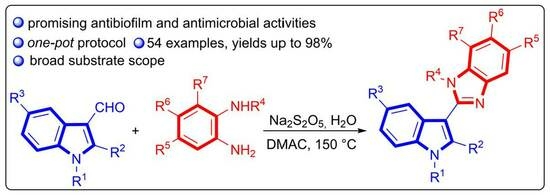
Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives
Abstract
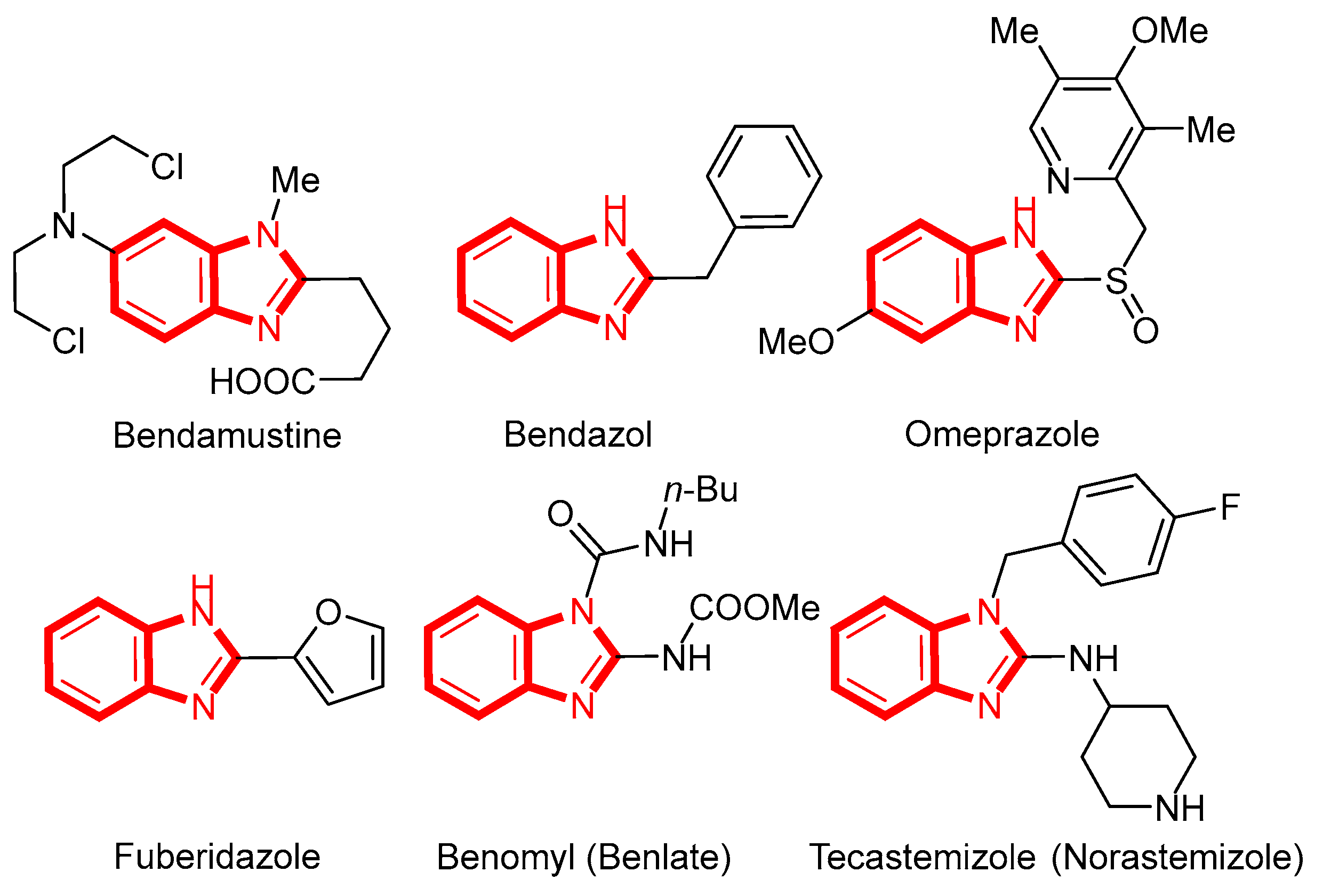
1. Introduction
2. Results and Discussion
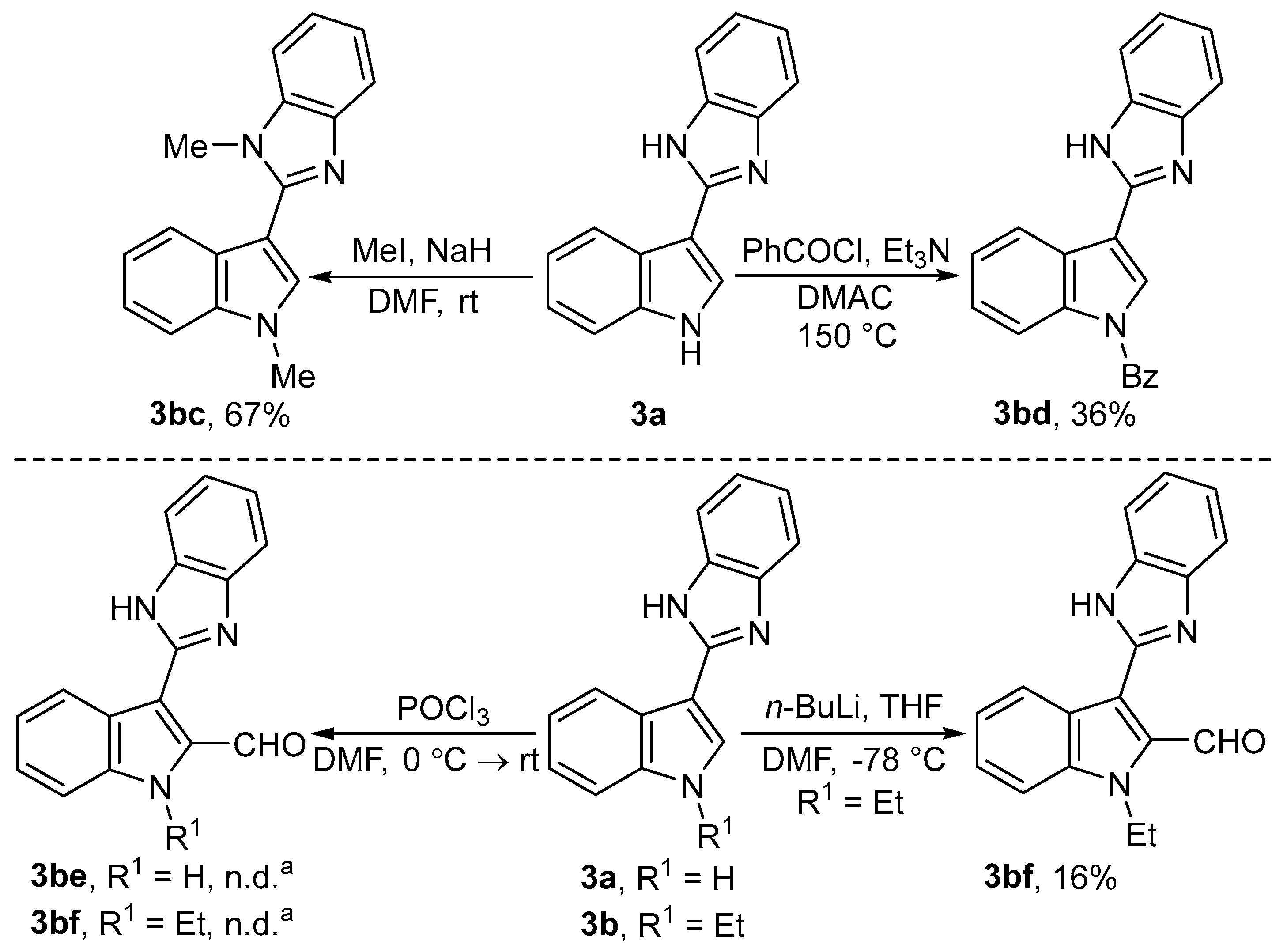
2.1. Chemistry
2.2. In Vitro Biological Evaluation
2.2.1. Antimicrobial Activity
2.2.2. Antibiofilm Activity
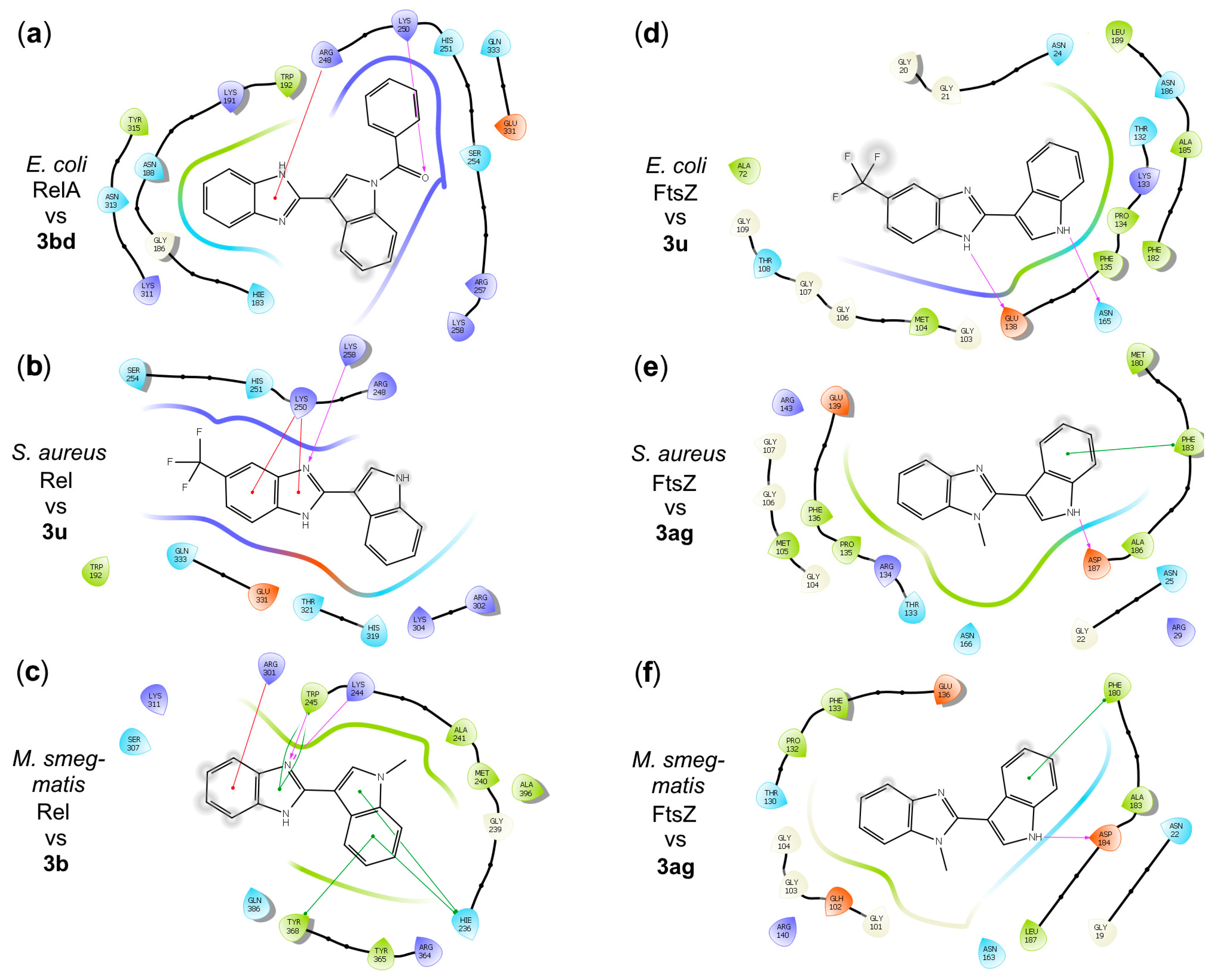
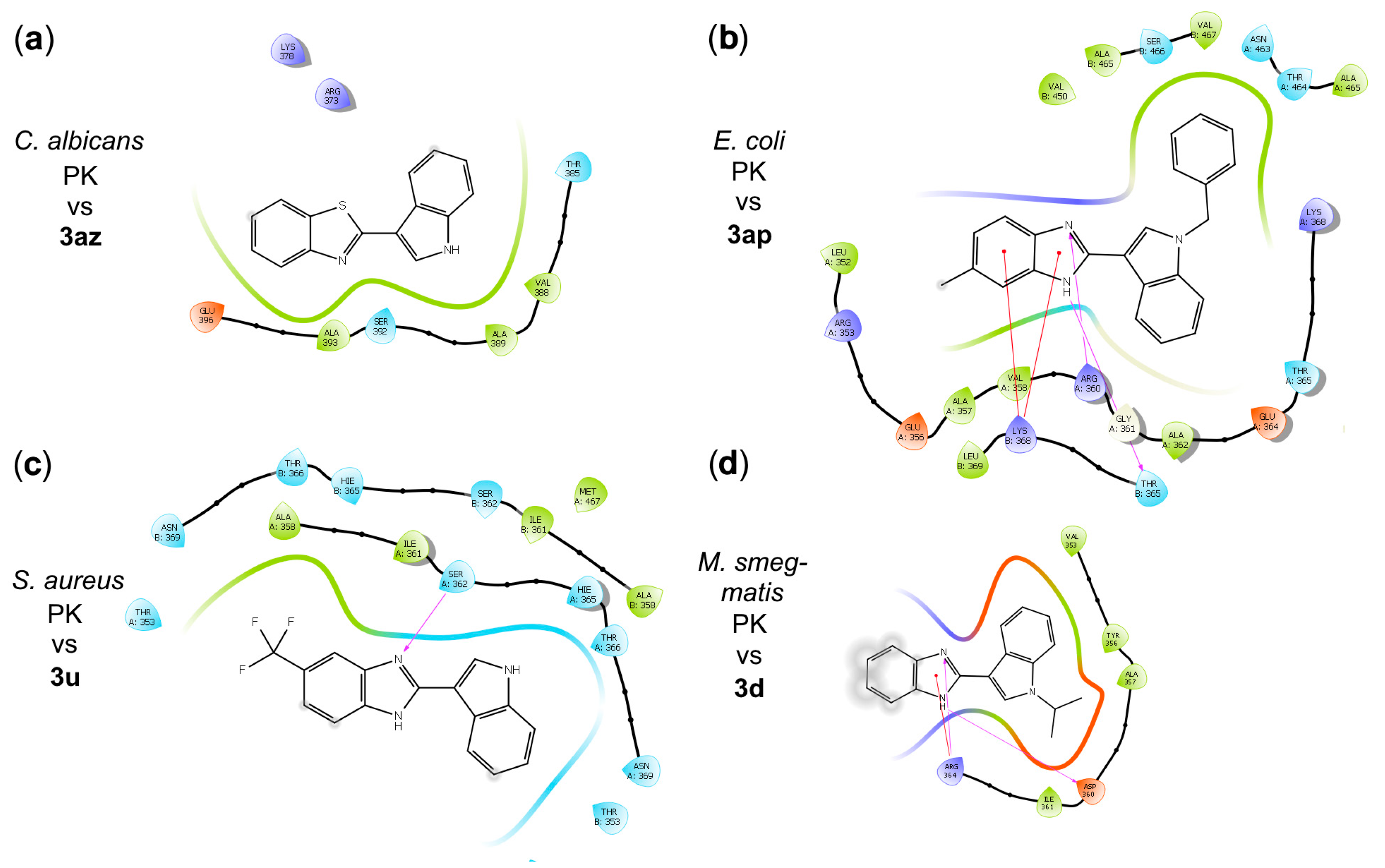
2.3. In Silico Characterization of Benzimidazole Derivatives Binding (p)ppGpp Synthetases/Hydrolases, Pyruvate Kinases, and FtsZ Proteins
Molecular Docking of Benzimidazole Derivatives
3. Materials and Methods
3.1. Instrumentation
3.2. Materials
3.3. Synthesis
3.3.1. Synthesis of the Starting Substrates
3.3.2. General Procedure for the Synthesis of 1H-Benzo[d]imidazoles 3a-bb
3.3.3. Synthesis of 1-Methyl-2-(1-methyl-1H-indol-3-yl)-1H-benzo[d]imidazole (3bc)
3.3.4. Synthesis of [3-(1H-Benzo[d]imidazol-2-yl)-1H-indol-1-yl]phenylmethanone (3bd)
3.3.5. Synthesis of 3-(1H-Benzo[d]imidazol-2-yl)-1-ethyl-1H-indole-2-carbaldehyde (3bf)
3.4. Antimicrobial Activity
3.5. Biofilm Assay
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- De Oliveira Santos, J.V.; da Costa Junior, S.D.; de Fatima Ramos Dos Santos Medeiros, S.M.; Cavalcanti, I.D.L.; de Souza, J.B.; Coriolano, D.L.; da Silva, W.R.C.; Alves, M.; Cavalcanti, I.M.F. Panorama of Bacterial Infections Caused by Epidemic Resistant Strains. Curr. Microbiol. 2022, 79, 175. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- World Health Organization. Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Duggan, S.; Leonhardt, I.; Hunniger, K.; Kurzai, O. Host response to Candida albicans bloodstream infection and sepsis. Virulence 2015, 6, 316–326. [Google Scholar] [CrossRef]
- Bansal, Y.; Kaur, M.; Bansal, G. Antimicrobial Potential of Benzimidazole Derived Molecules. Mini Rev. Med. Chem. 2019, 19, 624–646. [Google Scholar] [CrossRef]
- Keri, R.S.; Rajappa, C.K.; Patil, S.A.; Nagaraja, B.M. Benzimidazole-core as an antimycobacterial agent. Pharmacol. Rep. 2016, 68, 1254–1265. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Cohen, P.; Chen, L.; Robinson, K.S.; Forero-Torres, A.; La Casce, A.S.; Fayad, L.E.; Bessudo, A.; Camacho, E.S.; Williams, M.E.; et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: Results from a phase II multicenter, single-agent study. J. Clin. Oncol. 2008, 26, 204–210. [Google Scholar] [CrossRef]
- Vatolkina, O.E.; Plotkin, A.A.; Libinzon, R.E. Analysis of the relationship between the inhibition of the cyclic AMP phosphodiesterase activity and pharmacological action of drugs. Pharm. Chem. J. 1985, 19, 372–375. [Google Scholar] [CrossRef]
- Kearns, G.L.; Andersson, T.; James, L.P.; Gaedigk, A.; Kraynak, R.A.; Abdel-Rahman, S.M.; Ramabadran, K.; van den Anker, J.N. Omeprazole disposition in children following single-dose administration. J. Clin. Pharmacol. 2003, 43, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Elslahi, R.H.; Osman, A.G.; Sherif, A.M.; Elhussein, A.A. Comparative study of the fungicide Benomyl toxicity on some plant growth promoting bacteria and some fungi in pure cultures. Interdiscip. Toxicol. 2014, 7, 12–16. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of Fungicides. In Veterinary Toxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 569–580. [Google Scholar] [CrossRef]
