1. Introduction
Non-small cell lung cancer (NSCLC) is the most prevalent form of lung cancer, accounting for approximately 85% of all cases. It is a malignant disease primarily affecting the cells that line the lung airways. It involves the uncontrolled growth and spread of abnormal cells, resulting in tumor formation [
1]. The management of NSCLC depends on several factors, such as cancer stage, overall health of the patient, and specific tumor characteristics. In cases of advanced metastatic or localized NSCLC, the initial treatment option is chemotherapy using first-generation tyrosine kinase inhibitors (TKIs), like gefitinib and erlotinib [
2,
3,
4]. However, a considerable number of NSCLC cases exhibit a chromosomal rearrangement that fuses the EML4 (echinoderm microtubule-associated protein-like 4) gene with the ALK (anaplastic lymphoma kinase) gene. The resulting fusion gene leads to the reactivation of the kinase enzyme, contributing to increased cancer development and driving the malignant features of the cancer cells. Unfortunately, the first-generation TKIs are not effective in inhibiting the kinase activity of the EML4-ALK fusion protein [
4,
5]. Based on this finding, pharmaceutical companies have undertaken research programs to discover and develop effective ALK inhibitors.
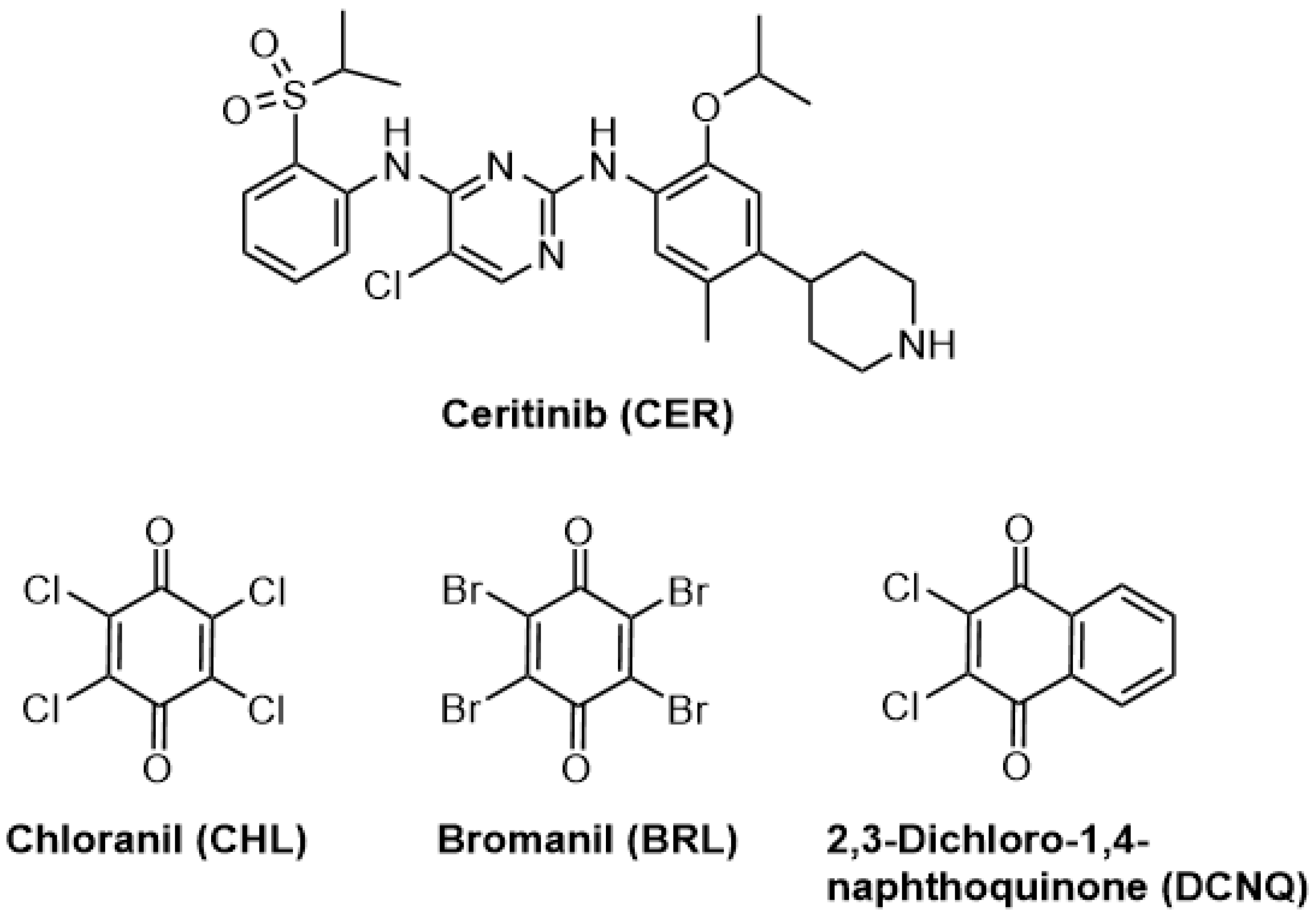
During the process of discovering new drugs, Novartis Pharmaceutical Corporation (located in Basel, Switzerland) identified ceritinib (CER) as a potent inhibitor of ALK. The chemical structure of CER is displayed in
Figure 1 [
6]. CER was specifically designed to overcome resistance to the earlier ALK inhibitor, crizotinib. It has received approval from the U.S. Food and Drug Administration (FDA) as a breakthrough treatment option for patients with ALK-positive NSCLC. CER is marketed under the trade name Zykadia
® capsules [
7]. Since its approval, CER has demonstrated remarkable effectiveness and safety, providing renewed hope for patients with this particular subtype of lung cancer. CER has the ability to inhibit multiple receptor tyrosine kinases, including ALK, ROS1, and insulin-like growth factor 1 receptor (IGF-1R). By specifically targeting and blocking the activity of these kinases, CER effectively suppresses the growth and survival of cancer cells that carry ALK gene rearrangements. This targeted approach makes CER an extraordinarily potent and highly specific therapy for ALK-positive NSCLC [
