The Dynamic Accumulation Rules of Chemical Components during the Medicine Formation Period of Angelica sinensis and Chemometric Classifying Analysis for Different Bolting Times Using ATR-FTIR
Abstract
:1. Introduction
2. Results and Discussion
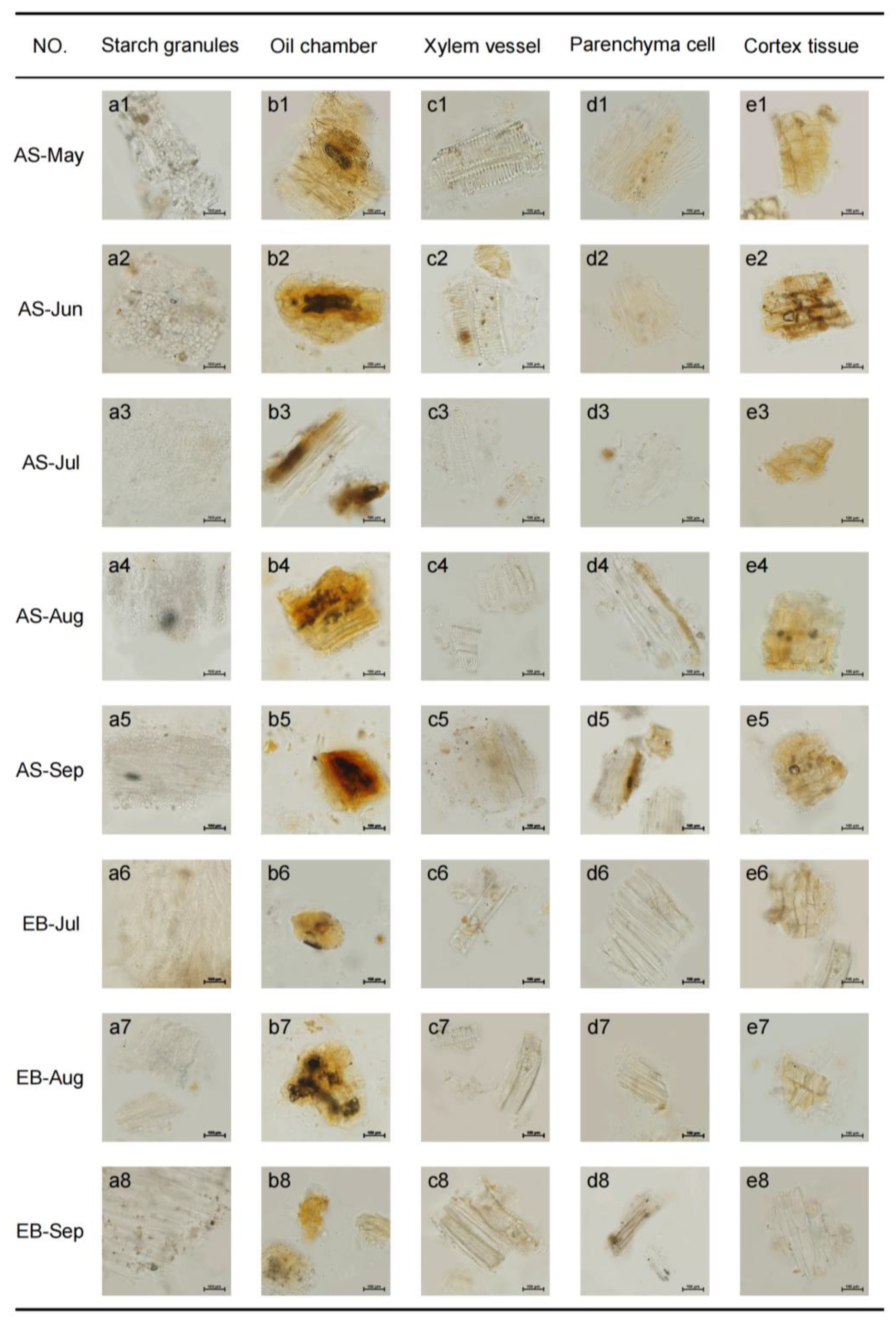
2.1. Microscopic Structure Features of Root Powders during Vegetative Growth Stage
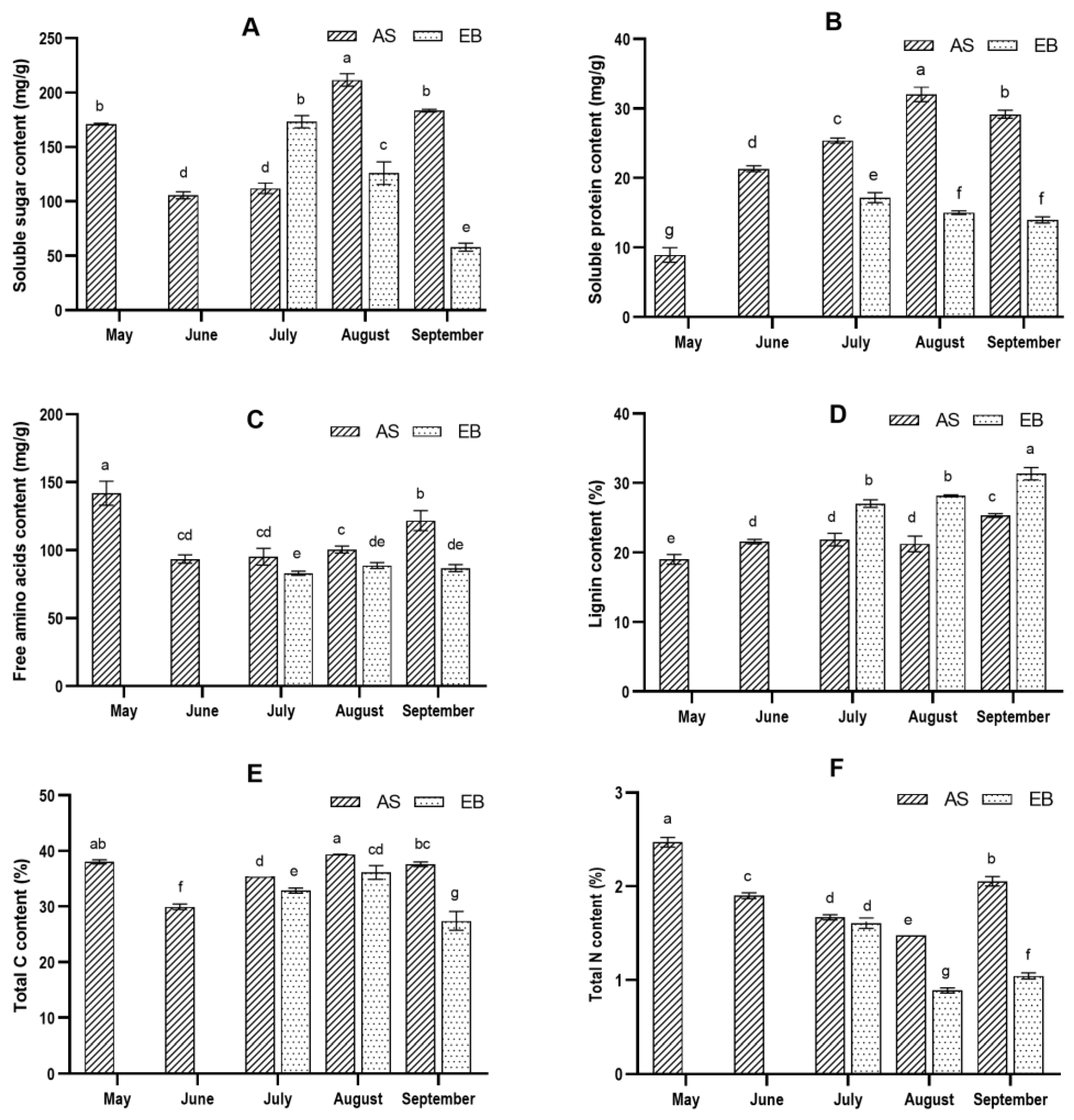
2.2. Accumulation of Nutrient Composition during Vegetative Growth Stage
2.3. Bolting Accompanied by Root Lignification during the Vegetative Growth Stage
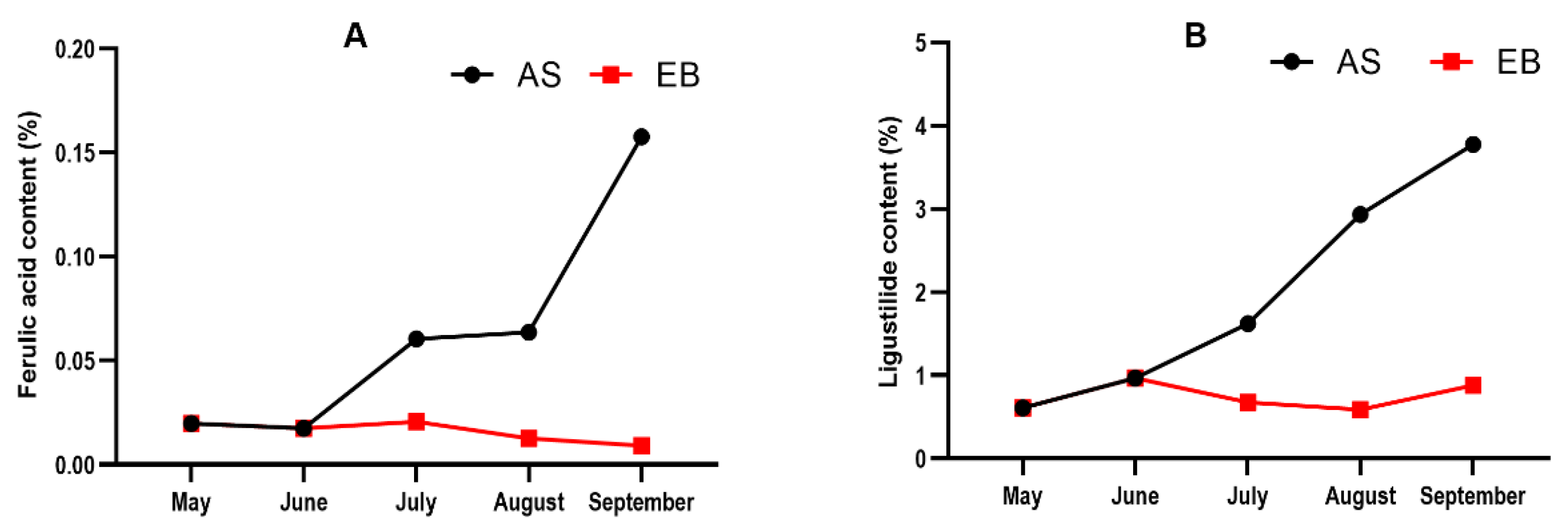
2.4. Bolting Reduces Ferulic Acid and Ligustilide Content during the Vegetative Growth Stage
2.5. Effect of Different Bolting Time on Morphological Characteristics of Root
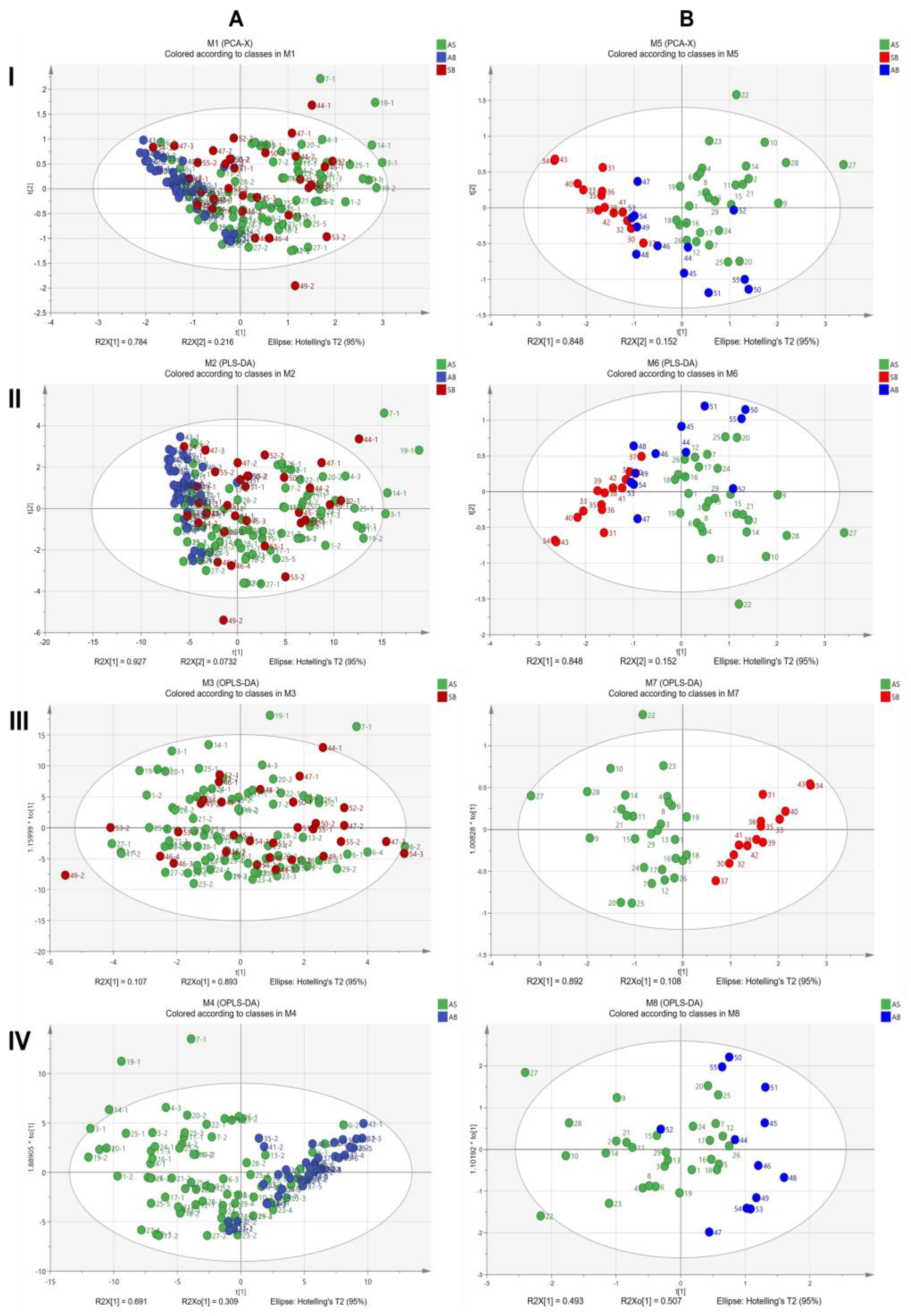
2.6. Effect of Different Bolting Times on Active Ingredient in Root
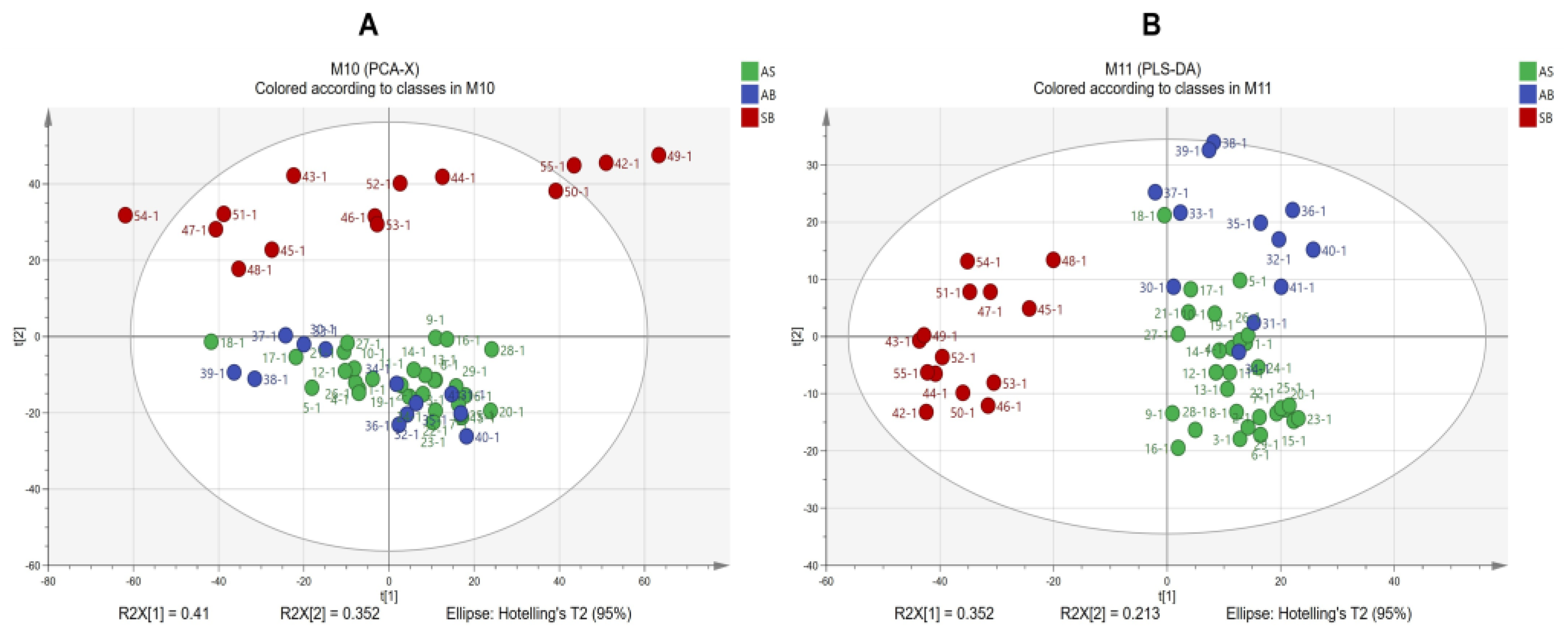
2.7. ATR-FTIR Characteristics of Three Types of Roots in the Harvest Period
3. Materials and Methods
3.1. Plant Materials
3.1.1. Dynamic Materials of A. sinensis during the Vegetative Growth Stage
3.1.2. Different Producing Areas Materials of A. sinensis during the Root Harvest Period
3.2. Microscopic Identification of Powdered Crude Root
3.3. Determination of Soluble Sugar Content
3.4. Determination of Soluble Protein Content
3.5. Extraction in the Identification of Free Amino Acids Contents
3.6. Determination of Total C and Total N Element Content
3.7. Determination of Lignin Content
3.8. Extraction in the Identification of Ferulic Acid and Ligustilide Contents
3.9. ATR-FTIR Spectroscopy