- Lever, R.; Hefni, A.; Moffatt, J.D.; Paul, W.; Page, C.P. Effect of tecastemizole on pulmonary and cutaneous allergic inflammatory responses. Clin. Exp. Allergy 2007, 37, 909–917. [Google Scholar] [CrossRef]
- Ameta, K.L.; Kavi, R.; Penoni, A.; Maspero, A.; Scapinello, L. N-Heterocycles: Synthesis and Biological Evaluation; Springer Nature: Singapore, 2022. [Google Scholar]
- Kudlickova, Z.; Michalkova, R.; Salayova, A.; Ksiazek, M.; Vilkova, M.; Bekesova, S.; Mojzis, J. Design, Synthesis, and Evaluation of Novel Indole Hybrid Chalcones and Their Antiproliferative and Antioxidant Activity. Molecules 2023, 28, 6583. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Moi, D.; Pedrini, M.; Pérez-Peña, H.; Pieraccini, S.; Dimasi, A.; Stagno, C.; Micale, N.; Schirmeister, T.; Sibille, G.; et al. Synthesis of SARS-CoV-2 Mpro inhibitors bearing a cinnamic ester warhead with in vitro activity against human coronaviruses. Org. Biomol. Chem. 2023, 21, 3811–3824. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, S.; Karpagam, S. Novel series of N-acyl substituted indole based piperazine, thiazole and tetrazoles as potential antibacterial, antifungal, antioxidant and cytotoxic agents, and their docking investigation as potential Mcl-1 inhibitors. J. Mol. Struct. 2023, 1271, 134013. [Google Scholar] [CrossRef]
- Park, B.; Awasthi, D.; Chowdhury, S.R.; Melief, E.H.; Kumar, K.; Knudson, S.E.; Slayden, R.A.; Ojima, I. Design, synthesis and evaluation of novel 2,5,6-trisubstituted benzimidazoles targeting FtsZ as antitubercular agents. Bioorg. Med. Chem. 2014, 22, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Zoraghi, R.; See, R.H.; Axerio-Cilies, P.; Kumar, N.S.; Gong, H.; Moreau, A.; Hsing, M.; Kaur, S.; Swayze, R.D.; Worrall, L.; et al. Identification of pyruvate kinase in methicillin-resistant Staphylococcus aureus as a novel antimicrobial drug target. Antimicrob. Agents Chemother. 2011, 55, 2042–2053. [Google Scholar] [CrossRef]
- Kumar, N.S.; Amandoron, E.A.; Cherkasov, A.; Finlay, B.B.; Gong, H.; Jackson, L.; Kaur, S.; Lian, T.; Moreau, A.; Labriere, C.; et al. Optimization and structure-activity relationships of a series of potent inhibitors of methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as novel antimicrobial agents. Bioorg. Med. Chem. 2012, 20, 7069–7082. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Boraston, A.B. (p)ppGpp and the Stringent Response: An Emerging Threat to Antibiotic Therapy. ACS Infect. Dis. 2019, 5, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Wexselblatt, E.; Oppenheimer-Shaanan, Y.; Kaspy, I.; London, N.; Schueler-Furman, O.; Yavin, E.; Glaser, G.; Katzhendler, J.; Ben-Yehuda, S. Relacin, a novel antibacterial agent targeting the Stringent Response. PLoS Pathog. 2012, 8, e1002925. [Google Scholar] [CrossRef]
- Dutta, N.K.; Klinkenberg, L.G.; Vazquez, M.J.; Segura-Carro, D.; Colmenarejo, G.; Ramon, F.; Rodriguez-Miquel, B.; Mata-Cantero, L.; Porras-De Francisco, E.; Chuang, Y.M.; et al. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci. Adv. 2019, 5, eaav2104. [Google Scholar] [CrossRef]