8].
While CER has shown significant clinical benefits, it is also associated with certain adverse events, including gastrointestinal disturbances, hepatotoxicity, fatigue, and edema. These side effects can be managed by adjusting the dosage; however, CER has also been linked to QT interval prolongation, which requires careful monitoring and critical dose management [
8]. Therefore, ensuring the precise control of CER content in its dosage form (Zykadia
® capsules) is crucial to achieve a therapy that is both effective and safe with CER. To accomplish this objective, the development of an efficient and reliable analytical tool is necessary for accurately quantifying the amount of CER present in the capsules.
There are limited analytical methods available for characterizing and quantifying CER in bulk drug or capsules. These methods include liquid chromatography coupled with an ultraviolet detector (HPLC-UV) [
9,
10] or a tandem mass spectrometric detector (LC-MS/MS) [
11], and voltammetry [
12]. HPLC-UV methods were primarily developed for stability testing of CER, whereas LC-MS/MS methods were designed for quantifying genotoxic impurities in CER bulk form [
11]. Although liquid chromatographic methods offer versatility in specific scenarios, they come with several disadvantages and limitations when applied in pharmaceutical quality control laboratories for characterizing CER formulation. The drawbacks of liquid chromatographic methods used in pharmaceutical quality control laboratories for drug formulation characterization include the following: (1) Complexity: The technique requires a high level of expertise to operate the instrument, optimize the method, and accurately interpret the results. (2) High cost: There are significant expenses involved in setting up and maintaining the system, as well as acquiring consumables. (3) Challenges in high-throughput situations: Liquid chromatography methods can be time-consuming, posing difficulties in rapidly processing numerous samples, such as in dosage form uniformity testing. (4) Solvent disposal challenges: The use of organic solvents in liquid chromatography methods presents issues related to hazardous waste disposal, which can be environmentally unfriendly. To overcome these disadvantages, it is crucial to develop alternative methodologies that can provide better solutions.
