A Bidens pilosa L. Non-Polar Extract Modulates the Polarization of Human Macrophages and Dendritic Cells into an Anti-Inflammatory Phenotype
Abstract
:1. Introduction
2. Results and Discussion
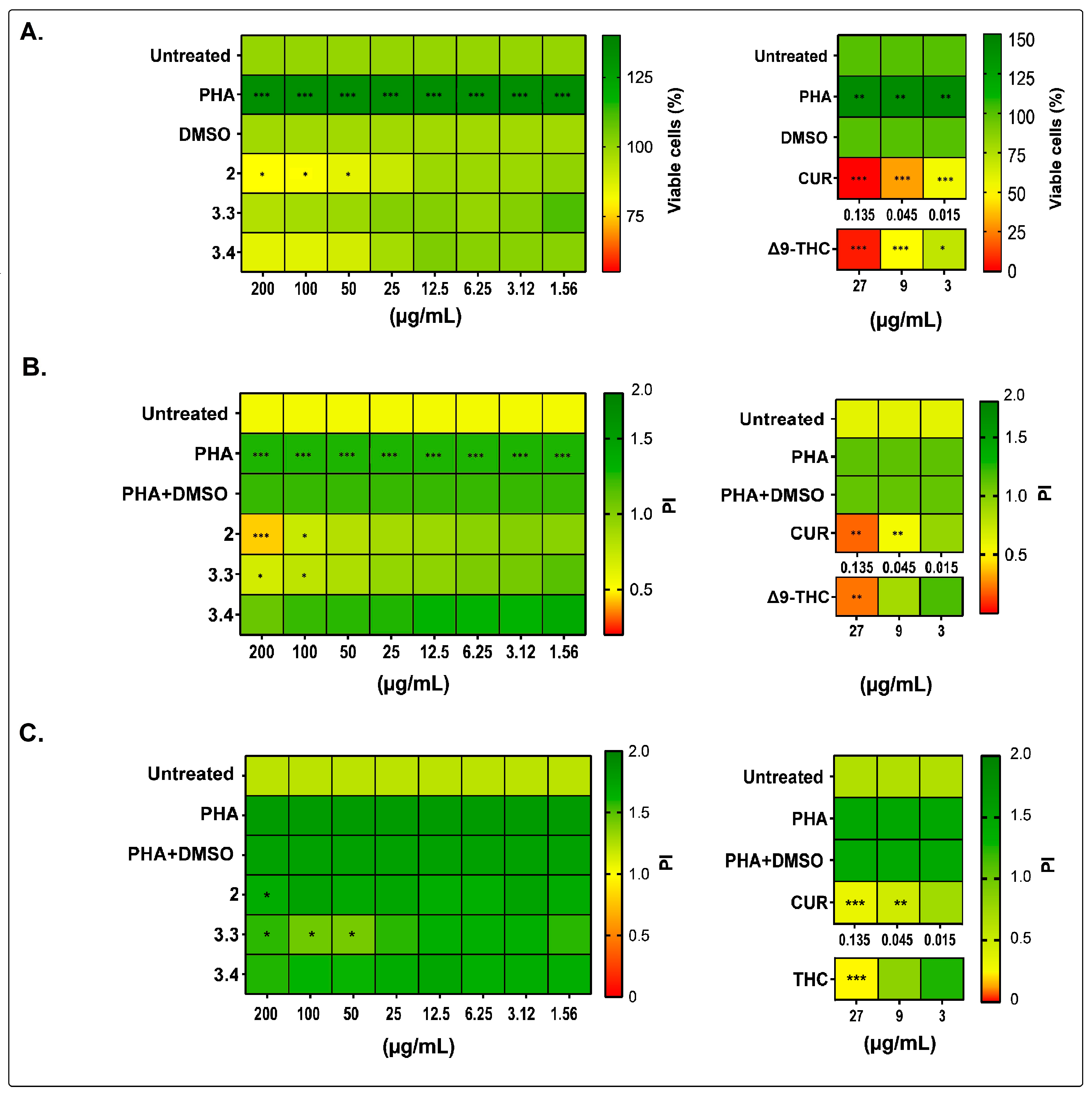
2.1. Bidens pilosa Extracts and Fractions Showed No Cytotoxic Activity towards Human Peripheral Blood Mononuclear Cells and Modulated Their Proliferation and Cytokine Secretion
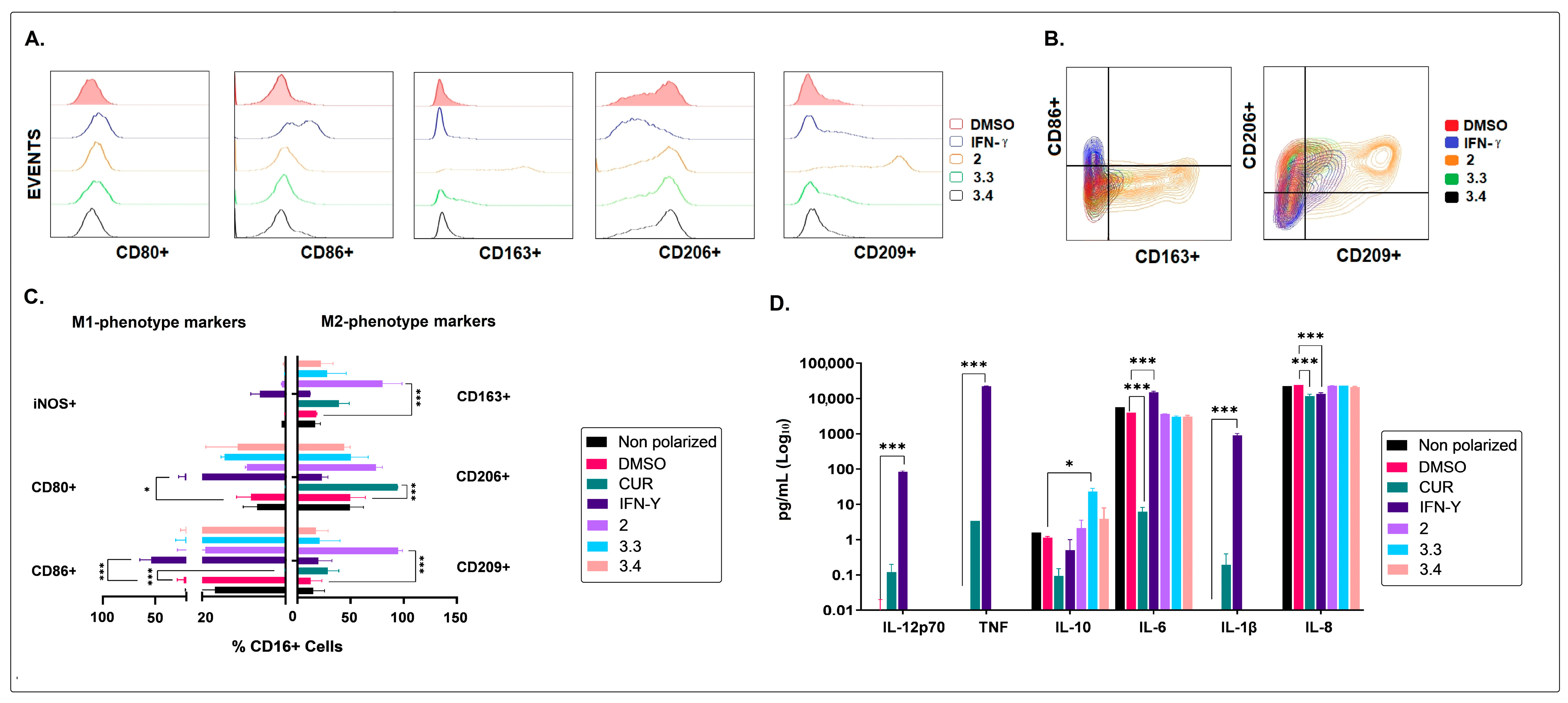
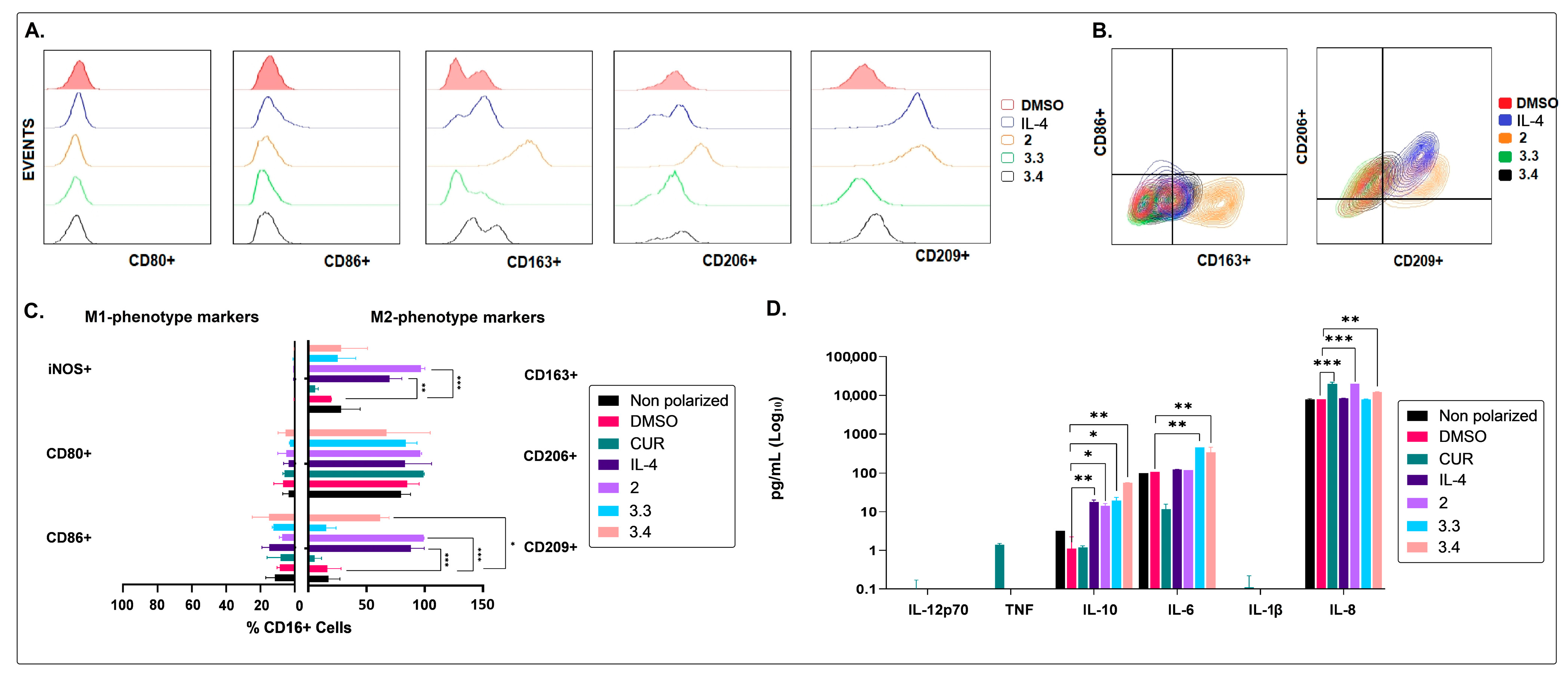
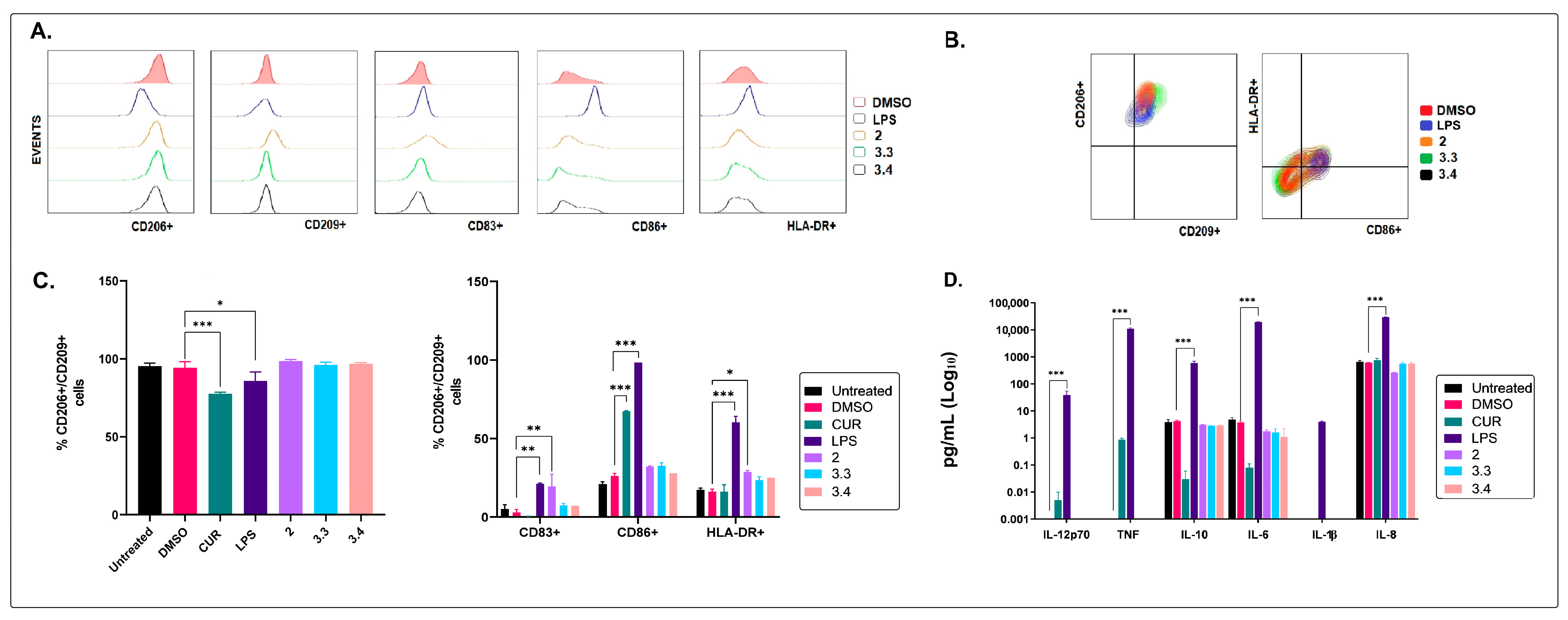
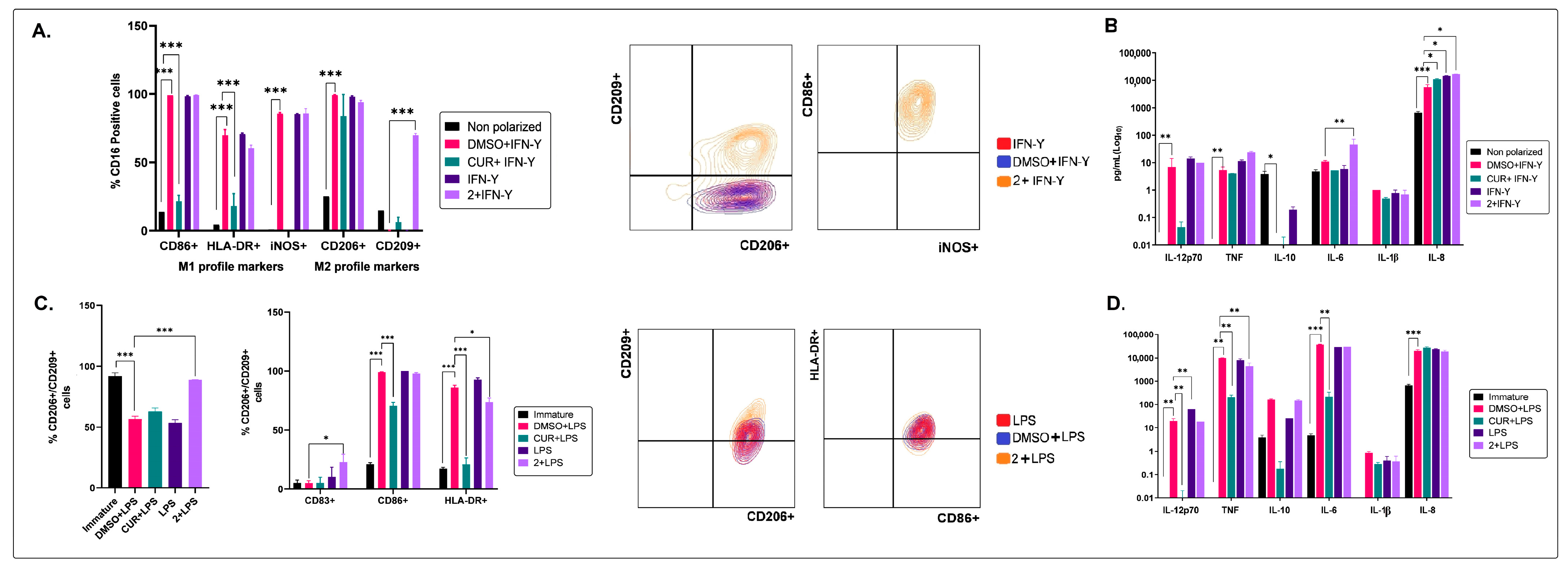
2.2. The Non-Polar Extract and the More Polar Fractions Obtained from B. pilosa Promoted an Anti-Inflammatory Profile of Human Macrophages and Dendritic Cells
2.3. The Medium–Low Polarity Compounds of the Petroleum Ether Extract of B. pilosa Would Be Associated with the Induction of Anti-Inflammatory Human Macrophages and Dendritic Cells
| RT (min) | Abundance (%) | Similarity (%) | Compound | Structure | Biological Activity | Reference |
|---|---|---|---|---|---|---|
| 8.677 | 1.16 | 92 | Germacrene D | 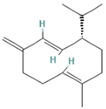 | Anti-inflammatory. Inhibits calcium mobilization, chemotaxis, and ROS production in neutrophils. | [73] |
| 9.993 | 2.60 | 89 | (+)-Spathulenol | 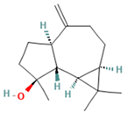 | Antinociceptive, antiproliferative activity against lymphocytes by inducing apoptosis. | [74] |
| 10.063 | 4.29 | 88 | Caryophyllene epoxide | 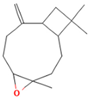 | Anti-tumor, anti-inflammatory, analgesic. | [75,76] |
| 10.273 | 1.74 | 91 | α-Humulene epoxide II | 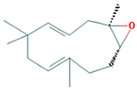 | Antioxidant, anti-inflammatory. | [77,78] |
| 13.337 | 3.25 | 90 | Ethyl palmitate | 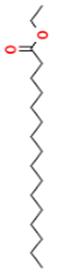 | Anti-inflammatory, anti-arthritic, and immunomodulatory. In vivo animal model: decreases PGE2 levels, plasma levels of TNF-α, and IL-6. Reduces NF-κB expression in liver and lung tissues and neutrophil tissue infiltration. | [79,80] |
| 14.797 | 1.18 | 85 | Methyl linolenate |  | Antioxidant and anti-inflammatory, decreases NO, iNOS, COX-2, and IL-1β synthesis in LPS-stimulated MØs. | [81,82] |
| 15.577 | 3.60 | 83 | Ethyl linoleate | 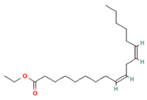 | Anti-inflammatory. Down-regulates iNOS and COX-2 expression, reduces NO and PGE2 production in LPS-activated RAW 264.7 cells. | [83] |
| 15.673 | 5.34 | 87 | Ethyl linolenate | 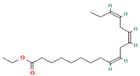 | - | - |
| 27.620 | 16.06 | 85 | Stigmasterol | 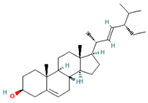 | Antimicrobial, antioxidant. In vitro anti-inflammatory activity against newborn mouse chondrocytes and primary cultures of IL-1β-stimulated patient-derived chondrocytes. Inhibits the NF-kB pathway and several proinflammatory mediators and mediators of matrix degradation. Anti-inflammatory activity for microglia. | [84,85] |
| 28.447 | 10.32 | 86 | Beta-Sitosterol | 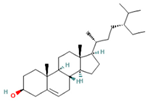 | Anti-inflammatory. Alleviates inflammatory response by inhibiting activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. Antiangiogenic activity in in vitro and in vivo models of rheumatoid arthritis. | [86,87] |