- Mendogralo, E.Y.; Nesterova, L.Y.; Nasibullina, E.R.; Shcherbakov, R.O.; Tkachenko, A.G.; Sidorov, R.Y.; Sukonnikov, M.A.; Skvortsov, D.A.; Uchuskin, M.G. The Synthesis and Biological Evaluation of 2-(1H-Indol-3-yl)quinazolin-4(3H)-One Derivatives. Molecules 2023, 28, 5348. [Google Scholar] [CrossRef]
- Phan, N.K.; Huynh, T.K.; Nguyen, H.P.; Le, Q.T.; Nguyen, T.C.; Ngo, K.K.; Nguyen, T.H.; Ton, K.A.; Thai, K.M.; Hoang, T.K. Exploration of Remarkably Potential Multitarget-Directed N-Alkylated-2-(substituted phenyl)-1H-benzimidazole Derivatives as Antiproliferative, Antifungal, and Antibacterial Agents. ACS Omega 2023, 8, 28733–28748. [Google Scholar] [CrossRef] [PubMed]
- Bougrin, K.; Soufiaoui, M. Nouvelle voie de synthèse des arylimidazoles sous irradiation micro-ondes en “milieu sec”. Tetrahedron Lett. 1995, 36, 3683–3686. [Google Scholar] [CrossRef]
- Arora, V.; Dutta, M.; Das, K.; Das, B.; Srivastava, H.K.; Kumar, A. Solvent-Free N-Alkylation and Dehydrogenative Coupling Catalyzed by a Highly Active Pincer-Nickel Complex. Organometallics 2020, 39, 2162–2176. [Google Scholar] [CrossRef]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1686. [Google Scholar] [CrossRef]
- Kucukbay, H.; Uckun, M.; Apohan, E.; Yesilada, O. Cytotoxic and antimicrobial potential of benzimidazole derivatives. Arch. Pharm. 2021, 354, e2100076. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.F.; Yan, W.; Cao, L.L.; Ye, Y.H. Synthesis and Biological Evaluation of Benzimidazole Phenylhydrazone Derivatives as Antifungal Agents against Phytopathogenic Fungi. Molecules 2016, 21, 1574. [Google Scholar] [CrossRef]
- Villa, P.; Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Mahalingam, S.M.; Maruoka, K.; Thangamani, S. Benzimidazole tethered pyrrolo[3,4-b]quinoline with broad-spectrum activity against fungal pathogens. Bioorg. Med. Chem. Lett. 2019, 29, 729–733. [Google Scholar] [CrossRef]
- Robinson, M.W.; McFerran, N.; Trudgett, A.; Hoey, L.; Fairweather, I. A possible model of benzimidazole binding to beta-tubulin disclosed by invoking an inter-domain movement. J. Mol. Graph. Model. 2004, 3, 275–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Zhu, Y.; Duan, Y.; Zhou, M. Mechanism of Action of the Benzimidazole Fungicide on Fusarium graminearum: Interfering with Polymerization of Monomeric Tubulin but Not Polymerized Microtubule. Phytopathology 2016, 106, 807–813. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin. Ther. Targets 2018, 22, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Obydennov, K.L.; Kalinina, T.A.; Galieva, N.A.; Beryozkina, T.V.; Zhang, Y.; Fan, Z.; Glukhareva, T.V.; Bakulev, V.A. Synthesis, Fungicidal Activity, and Molecular Docking of 2-Acylamino and 2-Thioacylamino Derivatives of 1H-benzo[d]imidazoles as Anti-Tubulin Agents. J. Agric. Food Chem. 2021, 69, 12048–12062. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Tarcomnicu, I.; Marinescu, M.; Nitulescu, G.M.; Tatia, R.; Moldovan, L.; Popa, M.; Chifiriuc, M.C. New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Hati, S.; Priyadarshini, R.; Sen, S. Transcriptome analysis predicts mode of action of benzimidazole molecules against Staphylococcus aureus UAMS-1. Drug Dev. Res. 2019, 80, 490–503. [Google Scholar] [CrossRef]