Microwell spectrophotometric methods (MW-SPMs), assisted with absorbance microplate readers, have gained significant importance in pharmaceutical analysis due to their exceptional advantages [
13,
14,
15]. One key benefit underlying the MW-SPMs is their ability to utilize smaller sample volumes compared to conventional spectrophotometric techniques, which rely on volumetric flasks and cuvettes. This approach reduces waste generation during the analytical process and lowers the cost of reagents. Additionally, it minimizes the use of hazardous solvents and chemicals, promoting an eco-friendly and sustainable approach. The versatility of the MW-SPMs allows for a wide range of applications in various pharmaceutical industry activities. These methods can be easily automated using robotic systems, enhancing their efficiency, reducing errors, and saving time and labor in the laboratory. By facilitating the rapid analysis of multiple samples, these methods contribute to achieving uniformity in pharmaceutical formulations and other pharmaceutical industry activities. These advantages have led to the increasing popularity of employing MW-SPMs not only in the pharmaceutical industry but also in other fields.
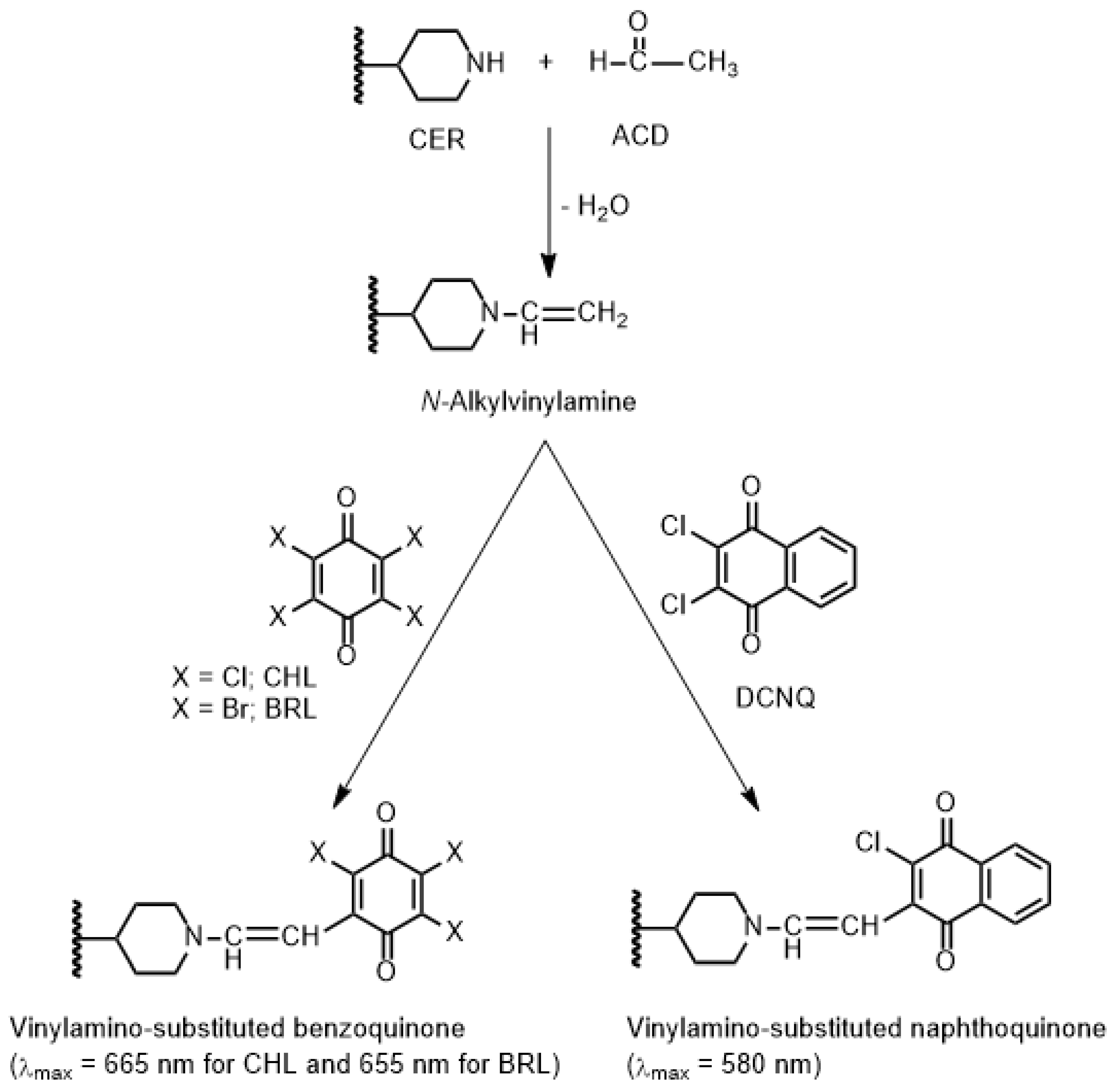
This study has presented the first-ever development of three distinct MW-SPMs for the quality control of CER in Zykadia® capsules. In these methods, colored N-vinylamino-substituted haloquinone derivatives of CER were formed within the microwells through reactions of CER with haloquinone reagents in the presence of acetaldehyde (ACD). These haloquinone reagents were chloranil (CHL), bromanil (BRL), and 2,3-dichloro-1,4-naphthoquinone reagents.
3. Experimental Procedure
3.1. Apparatus
The double-beam ultraviolet–visible spectrophotometer (model V-530) was a product of JASCO Co. Ltd. (Kyoto, Japan). It was used for scanning the absorption spectra of CER and its reaction mixtures. The absorbance microplate reader (model ELx808) used for these experiments was a product of Bio-Tek Instruments Inc. (Winooski, VT, USA). The instrument was controlled via KC junior software (version 2.0) provided with the reader. The reader was featured with a built-in temperature control system, which enables the heating and temperature maintenance