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feng, W.; Liu, P.; Yan, H.; Yu, G.; Zhang, S.; Jiang, S.; Shang, E.; Qian, D.; Duan, J. Investigation of enzymes in the phthalide biosynthetic pathway in angelica sinensis using integrative metabolite profiles and transcriptome analysis. Front. Plant Sci. 2022, 13, 928760. [Google Scholar] [CrossRef]
- Wei, W.; Zeng, R.; Gu, C.; Qu, Y.; Huang, L. Angelica sinensis in China—A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Jin, L.; White, J.F.; Kingsley, K.L.; Gou, W.; Cui, L.; Wang, F.; Wang, Z.; Wu, G. Validation and analysis of the geographical origin of Angelica sinensis (Oliv.) Diels using multi-element and stable isotopes. PeerJ 2021, 9, e11928. [Google Scholar] [CrossRef]
- Hou, C.; Yin, M.; Lan, P.; Wang, H.; Nie, H.; Ji, X. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: Extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 2021, 8, 13. [Google Scholar] [CrossRef]
- Choi, T.J.; Song, J.; Park, H.J.; Kang, S.S.; Lee, S.K. Anti-Inflammatory Activity of Glabralactone, a Coumarin Compound from Angelica sinensis, via Suppression of TRIF-Dependent IRF-3 Signaling and NF-κB Pathways. Mediat. Inflamm. 2022, 2022, 5985255. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, Y.; Hsu, Y.; Huang, C.; Wu, Y.; Hsu, M. Protective effects of the roots of Angelica sinensis on strenuous exercise-induced sports anaemia in rats. J. Ethnopharmacol. 2016, 193, 169–178. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Yang, H.; Mao, L. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity. Int. J. Biol. Macromol. 2016, 88, 44–50. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Tsang, C.; Yip, K.; Yeung, W.; Zhao, Z.; Zhu, S.; Fushimi, H.; Chang, H.; Chen, H. Comprehensive quality evaluation and comparison of Angelica sinensis radix and Angelica acutiloba radix by integrated metabolomics and glycomics. J. Food Drug Anal. 2018, 26, 1122–1137. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohyd. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, H.M.; Lee, K.H.; Kim, K.Y.; Huang, D.S.; Kim, J.H.; Seong, R.S. Quantitative analysis of marker compounds in Angelica gigas, Angelica sinensis, and Angelica acutiloba by HPLC/DAD. Chem. Pharm. Bull. 2015, 63, 504–511. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Lv, Y. In vivo macrophage activation and physicochemical property of the different polysaccharide fractions purified from Angelica sinensis. Carbohyd. Polym. 2008, 71, 372–379. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Bi, W.G.; Yu, Y.; Liao, W.B. Angelica sinensis (Oliv.) Diels in China: Distribution, cultivation, utilization and variation. Genet. Resour. Crop Evol. 2012, 59, 607–613. [Google Scholar] [CrossRef]
- Li, J.; Li, M.L.; Zhu, T.T.; Zhang, X.N.; Li, M.F.; Wei, J.H. Integrated transcriptomics and metabolites at different growth stages reveal the regulation mechanism of bolting and flowering of Angelica sinensis. Plant Biol. 2021, 23, 574–582. [Google Scholar] [CrossRef]
- Gao, X.; Guo, F.; Chen, Y.; Bai, G.; Liu, Y.; Jin, J.; Wang, Q. Full-length transcriptome analysis provides new insights into the early bolting occurrence in medicinal Angelica sinensis. Sci. Rep. 2021, 11, 13000. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Li, C.; Wang, Y.; Xu, L.; Muleke, E.M.; Tang, M.; Sun, X.; Liu, L. Transcriptomic Analysis Identifies Differentially Expressed Genes (DEGs) Associated with Bolting and Flowering in Radish (Raphanus sativus L.). Front. Plant Sci. 2016, 7, 682. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Cheng, D.; Liu, Q.; Cui, J.; Luo, C. Difference of proteomics vernalization-induced in bolting and flowering transitions of Beta vulgaris. Plant Physiol. Biochem. 2018, 123, 222–232. [Google Scholar] [CrossRef]
- Hernandez, R.; Spalholz, H. Characterization of solar radiation spectral contribution in lettuce bolting and flowering using LEDs in an indoor setting. Hortscience 2019, 54, S91–S92. [Google Scholar]
- Dong, H.; Li, M.; Jin, L.; Xie, X.; Li, M.; Wei, J. Cool temperature enhances growth, ferulic acid and flavonoid biosynthesis while inhibiting polysaccharide biosynthesis in angelica sinensis. Molecules 2022, 27, 320. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Li, M.; Wei, J. Depicting precise temperature and duration of vernalization and inhibiting early bolting and flowering of angelica sinensis by freezing storage. Front. Plant Sci. 2022, 13, 853444. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Li, M.; Wei, J. Transcriptomic Analysis Reveals LncRNAs Associated with Flowering of Angelica sinensis during vernalization. Curr. Issues Mol. Biol. 2022, 44, 1867–1888. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Xiao, Y.; Zhou, G. Early Bolting, Yield, and Quality of Angelica sinensis (Oliv.) Diels Responses to Intercropping Patterns. Plants 2022, 11, 2950. [Google Scholar] [CrossRef]
- Mei, Y.; Yao, Y.; Wang, X.; Zhang, T.; Zuo, X.; Zhang, G. Exploring the quality markers of the production region of Ligusticum chuanxiong through antiplatelet aggregation bioactivity and multiple active components, and correlation analysis of the quality markers and geographical factors. Ind. Crop. Prod. 2023, 200, 116845. [Google Scholar] [CrossRef]
