytochem. 2013, 2, 176–179. [Google Scholar]
- Adisakwattana, S.; Lerdsuwankij, O.; Poputtachai, U.; Minipun, A.; Suparpprom, C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Foods Hum. Nutr. 2011, 66, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, W.; Premakumara, G.; Ratnasooriya, W. In vitro antioxidant properties of leaf and bark extracts of ceylon cinnamon (Cinnamomum zeylanicum Blume). Trop. Agric. Res. 2013, 24, 128–138. [Google Scholar]
- Mushtaq, Z.; Tahir Nadeem, M.; Arshad, M.U.; Saeed, F.; Ahmed, M.H.; Bader Ul Ain, H.; Javed, A.; Anjum, F.M.; Hussain, S. Exploring the biochemical and antioxidant potential of ginger (Adric) and turmeric (Haldi). Int. J. Food Prop. 2019, 22, 1642–1651. [Google Scholar] [CrossRef]
- Somanawat, K.; Thong-Ngam, D.; Klaikeaw, N. Curcumin attenuated paracetamol overdose induced hepatitis. World J. Gastroenterol. WJG 2013, 19, 1962–1967. [Google Scholar] [CrossRef]
- Cheng, D.; Li, W.; Wang, L.; Lin, T.; Poiani, G.; Wassef, A.; Hudlikar, R.; Ondar, P.; Brunetti, L.; Kong, A.-N. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol. Pharm. 2019, 16, 1881–1889. [Google Scholar] [CrossRef]
- Kaushik, D.; O’Fallon, K.; Clarkson, P.M.; Patrick Dunne, C.; Conca, K.R.; Michniak-Kohn, B. Comparison of quercetin pharmacokinetics following oral supplementation in humans. J. Food Sci. 2012, 77, H231–H238. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 2E1 and its roles in disease. Chem. Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.T.; Buters, J.T.M.; Pineau, T.; Fernandez-Salguero, P.; Gonzalez, F.J. Role of CYP2E1 in the Hepatotoxicity of Acetaminophen (∗). J. Biol. Chem. 1996, 271, 12063–12067. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.N.; Bohner, A.; Dapito, D.H.; Schwabe, R.F.; Lammert, F. TLR4 deficiency protects against hepatic fibrosis and diethylnitrosamine-induced pre-carcinogenic liver injury in fibrotic liver. PLoS ONE 2016, 11, e0158819. [Google Scholar] [CrossRef]
- Kiziltas, S. Toll-like receptors in pathophysiology of liver diseases. World J. Hepatol. 2016, 8, 1354–1369. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Toledo-Pereyra, L.H. Toll-like receptor signaling in liver ischemia and reperfusion. J. Investig. Surg. 2012, 25, 271–277. [Google Scholar] [CrossRef] [PubMed]
- El Faras, A.A.; Elsawaf, A.L. Hepatoprotective activity of quercetin against paracetamol-induced liver toxicity in rats. Tanta Med. J. 2017, 45, 92. [Google Scholar]
- Husain, I.; Ahmad, R.; Chandra, A.; Raza, S.T.; Shukla, Y.; Mahdi, F. Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J. Ethnopharmacol. 2018, 219, 110–116. [Google Scholar] [CrossRef]
- Abdel-Lateef, E.; Mahmoud, F.; Hammam, O.; El-Ahwany, E.; El-Wakil, E.; Kandil, S.; Abu Taleb, H.; El-Sayed, M.; Hassenein, H. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2). Acta Pharm. 2016, 66, 387–398. [Google Scholar] [CrossRef]
- Ibrahim, J.; Kabiru, A.Y.; Abdulrasheed-Adeleke, T.; Lawal, B.; Adewuyi, A.H. Antioxidant and hepatoprotective potentials of curcuminoid isolates from turmeric (Curcuma longa) rhizome on CCl4-induced hepatic damage in Wistar rats. J. Taibah Univ. Sci. 2020, 14, 908–915. [Google Scholar] [CrossRef]