of solutions in microwell plates at the desired temperature up to 50 °C. A top-reading mode was employed for absorbance readings using the reader. Additionally, the reader can perform a 1 min shaking action to thoroughly mix the reagents with the analyte solutions. After shaking, a brief settling time of 300 milliseconds was allowed before measuring the absorbances.
3.2. Chemicals and Materials
The standard CER material was purchased from LC Laboratories (Woburn, MA, USA); its purity was ≥99%. Zykadia® capsules (Novartis Pharmaceutical Corporation (Basel, Switzerland) were kindly donated by Saudi FDA (Riyadh, Saudi Arabia), labeled to contain 150 mg CER per capsule. ACD, CHL, BRL, and DCNQ were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). The concentration of the ACD solution was 2% (v/v, in methanol). The concentrations of the CHL, BRL, and DCNQ solutions were 5% (w/v, in methanol). All solutions were prepared fresh in a working day. Transparent polystyrene Corning® 96-well plates were obtained from Merck & Co., Inc. (Rahway, NJ, USA). Adjustable single and 8-channel pipettes, branded Finnpipette®, were obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). To dispense solutions using the 8-channel pipettes, reagent reservoirs with cover lids were purchased from Merck KGaA (Darmstadt, Germany), and were of the BRAND® PP variety. All solvents used were of spectroscopic grade and were supplied by Merck (Darmstadt, Germany). All chemicals used in this study were of analytical grade.
3.3. Preparation of Standard and Capsule Solutions
For preparation of a standard CER solution, a weighed quantity (20 mg) of CER powder was dissolved in 10 mL of methanol. This stock solution (2 mg/mL) was diluted with methanol to yield working solutions in concentration ranges of 5–150 µg/mL.
For the preparation of the capsule solution, the contents of 10 Zykadia® capsules were collected, and an accurate weight of the contents equivalent to 50 mg of CER was transferred to a 25 mL calibrated flask. Following this step, 20 mL of methanol was added, and the contents were shaken well to ensure the complete dissolution of CER. The flask’s content was filtered via passing through 0.4 μm membrane filter. The filtrate was diluted with methanol for analysis via the MW-SPMs.