- Kong, Y.; Qi, Y.; Cui, N.; Zhang, Z.; Wei, N.; Wang, C.; Zeng, Y.; Sun, Y.; Kuang, H.; Wang, Q. The traditional herb Polygonum hydropiper from China: A comprehensive review on phytochemistry, pharmacological activities and applications. Pharm. Biol. 2023, 61, 799–814. [Google Scholar] [CrossRef]
- Tian, S.; Guo, L.; Song, Y.; Miao, J.; Peng, M.; Fang, X.; Bai, M.; Miao, M. Transcriptomic analysis the mechanisms of anti-osteoporosis of desert-living Cistanche herb in ovariectomized rats of postmenopausal osteoporosis. Funct. Integr. Genom. 2023, 23, 237. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, X.; Jin, L.; Li, M.; Wei, J. Bolting reduces ferulic acid and flavonoid biosynthesis and induces root lignification in Angelica sinensis. Plant Physiol. Biochem. 2022, 170, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, M.; Wang, L.; Li, M.; Wei, J. Apiaceae medicinal plants in China: A review of traditional uses, phytochemistry, bolting and flowering (BF), and BF control methods. Molecules 2023, 28, 4384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Zhao, Y.; Jin, Y.; Zhou, J.; Huang, L.; Zha, L.; Yuan, Y. Microscopic characterization of five Artemisia crude herbs using light microscopy, scanning electron microscopy, and microscopic quantitative analysis. Microsc. Res. Tech. 2022, 85, 2428–2437. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Zhou, Q.; Lu, G.; Chan, K. Application of mid-infrared spectroscopy in the quality control of traditional Chinese medicines. Planta Med. 2010, 76, 1987–1996. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Chen, Y.; Ji, F.; Li, X.; Yang, J.; Hong, S.; Zhu, Z.; Zang, Y. Optimization of near-infrared reflectance models in determining flavonoid composition of okra (Abelmoschus esculentus L.) pods. Food Chem. 2023, 418, 135953. [Google Scholar] [CrossRef]