- Ates-Alagoz, Z.; Kisla, M.M.; Goker, H.; Yildiz, S. Synthesis, Molecular Docking Studies and Antibacterial Activities of Novel Monocationic Indole-benzimidazole Derivatives. Med. Chem. 2021, 17, 699–706. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Singh, A.; Singh, V.K.; Verma, R.; Singh, N.; Singh, R.K. Synthesis, antibacterial activity, synergistic effect, cytotoxicity, docking and molecular dynamics of benzimidazole analogues. Comput. Biol. Chem. 2018, 76, 1–16. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 6-4. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Dos Santos Fontenelle, R.O.; de Brito, E.H.S.; de Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Kashevarova, N.M.; Sidorov, R.Y.; Nesterova, L.Y.; Akhova, A.V.; Tsyganov, I.V.; Vaganov, V.Y.; Shipilovskikh, S.A.; Rubtsov, A.E.; Malkov, A.V. A synthetic diterpene analogue inhibits mycobacterial persistence and biofilm formation by targeting (p)ppGpp synthetases. Cell Chem. Biol. 2021, 28, 1420–1432. [Google Scholar] [CrossRef]
- Tan, C.M.; Therien, A.G.; Lu, J.; Lee, S.H.; Caron, A.; Gill, C.J.; Lebeau-Jacob, C.; Benton-Perdomo, L.; Monteiro, J.M.; Pereira, P.M.; et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Sci. Transl. Med. 2012, 4, 126ra135. [Google Scholar] [CrossRef]
- Matsui, T.; Han, X.; Yu, J.; Yao, M.; Tanaka, I. Structural change in FtsZ Induced by intermolecular interactions between bound GTP and the T7 loop. J. Biol. Chem. 2014, 289, 3501–3509. [Google Scholar] [CrossRef]
- Ann, J.; Czikora, A.; Saini, A.S.; Zhou, X.; Mitchell, G.A.; Lewin, N.E.; Peach, M.L.; Blumberg, P.M.; Lee, J. alpha-Arylidene Diacylglycerol-Lactones (DAG-Lactones) as Selective Ras Guanine-Releasing Protein 3 (RasGRP3) Ligands. J. Med. Chem. 2018, 61, 6261–6276. [Google Scholar] [CrossRef] [PubMed]
- Bakherad, Z.; Safavi, M.; Sepehri, S.; Fassihi, A.; Sadeghi-Aliabadi, H.; Bakherad, M.; Rastegar, H.; Larijani, B.; Saghaie, L.; Mahdavi, M. Preparation of some novel imidazopyridine derivatives of indole as anticancer agents: One-pot multicomponent synthesis, biological evaluation and docking studies. Res. Chem. Intermed. 2019, 45, 5261–5290. [Google Scholar] [CrossRef]
- Van Baar, J.F.; Horton, A.D.; de Kloe, K.P.; Kragtwijk, E.; Mkoyan, S.G.; Nifant’ev, I.E.; Schut, P.A.; Taidakov, I.V. ansa-Zirconocenes Based on N-Substituted 2-Methylcyclopenta[b]Indoles: Synthesis and Catalyst Evaluation in Liquid Propylene Polymerization. Organometallics 2003, 22, 2711–2722. [Google Scholar] [CrossRef]
- Senaweera, S.; Weaver, J.D. S(N)Ar catalysis enhanced by an aromatic donor-acceptor interaction; facile access to chlorinated polyfluoroarenes. Chem. Commun. 2017, 53, 7545–7548. [Google Scholar] [CrossRef]
- Kumar, G.; Tanwar, O.; Kumar, J.; Akhter, M.; Sharma, S.; Pillai, C.R.; Alam, M.M.; Zama, M.S. Pyrazole-pyrazoline as promising novel antimalarial agents: A mechanistic study. Eur. J. Med. Chem. 2018, 149, 139–147. [Google Scholar] [CrossRef]
- Liu, X.G.; Sun, W. Synthesis, photophysics, and reverse saturable absorption of bipyridyl platinum(II) bis(acetylide) complexes bearing aromatic electron-withdrawing substituents on the acetylide ligands. J. Phys. Chem. A 2014, 118, 10318–10325. [Google Scholar] [CrossRef]
- Break, L. Synthesis of the Novel 3-Benzotriazole-5-yl difluoromethyl-5-trifluoromethyl benzotriazole Nucleosides. Int. J. Chem. 2015, 7, 99–105. [Google Scholar] [CrossRef][Green Version]