3.4. Optimization of Experimental Conditions
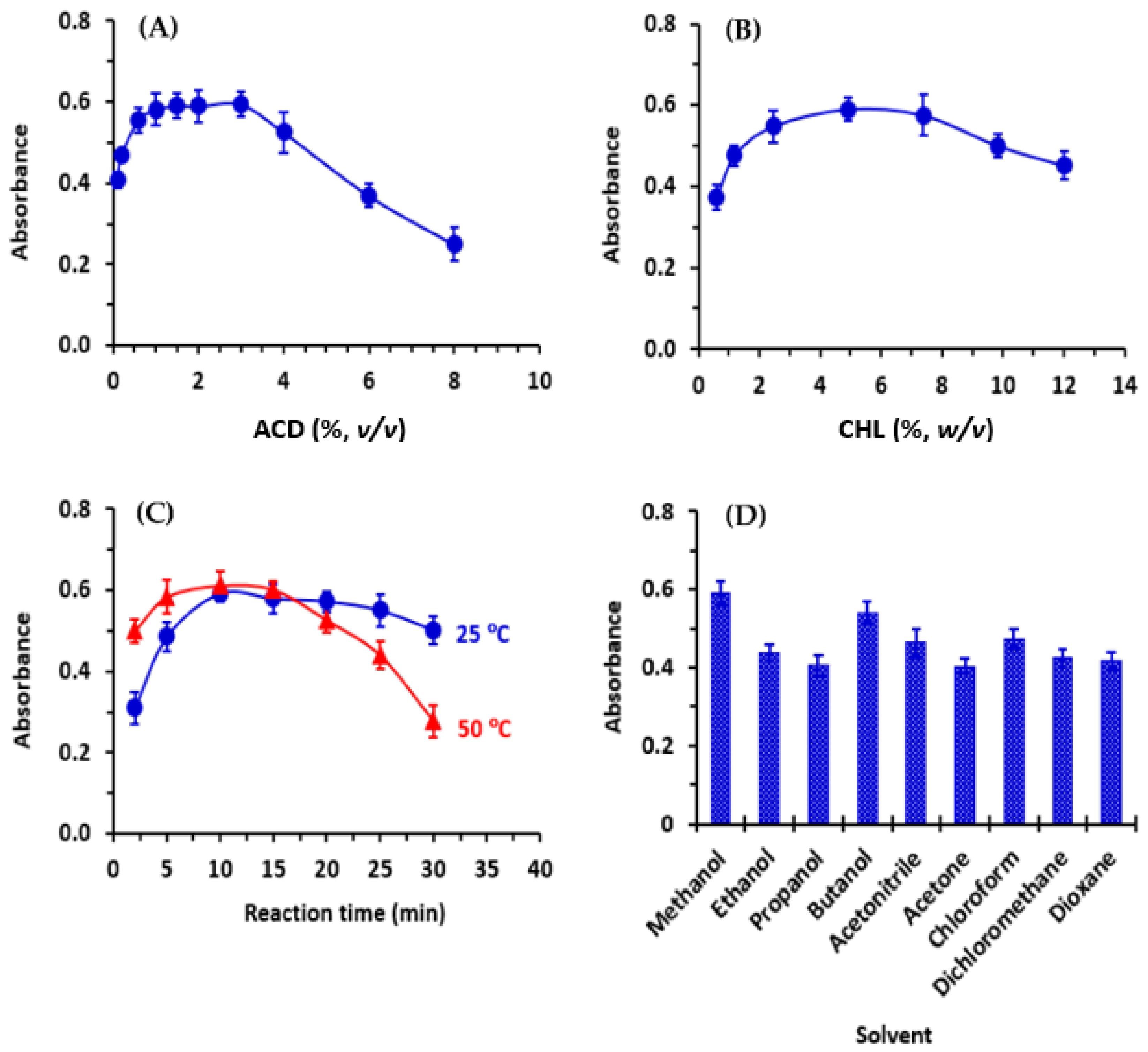
3.4.1. Concentration of ACD
The optimal concentration of ACD was determined by dispensing 50 µL of varying concentrations (0.05–8%, v/v in methanol) into each well of the 96-well plate that already contained 100 µL of the standard CER solution (25 μg/mL). In each well, CHL, BRL, or DCNQ reagent solution (5%, w/v) was added, and the reaction proceeded at room temperature (25 ± 2 °C) for 10 min. The absorbance of the colored mixed solution in each well was measured using the reader at 665 nm, 655 nm, and 580 nm for the reaction mixtures involving CHL, BRL, and DCNQ, respectively. To prepare the blank wells, the same procedure was followed, with the exception that 100 μL of methanol was dispensed into the wells instead of the standard CER solution.