- Pan, M.; Sun, S.; Zhou, Q.; Chen, J. A simple and portable screening method for adulterated olive oils using the Hand-Held FTIR spectrometer and chemometrics tools. J. Food Sci. 2018, 83, 1605–1612. [Google Scholar] [CrossRef]
- Ma, F.; Chen, J.; Wu, X.; Zhou, Q.; Sun, S. Rapid discrimination of Panax notogeinseng of different grades by FT-IR and 2DCOS-IR. J. Mol. Struct. 2016, 1124, 131–137. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, J.; Zhang, G.; Rong, L.; Wu, H.; Sun, S.; Guo, Y.; Yang, Y.; Lu, L.; Qu, L. Analysis and identification of two similar traditional Chinese medicines by using a three-stage infrared spectroscopy: Ligusticum chuanxiong, Angelica Sinensis and their different extracts. J. Mol. Struct. 2016, 1124, 164–172. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Anguebes, F.; Pat, L.; Ali, B.; Guerrero, A.; Cordova, A.V.; Abatal, M.; Garduza, J.P. Application of multivariable analysis and FTIR-ATR spectroscopy to the prediction of properties in campeche honey. J. Anal. Methods Chem. 2016, 2016, 5427526. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.F.; Zuo, Z.T.; Zhang, Q.Z.; Wang, Y.Z. Data Fusion of Fourier Transform Mid-Infrared (MIR) and Near-Infrared (NIR) Spectroscopies to Identify Geographical Origin of Wild Paris Polyphylla var. Yunnanensis. Molecules 2019, 24, 2559. [Google Scholar] [CrossRef]