| 32.44 | 29.10 | 85 | Friedelan-3-one | 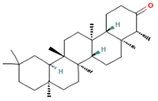 | Antioxidant, anti-inflammatory, and immunomodulatory activities. In vivo model: decreases DSS-induced colitis and regulates autophagy. It is associated with the inhibition of ROS production (intracellular/extracellular), TNF-α, IL-1β, and IL-6. It also inhibits cell proliferation. | [88,89] |
| RT (min) | Abundance (%) | Similarity (%) | Compound | Structure | Biological Activity | Reference |
|---|---|---|---|---|---|---|
| 9.610 | 0.64 | 93 | Dihydroactinidiolide | 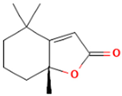 | Antioxidant activity. | [90] |
| 10.847 | 2.23 | 82 | ent-Germacra-4(15),5,10(14)-trien-1.beta.-ol |  | - | - |
| 11.833 | 1.29 | 88 | Neophytadiene | 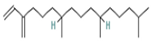 | Anti-inflammatory activity. Inhibits the production of NO and the inflammatory cytokines TNF-α, IL-6, and IL-10 in in vitro (RAW 264.7 cells) and in vivo (Sprague Dawley rats) models. | [91] |
| 22.897 | 1.95 | 85 | 1,1,6-trimethyl-3-methylene-2-(3,6,9,13-tetramethyl-6-ethenye-10,14-dimethylene-pentadec-4-enyl) cyclohexane |  | Antiproliferative activity against tumor cells. | [92] |
| 23.457 | 8.83 | 83 | Tetrapentacontane |  | Antioxidant and anti-inflammatory activities. | [93] |
| 29.123 | 5.15 | 89 | Methyl commate C |  | Antioxidant, anti-inflammatory, antidiabetic, and antihyperlipidemic activities. | [94,95] |
3. Materials and Methods
3.1. Obtaining Plant Material
3.2. Obtaining Extracts and Fractions
3.3. Screening of the Immunomodulatory Activity of Bidens pilosa L. in Human PBMCs
3.3.1. Isolation and Culture of Human Peripheral Blood Mononuclear Cells
3.3.2. Cytotoxicity Assays on PBMCs
3.3.3. Cell Proliferation Assays on PBMCs
3.4. Measurement of Cytokines in Culture Supernatants
3.5. Analysis of the Immunomodulatory Activity of B. pilosa L. on Human MØs and CDs
3.5.1. Culture and Differentiation of Human Monocyte-Derived MØs
3.5.2. Culture and Differentiation of Human Monocyte-Derived DCs
3.5.3. Characterization of the Phenotype of MØs and DCs
3.6. Chemical Characterization through Gas Chromatography Coupled with Mass Spectrometry (GC/MS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Gea-Banacloche, J.C. Immunomodulation. In Principles of Molecular Medicine; Runge, M.S., Patterson, C., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 893–904. [Google Scholar] [CrossRef]
- Masihi, K.N. Fighting infection using immunomodulatory agents. Expert. Opin. Biol. Ther. 2001, 1, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Schreiner, J.; Zippelius, A. Modulation of APC Function and Anti-Tumor Immunity by Anti-Cancer Drugs. Front. Immunol. 2015, 6, 501. [Google Scholar] [CrossRef] [PubMed]
- Bashaw, A.A.; Leggatt, G.R.; Chandra, J.; Tuong, Z.K.; Frazer, I.H. Modulation of antigen presenting cell functions during chronic HPV infection. Papillomavirus Res. 2017, 4, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Coutant, F.; Miossec, P. Altered dendritic cell functions in autoimmune diseases: Distinct and overlapping profiles. Nat. Rev. Rheumatol. 2016, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chow, A.; Greter, M.; Saenger, Y.; Kwan, W.H.; Leboeuf, M.; Ginhoux, F.; Ochando, J.C.; Kunisaki, Y.; van Rooijen, N.; et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 2011, 208, 1069–1082. [Google Scholar] [CrossRef]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Figdor, C.G.; Huijbens, R.; Mohan-Peterson, S.; Bennett, B.; Culpepper, J.; Dang, W.; Zurawski, G.; E de Vries, J. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 1993, 151, 6370–6381. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hong, H.; Kawakami, Y.; Lowell, C.A.; Kawakami, T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. J. Clin. Investig. 2008, 118, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Anthony, R.M.; Urban, J.F.; Alem, F.; Hamed, H.A.; Rozo, C.T.; Boucher, J.L.; Van Rooijen, N.; Gause, W.C. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006, 12, 955–960. [Google Scholar] [CrossRef]
- Bhatia, S.; Fei, M.; Yarlagadda, M.; Qi, Z.; Akira, S.; Saijo, S.; Iwakura, Y.; van Rooijen, N.; Gibson, G.A.; Croix, C.M.S.; et al. Rapid Host Defense against Aspergillus fumigatus Involves Alveolar Macrophages with a Predominance of Alternatively Activated Phenotype. PLoS ONE 2011, 6, e15943. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Harry, R.A.; McLeod, J.D. Apoptotic cells induce dendritic cell-mediated suppression via interferon-γ-induced IDO. Immunology 2008, 124, 89–101. [Google Scholar] [CrossRef]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yoon, Y.D.; Ahn, J.M.; Kang, J.S.; Park, S.K.; Lee, K.; Bin Song, K.; Kim, H.M.; Han, S.-B. Angelan isolated from Angelica gigas Nakai induces dendritic cell maturation through toll-like receptor 4. Int. Immunopharmacol. 2007, 7, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, M.K.; Yun, Y.P.; Kim, Y.; Kim, J.S.; Kim, Y.S.; Kim, K.; Han, S.S.; Lee, C.-K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharmacol. 2001, 1, 1275–1284. [Google Scholar] [CrossRef]
- Santander, S.P.; Aoki, M.; Hernandez, J.F.; Pombo, M.; Moins-Teisserenc, H.; Mooney, N.; Fiorentino, S. Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int. Immunopharmacol. 2011, 11, 652–660. [Google Scholar] [CrossRef]
- Bartolome, A.P.; Villaseñor, I.M.; Yang, W.-C. Bidens pilosa L. (Asteraceae): Botanical Properties, Traditional Uses, Phytochemistry, and Pharmacology. Evid. Based Complement. Altern. Med. ECAM 2013, 2013, 340215. [Google Scholar]
- Dos Santos Filho, E.X.; Da Silva, A.C.G.; De Ávila, R.I.; Batista, A.C.; Marreto, R.N.; Lima, E.M.; de Oliveira, C.M.A.; Mendonça, E.F.; Valadares, M.C. Chemopreventive effects of FITOPROT against 5-fluorouracil-induced toxicity in HaCaT cells. Life Sci. 2018, 193, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, A.E.V.; Cruz, V.M.; Almeida-Junior, L.D.; Costa, C.A.R.A.; Di Stasi, L.C. Bidens pilosa (Black Jack) Standardized Extract Ameliorates Acute TNBS-induced Intestinal Inflammation in Rats. Planta Med. 2020, 86, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, K.; Yang, Z.; Wang, Y.; Si, H. The Effect of the Aqueous Extract of Bidens Pilosa L. on Androgen Deficiency Dry. Eye in Rats. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2016, 39, 266–277. [Google Scholar] [CrossRef]
- Fotso, A.; Longo, F.; Djomeni, P.; Kouam, S.; Spiteller, M.; Dongmo, A.; Savineau, J.P. Analgesic and antiinflammatory activities of the ethyl acetate fraction of Bidens pilosa (Asteraceae). Inflammopharmacology 2014, 22, 105–114. [Google Scholar] [CrossRef]
- Pereira, R.L.; Ibrahim, T.; Lucchetti, L.; da Silva, A.J.; Gonçalves de Moraes, V.L. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology 1999, 43, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mesa, X.M.; Contreras Bolaños, L.A.; Mejía, A.; Pombo, L.M.; Modesti Costa, G.; Santander González, S.P. Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review. Pharmaceutics 2023, 15, 1491. [Google Scholar] [CrossRef] [PubMed]