- Ugwu, C.E.; Suru, S.M. Medicinal plants with hepatoprotective potentials against carbon tetrachloride-induced toxicity: A review. Egypt. Liver J. 2021, 11, 88. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, R.; Safdar, M.; Murtaza, S.; Mustafa, G.; Sajjad, M.; Bukhari, S.A.; Huma, T. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol. Biol. Rep. 2021, 48, 7703–7710. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid Based Complement Altern. Med. 2012, 2012, 519084. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef] [PubMed]
- Mahrosh, H.S.; Tanveer, M.; Arif, R.; Mustafa, G. Computer-aided prediction and identification of phytochemicals as potential drug candidates against MERS-CoV. BioMed Res. Int. 2021, 2021, 5578689. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Jiang, X.; Sun, Y.; Zhao, Z.; Li, S. Protective Effect of Flavonoids from Ziziphus jujuba cv. Jinsixiaozao against Acetaminophen-Induced Liver Injury by Inhibiting Oxidative Stress and Inflammation in Mice. Molecules 2017, 22, 1781. [Google Scholar] [CrossRef]
- Lee, N.-H.; Seo, C.-S.; Lee, H.-y.; Jung, D.-Y.; Lee, J.-K.; Lee, J.-A.; Song, K.Y.; Shin, H.-k.; Lee, M.-Y.; Seo, Y.B. Hepatoprotective and antioxidative activities of Cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid. Based Complement. Altern. Med. 2012, 2012, 804924. [Google Scholar]
- Saeedi Saravi, S.S.; Hasanvand, A.; Shahkarami, K.; Dehpour, A.R. The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm. Biol. 2016, 54, 2830–2837. [Google Scholar] [CrossRef]
- Eldin, D.N.; Fahim, H.I.; Ahmed, H.Y.; Abdelgawad, M.A.; Abourehab, M.A.S.; Ahmed, O.M. Preventive Effects of Mandarin Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin on Acetaminophen-Induced Hepatonephrotoxicity in Wistar Rats. Oxidative Med. Cell. Longev. 2022, 2022, 7065845. [Google Scholar] [CrossRef]
- Girish, C.; Pradhan, S.C. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J. Pharmacol. Pharmacother. 2012, 3, 149–155. [Google Scholar]
- Menezes, M.N.d.; Salles, É.M.; Vieira, F.; Amaral, E.P.; Luis, V.Z.; Cassado, A.A.; Epiphanio, S.; Mosig, J.M.A.; Alves Filho, J.C.F.; Mota, M.M. IL-1 alpha promotes liver inflammation and necrosis during blood-stage Plasmodium chabaudi malaria. Sci. Rep. 2019, 9, 7575. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; White, J.; Fang, J. Inhibitory effect of gallic acid on CCl 4-mediated liver fibrosis in mice. Cell Biochem. Biophys. 2014, 69, 21–26. [Google Scholar] [CrossRef]
| Sample Plant | Part of Plant | Solvent | % Yield ± SEM |
|---|---|---|---|
| C. longa | Rhizomes | 80% Ethanol | 12.7 ± 0.09 |
| C. zeylanicum | Bark | 80% Ethanol | 10.84 ± 0.09 |
| Sample | TPC (mg of Gallic Acid E/g of Extract) | TFC (mg QE/g of Extract) |
|---|---|---|
| C. longa | 68.0 ± 0.17 | 98.37 ± 0.27 |
| C. zeylanicum | 117.5 ± 0.39 | 86.11 ± 0.47 |
| Sample Name | DPPH % | Reducing Power % |
|---|---|---|
| C. longa | 27.89 ± 1.33 | 2.41 ± 0.17 |
| C. zeylanicum | 45.16 ± 0.66 | 1.88 ± 0.05 |
| Combination | 39.81 ± 0.73 | 2.49 ± 0.07 |
| Compound | MW | HBD | HBA | Nrotb | LogP | A | Violations |