3.4.2. Concentrations of CHL, BRL, and DCNQ
The optimal concentrations of the CHL, BRL, and DCNQ solutions were determined by adding 50 µL of varying concentrations (0.5–12%, w/v) of each reagent solution into each well of the plate containing 100 µL of CER solution (25 μg/mL) and 50 μL of ACD solution (2%, v/v). The plate was further manipulated using the same procedure described in optimizing the concentration of ACD.
3.4.3. Reaction Time and Temperature
The optimal amount of time required for completing the reaction was tested by checking the color development at room temperature (25 ± 2 °C) and at 50 °C, using a kinetic and temperature control feature of the reader. The concentrations of CER, ACD, and haloquinone reagents (CHL, BRL, and DCNQ) were 25 μg/mL, 2% (v/v), and 5% (w/v), respectively.
3.4.4. Solvent
The best solvent for conducting the reactions was selected by executing the reactions (using the above-mentioned CER and reagent concentrations) at room temperature (25 ± 2 °C) in different solvents. These solvents were methanol, ethanol, propanol, butanol, acetonitrile, acetone, chloroform, dichloroethane, and dioxane.
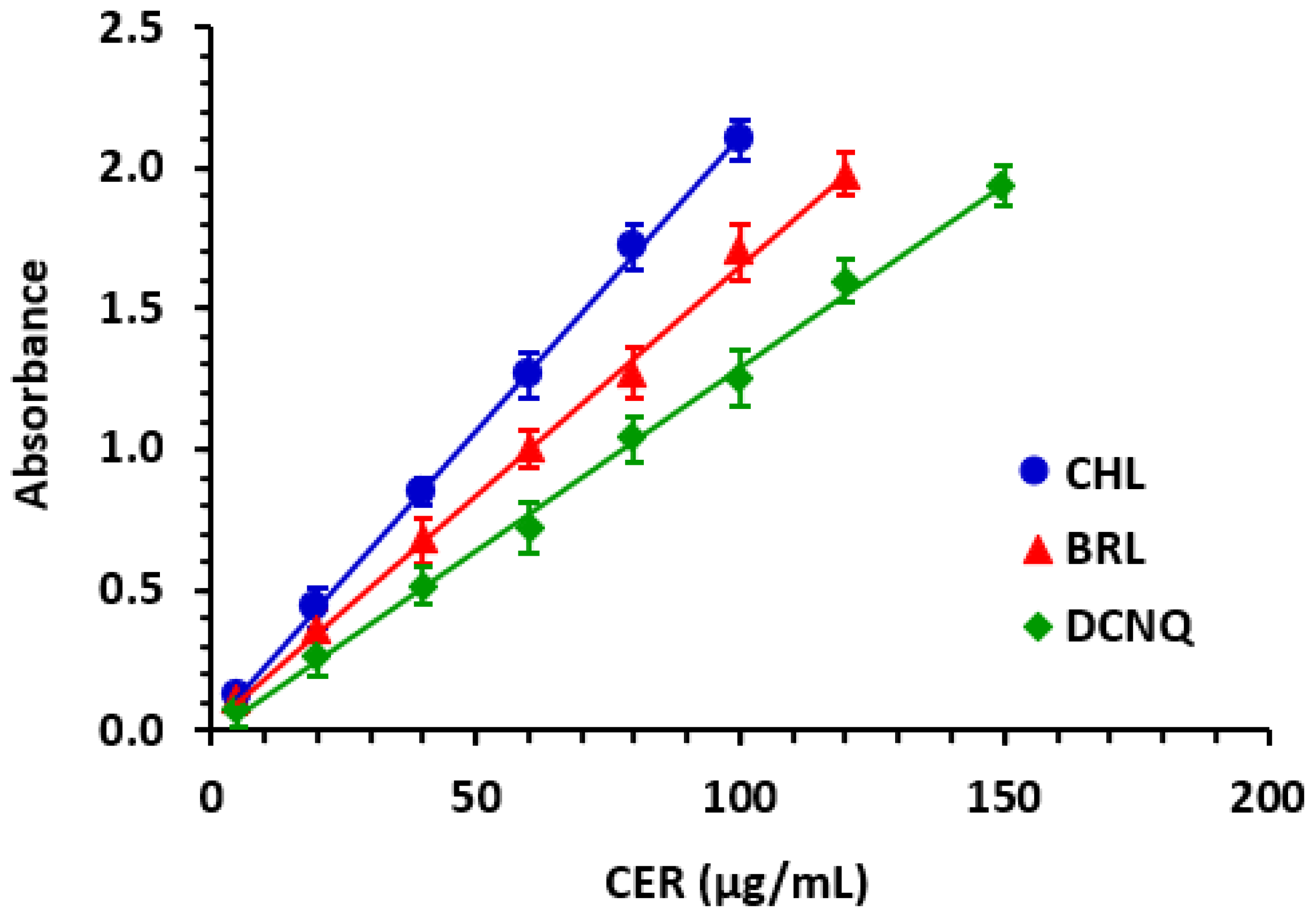
3.5. Procedures Underlying the MW-SPMs and Construction of Calibration Curves
Standard CER solution (100 µL) was transferred into each well of the plate. To each well, 50 μL of ACD solution (2%, v/v), followed by 50 μL of haloquinone reagent (CHL, BRL, or DCNQ) solution (5%, w/v) were added. The reaction proceeded at room temperature (25 ± 2 °C) for 10 min. The reader was then used to measure the absorbance in each well at 665 nm, 655 nm, and 580 nm for reactions with CHL, BRL, and DCNQ, respectively. The blank wells were treated in a similar way, with the exception that 100 μL of methanol was dispensed in each well instead of the standard CER solution. The absorbance readings of the blank wells were subtracted from those of the test solutions. The resulting difference in absorbances was plotted against the corresponding concentrations of CER to generate calibration curves. Regression analysis of the data was performed, and linear fitting equations were generated along with their parameters (intercepts, slopes, and determination coefficients). Capsule sample solutions were analyzed using the same procedures, and the linear fitting equations were utilized to determine the contents of CER in the capsule sample solutions.