- Kajino, A.; Bai, W.; Yoshimura, N.; Takayanagi, M. Identification of peach and apricot kernels for traditional Chinese medicines using near-infrared spectroscopy. Vib. Spectrosc. 2021, 113, 103202. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.T.; Wang, Y.Z. Identification of geographical origin and different parts of Wolfiporia cocos from Yunnan in China using PLS–DA and Res Net based on FT–NIR. Phytochem. Anal. 2022, 33, 792–808. [Google Scholar] [CrossRef]
- Guo, X.; Cai, R.; Wang, S.; Tang, B.; Li, Y.; Zhao, W. Non-destructive geographical traceability of sea cucumber (Apostichopus japonicus) using near-infrared spectroscopy combined with chemometric methods. R. Soc. Open Sci. 2018, 5, 170714. [Google Scholar] [CrossRef]
- Dunn, M.F. Key roles of microsymbiont amino acid metabolism in rhizobia-legume interactions. Crit. Rev. Microbiol. 2015, 41, 411–451. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.X.; Lyu, X.K.; Chen, T.Y.; Chen, J.; Chen, X.; Guo, S.X. Utilization of functional agro-waste residues for oyster mushroom production: Nutritions and active ingredients in healthcare. Front. Plant Sci. 2023, 13, 1085022. [Google Scholar] [CrossRef]
- Atkinson, R.R.L.; Burrell, M.; Osborne, C.P.; Rose, K.; Rees, M. A non-targeted metabolomics approach to quantifying differences in root storage between fast- and slow-growing plants. New Phytol. 2012, 196, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Ferrarese, M.D.; Siqueira, R.D.; Böhm, F.M.L.; Ferrarese-Filho, O. L-DOPA increases lignification associated with Glycine max root growth-inhibition. J. Chem. Ecol. 2007, 33, 265–275. [Google Scholar] [CrossRef]
- Chang, F.; Yu, D.; Wang, H.; Qu, S.; Wang, D.; Liu, X.; Pan, Y. Authentication of Saposhnikovia divaricata (Trucz.) Schischk and its two adulterants based on their macroscopic morphology and microscopic characteristics. Microsc. Res. Tech. 2021, 84, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Li, Q.; Yang, H.; Chen, H.; Zhao, Z.; Li, P. Identification of powdered Chinese herbal medicines by fluorescence microscopy, part 1: Fluorescent characteristics of mechanical tissues, conducting tissues, and ergastic substances. Microsc. Res. Tech. 2011, 74, 269–280. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Jiang, Y.; Li, B.; Zeng, Y.; Shang, H.; Wang, F.; Sun, Z. The Dynamic Accumulation Rules of Chemical Components during the Medicine Formation Period of Angelica sinensis and Chemometric Classifying Analysis for Different Bolting Times Using ATR-FTIR. Molecules 2023, 28, 7292. https://doi.org/10.3390/molecules28217292
Ma F, Jiang Y, Li B, Zeng Y, Shang H, Wang F, Sun Z. The Dynamic Accumulation Rules of Chemical Components during the Medicine Formation Period of Angelica sinensis and Chemometric Classifying Analysis for Different Bolting Times Using ATR-FTIR. Molecules. 2023; 28(21):7292. https://doi.org/10.3390/molecules28217292
Chicago/Turabian StyleMa, Fang, Yuan Jiang, Baoshan Li, Yuxin Zeng, Hushan Shang, Fusheng Wang, and Zhirong Sun. 2023. "The Dynamic Accumulation Rules of Chemical Components during the Medicine Formation Period of Angelica sinensis and Chemometric Classifying Analysis for Different Bolting Times Using ATR-FTIR" Molecules 28, no. 21: 7292. https://doi.org/10.3390/molecules28217292
APA StyleMa, F., Jiang, Y., Li, B., Zeng, Y., Shang, H., Wang, F., & Sun, Z. (2023). The Dynamic Accumulation Rules of Chemical Components during the Medicine Formation Period of Angelica sinensis and Chemometric Classifying Analysis for Different Bolting Times Using ATR-FTIR. Molecules, 28(21), 7292. https://doi.org/10.3390/molecules28217292