|---|---|---|---|---|---|---|---|
| Curcumin | 368.38 | 2 | 6 | 8 | 3.37 | 102.8 | 0 |
| Quercetin | 302.24 | 5 | 7 | 1 | 1.99 | 78.04 | 0 |
| Calebin A | 384.38 | 2 | 7 | 8 | 2.88 | 103.89 | 0 |
| Rutin | 610.52 | 10 | 16 | 6 | −1.51 | 141.38 | 3 |
| Ligand | Receptor/Enzyme | S-Score | Interacting Amino Acids |
|---|---|---|---|
| Quercetin | CYP2E1 | −7.4 | A chain (Asp 190, Glu 156), B chain (His 188, Asp B 190, Phe 189) |
| MAPK | −9.2 | B chain (Glu 36, Gln 90, Cys 102, Asp 89, Cys 50, Pro 33, Arg 32) C chain (Phe 103, Ser 31, Cys 50, Arg32, Pro 33) | |
| TLR4 | −8.2 | K chain (Gly 111, Gln 110, Pro 41, Gly 43, Val 93), L chain (Asp 41, Gln 42) | |
| Curcumin | CYP2E1 | −6.1 | A chain (Leu393, Thr 432, Ser 431, Lys 428, Arg 344, Arg 444, His 355, Phe 427, Leu 90, Val 436, Phe 430, Pro 429) |
| MAPK | −5.5 | C chain (Asn 38, Glu 44, Lys 25, Leu 27, Ile 46, Ala 73, His 41) | |
| TLR4 | −7.3 | Gln I:40, Ser N:12, J chain (Asp 41, Phe 10, Thr 85) | |
| Silymarin | CYP2E1 | −8.9 | A chain (Gly 8, Gly 10, Pro 207, Lys 206, Val 93, Tyr 95, Gly 111, Pro 154), Asp B:41 |
| MAPK | −9.5 | A chain (Leu 135, Glu 20, Phe 132), B chain (Arg 56, Leu 53, Lys 52, Asn 49), C chain (Phe 34, Cys 50) | |
| TLR4 | −9.0 | B chain (Tyr 398, Asp 394, Pro 54, Thr 58, Ala 61, His 81) |
| Group | Group Description | Treatment | Dose Conc./Duration Time | References |
|---|---|---|---|---|
| Group 1 | Control group | Normal saline | For three weeks | [64] |
| Group 2 | Induced toxicity group | Received APAP | APAP 200 mg/kg body weight | [62] |
| Group 3 | Protective group (Quercetin) | APAP + QUE | QUE (20) mg/kg for 21 days | [65] |
| Group 4 | Protective group (Curcumin) | APAP + CUR | CUR (50) mg/kg for 21 days | [66] |
| Group 5 | Protective group (Combination) | APAP + QUE + CUR | MIX (1:1) mg/kg for 21 days | -- |
| Group 6 | Protective group (Silymarin) | APAP + SILY | SILY (50) mg/kg for 21 days | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.; Asghar, E.; Ali, W.; Mustafa, G.; Ansari, I.A.; Zia, S.; Ansari, S.A.; Khan, S. Screening of Multitarget Compounds against Acetaminophen Hepatic Toxicity Using In Silico, In Vitro, and In Vivo Approaches. Molecules 2024, 29, 428. https://doi.org/10.3390/molecules29020428
Ali M, Asghar E, Ali W, Mustafa G, Ansari IA, Zia S, Ansari SA, Khan S. Screening of Multitarget Compounds against Acetaminophen Hepatic Toxicity Using In Silico, In Vitro, and In Vivo Approaches. Molecules. 2024; 29(2):428. https://doi.org/10.3390/molecules29020428
Chicago/Turabian StyleAli, Muhammad, Esha Asghar, Waqas Ali, Ghulam Mustafa, Irfan Aamer Ansari, Saadiya Zia, Siddique Akber Ansari, and Sumaiya Khan. 2024. "Screening of Multitarget Compounds against Acetaminophen Hepatic Toxicity Using In Silico, In Vitro, and In Vivo Approaches" Molecules 29, no. 2: 428. https://doi.org/10.3390/molecules29020428
APA StyleAli, M., Asghar, E., Ali, W., Mustafa, G., Ansari, I. A., Zia, S., Ansari, S. A., & Khan, S. (2024). Screening of Multitarget Compounds against Acetaminophen Hepatic Toxicity Using In Silico, In Vitro, and In Vivo Approaches. Molecules, 29(2), 428. https://doi.org/10.3390/molecules29020428