- Kohatsu, H.; Kamo, S.; Tomoshige, S.; Kuramochi, K. Total Syntheses of Pyocyanin, Lavanducyanin, and Marinocyanins A and B. Org. Lett. 2019, 21, 7311–7314. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.F.; Kher, S.M.; Cai, S.X.; Dinsmore, C.M.; Glenn, A.G.; Guastella, J.; Huang, J.C.; Ilyin, V.; Lu, Y.; Mouser, P.L.; et al. Synthesis and structure-activity relationships of substituted 1,4-dihydroquinoxaline-2,3-diones: Antagonists of N-methyl-D-aspartate (NMDA) receptor glycine sites and non-NMDA glutamate receptors. J. Med. Chem. 1995, 38, 4367–4379. [Google Scholar] [CrossRef] [PubMed]
- Biradar, J.S.; Sharanbasappa, B. MK-10 Clay–Catalyzed Synthesis of 2-(2′,5′-Disubstituted-1′H-indol-3′-yl)-1H-benzo[d]imidazoles under Conventional and Microwave Irradiation. Synth. Commun. 2011, 41, 885–890. [Google Scholar] [CrossRef]
- Lei, Z.; Xue, F.; Wang, B.; Wang, S.; Zhang, Y.; Xia, Y.; Jin, W.; Liu, C. A stable and recyclable Z-scheme g-C3N4/rGO/BiVO4 heterojunction photocatalyst for site-selective C-3 formylation of indoles with methanol as a formyl source under visible light. Green Chem. 2023, 25, 348–356. [Google Scholar] [CrossRef]
- Meng, Y.; Gui, Y.; Ji, Q.; Pan, Y.; Lin, Z.; Lü, L.; Zeng, X. Benzoimidazole Derivatives Containing Indole Unit: Activated Carbon/Air Promoted Synthesis and Spectral Properties. Chin. J. Org. Chem. 2016, 36, 384–392. [Google Scholar] [CrossRef]
- Sri Ramya, P.V.; Angapelly, S.; Rani, R.S.; Digwal, C.S.; Ganesh Kumar, C.; Nagendra Babu, B.; Guntuku, L.; Kamal, A. Hypervalent iodine(III) catalyzed rapid and efficient access to benzimidazoles, benzothiazoles and quinoxalines: Biological evaluation of some new benzimidazole-imidazo[1,2-a]pyridine conjugates. Arab. J. Chem. 2020, 13, 120–133. [Google Scholar] [CrossRef]
- Dzvinchuk, I.B.; Lozinskii, M.O. Synthesis of 2-(3-Indolyl)-1H-benzimidazoles from 2-Acylmethyl-1H-benzimidazoles. Chem. Heterocycl. Compd. 2005, 41, 177–180. [Google Scholar] [CrossRef]
- El-Nakkady, S.S.; Hanna, M.M.; Roaiah, H.M.; Ghannam, I.A. Synthesis, molecular docking study and antitumor activity of novel 2-phenylindole derivatives. Eur. J. Med. Chem. 2012, 47, 387–398. [Google Scholar] [CrossRef]
- Singh, K.S.; Joy, F.; Devi, P. Ruthenium(II)-catalyzed synthesis of 2-arylbenzimidazole and 2-arylbenzothiazole in water. Transit. Met. Chem. 2020, 46, 181–190. [Google Scholar] [CrossRef]
- Radha, Y.; Manjula, A.; Reddy, B.M.; Rao, B.V. Synthesis and biological activity of novel benzimidazoles. Indian J. Chem. 2011, 50B, 1762–1773. [Google Scholar]
- Nori, Z.Z.; Landarani-Isfahani, A.; Bahadori, M.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. Ultrafine Pt nanoparticles supported on a dendrimer containing thiol groups: An efficient catalyst for the synthesis of benzimidazoles and benzothiazoles from benzyl alcohol derivatives in water. RSC Adv. 2020, 10, 33137–33147. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Mirza, H.M.; Ansari, A.H.; Yasinzai, M.; Zaidi, S.Z.; Dilshad, S.; Ansari, F.L. Benzimidazole derivatives: Synthesis, leishmanicidal effectiveness, and molecular docking studies. Med. Chem. Res. 2012, 22, 3606–3620. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Mukherjee, R.; Karmakar, S.; Harigaya, Y. Tosic Acid-on-Silica Gel: A Cheap and Eco-friendly Catalyst for a Convenient One-pot Synthesis of Substituted Benzimidazoles. Monatsh. Chem. 2007, 138, 1279–1282. [Google Scholar] [CrossRef]
