Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Safety Evaluation of Hydroalcoholic Extracts of Tagetes erecta
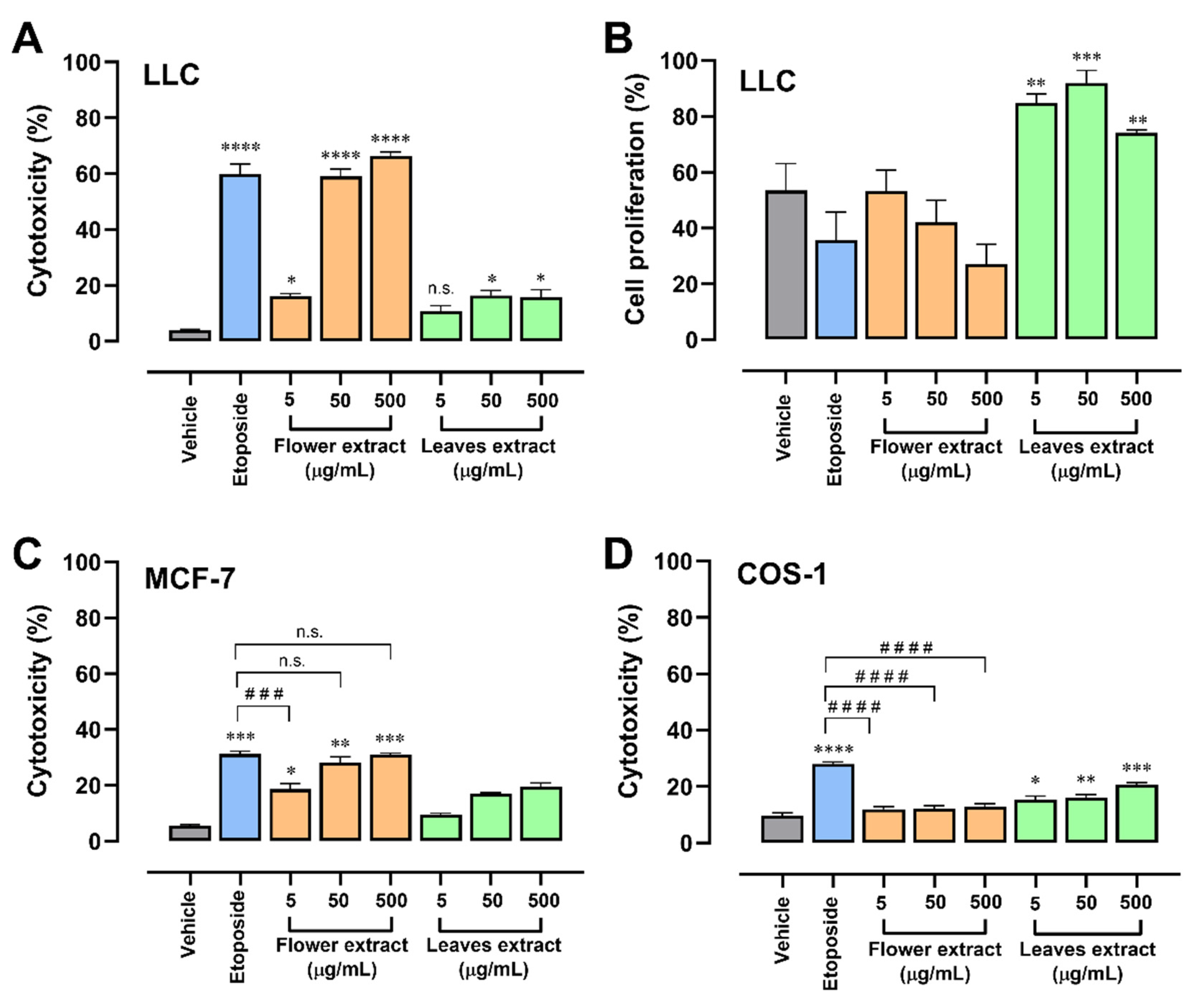
2.2. T. erecta Extract Induces Cytotoxicity in Tumor Cell Lines
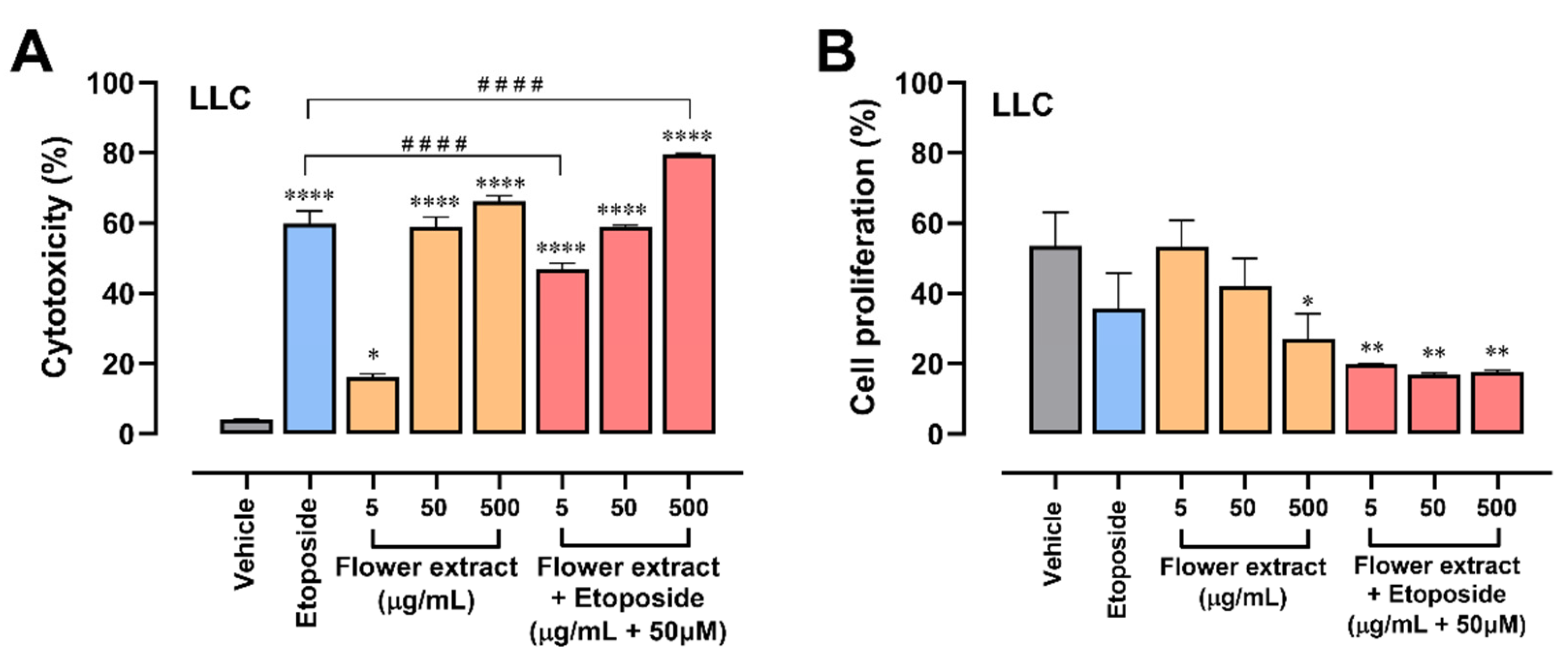
2.3. The Combination of T. erecta Extract and Etoposide Decreases Cell Proliferation
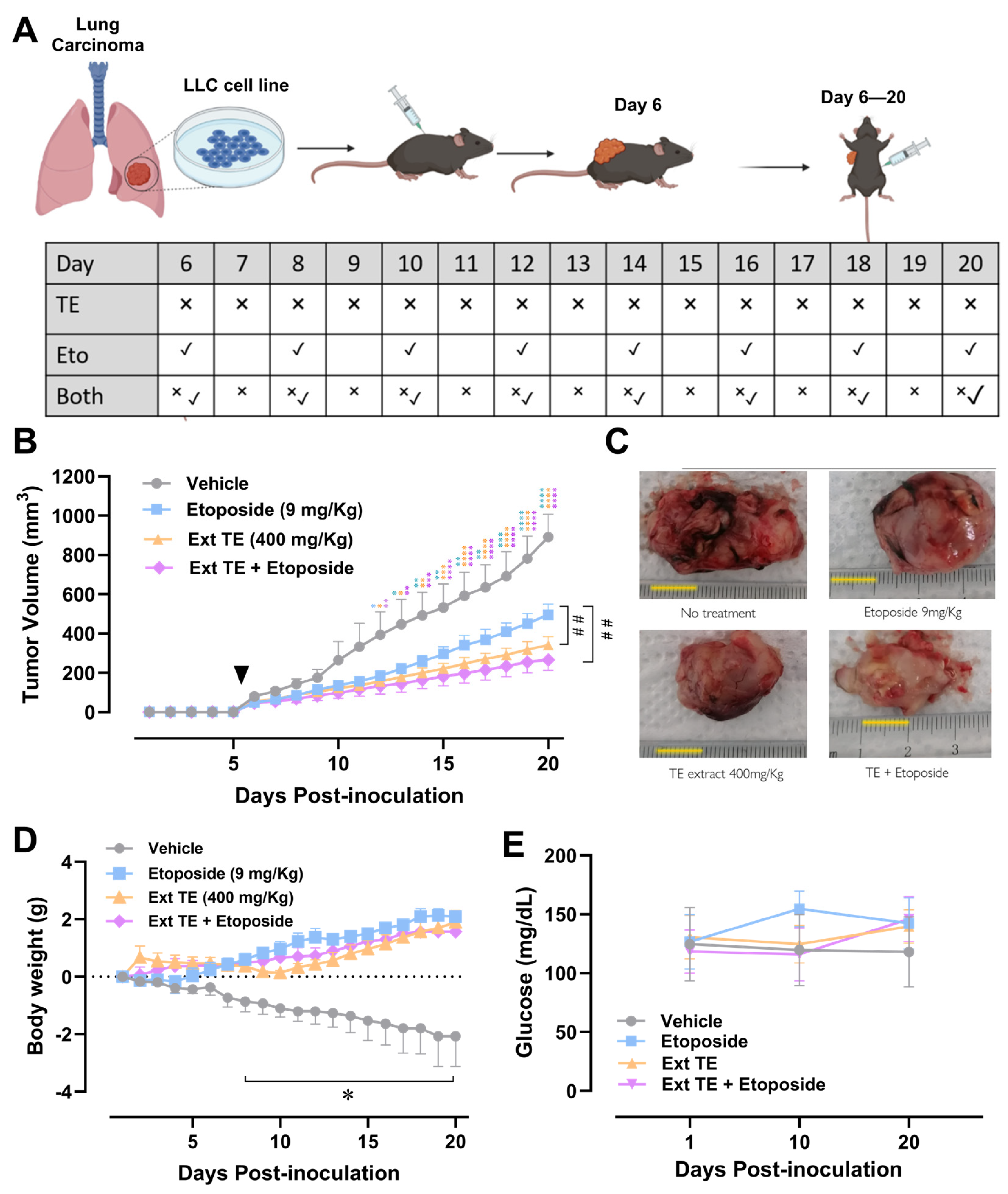
2.4. Tagetes erecta Extract Inhibits Tumor Growth
2.5. Tagetes erecta Extract Improves Body Weight Gain without Altering Glycemia in Tumor-Bearing Animal
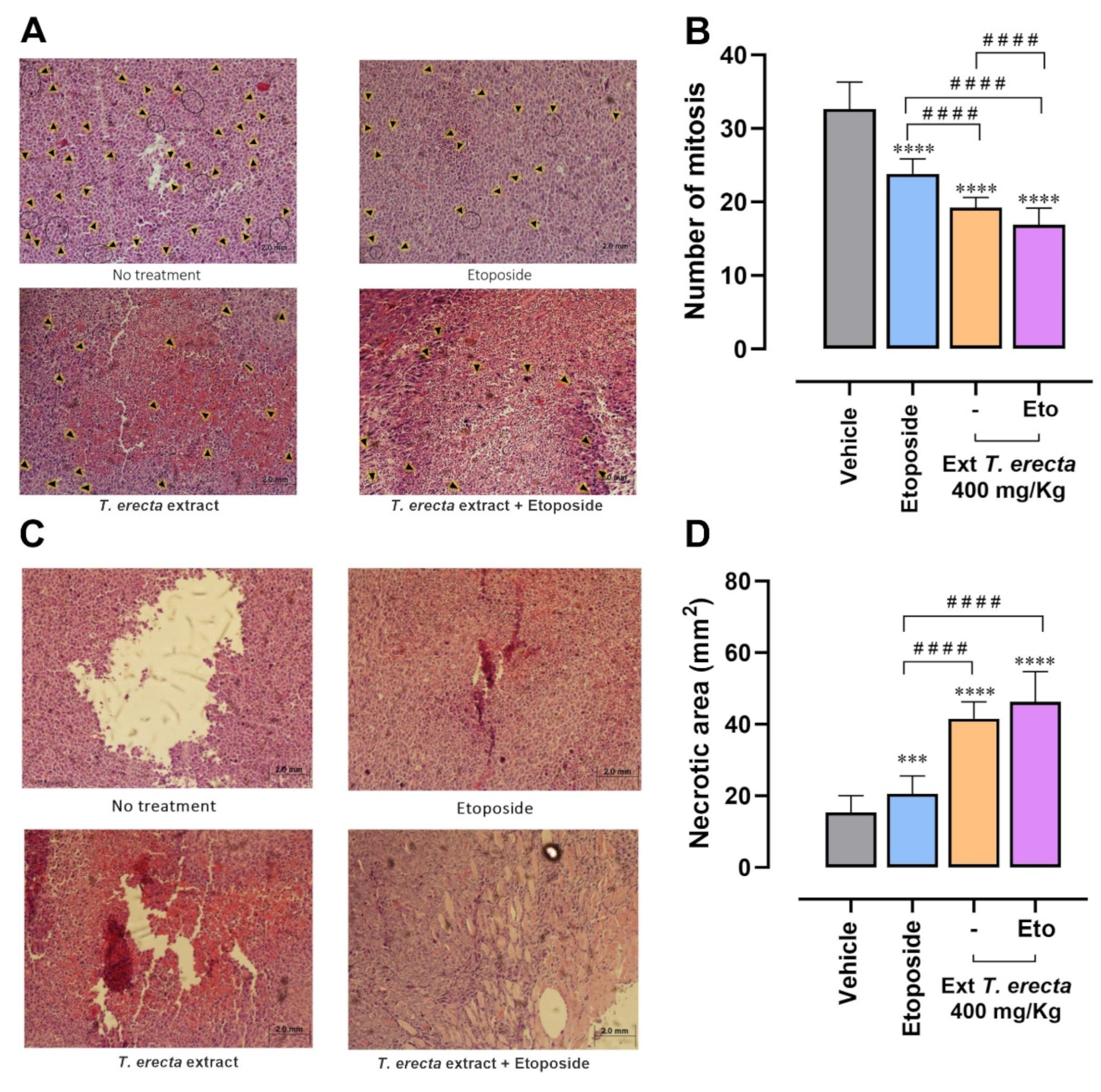
2.6. T. erecta Extract Administration Decreases Proliferation and Tumor Viability
2.7. GS–MS Study Showed the Presence of Various Bioactive Components in the Flowers of Tagetes erecta
3. Discussion
4. Methods
4.1. Plant Extract
4.2. Chromatography-Mass Spectrometry (GC/MS)
4.3. Toxicity Test in Artemia Salina
4.4. Cell Cultures
4.5. Cell Viability Assay
4.6. Proliferation Assay
4.7. In Vivo Studies
4.7.1. Animals
4.7.2. Tumor Murine Model
4.7.3. Monitoring Changes in Body Weight and Systemic Glucose
4.8. Ex Vivo Model