- Kviecinski, M.R.; Benelli, P.; Felipe, K.B.; Correia JF, G.; Pich, C.T.; Ferreira, S.R.S.; Pedrosa, R. SFE from Bidens pilosa Linné to obtain extracts rich in cytotoxic polyacetylenes with antitumor activity. J. Supercrit. Fluids. 2011, 56, 243–248. [Google Scholar] [CrossRef]
- Chang, J.S.; Chiang, L.C.; Chen, C.C.; Liu, L.T.; Wang, K.C.; Lin, C.C. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 2001, 29, 303–312. [Google Scholar] [CrossRef]
- Abajo, C.; Ángeles Boffill, M.; del Campo, J.; Alexandra Méndez, M.; González, Y.; Mitjans, M.; Vinardell, M.P. In vitro study of the antioxidant and immunomodulatory activity of aqueous infusion of Bidens pilosa. J. Ethnopharmacol. 2004, 93, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, W.C.; Liang, C.L.; Liu, H.Y.; Lai, S.K.; Chang, C.L.T. Data on the effect of Cytopiloyne against Listeria monocytogenes infection in mice. Data Brief 2016, 7, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, W.C.; Liang, C.L.; Liu, H.Y.; Lai, S.K.; Chang, C.L.T. Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidal agent via regulation of macrophages. J. Ethnopharmacol. 2016, 184, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Yang, C.Y.; Liang, Y.C.; Yang, C.W.; Li, W.Q.; Chung, C.Y.; Yang, M.-T.; Kuo, T.-F.; Lin, C.-F.; Liang, C.-L.; et al. Anti-coccidial properties and mechanisms of an edible herb, Bidens pilosa, and its active compounds for coccidiosis. Sci. Rep. 2019, 9, 2896. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Sosanya, A.S.; Nwadike, N.; Oshinloye, A.O. Beneficial effect of Bidens pilosa L. (Asteraceae) in a rat model of colitis. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Pineau, D.; Hermouet, S. Interleukin-8: An autocrine/paracrine growth factor for human hematopoietic progenitors acting in synergy with colony stimulating factor-1 to promote monocyte-macrophage growth and differentiation. Exp. Hematol. 1999, 27, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis1. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef]
- Zheng, T.; Ma, G.; Tang, M.; Li, Z.; Xu, R. IL-8 Secreted from M2 Macrophages Promoted Prostate Tumorigenesis via STAT3/MALAT1 Pathway. Int. J. Mol. Sci. 2018, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.; Garzetti, L.; Gatta, A.T.; Finardi, A.; Maiorino, C.; Ruffini, F.; Martino, G.; Muzio, L.; Furlan, R. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflamm. 2016, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M. Therapeutic Potential of Semi-Mature Dendritic Cells for Tolerance Induction. Front. Immunol. 2012, 3, 123. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013, 4, 438. [Google Scholar] [CrossRef] [PubMed]