- Kommi, D.N.; Kumar, D.; Bansal, R.; Chebolu, R.; Chakraborti, A.K. “All-water” chemistry of tandem N-alkylation–reduction–condensation for synthesis of N-arylmethyl-2-substituted benzimidazoles. Green Chem. 2012, 14, 3329–3335. [Google Scholar] [CrossRef]
- Secci, D.; Bolasco, A.; D’Ascenzio, M.; della Sala, F.; Yáñez, M.; Carradori, S. Conventional and Microwave-Assisted Synthesis of Benzimidazole Derivatives and TheirIn VitroInhibition of Human Cyclooxygenase. J. Heterocycl. Chem. 2012, 49, 1187–1195. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Li, P.; Wang, L. Highly efficient copper/palladium-catalyzed tandem Ullman reaction/arylation of azoles via C–H activation: Synthesis of benzofuranyl and indolyl azoles from 2-(gem-dibromovinyl)phenols(anilines) with azoles. Tetrahedron 2011, 67, 5913–5919. [Google Scholar] [CrossRef]
- Rastogi, R.; Sharma, S. Synthesis of 2-substituted benzofurans as potential anthelmintics. Indian J. Chem. B Org. Med. Chem. 1982, 21B, 485–487. [Google Scholar]
- Sharma, P.; Gupta, M. 1,3,5-Trimethylpyrazolium chloride based ionogel as an efficient and reusable heterogeneous catalyst for the synthesis of benzimidazoles. J. Chem. Sci. 2016, 128, 61–65. [Google Scholar] [CrossRef]
- Xiang, S.K.; Tan, W.; Zhang, D.X.; Tian, X.L.; Feng, C.; Wang, B.Q.; Zhao, K.Q.; Hu, P.; Yang, H. Synthesis of benzimidazoles by potassium tert-butoxide-promoted intermolecular cyclization reaction of 2-iodoanilines with nitriles. Org. Biomol. Chem. 2013, 11, 7271–7275. [Google Scholar] [CrossRef]
- Graml, A.; Ghosh, I.; Konig, B. Synthesis of Arylated Nucleobases by Visible Light Photoredox Catalysis. J. Org. Chem. 2017, 82, 3552–3560. [Google Scholar] [CrossRef]
- El’haninov, M.M.; Simonov, A.M.; Oleinikova, L.Y. Research on the chemistry of 2-hetarylbenzimidazoles. Chem. Heterocycl. Compd. 1980, 16, 59–61. [Google Scholar] [CrossRef]
- Kumar, S.; Ceruso, M.; Tuccinardi, T.; Supuran, C.T.; Sharma, P.K. Pyrazolylbenzo[d]imidazoles as new potent and selective inhibitors of carbonic anhydrase isoforms hCA IX and XII. Bioorg. Med. Chem. 2016, 24, 2907–2913. [Google Scholar] [CrossRef]
- Kaliyan, P.; Selvaraj, L.; Muthu, S.P. Water extract of onion catalyst: An economical green route for the synthesis of 2-substituted and 1,2-disubstituted benzimidazole derivatives with high selectivity. J. Heterocycl. Chem. 2020, 58, 340–349. [Google Scholar] [CrossRef]
- Stremski, Y.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I. Synthesis of Camalexin and Related Analogues. J. Heterocycl. Chem. 2018, 55, 1589–1595. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Li, Y. Copper-catalyzed synthesis of benzo[b]thiophenes and benzothiazoles using thiocarboxylic acids as a coupling partner. J. Org. Chem. 2013, 78, 8898–8903. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- AlphaFold Protein Structure Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 21 September 2023).
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 21 September 2023).