4.9. Histopathological Evaluation of Mitotic Cells and Necrotic Areas
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Pacheco, R.A.; González-León, M.; Arroyave-Loaiza, M.G.; Borja-Aburto, V.H. Incidencia, mortalidad y costos de la atención por cáncer de pulmón en el Instituto Mexicano del Seguro Social. Salud Pública Méx. 2019, 61, 257–264. [Google Scholar] [CrossRef] [PubMed]
- De Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.H.; Zuwaiel, A.A.; Moni, S.S.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M.E. Bioactive Principles and Potentiality of Hot Methanolic Extract of the Leaves from Artemisia absinthium L. “in vitro Cytotoxicity Against Human MCF-7 Breast Cancer Cells, Antibacterial Study and Wound Healing Activity”. Curr. Pharm. Biotechnol. 2020, 21, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Barhoi, D.; Upadhaya, P.; Barbhuiya, S.N.; Giri, A.; Giri, S. Extracts of Tagetes erecta exhibit potential cytotoxic and antitumor activity that could be employed as a promising therapeutic agent against cancer: A study involving in vitro and in vivo approach. Phytomed. Plus 2022, 2, 100187. [Google Scholar] [CrossRef]
- Quintanilla Carvajal, M.X.; Arenas Ocampo, M.L.A.; Campos Mendiola, R.; Camacho Díaz, B.H.; Jiménez Aparicio, A.R. Morphometric characterization of floral structures Tagetes erecta L. and Tagetes patula L. (Asteraceae) using digital image analysis and fractal dimension. Gayana Bot. 2015, 72, 137–144. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Valarmathy, N.; Kannan, S.; Jansirani, D.; Alsenaidy, A. Evaluation of cytotoxic properties of Curcuma longa and Tagetes erecta on cancer cell line (Hep2). Afr. J. Pharm. Pharmacol. 2013, 7, 736–739. [Google Scholar] [CrossRef]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.J.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, A.; Agarwal, K.; Tomar, P.; Satija, S. Antioxidant and cytotoxic potential of a new thienyl derivative from Tagetes erecta roots. Pharm. Biol. 2012, 50, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Vallisuta, O.; Nukoolkarn, V.; Mitrevej, A.; Sarisuta, N.; Leelapornpisid, P.; Phrutivorapongkul, A.; Sinchaipanid, N. In vitro studies on the cytotoxicity, and elastase and tyrosinase inhibitory activities of marigold (Tagetes erecta L.) flower