3.6. Content Uniformity Testing of Zykadia® Capsules
The procedure outlined in the United States Pharmacopeia (USP) [
31] was employed to conduct content uniformity testing on Zykadia
® capsules. The analysis involved using the proposed MW-SPMs to analyze 10 capsules individually, measuring the content of CER in each individual capsule, as well as determining the average content across the 10 capsules. Subsequently, the acceptance value was calculated and compared to the maximum allowable value specified for content uniformity testing of 10 units of solid dosage form [
31].
4. Conclusions
This study focused on the development and validation of three MW-SPMs for quantifying CER in capsule formulations. These methods involved the formation of colored N-vinylamino-substituted haloquinone derivatives of CER upon its reactions with CHL, BRL, and DCNQ, in the presence of ACD. Transparent 96-well plates were used to conduct the reactions, and the absorbances of the resulting-colored solutions were measured using an absorbance microplate reader. These methods were novel and specifically designed for CER, offering simple procedures that adhered to the requirements of GAC practices in quality control laboratories within the pharmaceutical industry in terms of their eco-friendliness and health safety. These three MW-SPMs were successfully applied to determine the CER content in capsules and perform drug uniformity testing. Additionally, they demonstrated high analytical throughput, allowing an analyst to comfortably analyze approximately 2400 samples per hour. Overall, this work expands the possibilities for the efficient utilization of microwell assays aided using plate readers in drug quantitation. It highlights the practical applications of these methods in the pharmaceutical industry, demonstrating their convenience and efficacy.