- Witherel, C.E.; Sao, K.; Brisson, B.K.; Han, B.; Volk, S.W.; Petrie, R.J.; Han, L.; Spiller, K.L. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials 2021, 269, 120667. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, J.E.; Crawford, R.B.; Kaminski, N.E. Suppression of CpG-ODN-mediated IFNα and TNFα response in human plasmacytoid dendritic cells (pDC) by cannabinoid receptor 2 (CB2)-specific agonists. Toxicol. Appl. Pharmacol. 2019, 369, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Mesa, X.M.; Moreno Vergara, A.F.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.D.; Dare, H.A.; Winnard, A.V.; Parker, J.M.; Miller, D.K. IL-1 beta-converting enzyme (ICE) is present and functional in human alveolar macrophages: Macrophage IL-1 beta release limitation is ICE independent. J. Immunol. 1997, 159, 5964–5972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, H.; Su, S.B. A critical role for interleukin-1β in the progression of autoimmune diseases. Int. Immunopharmacol. 2013, 17, 658–669. [Google Scholar] [CrossRef]
- Sparre, T.; Bjerre Christensen, U.; Gotfredsen, C.F.; Mose Larsen, P.; Fey, S.J.; Hjernø, K. Changes in expression of IL-1β influenced proteins in transplanted islets during development of diabetes in diabetes-prone BB rats. Diabetologia 2004, 47, 892–908. [Google Scholar]
- Corbett, J.A.; Kwon, G.; Turk, J.; McDaniel, M.L. IL-1.beta. induces the coexpression of both nitric oxide synthase and cyclooxygenase by Islets of Langerhans: Activation of cyclooxygenase by nitric oxide. Biochemistry 1993, 32, 13767–13770. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1β, IL-2, and IL-6 in Insulin-Dependent Diabetic Children. Mediat. Inflamm. 2006, 2006, e59206. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.; Oberholzer, C.; Moldawer, L.L. Cytokine signaling-regulation of the immune response in normal and critically ill states. Crit. Care Med. 2000, 28, N3. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1998, 14, 129–151. [Google Scholar] [CrossRef]
- Reddy, S.; Young, M. IL-1β Expression in Islet Cells of the NOD Mouse and Its Spatial Relationship to Beta Cells and Inducible Nitric Oxide Synthase. Ann. NY Acad. Sci. 2002, 958, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Cannella, B.; Raine, C.S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Olmos, G.; Lladó, J. Tumor Necrosis Factor Alpha: A Link between Neuroinflammation and Excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Bansard, C.; Lequerré, T.; Derambure, C.; Vittecoq, O.; Hiron, M.; Daragon, A.; Pouplin, S.; Daveau, M.; Boyer, O.; Tron, F.; et al. Gene profiling predicts rheumatoid arthritis responsiveness to IL-1Ra (anakinra). Rheumatology 2011, 50, 283–292. [Google Scholar] [CrossRef]
- Olsen, N.J.; Stein, C.M. New Drugs for Rheumatoid Arthritis. N. Engl. J. Med. 2004, 350, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New therapies for treatment of rheumatoid arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohns Colitis. 2016, 10, 989–997. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The Role of TNFα in Ulcerative Colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Kishimoto, T.; Kang, S.; Tanaka, T. IL-6: A New Era for the Treatment of Autoimmune Inflammatory Diseases. In Innovative Medicine; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015; pp. 131–147. [Google Scholar]