extracts. Exp. Ther. Med. 2014, 7, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Salehyar, S.M.; Mirza, M.; Dabiri, M. The essential oil of Tagetes erecta L. occurring in Iran. Flavour Fragr. J. 2004, 19, 579–581. [Google Scholar] [CrossRef]
- Reyhanoglu, G.; Tadi, P. Etoposide; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potential of Coumarin and its Derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Lan, L.; Ma, J.; Zeng, Y.; Xu, L.; Wu, D. Double-layered hyaluronic acid/stearic acid-modified polyethyleneimine nanoparticles encapsulating (-)-gossypol: A nanocarrier for chiral anticancer drugs. J. Mater. Chem. B 2014, 2, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Fortuna, C.G.; Salerno, L.; Modica, M.N.; Siracusa, M.A.; Cardile, V.; Romeo, G.; Bulbarelli, A.; Lonati, E.; Pittala, V. Antitumor properties of substituted (αE)-α-(1H-indol-3-ylmethylene)benzeneacetic acids or amides. Bioorg. Med. Chem. 2013, 21, 5233–5245. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Takeuchi, T.; Sugawara, F.; Yoshida, H. Anti-cancer targeting telomerase inhibitors: β-rubromycin and oleic acid. Mini Rev. Med. Chem. 2012, 12, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Shi, Y.; An, S.; Wan, Y.; Yang, F.; Liu, Q. How to best use acetic acid for the prevention of heart disease and cancer. Eur. J. Prev. Cardiol. 2019, 26, 437–438. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Hasan, I.; Chakrabortty, A.; Zaman, S.; Islam, S.S.; Ahmed, F.R.S.; Kabir, K.M.A.; Nurujjaman, M.; Uddin, M.B.; Alam, M.T.; et al. Moringa oleifera seed lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of Bak and NF-kappaB gene expression. Int. J. Biol. Macromol. 2018, 107 Pt B, 1936–1944. [Google Scholar] [CrossRef]
- Pouillart, P.R. Role of butyric acid and its derivatives in the treatment of colorectal cancer and hemoglobinopathies. Life Sci. 1998, 63, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wei, F.; Wei, M.; Xie, L.; Lin, Z.; Feng, X. Modulatory effect of Tagetes erecta flowers essential oils via Nrf2/HO-1/NF-κB/p65 axis mediated suppression of N-methyl-N’nitro-N-nitroguanidine (MNNG) induced gastric cancer in rats. Mol. Cell. Biochem. 2021, 476, 1541–1554. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Color-Aparicio, V.M.; Reyes-Cruz, G.; Vazquez-Prado, J. Protumoral bone marrow-derived cells migrate via Gbetagamma-dependent signaling pathways and exhibit a complex repertoire of RhoGEFs. J. Cell Commun. Signal. 2019, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]

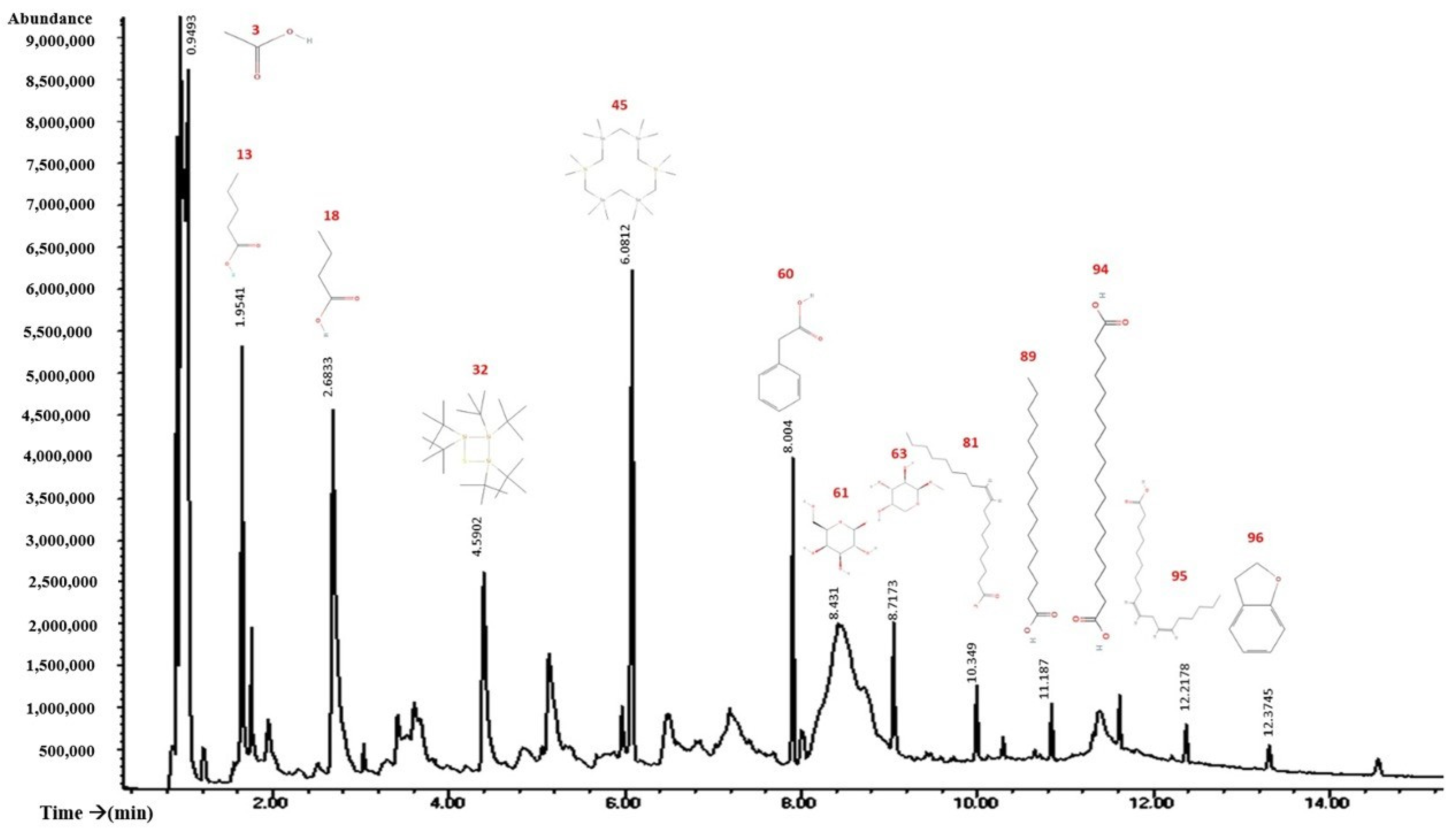
| Beak | Holding Time | % Area | Compound | Effect Reported in Cancer | Refs |
|---|---|---|---|---|---|
| 96 | 12.3745 | 21.13 | 2,3-Dihydro-benzofuran (coumaran) | Anticancer properties | [15] |
| 94 | 11.8127 | 15.32 | Octadecanoic acid | Nanocarrier for anticancer drugs | [16] |
| 60 | 8.004 | 12.1 | Benzeneacetic acid | Antiproliferative effect | [17] |
| 81 | 10.3487 | 11.23 | Oleic acid | Telomerase inhibitor | [18] |
| 95 | 12.2178 | 11.2 | Linoleic acid | Anticarcinogenic | [19] |
| 3 | 0.9493 | 9.49 | Acetic acid | Preventive function against cancer | [20] |
| 63 | 8.7173 | 6.96 | Methyl-β-d-galactopyranoside | Inhibits the growth of Ehrlich ascites carcinoma | [21] |
| 18 | 2.6833 | 6.61 | n-butyric acid | Colorectal cancer treatment | [22] |
| 61 | 8.431 | 6.34 | Methyl β-l-arabinopyranoside | Unknown | |
| 89 | 11.186 | 5.79 | n-hexadecanoic acid | Inhibits proliferation and metastasis in prostate cancer | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, A.S.C.; Valencia, M.G.; Cervantes-Villagrana, R.D.; Zapata, A.B.; Cervantes-Villagrana, A.R. Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells. Molecules 2023, 28, 7055. https://doi.org/10.3390/molecules28207055
González ASC, Valencia MG, Cervantes-Villagrana RD, Zapata AB, Cervantes-Villagrana AR. Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells. Molecules. 2023; 28(20):7055. https://doi.org/10.3390/molecules28207055
Chicago/Turabian StyleGonzález, Alma Sarahi Cuellar, Marisol Galván Valencia, Rodolfo Daniel Cervantes-Villagrana, Alondra Bocanegra Zapata, and Alberto Rafael Cervantes-Villagrana. 2023. "Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells" Molecules 28, no. 20: 7055. https://doi.org/10.3390/molecules28207055
APA StyleGonzález, A. S. C., Valencia, M. G., Cervantes-Villagrana, R. D., Zapata, A. B., & Cervantes-Villagrana, A. R. (2023). Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells. Molecules, 28(20), 7055. https://doi.org/10.3390/molecules28207055