- Maestro. Schrödinger Release 2018-1; Schrödinger, LLC: New York, NY, USA, 2018. [Google Scholar]



| Compounds | C.a. 10231 b | M. s. 70084 c | E. c. 25922 d | E. c. 8739 e | S. a. 25923 f | MRSA g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 3a | 15.6 | 15.6 | 125 | 250 | - h | - | 500 | 1000 | 125 | 125 | 125 | 125 |
| 3b | 125 | 125 | 62.5 | 125 | >500 | >500 | >500 | >500 | 31.3 | 31.3 | 62.5 | 62.5 |
| 3i | 31.3 | 62.5 | 7.8 | 62.5 | - | - | - | - | 62.5 | 62.5 | 125 | 125 |
| 3j | - | - | 1000 | 1000 | - | - | - | - | 15.6 | 62.5 | 125 | 125 |
| 3n | 31.3 | 31.3 | 125 | 125 | - | - | - | - | 15.6 | 15.6 | 15.6 | 31.3 |
| 3o | 65.5 | 62.5 | 125 | 125 | - | - | - | - | 31.3 | 31.3 | 31.3 | 31.3 |
| 3q | 62.5 | 62.5 | 250 | 250 | 125 | 125 | 125 | 125 | 62.5 | 62.5 | 31.3 | 62.5 |
| 3r | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| 3v | 250 | 250 | 125 | 125 | 250 | 250 | 125 | 250 | 31.3 | 62.5 | 62.5 | 125 |
| 3w | 62.5 | 62.5 | 62.5 | 62.5 | 125 | 125 | 125 | 125 | 62.5 | 62.5 | 31.3 | 62.5 |
| 3y | - | - | 125 | 250 | 1000 | - | 1000 | - | 125 | 125 | 125 | 125 |
| 3z | 62.5 | 62.5 | 125 | 125 | 250 | 250 | 250 | 250 | 62.5 | 62.5 | 62.5 | 125 |
| 3aa | 15.6 | 31.2 | 125 | 125 | - | - | - | - | 3.9 | 15.6 | 7.8 | 15.6 |
| 3ab | 31.3 | 62.5 | 125 | 125 | - | - | - | - | 15.6 | 31.2 | 15.6 | 31.2 |
| 3ac | 62.5 | 62.5 | 250 | 250 | - | - | - | - | 31.3 | 31.3 | 31.3 | 62.5 |
| 3ad | 7.8 | 15.6 | 62.5 | 125 | - | - | - | - | 7.8 | 7.8 | 3.9 | 7.8 |
| 3ae | 31.3 | 62.5 | 31.3 | 62.5 | - | - | - | - | 31.3 | 31.3 | 31.3 | 31.3 |
| 3af | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 125 | 62.5 | 250 |
| 3ag | 3.9 | 7.8 | 3.9 | 125 | - | - | - | - | 15.6 | 125 | 15.6 | 250 |
| 3al | - | - | 1000 | 1000 | - | - | - | - | 62.5 | 62.5 | 62.5 | 125 |
| 3ao | 15.6 | 15.6 | 125 | 125 | - | - | - | - | 0.98 | 7.8 | 1.98 | 7.8 |
| 3aq | 3.9 | 7.8 | 125 | 1000 | - | - | - | - | 0.98 | 3.9 | 1.95 | 3.9 |
| 3at | 250 | 250 | - | - | 250 | - | 250 | - | - | - | - | - |
| 3au | 250 | 250 | - | - | - | - | - | - | - | - | - | - |
| 3az | 15.6 | 15.6 | 62.5 | 250 | - | - | - | - | 15.6 | 250 | 31.2 | 125 |
| Cefotaxime | n.d. i | n.d. | n.d. | n.d. | 0.038 | 0.038 | 0.038 | 0.038 | 0.31 | 0.61 | 19.53 | 39.06 |
| Cefazolin | n.d. | n.d. | n.d. | n.d. | 2.44 | 2.44 | 2.44 | 2.44 | 0.15 | 0.61 | 9.77 | 39.06 |
| Amikacin | n.d. | n.d. | n.d. | n.d. | 19.53 | 19.53 | 19.53 | 19.53 | 4.88 | 9.77 | 9.77 | 9.77 |
| Fluconazole | 1.94 | 7.8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Isoniazid | n.d. | n.d. | 4.58 | 9.16 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rifampicin | n.d. | n.d. | 1.22 | 19.53 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Compounds | C. albicans ATCC 10231 | S. aureus ATCC 25923 | MRSA |
|---|---|---|---|
| 3aa | 125 | 250 | 250 |
| 3ad | 62.5 | 125 | 125 |
| 3ao | 125 | 125 | 125 |
| 3aq | 62.5 | 62.5 | 125 |
| Compounds | E. coli RelA (AF) | S. aureus Rel (AF) | M. smegmatis Rel (AF) | Compounds | E. coli RelA (AF) | S. aureus Rel (AF) | M. smegmatis Rel (AF) |
|---|---|---|---|---|---|---|---|
| 3a | −5.044 | −4.350 | −5.306 | 3y | −3.650 | −3.593 | −3.528 |
| 3b | −4.941 | −4.279 | −5.615 | 3z | −4.573 | −3.907 | −3.992 |
| 3c | −4.937 | −4.304 | −4.402 | 3aa | −4.337 | −4.085 | −4.953 |
| 3d | −5.058 | −4.277 | −4.722 | 3ab | −4.328 | −3.940 | −3.956 |
| 3e | −4.673 | −3.836 | −5.021 | 3ac | −4.395 | −3.854 | −4.141 |
| 3f | −5.532 | −4.298 | −4.259 | 3ad | −4.143 | −4.052 | −4.525 |
| 3g | −4.547 | −4.486 | −4.914 | 3ae | −4.079 | −3.984 | −4.321 |
| 3h | −4.394 | −3.833 | −4.629 | 3af | −5.081 | −4.601 | −5.327 |
| 3i | −5.181 | −4.308 | −4.485 | 3ag | −4.694 | −4.200 | −3.808 |
| 3j | −4.485 | −4.281 | −4.313 | 3ah | −4.698 | −3.483 | −4.638 |
| 3k | −3.864 | −4.241 | −4.405 | 3ai | −3.982 | −3.554 | −4.201 |
| 3l | −4.631 | −3.946 | −5.017 | 3aj | −4.823 | −3.835 | −4.589 |
| 3m | −4.506 | −4.149 | −4.362 | 3ak | −4.348 | −3.797 | −4.209 |
| 3n | −4.422 | −4.116 | −4.893 | 3al | −4.559 | −2.974 | −3.484 |
| 3o | −4.729 | −4.433 | −5.199 | 3am | −4.854 | −4.112 | −5.176 |
| 3p | −4.497 | −4.171 | −4.094 | 3an | −4.154 | −3.660 | −3.548 |
| 3q | −4.904 | −4.175 | −4.724 | 3ao | −3.837 | −3.847 | −4.405 |
| 3r | −4.288 | −4.294 | −4.948 | 3ap | −3.569 | −3.846 | −3.898 |
| 3s | −4.204 | −3.921 | −4.595 | 3aq | −4.566 | −3.816 | −4.095 |