- Geissberger, P.; Séquin, U. Constituents of Bidens pilosa L.: Do the components found so far explain the use of this plant in traditional medicine? Acta Trop. 1991, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical Composition and Immunomodulatory Activity of Hypericum perforatum Essential Oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, A.; Ramezani, M.; Wright, L.; Paetz, C.; Schneider, B.; Amirghofran, Z. Identification of spathulenol in Salvia mirzayanii and the immunomodulatory effects. Phytother. Res. PTR. 2011, 25, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, anti-inflammatory, and cytotoxic properties of Origanum vulgare essential oil, rich with β-caryophyllene and β-caryophyllene oxide. Korean J. Pain 2022, 35, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shah, G.C. Composition and antioxidant activity of Senecio nudicaulis Wall. ex DC. (Asteraceae): A medicinal plant growing wild in Himachal Pradesh, India. Nat. Prod. Res. 2015, 29, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Shebaby, W.; Saliba, J.; Faour, W.H.; Ismail, J.; El Hage, M.; Daher, C.F.; Taleb, R.I.; Nehmeh, B.; Dagher, C.; Chrabieh, E.; et al. In vivo and in vitro anti-inflammatory activity evaluation of Lebanese Cannabis sativa L. ssp. indica (Lam.). J. Ethnopharmacol. 2021, 270, 113743. [Google Scholar] [CrossRef]
- Jiménez-Ferrer, E.; Vargas-Villa, G.; Martínez-Hernández, G.B.; González-Cortazar, M.; Zamilpa, A.; García-Aguilar, M.P.; Arenas-Ocampo, M.L.; Herrera-Ruiz, M. Fatty-Acid-Rich Agave angustifolia Fraction Shows Antiarthritic and Immunomodulatory Effect. Molecules 2022, 27, 7204. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Yuan, H.; Xu, Y.; Liu, R.M.; Luo, Y.; Xiao, J.H. Protective effects of Ligularia fischeri root extracts against ulcerative colitis in mice through activation of Bcl-2/Bax signalings. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 99, 154006. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Truong, V.L.; Jeong, W.S. Phytochemical Composition and Antioxidant and Anti-Inflammatory Activities of Ligularia fischeri Turcz: A Comparison between Leaf and Root Extracts. Plants 2022, 11, 3005. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Seetharaman, R.; Ko, M.J.; Kim, D.Y.; Kim, T.H.; Yoon, M.K.; Kwak, J.H.; Lee, S.J.; Bae, Y.S.; Choi, Y.W. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int. Immunopharmacol. 2014, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Arumugam, G.; Kaur, R.; Ghasemzadeh, A.; Yusoff, M.M.; Sinniah, U.R. GC-MS Based Metabolite Profiling, Antioxidant and Antimicrobial Properties of Different Solvent Extracts of Malaysian Plectranthus amboinicus Leaves. Evid. Based Complement. Alternat Med. 2017, 2017, e1517683. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Zheng, X.X.; Wang, C.; Huang, W.G.; Liu, X.B.; Xu, S.D.; Liu, D.-K.; Liu, M.-Y.; Lin, C.-S. β-Sitosterol Inhibits Rheumatoid Synovial Angiogenesis Through Suppressing VEGF Signaling Pathway. Front. Pharmacol. 2022, 12, 816477. [Google Scholar] [CrossRef] [PubMed]
- Feigni, O.Y.M.; Mbiantcha, M.; Yousseu, W.N.; Tsafack, G.E.; Djuichou, F.N.S.; Noungoua, C.M.; Atsafack, C.M.L.; Ateufack, G. Immunomodulatory, anti-infammatory and antioxidant activities of aqueous and ethanolic extracts of Cissus quadrangularis Linn. (Vitaceae) in chronic pai. Eur. PMC 2022. [Google Scholar] [CrossRef]