| 3t | −4.778 | −4.328 | −4.591 | 3ar | −3.409 | −2.410 | −2.760 |
| 3u | −4.563 | −4.719 | −5.054 | 3az | −5.005 | −3.927 | −5.166 |
| 3v | −4.696 | −3.701 | −4.011 | 3bd | −6.101 | −4.656 | −4.254 |
| 3w | −4.346 | −3.993 | −4.244 | 3bf | −5.454 | −4.274 | −4.784 |
| 3x | −4.619 | −4.234 | −5.299 | relacin a | −6.757 | −6.880 | −7.457 |
| DMNP a,b (COO—) | −3.898 | −4.612 | −3.309 | indole-5-carboxylic acid a (I2) (COO—) | −5.461 | −5.883 | −5.283 |
| Pyruvate Kinase | FtsZ | ||||||
|---|---|---|---|---|---|---|---|
| Compounds | C. albicans (AF) | E. coli (PDB 1PKY) | S. aureus (PDB 3T0T) | M. smegmatis (AF) | E. coli (AF) | S. aureus FtsZ (AF) | M. smegmatis (AF) |
| 3a | −5.803 | −6.356 | −8.309 | −3.881 | −6.178 | −5.650 | −5.944 |
| 3n | −5.095 | −6.303 | −7.491 | −4.317 | −5.996 | −4.969 | −5.614 |
| 3o | −5.591 | −6.255 | −7.751 | −4.843 | −6.089 | −5.197 | −5.765 |
| 3b | −5.376 | −7.073 | −8.194 | −2.248 | −5.488 | −5.573 | −5.538 |
| 3c | −5.479 | −3.345 | −8.277 | −4.776 | −4.593 | −5.008 | −5.579 |
| 3d | −5.516 | −6.797 | −8.508 | −5.982 | −4.982 | −5.198 | −5.488 |
| 3g | −5.287 | −6.462 | −8.841 | −3.059 | −5.407 | −4.639 | −5.677 |
| 3f | −6.058 | −3.848 | −7.638 | −3.480 | −5.186 | −4.240 | −4.881 |
| 3q | −5.452 | −5.591 | −8.257 | −2.944 | −5.971 | −4.877 | −5.694 |
| 3r | −5.177 | −5.529 | −7.835 | −3.084 | −5.703 | −4.939 | −5.492 |
| 3s | −5.057 | −5.598 | −7.974 | −3.998 | −5.685 | −4.922 | −5.448 |
| 3y | −4.361 | −5.915 | −6.917 | −3.147 | −5.361 | −4.471 | −4.852 |
| 3u | −4.524 | −7.168 | −8.628 | −4.140 | −6.546 | −5.452 | −5.694 |
| 3v | −4.624 | −5.391 | −7.663 | −2.867 | −5.561 | −4.858 | −5.098 |
| 3ae | −5.288 | −5.856 | −7.707 | −3.257 | −5.602 | −5.155 | −5.691 |
| 3t | −5.443 | −6.320 | −8.256 | −3.528 | −6.209 | −5.556 | −5.704 |
| 3w | −5.358 | −5.929 | −8.052 | −3.051 | −5.665 | −4.918 | −5.534 |
| 3h | −6.084 | −4.775 | −7.679 | −2.959 | −5.078 | −4.497 | −5.073 |
| 3i | −4.606 | −3.271 | −7.804 | −3.009 | −4.595 | −4.703 | −5.015 |
| 3al | −3.258 | −2.866 | −5.359 | n.d. a | n.d. | −2.465 | −4.450 |
| 3ag | −5.493 | −7.666 | −8.086 | −2.382 | −5.552 | −5.756 | −6.139 |
| 3x | −6.043 | −6.111 | −8.326 | −3.543 | −6.297 | −5.178 | −6.116 |
| 3ab | −4.610 | −6.001 | −7.703 | −3.223 | −6.129 | −5.334 | −5.640 |
| 3ac | −5.317 | −5.406 | −7.721 | −4.100 | −6.052 | −5.182 | −5.551 |
| 3aa | −5.302 | −5.352 | −7.314 | −4.012 | −6.220 | −5.405 | −5.772 |
| 3ao | −4.632 | −5.325 | −7.520 | −2.945 | −6.060 | −4.931 | −5.243 |
| 3z | −5.180 | −5.759 | −7.497 | −2.915 | −5.708 | −5.119 | −5.256 |
| 3ap | −5.011 | −8.114 | −8.184 | −2.191 | −5.035 | −4.565 | −4.203 |
| 3af | −5.182 | −5.211 | −8.506 | −4.393 | −6.308 | −5.146 | −6.087 |
| 3j | −4.627 | −5.168 | −7.640 | −2.895 | −5.004 | −4.315 | −4.970 |
| 3aq | −5.470 | −1.811 | −7.425 | −3.099 | −5.686 | −4.736 | −5.279 |
| 3ad | −5.126 | −5.177 | −7.927 | −1.929 | −5.092 | −4.522 | −5.348 |
| 3p | −5.063 | −6.641 | −7.668 | −2.930 | −4.833 | −5.478 | −4.828 |
| 3az | −6.224 | −6.985 | −7.912 | −3.907 | −5.315 | −5.263 | −5.647 |
| Reference ligands | |||||||
| GTP b | - | - | - | - | −7.548 | −7.705 | −7.385 |
| IS-130 c | −3.179 | −2.543 | −6.933 | −3.488 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendogralo, E.Y.; Nesterova, L.Y.; Nasibullina, E.R.; Shcherbakov, R.O.; Myasnikov, D.A.; Tkachenko, A.G.; Sidorov, R.Y.; Uchuskin, M.G. Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives. Molecules 2023, 28, 7095. https://doi.org/10.3390/molecules28207095
Mendogralo EY, Nesterova LY, Nasibullina ER, Shcherbakov RO, Myasnikov DA, Tkachenko AG, Sidorov RY, Uchuskin MG. Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives. Molecules. 2023; 28(20):7095. https://doi.org/10.3390/molecules28207095
Chicago/Turabian StyleMendogralo, Elena Y., Larisa Y. Nesterova, Ekaterina R. Nasibullina, Roman O. Shcherbakov, Danil A. Myasnikov, Alexander G. Tkachenko, Roman Y. Sidorov, and Maxim G. Uchuskin. 2023. "Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives" Molecules 28, no. 20: 7095. https://doi.org/10.3390/molecules28207095
APA StyleMendogralo, E. Y., Nesterova, L. Y., Nasibullina, E. R., Shcherbakov, R. O., Myasnikov, D. A., Tkachenko, A. G., Sidorov, R. Y., & Uchuskin, M. G. (2023). Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives. Molecules, 28(20), 7095. https://doi.org/10.3390/molecules28207095