- Shi, B.; Liu, S.; Huang, A.; Zhou, M.; Sun, B.; Cao, H.; Shan, J.; Sun, B.; Lin, J. Revealing the Mechanism of Friedelin in the Treatment of Ulcerative Colitis Based on Network Pharmacology and Experimental Verification. Evid. Based Complement. Altern. Med. ECAM. 2021, 2021, 4451779. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Devi, K.P. Dihydroactinidiolide regulates Nrf2/HO-1 expression and inhibits caspase-3/Bax pathway to protect SH-SY5Y human neuroblastoma cells from oxidative stress induced neuronal apoptosis. Neurotoxicology 2021, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Arya, J.S.; Maiti, K.K.; Gupta, S.C. Curcuma raktakanda Induces Apoptosis and Suppresses Migration in Cancer Cells: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Tanod, W.A.; Yanuhar, U.; Maftuch Putra, M.Y.; Risjani, Y. Screening of NO Inhibitor Release Activity from Soft Coral Extracts Origin Palu Bay, Central Sulawesi, Indonesia. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 18, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Gairola, K.; Gururani, S.; Kumar, R.; Prakash, O.; Agarwal, S.; Dubey, S. Phytochemical composition antioxidant and anti-inflammatory activities of essential oil of Acmella uliginosa (Sw.) Cass. grown in North India Terai region of Uttarakhand. Trends Phytochem Res. 2021, 5, 44–52. [Google Scholar] [CrossRef]
- Vats, S.; Gupta, T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants 2017, 23, 239–248. [Google Scholar] [CrossRef]
- Antonisamy, P.; Duraipandiyan, V.; Ignacimuthu, S. Anti-inflammatory, analgesic and antipyretic effects of friedelin isolated from Azima tetracantha Lam. in mouse and rat models. J. Pharm. Pharmacol. 2011, 63, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Deci, M.B.; Ferguson, S.W.; Scatigno, S.L.; Nguyen, J. Modulating Macrophage Polarization through CCR2 Inhibition and Multivalent Engagement. Mol. Pharm. 2018, 15, 2721–2731. [Google Scholar] [CrossRef]
- Jimenez, F.; Quinones, M.P.; Martinez, H.G.; Estrada, C.A.; Clark, K.; Garavito, E.; Ibarra, J.; Melby, P.C.; Ahuja, S.S. CCR2 Plays a Critical Role in Dendritic Cell Maturation: Possible Role of, C.C.L.2.; NF-κB. J. Immunol. 2010, 184, 5571–5581. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Gaffal, E.; Kemter, A.M.; Scheu, S.; Leite Dantas, R.; Vogt, J.; Baune, B.; Tüting, T.; Zimmer, A.; Alferink, J. Cannabinoid Receptor 2 Modulates Maturation of Dendritic Cells and Their Capacity to Induce Hapten-Induced Contact Hypersensitivity. Int. J. Mol. Sci. 2020, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Balic, J.J.; Albargy, H.; Luu, K.; Kirby, F.J.; Jayasekara, W.S.N.; Mansell, F.; Garama, D.J.; De Nardo, D.; Baschuk, N.; Louis, C.; et al. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1β expression. Nat. Commun. 2020, 11, 3816. [Google Scholar] [CrossRef]
- Sulczewski, F.B.; Martino, L.A.; Salles, D.; Yamamoto, M.M.; Rosa, D.S.; Boscardin, S.B. STAT3 signaling modulates the immune response induced after antigen targeting to conventional type 1 dendritic cells through the DEC205 receptor. Front. Immunol. 2022, 13, 1006996. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Fu, X.; Shi, H.; Chen, G.; Dong, P.; Zhang, W. Induction of Murine Macrophage M2 Polarization by Cigarette Smoke Extract via the JAK2/STAT3 Pathway. PLoS ONE 2014, 9, e107063. [Google Scholar] [CrossRef] [PubMed]
- Jie, F.; Yang, X.; Yang, B.; Liu, Y.; Wu, L.; Lu, B. Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed. Pharmacother. 2022, 153, 113317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols Suppress Phagocytosis and Inhibit Inflammatory Mediators via ERK Pathway on LPS-Triggered Inflammatory Responses in RAW264.7 Macrophages and the Correlation with Their Structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef]
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019, 57, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, L.; Lin, T. β-sitosterol targets glucocorticoid receptor to reduce airway inflammation and remodeling in allergic asthma. Pulm. Pharmacol. Ther. 2023, 78, 102183. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.; Drebant, J.; Hägele, T.; Burger, L.; Serger, C.; Lauenstein, C.; Dudys, P.; Erdmann, G.; Offringa, R. p38 MAPK signaling in M1 macrophages results in selective elimination of M2 macrophages by MEK inhibition. J. Immunother. Cancer 2021, 9, e002319. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Brown, D.E.; Brewer, J.A.; Vogt, S.K.; Muglia, L.J. Macrophage glucocorticoid receptors regulate Toll-like receptor 4–mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 2007, 109, 4313–4319. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Ni, X.X.; Xu, Q.Y.; Wang, Q.; Li, X.Y.; Hua, J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway. J. Gastroenterol. Hepatol. 2020, 35, 1998–2008. [Google Scholar] [CrossRef]
- Velandia, S.A.; Quintero, E.; Stashenko, E.E.; Ocazionez, R.E. Actividad antiproliferativa de aceites esenciales de plantas cultivadas en Colombia. Acta Biológica Colomb. 2018, 23, 189–198. [Google Scholar] [CrossRef]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomed. Int. J. Phytother. Phytopharm. 2013, 20, 615–621. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F.A. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother. Res. PTR 2009, 23, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Zarif, J.C.; Hernandez, J.R.; Verdone, J.E.; Campbell, S.P.; Drake, C.G.; Pienta, K.J. A phased strategy to differentiate human CD14+monocytes into classically and alternatively activated macrophages and dendritic cells. BioTechniques 2016, 61, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mia, S.; Warnecke, A.; Zhang, X.M.; Malmström, V.; Harris, R.A. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Mesa, X.M.; Contreras Bolaños, L.A.; Modesti Costa, G.; Mejia, A.L.; Santander González, S.P. A Bidens pilosa L. Non-Polar Extract Modulates the Polarization of Human Macrophages and Dendritic Cells into an Anti-Inflammatory Phenotype. Molecules 2023, 28, 7094. https://doi.org/10.3390/molecules28207094
Rodríguez Mesa XM, Contreras Bolaños LA, Modesti Costa G, Mejia AL, Santander González SP. A Bidens pilosa L. Non-Polar Extract Modulates the Polarization of Human Macrophages and Dendritic Cells into an Anti-Inflammatory Phenotype. Molecules. 2023; 28(20):7094. https://doi.org/10.3390/molecules28207094
Chicago/Turabian StyleRodríguez Mesa, Xandy Melissa, Leonardo Andres Contreras Bolaños, Geison Modesti Costa, Antonio Luis Mejia, and Sandra Paola Santander González. 2023. "A Bidens pilosa L. Non-Polar Extract Modulates the Polarization of Human Macrophages and Dendritic Cells into an Anti-Inflammatory Phenotype" Molecules 28, no. 20: 7094. https://doi.org/10.3390/molecules28207094
APA StyleRodríguez Mesa, X. M., Contreras Bolaños, L. A., Modesti Costa, G., Mejia, A. L., & Santander González, S. P. (2023). A Bidens pilosa L. Non-Polar Extract Modulates the Polarization of Human Macrophages and Dendritic Cells into an Anti-Inflammatory Phenotype. Molecules, 28(20), 7094. https://doi.org/10.3390/molecules28207094








